Abstract
• Background and Aims Ovary abortion can occur in maize (Zea mays) if water deficits lower the water potential (ψw) sufficiently to inhibit photosynthesis around the time of pollination. The abortion decreases kernel number. The present work explored the activity of ovary acid invertases and their genes, together with other genes for sucrose-processing enzymes, when this kind of abortion occurred. Cytological evidence suggested that senescence may have been initiated after 2 or 3 d of low ψw, and the expression of some likely senescence genes was also determined.
• Methods Ovary abortion was assessed at kernel maturity. Acid invertase activities were localized in vivo and in situ. Time courses for mRNA abundance were measured with real time PCR. Sucrose was fed to the stems to vary the sugar flux.
• Key Results Many kernels developed in controls but most aborted when ψw became low. Ovary invertase was active in controls but severely inhibited at low ψw for cell wall-bound forms in vivo and soluble forms in situ. All ovary genes for sucrose processing enzymes were rapidly down-regulated at low ψw except for a gene for invertase inhibitor peptide that appeared to be constitutively expressed. Some ovary genes for senescence were subsequently up-regulated (RIP2 and PLD1). In some genes, these regulatory changes were reversed by feeding sucrose to the stems. Abortion was partially prevented by feeding sucrose.
• Conclusions A general response to low ψw in maize ovaries was an early down-regulation of genes for sucrose processing enzymes followed by up-regulation of some genes involved in senescence. Because some of these genes were sucrose responsive, the partial prevention of abortion with sucrose feeding may have been caused in part by the differential sugar-responsiveness of these genes. The late up-regulation of senescence genes may have caused the irreversibility of abortion.
Key words: Zea mays, abortion, water potential, gene expression, senescence, sucrose, invertase, pollination, ovary development, sugar-responsive genes, ribosome inactivating protein, phospholipase D
INTRODUCTION
Increasing evidence indicates that ovary abortion can account for substantial kernel losses when maize experiences low water potentials (low ψw) around the time of pollination (Claassen and Shaw, 1966; Westgate and Boyer, 1985, 1986; Boyle et al., 1991; Zinselmeier et al., 1995a, b, 1999; Andersen et al., 2002). The abortion can be caused by only 2 or 3 d of ψw low enough to inhibit photosynthesis (Westgate and Boyer, 1986). Feeding sucrose to the stems at low ψw can prevent many ovaries from aborting (Boyle et al., 1991; Zinselmeier et al., 1995a, 1999). Sufficient sucrose must be fed to replace the photosynthate missing during the exposure to low ψw.
In addition to changes in photosynthetic activity, many other metabolic changes occur at low ψw, and large numbers of enzymes exhibit altered activities (Kramer and Boyer, 1995). In maize, hundreds of genes are altered in expression when low ψw occurs during pollination and early kernel development (Zinselmeier et al., 2002; Yu and Setter, 2003). Some of the altered expression could be responsible for the metabolic and enzymatic changes taking place. The genes coding for invertases are candidates because Zinselmeier et al. (1995b, 1999) implicated acid invertases as possible enzymes limiting the flow of carbon into the developing ovaries and kernels at low ψw. In support of this finding, McLaughlin and Boyer (2004) reported that glucose, an immediate product of sucrose hydrolysis by invertase, was depleted in ovaries undergoing abortion at low ψw.
The prevention of abortion with sucrose (Boyle et al., 1991; Zinselmeier et al., 1995a, 1999) suggests that certain abortion-inducing genes could be sugar-responsive. Koch (1996) and Sheen et al. (1999) identified a number of sugar-responsive genes in plants. Therefore, the following experiments were undertaken to determine whether the transcriptional activity of invertase genes and other sucrose-processing genes were sugar-responsive when maize was subjected to an abortion-inducing water deficit. A search also was made for events that might contribute to the irreversibility of abortion.
MATERIALS AND METHODS
Plant material and growth conditions
This study is part of a larger one using the same maize plants (Zea mays L. ‘DE2 X H99’) and thus identical conditions as in McLaughlin and Boyer (2004). The plants were grown in soil in controlled environments at the plant density used in agricultural conditions. All plants were maintained at high ψw except for a short period during flowering. At the appearance of the first silks, the soil was brought to field capacity with water. Water was withheld from some of the plants for 6 d, causing low ψw. Water was then re-supplied. The treatments were low ψw for one-third of the plants, low ψw + sucrose infusion for one-third of the plants, and high ψw for the remaining one-third of the plants. Leaf ψw and net photosynthesis were measured on the unshaded third leaf from the top of the plant and are reported in McLaughlin and Boyer (2004). During the 6-d treatments, plants were hand-pollinated on the fifth day. According to this schedule, the first day of silking was designated day −5 before pollination, the day of pollination was day 0, and the day of rewatering the plants at low ψw was day 1 following pollination.
Stem infusions
Sucrose from sugarcane (0·438 m, ψS = −1·1 MPa) was infused into the stems of the plants in the treatment designated low ψw + sucrose infusion according to McLaughlin and Boyer (2004). Starting on day −4, the infusion was carried out at one internode each day. The plants received between 35 and 45 mL of sucrose solution (5·3–6·8 g of sucrose) each day, which was sufficient to completely replace that normally produced by photosynthesis (Jurgens et al., 1978).
In vivo and in situ invertase localization
Activities of insoluble (cell wall-bound) and soluble invertases (cytoplasmic) were localized as in McLaughlin and Boyer (2004).
Evans Blue assay
The cell membrane integrity in developing ovary tissue was tested with Evans Blue (Gaff and Okong 'O-Ogola, 1971; Young et al., 1997). A 0·1 % (w/v) solution of the stain (Evans Blue; Sigma E-2129) was prepared in water. Each ovary was cut in half longitudinally and the cut surface placed face down in a glass Petri dish containing the stain. After incubating for 2 min at room temperature, the sections were washed with water for 10 min, fixed in a 4 % solution of formaldehyde, viewed under a dissecting microscope, and imaged with a digital camera (Nikon CoolPix 990). Cells with blue stain were considered to have lost integrity of the plasma membrane.
RNA extraction and quantification
Ovary or young kernel tissues (including both the pedicel and ovary) were harvested swiftly, placed in cold 2-mL cyrotubes (Corning, Corning, NY, USA), submerged in liquid N2, and stored at −80 °C. From about 500 mg (f. wt) of these tissues, RNA, DNA and protein were extracted with TRI Reagent (Molecular Research Center, Cincinnati, OH, USA) (Chomczynski and Sacchi, 1987) by grinding in liquid nitrogen (mortar and pestle), placing into 6 mL of cold TRI Reagent, further homogenizing to completion with a Brinkmann homogenizer (Brinkmann Instruments, Westbury, NY, USA), and centrifuging at 12 000 g for 10 min at 4 °C. As recommended by the protocol, 1-bromo-3-chloropropane (BCP) was used as a replacement for chloroform in the phase separation stage of the extraction.
In addition, 0·8 m Na citrate and 1·2 m NaCl were added to the isopropanol RNA precipitation step (for every 2·5 mL of RNA supernatant isolated, 1·25 mL of isopropanol and 1·25 mL of the high salt solution were added) to remove additional polysaccharides and proteoglycans (Chomczynski and Mackey, 1995). The RNA pellet was cleaned twice by adding 8 mL of 70 % ethanol, vortexing, and centrifuging at 7500 g. The RNA pellet was re-suspended in diethyl pyrocarbonate-treated water. Residual genomic DNA was removed by incubating the RNA solution with DNase I obtained from the DNA-free™ kit (Ambion, Austin, TX, USA). The DNase enzyme was subsequently removed by the addition of a DNase inactivation binding agent according to the manufacturer's instructions. The quality of the RNA was assessed from the integrity of the 16S and 23S rRNA bands on a formaldehyde-agarose gel.
Total maize RNA was quantified against a standard curve of total yeast RNA (Worthington Biochemical Corp., Lakewood, NJ, USA) using a fluorescence-based assay developed by Schmidt and Ernst (1995). From the solution of total maize RNA (10 μL RNA extract in 490 μL of 10 mm Tris, 1 mm EDTA, pH 8·0), 10 μL was added to 990 μL of 60 μm SYBR Green II (Molecular Probes, Inc., Eugene, OR, USA) in 10 mm Tris, 1 mm EDTA, pH 8·0. The assay was linear from <10 ng to 1000 ng total RNA using SYBR Green II at this concentration. The assay was quantified using a Shimadzu RF-1501 spectrofluorophotometer (Shimadzu, Kyoto, Japan) with excitation at 468 nm and emission at 525 nm.
Reverse transcription
First strand cDNAs were synthesized from 10 µg of total RNA using gene-specific primers (Table 1; 100 ng per 50 μL reaction) and Stratascript reverse transcriptase (Stratagene, La Jolla, CA, USA) according to the manufacturer's instructions. The first strand cDNA product was treated with RNase H (Promega, Madison, WI, USA). The cDNA samples were pipetted into separate tubes and stored at −80 °C.
Table 1.
Primers and GenBank accessions for genes investigated in the present study
| Gene |
Symbol |
GenBank gene |
GenBank mRNA |
Forward primer (seq. 5′–3′) |
Reverse primer (seq. 5′–3′) |
|---|---|---|---|---|---|
| Cell-wall acid invertase 1 (short) | Incw1 (S) | AF050129 | U17695 | CCGTCTTCTTCAGGGTGTTC | CGTAGAGGTGAGCGTCCTTC |
| Cell-wall acid invertase 1 (long) | Incw1 (L) | AF050129 | U17695 | CCGTCTTCTTCAGGGTGTTC | AGCGTAAACAACATGCTCAAG |
| Cell-wall acid invertase 2 | Incw2 | AF050128 | AF050631 | CTAAAGGCTGGGCTGGAAT | GTGACCGACTTTGCTCTCAG |
| Soluble acid invertase 1 | Ivr1 | U16123 | AF171874 | GTGCTCCTGCTCGTCCTC | AGCCAACAACTTACCGTTCG |
| Soluble acid invertase 2 | Ivr2 | AAA74584 (protein) | U31451 | ACGACCGCCACGACTACTAC | GCCCATCCCTTGGACAC |
| Invertase inhibitor 1 | Zminh1 | AX21433 | TTGGATTTGCATTGTCAGTCAG | CTTGTAGGACGGAAGCGTTG | |
| Sucrose synthase 1 (Sh1) | SS1 | X02382 | X02400 | CACGACGATGATGTTGAATGAC | CGAGAAGCAAGTGGAGTGTGTC |
| Sucrose synthase 2 (Sus1) | SS2 | L33244 | L22296 | CCTGTCCATCTACTTCCCGTA | GATTGGCTTGTTCCTGTCGT |
| Ribosome-inactivating protein 2 | RIP2 | AJ300265 | AF233881 | GGACCTCATCGGCAGTAAG | CTCTTCTCCTCGGCTTTGG |
| Bifunctional nuclease 1 | ZmBFN1 | AY105439/AI881514 | CTGACGGAGAGCCTGATGTT | CGTGATGGAGGTTTGCTTT | |
| Cysteine protease 1 | CCP1 | BAA08244 (protein) | D45402 | GTCTCTACCGTGTCCGCAGT | TTACAGCAAGCATCCCATTG |
| Phospholipase D | PLD1 | BAA11135 (protein) | D73410 | CGAGGAGGACGAGACGAG | CGCAGTAGATGTGGAAGGAC |
All sequences are from maize.
Preparation of oligonucleotides
Gene specific primers were designed using Primer3 (Rozen and Skaletsky, 1998) from selected gene sequences obtained from GenBank (Table 1). When both genomic and cDNA sequence information were available for the gene of interest, primers were designed to include an intron to help distinguish between genomic DNA and cDNA following PCR amplification. All primers were synthesized by Qiagen (Valencia, CA, USA).
Real-time quantitative PCR
Gene-specific mRNA quantification was performed by real-time quantitative PCR using the ABI 7700 Sequence Detection System (PE Applied Biosystems, Foster City, CA, USA). SYBR Green chemistry was used for cDNA quantification using the SYBR Green Mastermix (PE Applied Biosystems) as recommended by the manufacturer. Along with the SYBR Green Master Mix, the PCR mixture contained 200 nm forward and reverse gene-specific primers (Table 1), 5 μL of a 1 : 10 dilution of the cDNA, and water to a total volume of 25 μL. A standard curve was developed as a reference by using a serial dilution of a day 2, high ψw sample. The serial dilution consisted of 0·2, 0·1, 0·05, 0·0125 and 0·00625 dilutions of the original cDNA first strand preparation. All cycles began with a step for 10 min at 95 °C, followed by 40 cycles of 5 min at 95 °C and 1 min at 60 °C. Following the final cycle of PCR, the reactions were heat denatured in a thermal gradient from 60 °C to 95 °C at 0·03 °C/s to check for the purity of the amplified product. In some runs, this gradient was narrowed to reveal more detail about each individual peak. In addition, PCR amplification products were checked for purity in a 2 % agarose gel by electrophoresis that detected contaminating primer-dimers or amplified genomic DNA.
Mean Ct values were compared with the standard curve obtained from the serial dilution series and used to estimate the relative transcript copy number. For the standard curve, a linear regression equation for each PCR run was developed between the log concentration of the serial dilution series (x-axis) and the associated mean Ct values (y-axis). This regression equation was used to convert Ct values of the unknowns to relative mRNA concentrations. The Ct value represented the amplicon accumulation within a reaction, i.e. the copy number of cDNA and thus the amount of mRNA originally present in the tissue extract for a specific gene. By comparing the Ct values, the relative differences in mRNA abundance was determined (Morrison et al., 1998). The relative mRNA abundance calculated by this method was set to unity on day −5 before treatments were imposed. Every sample from the subsequent treatments was compared with this initial abundance.
Ampilicon sequencing
In addition to checking the estimated amplicon base-pair size by electrophoresis and gel separation, the fidelity of the PCR products was verified by dideoxy sequencing. Amplicons from the quantitative PCR experiments were prepared for sequencing using the QIAprep Spin Miniprep Kit (Qiagen) according to the manufacturer's instructions. Labelling and sequencing of the amplicons were performed using the ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction Kit (Perkin Elmer, Norwalk, CT, USA) and the ABI 310 automatic DNA sequencer (Perkin Elmer).
RESULTS
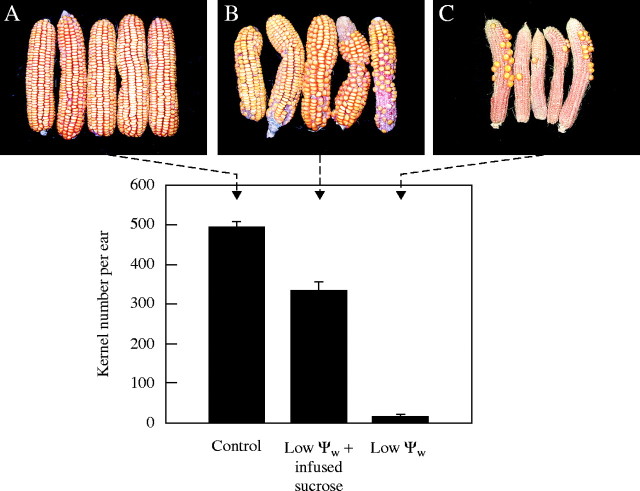
McLaughlin and Boyer (2004) reported that the controls had high ψw throughout their life cycle, generally −0·45 MPa during mid-day. At maturity, they had fully formed ears with approx. 500 kernels per ear (Fig. 1, Control). When water was withheld for 6 d starting on day −5, leaf ψw decreased until it reached −1·45 MPa on day 0 (pollination). Photosynthesis and plant water use were severely inhibited. The plants were re-watered on day 1 (allowing 1 d for pollen tubes to grow and fertilize the egg and polar nuclei at low ψw), and ψw recovered to the control level by day 2. Under these conditions, nearly all of the kernels failed to develop (Fig. 1, Low ψw). Westgate and Boyer (1986) found undeveloped embryos and endosperm in each floret after a similar treatment, indicating that reproduction had been completed in every other respect. If sucrose was fed to the stems, starting on day −4 and continuing each day through day 0, nearly 68 % of the kernels developed normally (Fig. 1, Low ψw + sucrose infusion).
Fig. 1.
Ear development and kernel numbers at maturity in maize subjected to low ψw around the time of pollination. (A) Controls at high ψw, (B) low ψw plus sucrose infused into stems, (C) low ψw. Ears are somewhat crooked because ovaries were sampled in mid-ear around the time of pollination, which caused slight deformation at maturity. Sampled areas are on back of ear and not visible. Histogram indicates means ± s.e. for 9–11 plants assuming development in sampled areas was similar to that in unsampled areas.
Ovary invertase activity
There is evidence that acid invertases are inhibited in maize ovaries at low ψw (Zinselmeier et al., 1995b, 1999; Andersen et al., 2002). The inhibition is observed in vitro in both the insoluble, cell wall-bound form of the enzyme and in a soluble form thought to reside in the cytoplasm (Doehlert and Felker, 1987; Sturm and Chrispeels, 1990). Maize produces an inhibitor peptide (Jaynes and Nelson, 1971; Bate et al., 2004) that could contribute to the inhibition in vitro (Pressey, 1967; Bate et al., 2004). Therefore, the enzyme activity was measured in vivo in tissue slices for the cell wall-bound form according to the method of McLaughlin and Boyer (2004). The in vivo assay detected activity only outside of the plasma membrane. Because the assay was preceded by an extensive water rinse, the activity was bound to the cell walls. This form of invertase was present in the upper pedicel tissue but not the nucellus (Fig. 2A–D). Activity increased as the ovaries developed but at low ψw, no activity was detected, regardless of development (Fig. 2H–K). If the 1-h incubation time was extended to as much as 24 h, activity was sometimes detected at low ψw (data not shown), indicating that the assay was active but cell wall-bound invertase was very inactive. If sucrose was fed to the stems at low ψw, activity recovered partially but was more variable than in controls (Fig. 2F and G), probably because some ovaries were going to develop normally (68 %, Fig. 1) and others were not (32 %). Figure 2L indicates that the in vitro assays of cell wall-bound invertase from Zinselmeier et al. (1999) compared closely with the in vivo assays in Fig. 2A–K.
Fig. 2.
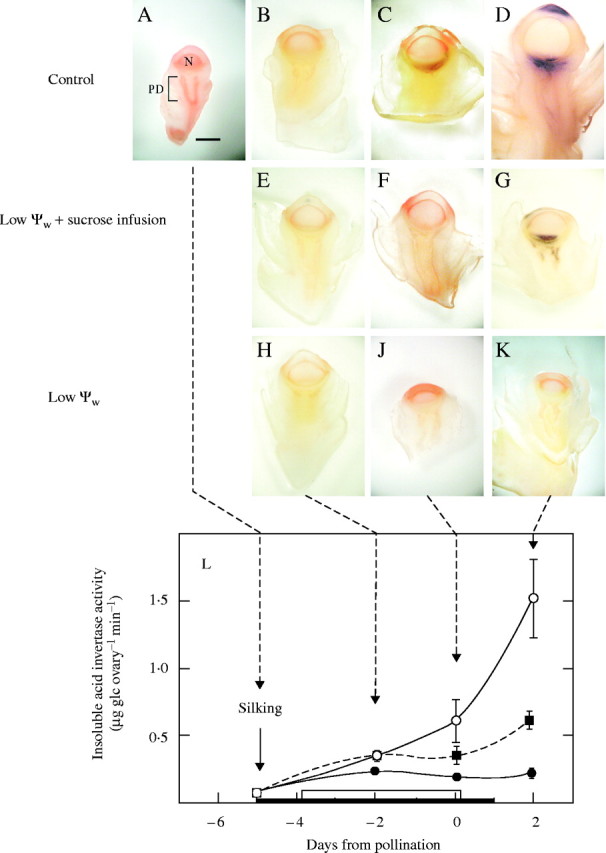
Cell wall-bound acid invertase activity in vivo in maize ovaries when low ψw occurred around the time of pollination for the ears in Fig. 1. Invertase shown as dark areas. (A–D) Control: high ψw. Activity increased from (A) to (D) and was located in upper pedicel (PD) tissue below nucellus (N). Ovaries became substantially larger at later times. (E–G) Low ψw + sucrose infusion: images show less activity than in controls, barely visible in (F) and apparent in (G). Activity in (G) was visible in two out of every three samples. (H–K) Low ψw: no activity was detected. No enlargement of ovaries. Scale bar = 1 mm. (L) For comparison with images, in vitro assays are shown for cell wall-bound acid invertase from Zinselmeier et al. (1999). Conditions for (L) were identical to those for (A–K) except for maize genotype. Black bar on abscissa indicates water was withheld on day −5 and resupplied on day 1. White bar on abscissa indicates sucrose was infused into stem each day beginning on day −4 and continuing through day 0.
The soluble form of acid invertase was detected with the same assay but in situ after a freezing and thawing pretreatment. As described by McLaughlin and Boyer (2004), the assay relied on the entrapment of the large soluble enzyme inside the cells after freezing/thawing. The freeze/thaw treatment allowed small solutes to diffuse from the cell interior into the walls, and the salts included in the solute appeared to release the cell wall-bound form of the enzyme, which was washed away during the subsequent rinsing in preparation for the assays. The soluble activity remained in the tissue and was in the nucellus rather than the pedicel in controls (Fig. 3A–D). Because of the hand sections, the cup-shaped upper pedicel tended to overlap the nucellus, blurring the difference between the tissues, but occasional sections clearly distinguished the two kinds of tissues, as shown by McLaughlin and Boyer (2004), confirming that the soluble activity was restricted to the nucellus. The activity increased markedly with ovary development, so much so by day 2 (Fig. 3D) that the assay product spread over the tissue surface and the test tube walls, becoming very nonspecific for location as discussed in McLaughlin and Boyer (2004). A shorter assay on day 2 limited the spreading and indicated that the enzyme activity was restricted to the nucellus as in Fig. 3C (data not shown). As with the insoluble activity in Fig. 2, soluble activity was lacking at low ψw (Fig. 3H–K) but was present in sucrose-fed plants, although more variable than in controls or at low ψw (Fig. 3E–G). There was a close relationship between the in vitro activity reported by Zinselmeier et al. (1999) shown in Fig. 3L and the in situ activities in Fig. 3A–K.
Fig. 3.
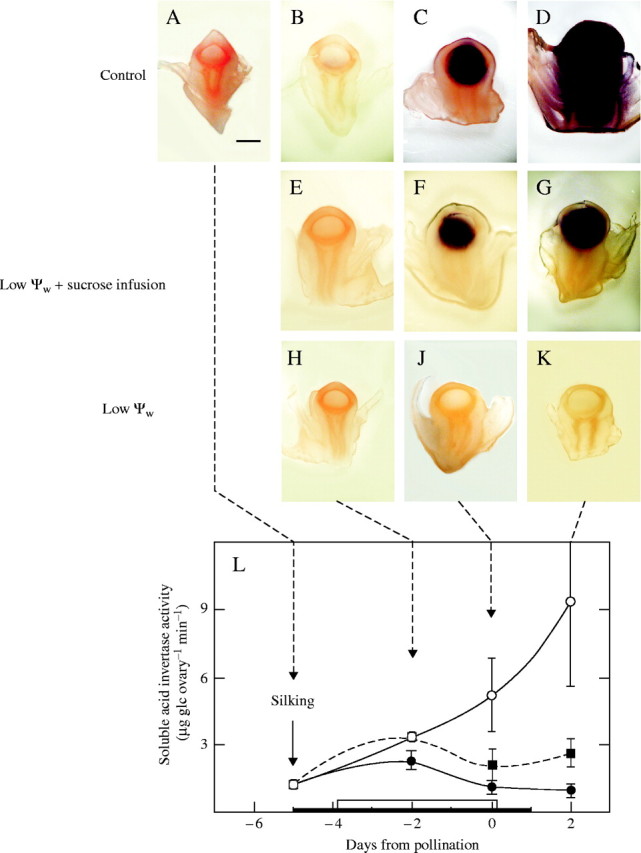
Same as Fig. 2 but for soluble acid invertase in situ. (A–D) Control: high ψw. Soluble activity was associated with nucellus and increased markedly at later times. Because of high activity at (D), there was over-development and non-specific staining in this image. (E–G) Low ψw + sucrose infusion: activity was less than in controls and more variable. Activity in (F) and (G) was apparent in two out of three samples. (H–K) Low ψw: activity was undetectable. Scale bar = 1 mm. (L) For comparison with images, in vitro assays are shown for soluble acid invertase from Zinselmeier et al. (1999). Note higher activity in (L) than for cell wall-bound invertase in Fig. 2L.
mRNA abundance for sucrose processing enzymes
The changes in invertase activity at low ψw indicate that the enzymes were highly regulated in maize ovaries. In order to determine whether transcription might be involved, mRNA abundance was measured for genes Incw1 and Incw2 of the cell wall acid invertases (Shanker et al., 1995; Cheng et al., 1996; Taliercio et al., 1999), Ivr1 and Ivr2 of the soluble acid invertases (Koch and Nolte, 1995; Xu et al., 1995), and the maize invertase inhibitor peptide Zminh1 (Allen et al., 2001; Bate et al., 2004). In addition, mRNA abundance was measured for the two sucrose synthases in maize, SS1 and SS2, encoded by genes SS1 and SS2 (sometimes called shrunken1 (Sh1) and sucrose synthase1 (Sus1) from the endosperm mutants), respectively (Echt and Chourey, 1985; Werr et al., 1985). The enzymes SS1 and SS2 become most active in the developing kernel 10–12 d after pollination (Chourey and Nelson, 1976), but activity was reported in the nucellus, antipodal cells and integuments of the unpollinated ovary (Wittich and Vreugdenhil, 1998).
Typically, the PCR assays of each sample run in triplicate provided close replication of the amplification profiles (similar Ct values obtained; Fig. 4A) and the resulting amplicons were of high purity (Fig. 4B). There were significant differences in cycle numbers and hence mRNA abundance between treatments. Figure 4A shows that mRNA abundance for Incw2 was less at low ψw than in controls on day 2. It was at an intermediate level if the stems were fed sucrose. After each PCR analysis, the amplicons were sequenced for comparison with the native mRNA for each gene. The sequence identity averaged 97 % (range = 93–99 %) for all amplicons except the long version of Incw1. This sequence had 87 % identity to the full version of the gene (AF050129), but a 39 bp segment gave a 100 % match to a region of the long (large) version of Incw1 and was completely absent in the short version (Cheng et al., 1999), which gave increased confidence that the long and short version could be distinguished.
Fig. 4.
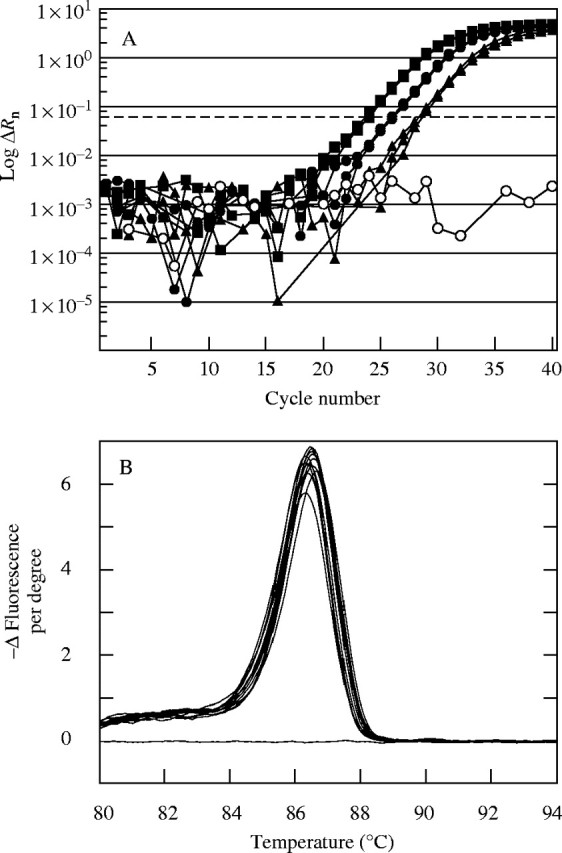
Typical analysis of mRNA abundance using real-time PCR. (A) Progress of PCR amplification expressed as log ΔRn in various PCR cycles using SYBR Green and gene-specific primer for maize ovary Incw2 on day 2 of Fig. 2. Three replicate extracts were made from ovaries from three treatments: control plants (filled squares), plants at low ψw plus sucrose infusion (filled circles), plants at low ψw (filled triangles). Also shown is a no template control (open circles). The dashed line represents the selected Ct threshold at which mRNA abundance was assessed. Greater cycle number to reach the threshold indicates lower abundance of mRNA. Three replicates are superimposed for each treatment and are often too close to be distinguished in this figure. (B) Thermal denaturation of amplicons produced in (A). Following the final real-time PCR analysis, each sample was subjected to thermal denaturation to test the purity of amplification, judged from the number of peaks in the denaturation response. Each denaturation curve represents one amplicon from (A), and the single symmetrical peak indicates high purity. Denaturation shown as decrease in fluorescence of SYBR Green as temperature increased.
For the short form of Incw1, Fig. 5A indicates that the mRNA abundance increased approximately seven-fold from −5 d to +2 d in control ovaries at high ψw. At low ψw, the mRNA abundance was significantly reduced compared with the controls whether sucrose was fed to the stems or not. Incw1 appeared to increase in transcriptional activity after rewatering. Transcript abundance was not tested for the long version of Incw1.
Fig. 5.
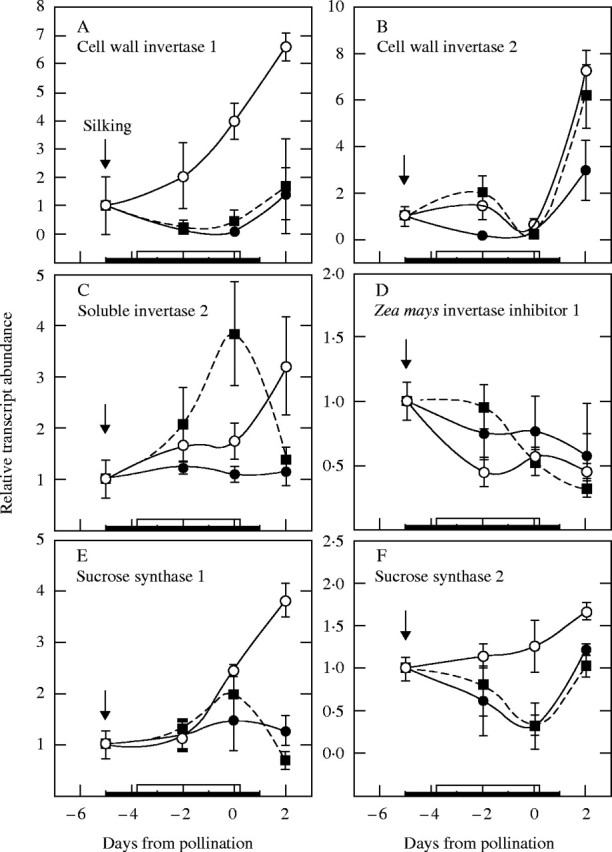
mRNA abundance profile for genes of sucrose processing enzymes in maize ovaries at various times when low ψw occurred around pollination. See Fig. 1 for sampled ears at maturity. Real-time PCR analysis as in Fig. 4 for (A) cell-wall invertase 1 (Incw1), (B) cell-wall invertase 2 (Incw2), (C) soluble invertase 2 (Ivr2), (D) Zea mays invertase inhibitor 1 (Zminh1), (E) sucrose synthase 1 (SS1), and (F) sucrose synthase 2 (SS2). Control at high ψw (open circles), low ψw plus sucrose infusion into the stems (filled squares), low ψw (filled circles). The black bar on the abscissa indicates when water was withheld from the soil. The white bar indicates when sucrose was infused into stems starting on day −4 and continuing each day to include day 0. Data are means ± s.e. for three separate plants relative to mRNA abundance on day −5 before treatments were imposed.
For Incw2, Fig. 5B indicates that mRNA abundance at low ψw was generally less than in control ovaries unless sucrose was fed to the stems, which maintained mRNA abundance nearly at control levels. As with Incw1, the mRNA abundance appeared to increase on day 2 after rewatering. Curiously, on the day of pollination, there was no difference among the treatments, for reasons that remain obscure.
The soluble invertase Ivr1 did not generate enough fluorescent signal to detect mRNA, but Ivr2 mRNA was detected throughout early ovary development (Fig. 5C). Low ψw decreased the abundance of Ivr2 mRNA, and sucrose feeding increased the abundance above control levels except on day 2. It should be noted that the last sucrose was fed on day 0, and continued feeding until day 2 might have maintained Ivr2 above the controls.
The mRNA abundance for the maize inhibitor peptide Zminh1 decreased slightly from day −5 (Fig. 5D). There were no significant differences between any of the treatments, all of which displayed a similar declining pattern.
SS1 mRNA was detected in the ovaries (Fig. 5E) and the abundance was initially unaffected at low ψw but was clearly diminished on day 2 compared with the controls. SS2 mRNA behaved similarly but differences appeared earlier than for SS1 (Fig. 5F). However, the mRNA abundance for SS2 increased on day 2 after rewatering. Sucrose feeding had little effect on the expression of SS1 or SS2.
mRNA abundance for possible senescence enzymes
The recovery of Incw1, Incw2 and SS2 after rewatering suggests that some of the genes for sucrose processing were reversibly affected at low ψw. Because abortion is irreversible, it seemed possible that additional irreversible steps might be involved. Using Evans Blue to test whether plasma membrane integrity was intact (Gaff and Okong 'O-Ogola, 1971; Young et al., 1997), control ovaries did not stain (Fig. 6A and B). Ovaries at low ψw also did not stain when sucrose was fed to the stems (Fig. 6C and D). However, at low ψw without fed sucrose, staining appeared on day 2 in the nucellus and pedicel (Fig. 6E and F). The staining occurred in individual cells (Fig. 6H) scattered around the phloem termini and in the nucellus.
Fig. 6.
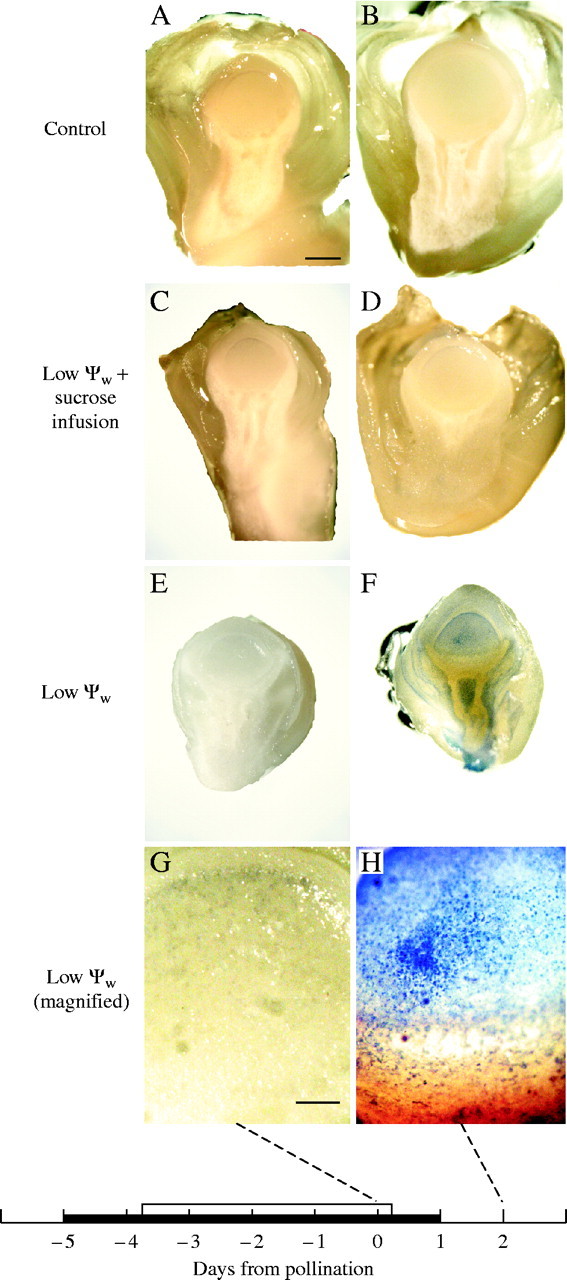
Evans Blue staining of maize ovaries when low ψw occurred around the time of pollination, for ears in Fig. 1. (A and B) Control: high ψw. No stain visible. (C and D) Low ψw plus sucrose infusion. No stain visible. (E and F) Low ψw. No stain detectable in (E) but stain is apparent in (F). Stain in (F) is present in nucellus and around vascular tissue in upper pedicel 2 d after pollination. (G and H) Magnified view of nucellus in (E and F). No stain detected in (G) but present in individual cells in (H). The black bar on the abscissa indicates when water was withheld from the soil. Plants were rewatered on day 0. The white bar indicates when sucrose was infused into stems starting on day −4 and continuing each day to include day 0. Scale bars: A–F = 1 mm; G and H = 0·1 mm.
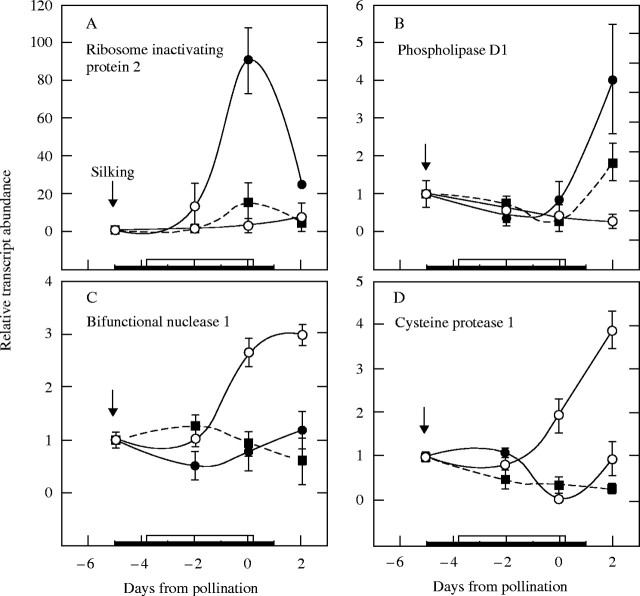
Because of this loss in membrane integrity, four genes for putative senescence enzymes were selected for testing: ribosome-inactivating protein 2 (RIP2), phospholipase D1 (PLD1), a putative maize homologue to the arabidopsis (and rice, barley, Zinnia) bifunctional nuclease 1 (BFN1), and cysteine protease 1 (CCP1). Each gene was previously reported to be up-regulated during senescence, drought, or other abiotic or biotic stresses (Perez-Amador et al., 2000; Wang, 2000; Zinselmeier et al., 2002).
At low ψw, the mRNA abundance for RIP2 became approx. 30-fold higher than in the high ψw control, reaching a maximum on day 0 (Fig. 7A). This large up-regulation was mostly eliminated by feeding sucrose to the stems at low ψw. After rewatering on day 1, mRNA abundance fell nearly to control levels on day 2. For PLD1, low ψw had no effect until day 2 when the low ψw treatment displayed a four-fold up-regulation (Fig. 7B). Feeding sucrose at low ψw partially returned the mRNA abundance to control levels. BFN1 (Fig. 7C) and CCP1 (Fig. 7D) were not up-regulated and instead displayed a down-regulation pattern resembling Incw1 or SS1 at low ψw (Fig. 5). Sucrose feeding made scarcely any difference to either gene.
Fig. 7.
mRNA abundance for genes coding for enzymes that breakdown ribosomes, phospholipids, nucleic acids, or proteins in maize ovaries at various times when low ψw occurred around pollination. See Fig. 1 for ears sampled at maturity. Real-time PCR analysis as in Fig. 4 for (A) Ribosome inactivating protein 2 (RIP2), (B) phospholipase D (PLD1), (C) bifunctional nuclease 1 (BFN1), (D) cysteine protease 1 (CCP1). Control at high ψw (open circles), low ψw plus sucrose infusion into the stems (filled squares), low ψw (filled circles). The black bar on the abscissa indicates when water was withheld from the soil. The white bar indicates when sucrose was infused into stems starting on day −4 and continuing each day to include day 0. Data are means ± s.e. for three separate plants relative to mRNA abundance on day −5 before treatments were imposed.
DISCUSSION
The treatments caused low ψw, diminished photosynthesis and nearly complete ovary abortion resembling the effects reported by Westgate and Boyer (1986), Boyle et al. (1991) and Zinselmeier et al. (1995a, 1999) for maize. Although these conditions alter the expression of hundreds of genes in maize ovaries and young kernels (Zinselmeier et al., 2002; Yu and Setter, 2003), sucrose feeding allowed many of the ovaries to develop normally. In effect, the key to irreversible abortion appeared to lie in the changes caused by sucrose feeding. The sugar responsiveness of these genes is thus of great interest.
Incw2 and Ivr2 clearly were sugar-responsive but Incw1 was not. Considering Incw2 and Incw1, the mix of sugar-responsiveness of the genes might give a resulting mix of cell wall-bound enzyme activities that would be intermediate when sucrose was fed at low ψw. Likewise for Ivr2, the active transcription on day 0 but inactive transcription on day 2 might give an intermediate soluble enzyme activity in the sucrose-fed plants. Intermediate invertase activities were observed for both forms of the enzymes in vivo and/or in situ when sucrose was fed.
Because Zinselmeier et al. (1999) found normal amounts of sucrose but partial activities of invertase in the ovaries when sucrose was fed at low ψw, attention in this study focused on gene expression for the invertases and related sucrose-processing enzymes. Zinselmeier et al. (1999) proposed that invertase may not have responded fully to fed sucrose because the sugar may not have been distributed normally within the pedicel/ovary structure. However, McLaughlin and Boyer (2004) found no evidence of abnormal distribution. Andersen et al. (2002) reported a correlation between invertase activities and transcriptional activities of Incw2 and Ivr2 genes in maize ovaries at low ψw. Some of these genes are considered to be sugar-responsive (Koch, 1996; Sheen et al., 1999), which is consistent with the present results.
Perhaps because some but not all of the genes were sugar-responsive, abortion was prevented only partially by sucrose feeding at low ψw.
Expression of sucrose processing genes in maize ovaries
All of the sucrose-processing enzymes explored in our work were rapidly down-regulated at low ψw. The cell wall-bound, insoluble forms of invertases are coded by a four-gene family expressed at various times and in different tissues during development, with Incw1 transcript identified in kernels late in their development (Taliercio et al., 1999) and mutants of Incw2 affecting activity in kernels early in their development (Miller and Chourey, 1992; Cheng et al., 1996). The ovaries of the present study were several days younger than the youngest kernels used by most other workers, and embryos and endosperm were absent until day 0. Despite these differences, Incw1 mRNA was clearly detected along with Incw2. Andersen et al. (2002) investigated invertases in ovaries similar in age to those in our work and did not detect Incw1 mRNA in their material. However, sequence analysis of the Incw1 and Incw2 amplicons revealed sequences unique to each gene, and Incw1 was further confirmed by amplifying the long version described by Cheng et al. (1999) (see Table 1). Although the work of Cheng et al. (1996) points toward Incw2 as the dominant gene responsible for wall-bound invertase in kernels at day 12, in the young ovaries investigated here Incw1 could have played a role. Incw3 and Incw4 were not studied because Kim et al. (2000) were unable to detect Incw3 in maize ovary tissue and Incw4 was expressed at a minor level that did not appear to contribute significantly to invertase activity in the Incw2 miniature mutant a few days after pollination (Cheng et al., 1996).
As with the wall-bound invertases, members of the two-gene family of soluble invertases appeared to be expressed differentially during maize reproduction. Ivr1 was up-regulated in sucrose-depleted cell suspension cultures of maize (Carlson and Chourey, 1999) and detected in kernel tissue on day 2 and day 8 by Northern analysis (Xu et al., 1996), but later reports (Carlson and Chourey, 1999; Andersen et al., 2002) including this one did not detect Ivr1 mRNA during early ovary/kernel development. As a consequence, among the soluble invertases, Ivr2 likely contributed most of the soluble enzyme activity in the nucellus in this study.
Because Andersen et al. (2002) found activities of the soluble and cell wall-bound enzymes to be correlated with mRNA abundance for Incw2 and Ivr2, the coordinate control discovered by Cheng et al. (1996) between cell wall-bound and soluble forms of the enzyme might be explained. In the present work, both mRNA abundance and enzymatic activities decreased for the invertases when ψw were low enough to inhibit photosynthesis (and thus the delivery of sucrose) and both responded to increased sucrose in the ovaries when sucrose was fed to the stems. This similarity in sugar-responsiveness at the gene level might cause enzyme activity to be similarly expressed and thus display coordinate control.
In addition, however, other forms of enzyme regulation might occur. Bate et al. (2004) found that recombinant inhibitor produced in Escherichia coli from Zminh1 decreased the activity of insoluble invertase from maize in vitro. Several peptide inhibitors have been described for invertase in maize and may play roles at various developmental stages (Allen et al., 2001). Krausgrill et al. (1996) and Greiner et al. (1998) considered invertase inhibitors to regulate invertase activity during plant development. Zminh1 was active during early kernel growth (Allen et al., 2001; Bate et al., 2004) and appeared to be constitutively expressed in the ovaries of the present work.
Weil et al. (1994) and Sander et al. (1996) report that invertase inhibitor may bind non-competitively to invertase when sucrose concentrations are very low, inhibiting the enzyme activity. In the present work, the in vivo and in situ invertase assays maintained endogenous sugars until rinsing of the tissue slices began. The lowest endogenous sucrose reported by Zinselmeier et al. (1999) was 0·1 mg in ovaries containing about 22 μL of water, i.e. endogenous sucrose concentrations were 13 mm at minimum. This concentration is well above the 1–3 mm for inhibitor binding to invertase (Weil et al., 1994). Consequently, the inhibitor peptide should have remained unbound and soluble. Its small size (17·7 kDa; Bate et al., 2004) should have allowed any unbound inhibitor to be removed from the enzyme environment during rinsing. Without inhibitor, no binding would occur during the assays, and any loss in invertase activity at low ψw would be attributable to factor(s) other than assay-induced inhibitor binding. Assay-induced binding also is unlikely to explain the activity losses during enzyme extraction, because the in vitro data of Zinselmeier et al. (1999) corresponded closely with the in vivo (cell wall-bound form) and in situ (soluble form) images.
If some of the sucrose was sequestered away from the enzyme in vivo, the inhibitor might have bound to the enzyme in vivo despite the high overall concentrations of sucrose. Accordingly, there is a possibility that inhibitor binding in vivo could have contributed to the activity losses in the invertase assays of this study at low ψw. But there was little likelihood for additional inhibitor binding during the assays themselves, and a role for Zminh1 and its peptide product for post-transcriptional control in vivo remains to be convincingly demonstrated.
For sucrose synthase, transcripts of SS1 and SS2 were detected in agreement with the enzyme activities detected by Wittich and Vreugdenhil (1998). Although both synthases of this two-gene family decreased at low ψw, neither was sugar responsive. Koch et al. (1992) and Xu et al. (1996) classified the maize sucrose synthase genes, as well as the soluble invertase Ivr1 and Ivr2, as part of a ‘feast-and-famine’ model of gene expression based on the sugar status of the tissue or cell. SS1 was classified as a sugar-repressed starvation-tolerant gene and SS2 as a sugar-enhanced gene using maize root tip assays. In addition, the SS1 promoter was repressed by sucrose in transient expression assays (Maas et al., 1990). Although the present results seem at odds with this classification, differences in sugar responsiveness might arise in different tissues (e.g. root vs. ovary) or stages of development (e.g. vegetative vs. reproductive).
With this survey, changes in the amount of mRNA were examined for nearly all of the genes known to be involved in sucrose breakdown in young maize ovaries. The mRNA amounts responded early to low ψw, and were down-regulated for all the genes except Zminh1. The stability in abundance of Zminh1 mRNA together with the decreased abundance for the other genes indicates that the changes were gene-specific. The relationship between gene expression and mRNA abundance depends on the rate of transcription and mRNA degradation. Both processes can lead to altered transcript levels and thus influence development. Recognizing this, it seems safe to conclude that a major early response to low ψw was a decreased expression of the sucrose-processing genes, sometimes in response to less sugar. This probably contributed to the general slowdown in enzyme activities for processing sucrose, decreased sucrose use (Schussler and Westgate, 1991; Zinselmeier et al., 1999), and less biosynthetic activity for ovary structures (e.g. for starch shown by Zinselmeier et al., 1999).
Role of invertase
Much evidence indicates that acid invertase is the main enzyme processing sucrose in developing maize kernels (Shannon 1972; Felker and Shannon, 1980; Doehlert and Felker, 1987; Miller and Chourey, 1992; Xu et al., 1995; Cheng et al., 1996). In the present study, invertases were detected in young ovaries before kernels were present and, judging from the location of glucose (McLaughlin and Boyer, 2004), the cell wall (insoluble) acid invertases hydrolyse sucrose in the apoplast of the upper pedicel tissues surrounding the phloem termini. The activity of invertase creates a steep gradient in glucose concentration between the upper pedicel and the nucellus in the young maize ovary. These biochemical properties in ovaries resemble those later when endosperm and embryo are present (Shannon, 1972; Felker and Shannon, 1980; Doehlert and Felker, 1987; Miller and Chourey, 1992; Xu et al., 1995; Cheng et al., 1996).
Some of the glucose is deposited as starch in the pedicel and ovary wall tissues (Zinselmeier et al., 1999; Andersen et al., 2002; McLaughlin and Boyer, 2004). Some also makes its way into the ovary, probably by way of the nucellus, and is utilized for ovary growth and to synthesize a small amount of starch in the embryo sac (Diboll and Larson, 1966). The gradient may enhance the movement of glucose into the nucellus, where only moderate glucose concentrations reside.
At low ψw, the concentration of glucose decreased in the upper pedicel tissues (McLaughlin and Boyer, 2004), and insoluble invertase activity decreased there as well. These changes decreased the gradient in ovary glucose, which may have inhibited sugar transport (McLaughlin and Boyer, 2004). This supports the notion that relatively inactive invertase can limit carbon metabolism by blocking glucose production and transport.
Role of senescence
After these changes took place at low ψw, the ovaries appeared to undergo some steps towards senescence. On day 2 in the low ψw treatment, staining indicated that cell membranes were losing integrity while PLD1 was up-regulated, which suggests that phospholipase D could have been increasing and degrading phospholipid membranes. The up-regulation was reversed by sucrose feeding and was thus sugar-responsive. It is noteworthy that the up-regulation occurred on day 2, after the plants had been rewatered. This suggests that the activation of PLD1 was not quickly reversed, which could be a manifestation of the irreversible loss of development resulting in abortion.
Phospholipase D and its breakdown products were involved in responses to water deficits, cold, carbon starvation, wounding and pathogen invasion (Ueki et al., 1995; Lee et al., 1998; El Maarouf et al., 1999; Wang, 2000). Thompson et al. (1998) linked increased lipid metabolism to plant senescence, and Aubert et al. (1996) established that plant leaves or suspension cells sacrifice their cellular membrane phospholipids to generate fatty acids and the downstream metabolites upon glucose or sucrose starvation. Fatty acid β-oxidation was increased in glucose-starved maize root tips (Dieuaide-Noubhani et al., 1997).
Similar links may exist for RIP2 between sugar-responsiveness and senescence. There were especially dramatic increases in RIP2 transcriptional activity late in the development of low ψw, and these were nearly abolished by an increased sugar flux arriving at the ovaries because of sucrose feeding. The trigger for RIP2 was earlier than for PLD1. RIP genes code for N-glycosidases that modify large ribosomal RNAs (28S), rendering them incapable of further translation (Nielsen and Boston, 2001). The enzymes remove a specific adenine in the rRNA, preventing binding of elongation factor EF2 and blocking translation. Three types have been identified with type 2 being highly cytotoxic. They are thought to function in defense against bacterial, fungal and insect attack, and may be senescence inducers (programmed cell death) (Stirpe et al., 1996; Rippmann et al., 1997; Nielsen and Boston, 2001; Zinselmeier et al., 2002). RIP2 was identified in maize by Bass et al. (1995) and reported to be strongly induced in the developing maize kernel during water deficits (Nielsen and Boston, 2001; Zinselmeier et al., 2002). Given these roles for RIP2, the transcriptional changes for RIP2 at low ψw may have a global effect on translation in maize ovaries.
The other gene candidates for senescence ZmBFN1 and CCP1 were not up-regulated at low ψw nor were they sugar-responsive. Because they were down-regulated with behaviour resembling that of some of the sugar processing genes, they may play a role in the normal development of the maize ovaries, with low ψw interrupting that role.
ZmBFN1 was investigated because nuclease activity increased in cultured maize endosperm cells following sucrose starvation (Gallie et al., 2002), and a nuclease was induced during tissue senescence in arabidopsis (bifunctional nuclease 1, BFN1, Accession no. U90264; Perez-Amador et al., 2000). The BFN1 gene from maize (AY105439 and the related partial cDNA sequence A1881514) was identified from substantial homology to several bifunctional nuclease sequences (DNA and protein) identified in arabidopsis (AAG52597), Hordeum vulgare (BAA82696), Oryza sativa (BAB18300) and Zinnia elegans (AAD00695) in a cDNA library developed from immature ear tissue. Likewise, cysteine protease genes such as CCP1 were included because they are involved in many forms of plant senescence (del Pozo and Lam, 1998) and often were up-regulated in response to unfavourable environments (Koizumi et al., 1993; Stroeher et al., 1997; Harrak et al., 2001).
Significance for plant performance
These experiments tested for sugar responsiveness in the intact plant by feeding photosynthetic quantities of sucrose without changing ψw, and the identification of sugar-responsive genes thus seems unequivocal. Sucrose had to be fed to the stems at a rate comparable to its production by photosynthesis, perhaps because diverse sinks were being fed. With control ovaries gaining about 1 mg of dry mass per day (Zinselmeier et al., 1999) and 500 ovaries per ear, only 0·5 g was needed to maintain ovary growth each day. Photosynthesis normally deposited considerably in excess of this amount — about 5 g of dry mass each day throughout the parent plant — and as a result about 90 % of the sinks for dry mass appeared to be elsewhere in the plant. These sinks may have needed to be satisfied before the sucrose stream to the ovaries could be returned to control levels by feeding.
The exposure to low ψw ensured that virtually all the ovaries would abort if the plants were not fed sucrose. This allowed the triggering events to be studied despite the invisibility of abortion until several days later when developmental differences could be detected. The predictable development allowed early molecular events to be reliably separated from later ones.
The first separable events were the inhibited acid invertase activity together with a down-regulation of genes coding for sucrose-processing enzymes often apparent by day −2. Starch breakdown was apparent by day 0 (Zinselmeier et al., 1999) and probably helped maintain glucose but only for a short time. On day 0, the ovaries normally accumulated 1 mg of dry mass per day (Zinselmeier et al., 1999; Andersen et al., 2002) and contained about 0·4 mg of starch. Because this amount of starch was small compared with the dry mass delivered to the ovaries, starch breakdown could supply glucose for only about half a day. Glucose was depleted soon after day 0 (McLaughlin and Boyer, 2004). About this time, sugar-responsive genes such as RIP2 and then PLD1 were up-regulated. Therefore, the up-regulation of these genes may have been triggered by glucose depletion in the ovaries.
This sequence, summarized in Fig. 8, suggests that abortion is a two-step process. The first (Fig. 8B and C) occurs when the sugar stream to the ovaries diminishes because of inhibited photosynthesis, triggering a down-regulation of sucrose-processing enzymes [Down-regulate (Sucrose)]. The second occurs later, perhaps after the depletion of glucose (Fig. 8D and E), when sugar-responsive genes for senescence are activated (starting with RIP2 and continuing to other senescence genes such as PLD1). Zinselmeier et al. (1999) observed starch depletion in ovaries under these conditions and concluded that the ovaries were starved of substrates for growth at low ψw but adequate carbon is clearly available to up-regulate RIP2 and PLD1, which argues against a simple starvation hypothesis. The two-step sequence emphasizes the primary importance of the sugar stream to the development of the ovaries, as pointed out by Schussler and Westgate (1994, 1995), and further suggests that the irreversible step is likely to be the second one in view of the eventual irreversibility of senescence.
Fig. 8.
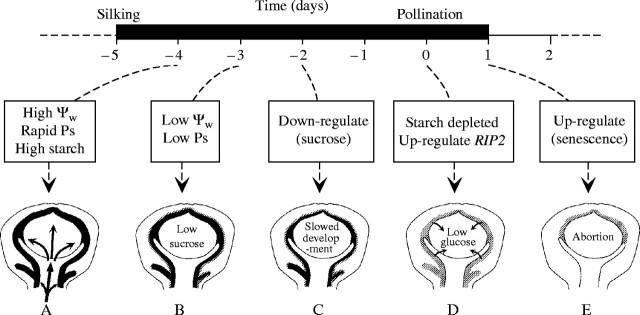
Summary of events leading to abortion of maize ovaries when plants are subjected to low ψw around the time of pollination. (A) Photosynthesis providing sucrose to give about 1 mg of dry mass on the day of pollination to ovaries containing 3 mg of dry mass. About 0·4 mg of the dry mass is starch shown as black area in ovary wall. (B) ψw low enough to inhibit photosynthesis curtails sucrose delivery. (C) Genes for sucrose processing are down-regulated. (D) Lack of sucrose triggers starch breakdown, maintaining glucose for a short time. About the time glucose concentrations fall, RIP2 is up-regulated. (E) With a continued lack of glucose, certain senescence genes are up-regulated, leading to irreversible loss in development.
If this model has validity, eliminating or delaying the activation of senescence genes might result in a reversible quiescence at low ψw rather than an irreversible abortion. Rewatering would then allow ψw to rise, gene function to return to normal, and ovaries to resume growth. The fact that the parent also survives and resumes metabolic activity in response to rewatering illustrates a form of quiescence that is in marked contrast to the developmental fate of the ovary around the time of pollination. At later stages of kernel development, abortion is much less frequent and the kernel grows during exposure to low ψw (McPherson and Boyer, 1977; Jurgens et al., 1978; Westgate and Boyer, 1985). From an agricultural standpoint, incorporating this quiescent ability in ovaries and young kernels might have value.
CONCLUSIONS
All of the tested ovary genes for sucrose processing enzymes were down-regulated at low ψw.
After this down-regulation, some of the ovary genes for putative senescence enzymes were up-regulated at low ψw.
Some of the ovary genes for sucrose processing and senescence were sugar-responsive. Others were not and responded instead to other signals associated with low ψw.
Because some of the sucrose processing genes were sugar-responsive and others were not, sucrose feeding probably left a mix of recovered and non-recovered enzyme activities at low ψw, perhaps explaining why invertase activity responded only partially to the feeding, and abortion was only partially prevented.
The sequence in gene expression may have been determined by the sequence in sugar signals. Sucrose delivery decreased first, followed by depletion of glucose a few days later. The sucrose signal may have triggered an early down-regulation of genes coding for sucrose-processing enzymes (e.g. INCW2, IVR2), while the glucose signal may have triggered a later up-regulation of putative senescence genes (e.g. RIP2, PLD1).
The late up-regulation of senescence genes may be the irreversible component of abortion.
Supplementary Material
Acknowledgments
We thank James A. Hawk and Teclemariam Weldekidan for making the crosses and supplying the seed for the hybrid used in this study, Karen E. Koch for encouragement and suggesting the invertase assay, Kathy Coyne and Barbara Campbell for help with PCR techniques, Craig Cary for the use of PCR instruments, Nick Bate for guidance with Zminh1, Tim Helentjaris and Chris Zinselmeier for discussing their results from microarray analysis of maize gene expression, and An-Ching Tang for help with the artwork. This work was supported by grant number 2002-01052 to J.S.B. from the US Department of Agriculture National Research Initiative Competitive Grants Program.
LITERATURE CITED
- Allen SM, Helentjaris T, Bate NJ. 2001. Novel invertase inhibitors and methods of use. Pioneer Hi-Bred Inc. (US); E.I. Du Pont De Nemours and Company (US). WO Patent 0158939-A. Date issued: 16 August. [Google Scholar]
- Andersen MN, Asch F, Wu Y, Jensen CR, Naested H, Mogensen VO, Koch KE. 2002. Soluble invertase expression is an early target of drought stress during the critical, abortion-sensitive phase of young ovary development in maize. Plant Physiology 130: 591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert S, Gout E, Bligny R, Marty-Mazars D, Barrieu F, Alabouvette J, Marty F, Douce R. 1996. Ultrastructural and biochemical characterization of autophagy in higher plant cells subjected to carbon deprivation: control by the supply of mitochondria with respiratory substrates. Journal of Cell Biology 133: 1251–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass HW, OBrian GR, Boston RS. 1995. Cloning and sequencing a second ribosome-inactivating protein gene from maize (Zea mays L.). Plant Physiology 107: 661–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bate NJ, Niu X, Wang Y, Reimann KS, Helentjaris TG. 2004. An invertase inhibitor from maize localizes to the embryo surrounding region during early kernel development. Plant Physiology 134: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle MG, Boyer JS, Morgan PW. 1991. Stem infusion of liquid culture medium prevents reproductive failure of maize at low water potential. Crop Science 31: 1246–1252. [Google Scholar]
- Carlson SJ, Chourey PS. 1999. A re-evaluation of the relative roles of two invertases, INCW2 and IVR1, in developing maize kernels and other tissues. Plant Physiology 121: 1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W-H, Taliercio EW, Chourey PS. 1996. The Miniature1 seed locus of maize encodes a cell wall invertase required for normal development of endosperm and maternal cells in the pedicel. Plant Cell 8: 971–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng W-H, Taliercio EW, Chourey PS. 1999. Sugars modulate an unusual mode of control of the cell-wall invertase gene (Incw1) through its 3′ untranslated region in a cell suspension culture of maize. Proceedings of the National Academy of Sciences of the USA 96: 10512–10517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P, Mackey K. 1995. Modification of the TRI Reagent™ procedure for isolation of RNA from polysaccharide- and proteoglycan-rich sources. Biotechniques 19: 942–945. [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analytical Biochemistry 162: 156–159. [DOI] [PubMed] [Google Scholar]
- Chourey PS, Nelson OE. 1976. The enzymatic deficiency conditioned by the shrunken-1 mutations in maize. Biochemical Genetics 14: 1041–1055. [DOI] [PubMed] [Google Scholar]
- Claassen MM, Shaw RH. 1970. Water deficit effects on corn. II. Grain components. Agronomy Journal 62: 652–655. [Google Scholar]
- del Pozo O, Lam E. 1998. Caspases and programmed cell death in the hypersensitive response of plants to pathogens. Current Biology 8: 1129–1132. [DOI] [PubMed] [Google Scholar]
- Diboll AG, Larson DA. 1966. An electron microscope study of the mature megagametophyte in Zea mays American Journal of Botany 53: 391–402. [PubMed] [Google Scholar]
- Dieuaide-Noubhani M, Canioni P, Raymond P. 1997. Sugar-starvation-induced changes of carbon metabolism in excised maize root tips. Plant Physiology 115: 1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehlert DC, Felker FC. 1987. Characterization and distribution of invertase activity in developing maize (Zea mays) kernels. Physiologia Plantarum 70: 51–57. [Google Scholar]
- Echt CS, Chourey PS. 1985. A comparison of two sucrose synthetase isozymes from normal and shrunken-1 maize. Plant Physiology 79: 530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Maarouf H, Zuily-Fodil Y, Gareil M, d'Arcy-Lameta A, Pham-Thi AT. 1999. Enzymatic activity and gene expression under water stress of phospholipase D in two cultivars of Vigna unguiculata L. Walp. differing in drought tolerance. Plant Molecular Biology 39: 1257–1265. [DOI] [PubMed] [Google Scholar]
- Felker FC, Shannon JC. 1980. Movement of 14C-labelled assimilates into kernels of Zea mays L. An anatomical examination and microautoradiographic study of assimilate transfer. Plant Physiology 65: 864–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaff DF, Okong 'O-Ogola O. 1971. The use of non-permeating pigments for testing the survival of cells. Journal of Experimental Botany 22: 756–758. [Google Scholar]
- Gallie DR, Chang S, Young TE. 2002. Induction of RNase and nuclease activity in cultured maize endosperm cells following sucrose starvation. Plant Cell, Tissue and Organ Culture 68: 163–170. [Google Scholar]
- Greiner S, Krausgrill S, Rausch T. 1998. Cloning of a tobacco apoplasmic invertase inhibitor: proof of function of the recombinant protein and expression analysis during plant development. Plant Physiology 116: 733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrak H, Azelmat S, Baker EN, Tabaeizadeh Z. 2001. Isolation and characterization of a gene encoding a drought-induced cysteine protease in tomato (Lycopersicon esculentum). Genome 44: 368–374. [DOI] [PubMed] [Google Scholar]
- Jaynes TA, Nelson OE. 1971. An invertase inactivator in maize endosperm and factors affecting inactivation. Plant Physiology 47: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens SK, Johnson RR, Boyer JS. 1978. Dry matter production and translocation in maize subjected to drought during grain fill. Agronomy Journal 70: 678–682. [Google Scholar]
- Kim JY, Mahe A, Guy S, Brangeon J, Roche O, Chourey PS, Prioul JL. 2000. Characterization of two members of the maize gene family, Incw3 and Incw4, encoding cell-wall invertases. Gene 245: 89–102. [DOI] [PubMed] [Google Scholar]
- Koch KE. 1996. Carbohydrate-modulated gene expression in plants. Annual Review of Plant Physiology and Plant Molecular Biology 47: 509–540. [DOI] [PubMed] [Google Scholar]
- Koch KE, Nolte KD. 1995. Sugar-modulated expression of genes for sucrose metabolism and their relationship to transport pathways. In: Madore MA, Lucas WJ, eds. Carbon partitioning and source-sink interactions in plants. Rockville, MD: American Society of Plant Physiologists, 141–155. [Google Scholar]
- Koch KE, Nolte KD, Duke ER, McCarty DR, Avigne WT. 1992. Sugar levels modulate differential expression of maize sucrose synthase genes. Plant Cell 4: 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi M, Yamaguchi-Shinozaki K, Tsuji H, Shinozaki K. 1993. Structure and expression of two genes that encode distinct drought-inducible cysteine proteinases in Aradidopsis thaliana Gene 129: 175–182. [DOI] [PubMed] [Google Scholar]
- Kramer PJ, Boyer JS. 1995.Water relations of plants and soils. San Diego: Academic Press. [Google Scholar]
- Krausgrill S, Sander A, Greiner S, Weil M, Rausch T. 1996. Regulation of cell wall invertase by a proteinaceous inhibitor. Journal of Experimental Botany 47: 1193–1198. [DOI] [PubMed] [Google Scholar]
- Lee SH, Chae HS, Lee TK, Kim SH, Shin SH, Cho BH, Cho SH, Kang BG, Lee WS. 1998. Ethylene-mediated phospholipid catabolic pathway in glucose-starved carrot suspension cells. Plant Physiology 116: 223–229. [Google Scholar]
- McLaughlin JE, Boyer JS. 2004. Glucose localization in maize ovaries when kernel number decreases at low water potential and sucrose is fed to the stems. Annals of Botany 94: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson HG, Boyer JS. 1977. Regulation of grain yield by photosynthesis in maize subjected to a water deficiency. Agronomy Journal 69: 714–718. [Google Scholar]
- Maas C, Schaal S, Werr W. 1990. A feedback control element near the transcription start site of the maize shrunken-1 gene determines promoter activity. EMBO Journal 9: 3447–3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ME, Chourey PS. 1992. The maize invertase-deficient miniature-1 seed mutation is associated with aberrant pedicel and endosperm development. Plant Cell 4: 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison TB, Weis JJ, Wittwer CT. 1998. Quantification of low-copy transcripts by continuous SYBR Green I monitoring during amplification. Biotechniques 24: 954–962. [PubMed] [Google Scholar]
- Nielsen K, Boston RS. 2001. Ribosome-inactivating proteins: a plant perspective. Annual Review of Plant Physiology and Plant Molecular Biology 52: 785–816. [DOI] [PubMed] [Google Scholar]
- Perez-Amador MA, Abler ML, De Rocher EJ, Thompson DM, van Hoof A, LeBrasseur ND, Lers A, Green PJ. 2000. Identification of BFN1, a bifunctional nuclease induced during leaf and stem senescence in Arabidopsis. Plant Physiology 122: 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressey R. 1967. Invertase inhibitors from potatoes: purification, characterization, and reactivity with plant invertases. Plant Physiology 42: 1780–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippmann JF, Michalowski CB, Nelson DE, Bohnert HJ. 1997. Induction of a ribosome-inactivating protein upon environmental stress. Plant Molecular Biology 35: 701–709. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky HJ. 1998. PRIMER 3. Code available at: http://frodo.wi.mit.edu/primer3/primer3_code.html. [Google Scholar]
- Sander A, Krausgrill S, Greiner S, Weil M, Rausch T. 1996. Sucrose protects cell wall invertase but not vacuolar invertase against proteinaceous inhibitors. Federation of European Biochemical Societies Letters 385: 171–175. [DOI] [PubMed] [Google Scholar]
- Schmidt DM, Ernst JD. 1995. A fluorometric assay for the quantification of RNA in solution with nanogram sensitivity. Analytical Biochemistry 232: 144–146. [DOI] [PubMed] [Google Scholar]
- Schussler JR, Westgate ME. 1991. Maize kernel set at low water potential. II. Sensitivity to reduced assimilates at pollination. Crop Science 31: 1196–1203. [Google Scholar]
- Schussler JR, Westgate ME. 1994. Increasing assimilate reserves does not prevent kernel abortion at low water potential in maize. Crop Science 34: 1569–1576. [Google Scholar]
- Schussler JR, Westgate ME. 1995. Assimilate flux determines kernel set at low water potential in maize. Crop Science 35: 1074–1080. [Google Scholar]
- Shanker S, Salazar RW, Taliercio EW, Chourey PS. 1995. Cloning and characterization of full-length cDNA encoding cell-wall invertase from maize. Plant Physiology 108: 873–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon JC. 1972. Movement of 14C-labeled assimilates into kernels of Zea mays L. I. Pattern and rate of sugar movement. Plant Physiology 49: 198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J, Zhou L, Jang JC. 1999. Sugars as signaling molecules. Current Opinion in Plant Biology 2: 410–418. [DOI] [PubMed] [Google Scholar]
- Stirpe F, Barbieri L, Gorini P, Valbonesi P, Bolognesi A, Polito L. 1996. Activities associated with the presence of ribosome-inactivating proteins increase in senescent and stressed leaves. Federation of European Biochemical Societies Letters 382: 309–312. [DOI] [PubMed] [Google Scholar]
- Stroeher VL, Maclagan JL, Good AG. 1997. Molecular cloning of a Brassica napus cysteine protease gene inducible by drought and low temperature stress. Physiologia Plantarum 101: 389–397. [Google Scholar]
- Sturm A, Chrispeels MJ. 1990. cDNA cloning of carrot extracellular β-fructosidase and its expression in response to wounding and bacterial infection. Plant Cell 2: 1107–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taliercio EW, Kim JY, Mahe A, Shanker S, Choi J, Cheng WH, Prioul JL, Chourey PS. 1999. Isolation, characterization and expression analysis of two cell wall invertase genes in maize. Journal of Plant Physiology 155: 197–204. [Google Scholar]
- Thompson JE, Froese CD, Madey E, Smith MD, Hong Y. 1998. Lipid metabolism during plant senescence. Progress in Lipid Research 37: 119–141. [DOI] [PubMed] [Google Scholar]
- Ueki J, Morioka S, Komari T, Kumashiro T. 1995. Purification and characterization of phospholipase D (PLD) from rice (Oryza sativa L.) and cloning of cDNA for PLD from rice and maize (Zea mays L.). Plant and Cell Physiology 36: 903–914. [DOI] [PubMed] [Google Scholar]
- Wang XM. 2000. Multiple forms of phospholipase D in plants: the gene family, catalytic and regulatory properties, and cellular functions. Progress in Lipid Research 39: 109–149 [DOI] [PubMed] [Google Scholar]
- Weil M, Krausgrill S, Schuster A, RauschT. 1994. A 17-kDa Nicotiana tabacum cell-wall peptide acts as an in-vitro inhibitor of the cell-wall isoform of acid invertase. Planta 193: 438–445. [DOI] [PubMed] [Google Scholar]
- Werr W, Frommer WB, Maas C, Starlinger P. 1985. Structure of the sucrose synthase gene on chromosome 9 of Zea mays EMBO Journal 4: 1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westgate ME, Boyer JS. 1985. Carbohydrate reserves and reproductive development at low leaf water potentials in maize. Crop Science 25: 762–769. [Google Scholar]
- Westgate ME, Boyer JS. 1986. Reproduction at low silk and pollen water potentials in maize. Crop Science 26: 951–956. [Google Scholar]
- Wittich KE, Vreugdenhil D. 1998. Localization of sucrose synthase activity in developing maize kernels by in situ enzyme histochemistry. Journal of Experimental Botany 49: 1163–1171. [Google Scholar]
- Xu J, Avigne WT, McCarty DR, Koch KE. 1996. A similar dichotomy of sugar modulation and developmental expression affects both paths of sucrose metabolism: evidence from a maize invertase gene family. Plant Cell 8: 1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Pemberton GH, Almira EC, McCarty DR, Koch KE. 1995. The Ivr1 gene for invertase in Zea mays L. Plant Physiology 108: 1293–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young TE, Gallie DR, DeMason DA. 1997. Ethylene-mediated programmed cell death during maize endosperm development of wild-type and shrunken2 genotypes. Plant Physiology 115: 737–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Setter TL. 2003. Comparative transcriptional profiling of placenta and endosperm in developing maize kernels in response to water deficit. Plant Physiology 131: 568–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinselmeier C, Jeong BR, Boyer JS. 1999. Starch and the control of kernel number in maize at low water potentials. Plant Physiology 121: 25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinselmeier C, Lauer MJ, Boyer JS. 1995. Reversing drought-induced losses in grain yield: sucrose maintains embryo growth in maize. Crop Science 35: 1390–1400. [Google Scholar]
- Zinselmeier C, Sun YJ, Helentjaris T, Beatty M, Yang S, Smith H, Habben J. 2002. The use of gene expression profiling to dissect the stress sensitivity of reproductive development in maize. Field Crops Research 75: 111–121. [Google Scholar]
- Zinselmeier C, Westgate ME, Schussler JR, Jones RJ. 1995. Low water potential disrupts carbohydrate metabolism in maize (Zea mays L.) ovaries. Plant Physiology 107: 385–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.