Abstract
• Background and Aims Pseudopollen is a whitish, mealy material produced upon the labella of a number of orchid species as labellar hairs either become detached or fragment. Since individual hair cells are rich in protein and starch, it has long been speculated that pseudopollen functions as a reward for visiting insects. Although some 90 years have passed since Beck first described pseudopollen for a small number of Eria spp. currently assigned to section Mycaranthes Rchb.f., we still know little about the character of pseudopollen in this taxon. The use of SEM and histochemistry would re-address this deficit in our knowledge whereas comparison of pseudopollen in Eria (S.E. Asia), Maxillaria (tropical and sub-tropical America), Polystachya (largely tropical Africa and Madagascar) and Dendrobium unicum (Thailand and Laos) would perhaps help us to understand better how this feature may have arisen and evolved on a number of different continents.
• Methods Pseudopollen morphology is described using light microscopy and scanning electron microscopy. Hairs were tested for starch, lipid and protein using IKI, Sudan III and the xanthoproteic test, respectively.
• Key Results and Conclusions The labellar hairs of all eight representatives of section Mycaranthes examined are identical. They are unicellular, clavate with a narrow ‘stalk’ and contain both protein and starch but no detectable lipid droplets. The protein is distributed throughout the cytoplasm and the starch is confined to amyloplasts. The hairs become detached from the labellar surface and bear raised cuticular ridges and flaky deposits that are presumed to be wax. In that they are unicellular and appear to bear wax distally, the labellar hairs are significantly different from those observed for other orchid species. Comparative morphology indicates that they evolved independently in response to pollinator pressures similar to those experienced by other unrelated pseudopollen-forming orchids on other continents.
Key words: Evolution, food-hairs, histochemistry, labellum, light microscopy, papillae, pollinators, pseudopollen, scanning electron microscopy, wax
INTRODUCTION
The flowers of many epidendroid orchid species are visited by insects searching for nectar, oils or droplets of fragrance (van der Pijl and Dodson, 1969; Arditti, 1992; Dressler, 1990, 1993) and it has been shown that orchid species that reward pollinators in this way often double their chances of fruiting (Neiland and Wilcock, 1998). A significant number of epidendroid orchids, however, reward potential pollinators with food-laden pseudopollen and these species tend not to produce nectar (van der Pijl and Dodson, 1969).
Pseudopollen is a mealy substance and is usually formed by the fragmentation of uniseriate, multicellular, labellar trichomes into individual component cells or short chains of cells. Some food-hairs, however, simply become detached from the labellar surface. Species that produce pseudopollen are to be found in the genera Maxillaria Ruiz & Pav. (Janse, 1886; Porsch, 1905; van der Pijl and Dodson, 1969; Davies and Winters, 1998; Davies et al., 2000, 2003a; Davies and Turner, 2004a), Polystachya Hook. (Porsch, 1906; Beck, 1914; Davies et al., 2002), Dendrobium Sw. (Kjellsson and Rasmussen, 1987; Davies and Turner, 2004b) and Eria Lindl. (Beck, 1914). In Maxillaria and Polystachya section Polystachya, pseudopollen is formed by fragmentation of moniliform trichomes. Other species of Polystachya possess unicellular or 2–4-celled, uniseriate food-hairs with a clavate or sub-clavate terminal cell or have bristle-like hairs with a tapering or fusiform terminal cell and here, the complete hair often becomes detached from the labellum (Davies et al., 2000, 2002, 2003a; Davies and Turner, 2004a). The pseudopollen-forming labellar hairs of Dendrobium unicum Seidenf., however, are really quite different, comprising a multicellular ‘head’ arising from a ‘stalk’ cell. Here, pseudopollen is formed by the fragmentation of the ‘head’ into individual or small clusters of cells called ‘granulae’ (Kjellsson and Rasmussen, 1987; Davies and Turner, 2004b).
Pseudopollen is usually rich in protein but starch is also often present and occasionally a little lipid (Davies et al., 2000, 2002, 2003a; Davies and Turner, 2004a). The protein may occur in discrete protein bodies as in Maxillaria (Davies et al., 2000, 2003a; Davies and Turner, 2004a) but is frequently distributed throughout the cytoplasm as in Polystachya (Davies et al., 2002) and D. unicum (Davies and Turner, 2004b). Protein, therefore, is the main food reserve found in pseudopollen. However, an exception to this general rule occurs in D. unicum. In this species, the main food material is starch not protein and, whereas the pseudopollen of the other orchid species hitherto examined often have numerous small amyloplasts, each containing several grains of starch, the component cells of D. unicum each contain a single, relatively large starch grain (Davies and Turner, 2004b). Although it has generally been assumed that pseudopollen functions in the rewarding of potential pollinators, Vogel (1979) has argued that pseudopollen devoid of food material can still attract insects solely by deceit. Meliponini (stingless bees) and halictid bees are the main pollinators of Maxillaria and Polystachya spp., respectively (Goss, 1977; Petterrson and Nilsson, 1993; Singer and Cocucci, 1999; Roubik, 2000) but, unfortunately, records of insects actually gathering pseudopollen are rare. Nevertheless, old records report that euglossine bees have been observed collecting pseudopollen from Maxillaria flowers (Dodson and Frymire, 1961; Dodson, 1962). More recently, Trigona spp. (meliponini) have been observed collecting hairs, presumably pseudopollen, from the labella of M. ochroleuca Lodd. ex Lindl. and M. brasiliensis Brieger & Bicalho (Singer, 2003; Singer and Koehler, 2004). Moreover, Davies and Turner (2004b) have suggested that the pseudopollen of D. unicum is gathered by small eusocial bees since the main food here is starch and starch alone is unlikely to satisfy the nutritional requirements of solitary bees. Furthermore, as pollen is not used by wasps, these insects are unlikely to visit pseudopollen-producing flowers. Our knowledge of the pollination biology of Eria is even more vague and it has been speculated that beetles (Beck, 1914) or small bees (Dressler, 1990) may pollinate these flowers.
Beck (1914) was the first to study the pseudopollen of Eria in any detail. He examined E. monostachya Lindl. var. pleiostachya Beck & Lerchen and E. paniculata Lindl., both of which are currently placed in section Mycaranthes Rchb.f. This section, which contains about 20 species (Seidenfaden, 1982), is well represented in Sumatra and Borneo with a few species reaching Thailand, Indochina, New Guinea, the Philippines and Malaya. Species assigned to this section tend to be non-pseudobulbous mountain epiphytes but some are lowland plants. They have fairly long, thick (but not fleshy), leafy stems with narrow, lanceolate leaves. Usually three or four terminal or sub-terminal inflorescences arise together and these are covered with short woolly hairs. Each inflorescence bears numerous, small flowers with widely spreading tepals, tomentose on the outer surface, and a labellum with well-developed side lobes. The labellum has a farinaceous median ridge connecting a higher callus to a large mealy callus on the mid-lobe and the column-foot is relatively long (Seidenfaden, 1982; Seidenfaden and Wood, 1992).
Beck (1914), in describing E. monostachya var. pleiostachya, claimed that the part of the labellum that produces pseudopollen lacks a cuticle and that epidermal cells swell early in the development of pseudopollen. The papillae thus formed are vacuolate with a little peripheral cytoplasm, contain nuclei and become ellipsoid, pyriform or clavate. The base of each papilla has a short ‘stalk’. Soon, starch grains develop within the papillae but these may be absent. The papillae now measure some 30–92 µm × 30–40 µm although most are 50–60 µm in length and have a very characteristic cuticle of fine, wavy ‘lines’. Beck also reported that the ‘stalk’ is very fragile and that the papillae are detached by passing insects. His histochemical tests showed that whereas the swollen part of the papilla stained for cellulose with chlorzinc iodide (Schulze's solution), the ‘stalk’ did not and therefore must have had a different chemical composition. Histochemical tests did not reveal the presence of sugars within the pseudopollen. Furthermore, Beck proposed that during the course of evolution, pseudopollen gradually replaced nectar in these flowers and that only relatively large insects, probably beetles, attracted by scent, could possibly pollinate the flower since the reproductive organs occur some 2·5 mm above the pseudopollen. As only herbarium (presumably dried and pressed) material of E. paniculata was available to Beck, he could not be certain that the subject of his study was actually this species. However, the structure and detachment of pseudopollen here was similar to E. monostachya. The papillae measured 55–88 µm in length and contained much starch, as indeed did the pseudopollen of E. stricta Lindl.
The aim of the present paper is to examine the range of pseudopollen morphology found within Eria section Mycaranthes based on a greater number of taxa and to compare the results with those obtained by Beck (1914). Scanning electron microscopy (SEM) studies, coupled with histochemical analyses at light microscopy level, would perhaps better allow us to speculate as to what the pollinators could be. Finally, since Eria spp. and D. unicum occur exclusively in Asia, whereas Maxillaria spp. grow solely in the American tropics and subtropics and Polystachya spp. occur largely in tropical Africa and Madagascar with some representatives in southern Africa, Asia, Australia and central South America, comparison of their pseudopollen could perhaps yield useful information about the way this feature may have arisen and evolved in a number of unrelated genera on different continents.
MATERIALS AND METHODS
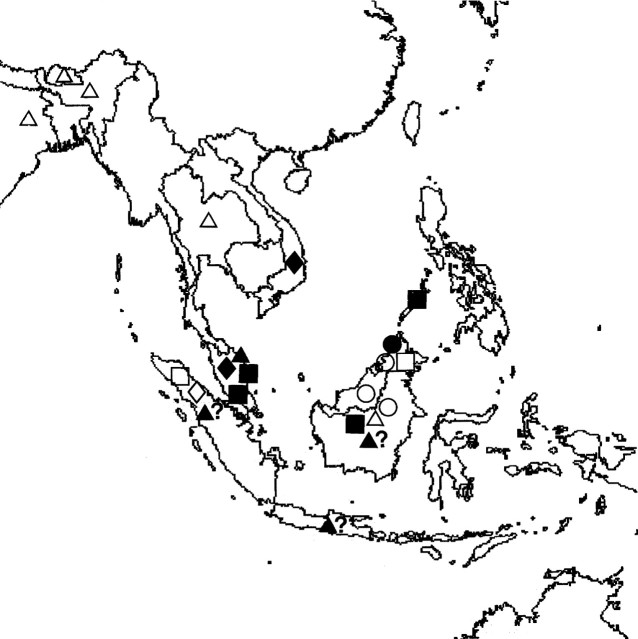
Twenty-eight samples of spirit-preserved flowers representing eight species of Eria currently assigned to section Mycaranthes (Table 1) and collected from a number of localities in south-east Asia (Fig. 1) were examined. Those specimens whose accession numbers are prefixed ‘K’ were obtained from the herbarium of the Royal Botanic Gardens, Kew, UK, whereas those prefixed ‘SBG’ were obtained from the Singapore Botanic Gardens. The former were kept in ‘Copenhagen mix’ (70 cm3 industrial methylated spirit : 2 cm3 glycerol : 28 cm3 water) but as some of these specimens were collected as long ago as 1929, many had formerly been stored in a range of preservatives that contained formalin. The specimens obtained from the Singapore Botanic Gardens, however, were collected much more recently, sent to us in and subsequently stored in 5 % formalin. The authorities for plant names follow Brummitt and Powell (1992).
Table 1.
Specimens examined and their provenance
| Taxon |
Accession no.* |
Collector |
Collector no. |
Date collected |
Provenance |
|---|---|---|---|---|---|
| E. citrina Ridl. | K18306·000 | Malay Peninsula | |||
| E. citrina Ridl. | K29047·683 | Palawan Botanical Expedition | 126 | Palawan. Philippines | |
| E. citrina Ridl. | K43294·000 | Lewis G.P. | 147 | 1977 | Terengganu, Malaysia |
| E. citrina Ridl. | K55329·000 | Lamb A. | AL 1185/89 | 1988 | Gunung Trus Madi, Malaysia |
| E. citrina Ridl. | K6132·000 | Borneo | |||
| E. citrina Ridl. | K63637·000 | de Vogel E.F. | 842 | Kalimantan Sangkuliran Timur, Malaysia | |
| E. citrina Ridl. | K70200·000 | Ng Y.P. | 1999 | ||
| E. iridifolia Hook.f. | K48356·000 | Bailes C., Cribb P. | 712 | Sabah | |
| E. iridifolia Hook.f. | K56037·000 | Wood J.J. | 947 | 1990 | North Sumatra |
| E. monostachya Lindl. | K24885·000 | ||||
| E. monostachya Lindl. | K32253·000 | near Mt Kinabalu, Malaysia | |||
| E. obliqua (Lindl.) Lindl. | K62046·000 | Chan C.L. | 2000 | Malaysia | |
| E. obliqua (Lindl.) Lindl. | K43152·000 | Lewis G.P. | 287 | 1977 | Sarawak |
| E. obliqua (Lindl.) Lindl. | K56799·000 | de Vogel E.F., Cribb P. | 9043 | 1991 | E. Kalimantan Apokayan, Malaysia |
| E. obliqua (Lindl.) Lindl. | SBG 04199 | Vermeulen J.J. & Lamb A. | 2003 | Sabah, Malaysia | |
| E. oblitterata (Blume) Rchb.f. | K20105·000 | Courtauld (cult.) | 1940 | Indonesia | |
| E. oblitterata (Blume) Rchb.f. | SBG 00459 | Heok Hui Tan | 2000 | Pahang, Malaysia | |
| E. paniculata Lindl. | K18320·000 | Kalimpong, Bengal | |||
| E. paniculata Lindl. | K18323·000 | Assam | |||
| E. paniculata Lindl. | K18325·000 | 1958 | Borneo | ||
| E. paniculata Lindl. | K20109·000 | Mrs Rothschild (cult.) | |||
| E. paniculata Lindl. | K21306·000 | 1958 | Borneo | ||
| E. paniculata Lindl. | K21308·000 | Bhutan | |||
| E. paniculata Lindl. | K47852·000 | Menzies, Du Puy | 394 | 1983 | Loei Prov., Thailand |
| E. ridleyi Rolfe | K13608·000 | Vietnam | |||
| E. ridleyi Rolfe | K18303·000 | Carr C.E. | K 119 | 1929 | Pahang, Fraser Hill, Malaysia |
| E. ridleyi Rolfe | K26178·000 | ||||
| E. tjadasmalangensis J.J. Sm. | K56038·000 | Wood J.J. | 924 | 1990 | North Sumatra |
The prefixes indicate where the material was obtained: ‘K’, the Royal Botanic Gardens, Kew; ‘SBG’, the Singapore Botanic Gardens.
Fig. 1.
Map of south-east Asia showing approximate distribution of Eria specimens examined: E. citrina, filled squares; E. iridifolia, open squares; E. monostachya, filled circles; E. obliqua, open circles; E. oblitterata, filled triangles; E. paniculata, open triangles; E. ridleyi, filled diamonds; E. tjadasmalangensis, open diamonds.
Scanning electron microscopy
Labella were transferred to and stored in tubes of 70 % (v/v) ethanol. They were dehydrated in 90 % (v/v) ethanol (15 min at room temperature) followed by two changes of 100 % ethanol (30 min each at room temperature) and subjected to critical-point drying (Balzers 030 CPD) using liquid CO2. The specimens were then mounted on stubs by means of double-sided carbon adhesive tabs, coated with gold (Edwards S150B sputter coater) and examined using a JSM 5200 LV-SEM at an accelerating voltage of 20 kV.
Histochemistry
Pseudopollen was tested for starch using a dilute iodine/potassium iodide (IKI) solution, for lipids using a saturated ethanolic solution of Sudan III and for protein by means of the xanthoproteic test as outlined in our previous papers (Davies et al., 2000, 2003a, b).
RESULTS
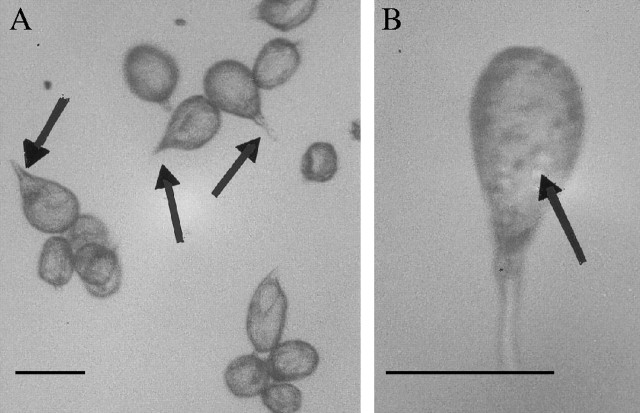
Light microscopy revealed that pseudopollen of all species of Eria section Mycaranthes examined is very similar in form in that the labellar trichomes are unicellular and clavate with ‘stalks’ of varying lengths. Moreover, these hairs easily become detached from the surface of the labellum (Fig. 2A). A number of small spherical cytoplasmic organelles are visible within the swollen tip of each hair, even in the unstained state (Fig. 2B). In common with D. unicum and pseudopollen-forming species of Maxillaria and Polystachya, those species that have been observed in vivo by earlier authors appear not to produce nectar.
Fig. 2.
Unstained light microscopy preparations of dispersed pseudopollen of (A) E. paniculata K47852·000 and (B) E. monostachya K24885·000 showing hair ‘stalks’ and amyloplasts (arrows), respectively. Scale bar = 50 µm.
Examination by SEM further revealed that the labellar trichomes, regardless of species, are striated (Figs 3A–C and 4A–E) and that these raised cuticular ridges tend to be shorter and more densely arranged towards the apex of the hair (Fig. 4C and E). Furthermore, the apices often have a flaky appearance, possibly due to wax deposits (Fig. 4A and B) and both apices and ridges stained intensely with Sudan III indicating high lipid content.
Fig. 3.
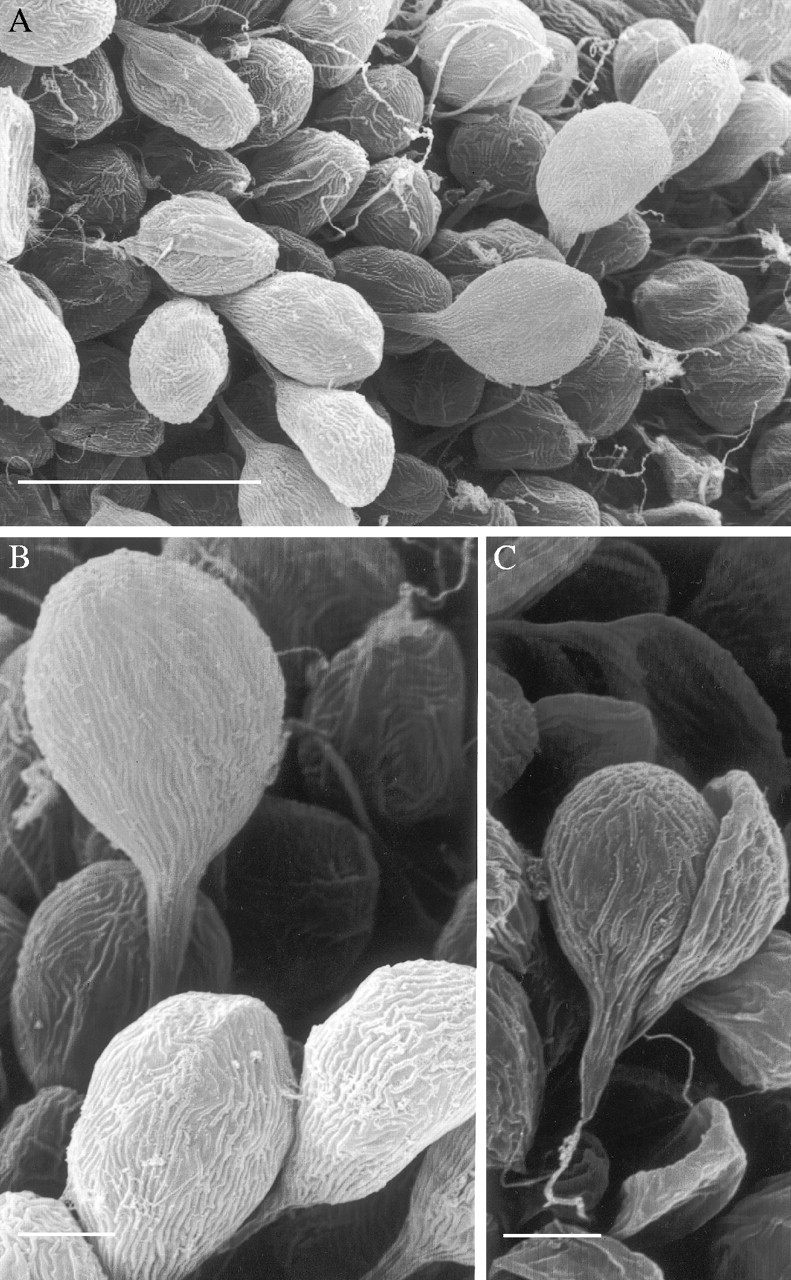
SEM studies of clavate, unicellular labellar hairs of (A) E. paniculata K20109·000 showing (B) cuticular ridges and pronounced flaky deposits. Trichome of E. paniculata K18323·000 (C) becoming detached from labellar surface. Note the associated filamentous structure at the base of the trichome. An abundance of such structures (see Fig. 3A) may account for earlier reports that, on dispersal of pseudopollen, the labellum becomes ‘hairy’ (Beck, 1914). Scale bars: A = 50 µm; B and C = 10 µm.
Fig. 4.
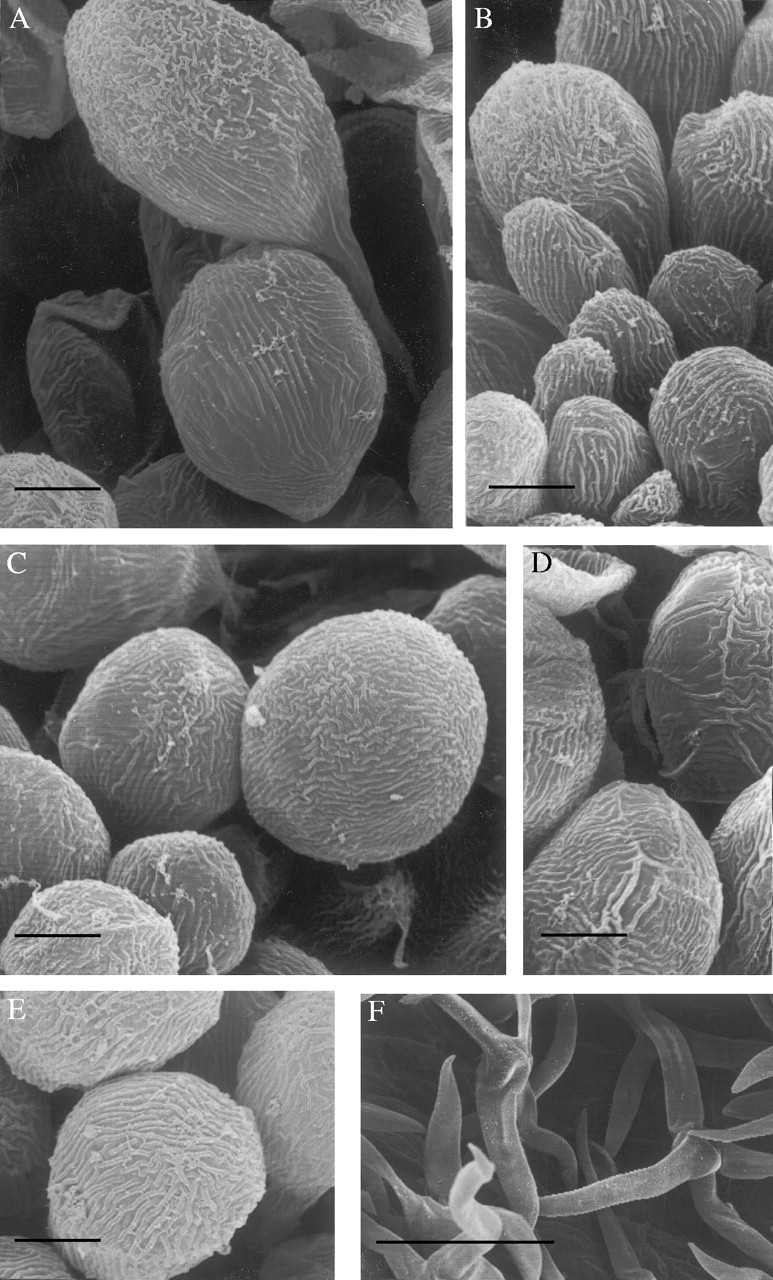
SEM studies of unicellular, labellar hairs of (A) E. oblitterata K20105·000, (B) E. ridleyi K13608·000, (C) E. citrina K63637·000, (D) E. iridifolia K48356·000 and (E) E. monostachya K24885·000, showing cuticular ridges and flaky deposits. Multicellular, branched hairs of E. ridleyi K26178·000 (F) as found on the reverse surfaces of tepals and upon the pedicellate ovary. Scale bars: A–E = 10 µm; F = 100 µm.
Histochemistry also showed that the labellar hairs are rich in aromatic amino acids and starch grains (Table 2). The former are distributed throughout the cytoplasm, whereas the latter are confined to amyloplasts that correspond in shape, size and position to the small spherical organelles visible in unstained preparations. Lipid droplets were not detected in the labellar hairs of any species, regardless of whether the flowers were preserved in solutions containing alcohol or formalin.
Table 2.
Histochemical analysis of labellar trichomes: foods present in trichomes
| Taxon |
Accession no.* |
Protein |
Starch |
Lipid |
|---|---|---|---|---|
| E. citrina Ridl. | K18306·000 | + | + | − |
| E. citrina Ridl. | K6132·000 | + | + | − |
| E. iridifolia Hook.f. | K48356·000 | + | + | − |
| E. monostachya Lindl. | K32253·000 | + | + | − |
| E. obliqua (Lindl.) Lindl. | K43152·000 | + | + | − |
| E. obliqua (Lindl.) Lindl. | SBG 04199 | + | + | − |
| E. oblitterata (Blume) Rchb.f. | K20105·000 | + | + | − |
| E. oblitterata (Blume) Rchb.f. | SBG 00459 | + | + | − |
| E. paniculata Lindl. | K18320·000 | + | + | − |
| E. paniculata Lindl. | K20109·000 | + | + | − |
| E. ridleyi Rolfe | K13608·000 | + | + | − |
| E. ridleyi Rolfe | K26178·000 | + | + | − |
| E. tjadasmalangensis J.J. Sm. | K56038·000 | + | + | − |
The prefixes indicate where the material was obtained: ‘K’, the Royal Botanic Gardens, Kew; ‘SBG’, the Singapore Botanic Gardens.
Characteristic multicellular, branched hairs were observed on the reverse of the tepals and upon the pedicellate ovary of all species (Fig. 4F).
DISCUSSION
Comparative morphology and histochemistry
Despite their wide geographical distribution, the pseudopollen of all eight taxa showed considerable uniformity, in as much as it was equally difficult to distinguish between the pseudopollen of well-defined species (Figs 3A–C and 4C–E) as it was to distinguish between that of taxa such as E. oblitterata (Blume) Rchb.f. (Fig. 4A) and E. ridleyi Rolfe (Fig. 4B) which are considered by some authors to be conspecific (Seidenfaden and Wood, 1992). Consequently, pseudopollen in Eria section Mycaranthes is a highly conservative character and is thus of little value in taxonomy at species level. Indeed, data relating to pseudopollen alone would support the concept that these taxa form a species complex. Seidenfaden (Seidenfaden, 1982; Seidenfaden and Wood, 1992) states that Mycaranthes is well separated on morphological grounds from all other sections of Eria and that several of its species are morphologically very variable.
It would appear that in the specimens examined here, unicellular, clavate, pseudopollen-forming hairs develop from papillae as described by Beck (1914). This is supported by the presence of cuticular ridges and flaky deposits upon both trichomes and papillae. The multicellular, moniliform, pseudopollen-forming hairs of M. sanderiana Rchb.f. also arise in like manner (Davies et al., 2000), although it is speculated that such hairs may have evolved in the M. discolor (Lodd. ex Lindl.) Rchb.f. alliance from simple uniseriate, multicellular trichomes (Davies et al., 2003a).
In Eria, pseudopollen is formed as clavate hairs become detached from the labellar surface. These pseudopollen-forming hairs are very different from those found in Maxillaria, Polystachya and Dendrobium in that they are unicellular, not multicellular. Moreover, unlike the pseudopollen-forming hairs of Maxillaria and Dendrobium, they do not fragment, rather, the complete hair becomes detached from the surface of the labellum as occurs in certain species of Polystachya. Indeed, these hairs are very similar in shape to those of P. campyloglossa Rolfe. Also, the hairs are rich in aromatic amino acids, and protein forms the main food reserve here as in Maxillaria and Polystachya although, unlike the former genus, the protein in Eria does not occur in a well-defined protein-body, but is distributed throughout the cytoplasm as in Polystachya spp. and D. unicum. Starch is also present within the pseudopollen of Eria and occurs in numerous small amyloplasts, as in Maxillaria and Polystachya spp., unlike D. unicum where each component cell contains a single, relatively large starch grain (Kjellsson and Rasmussen, 1987; Davies and Winters, 1998; Davies et al., 2000, 2002, 2003a; Davies and Turner, 2004a, b).
The pseudopollen-forming hairs of the Eria spp. examined are significantly different from those hitherto described for other orchid genera in that they bear pronounced cuticular ridges and possibly wax deposits. These ridges closely resemble the waxy striations found upon the labellar papillae of M. cerifera Barb. Rodr. (Senghas, 1993). Labellar wax in Maxillaria is generally thought to be gathered by meliponini (Flach et al., 2004) and may perhaps be used for nest-building (van der Pijl and Dodson, 1969). D. W. Roubik (pers. comm.) remarks that if this is true, it would be ironic since meliponini, unlike euglossine and halictid bees, are capable of making their own wax. Lipoidal material also occurs upon the labella of other Maxillaria spp. closely related to M. cerifera, but here the secretion has a more resinous consistency and probably also has a nutritive function since it is rich in lipids and aromatic amino acids (Davies et al., 2003b). A similar lipoidal, protein-rich, resin-like material also occurs upon the labella of certain members of the M. discolor and M. rufescens Lindl. alliances and this too may function as a reward (Davies et al., 2003a; Davies and Turner, 2004a). Flach et al. (2004) have recently analysed this material and report that triterpenoids form the major class of compound present. Von Kirchner (1925) also described a tough, thick and mucilaginous mass not unlike rubber in appearance and consistency upon the labellum of E. vulpina Rchb.f. [now Trichotosia vulpina (Rchb.f.) Kraenzl.] and this was associated with a glossy, varnish-like material. This substance reacted in similar manner to the resin-like material found in certain Maxillaria spp. (Davies et al., 2003a, b) in that it produced an intense yellow colour with KOH and concentrated sulphuric acid (cf. xanthoproteic test) and stained black and red with osmium tetroxide and Sudan-glycerin, respectively.
No lipid droplets were detected within the cytoplasm of our material when pseudopollen-forming hairs were treated with Sudan III. Indeed, it had initially been feared that preserving flowers in spirit may have dissolved and leached out any lipids that might have originally been present. Historically, our material had been fixed in a range of mixtures containing formalin and then, in recent years, stored in Copenhagen mix—a preservative that consists mainly of alcohol. Although it is acknowledged that intracellular substances, in particular electrolytes, can be leached from tissue or translocated during fixation (Hayat, 1981; Coetzee and van der Merwe, 1984), it is now strongly felt that, had the material originally contained lipids, sufficient amounts of this substance would have remained and would have been detected by histochemical means. Our reasons for this are as follows: Firstly, formalin does not dissolve lipids (Bancroft, 1967) and in fact is an excellent preservative of phospholipids (Johansen, 1940). Furthermore, flowers of E. oblitterata and E. obliqua (Lindl.) Lindl. preserved in aqueous formalin solution, when compared with spirit-preserved material, yielded identical histochemical results and, in both cases, the lipid-rich, flaky deposits remained intact. Similarly, comparison of fresh material with spirit-preserved material of members of the M. acuminata Lindl. alliance indicated that storage in alcohol has little effect on intracellular lipid and this is confirmed by TEM. Here, sections of fixed labellar material clearly show globular, intracellular lipid bodies following dehydration in ethanol (Davies et al., 2003b). Members of this alliance often produce a viscid labellar secretion rich in lipids and protein and, although again it had been anticipated that prolonged preservation in 70 % ethanol would dissolve this seemingly delicate film, this was not the case. Indeed, subsequent staining with Sudan III and the xanthoproteic test revealed that the film remained intact and that the lipids and protein present in the living flower stained equally intensely after several years of storage in ethanol. Similar results have been obtained for a range of other orchid species fixed and stored in various combinations of formalin and ethanol (K. L. Davies, unpubl. res.). As a result, it is very likely that the pseudopollen of Eria spp. did not contain high lipid levels in vivo. This agrees with results obtained for living tissue samples from most of the orchid taxa studied to date (Davies et al., 2000, 2002, 2003a; Davies and Turner, 2004a, b). In short, lipid-rich surface deposits, protein and starch had been preserved in all specimens of Eria tested, indicating that conventional preservation methods were adequate and that material kept in this manner could still be used successfully for histochemical analyses even after some 60 years of preservation in fluid!
However, the presence of food materials alone is not sufficient evidence that labellar hairs function as pseudopollen. For example, E. pilifera Ridl. has unicellular, clavate hairs not unlike those observed for Eria spp. assigned to section Mycaranthes, yet, although the cytoplasm of this species is rich in protein and numerous small amyloplasts containing starch, it appears that the hairs do not become detached and therefore cannot function as pseudopollen (K. L. Davies, unpubl. res.). Even so, this does not preclude the possibility that insect pollinators may nibble at the hairs for the food they contain.
Pollination biology
Current knowledge of the pollination biology of Eria spp. is poor. Beck (1914), basing his argument on the relative dimensions and position of floral parts, speculated that Eria flowers may be pollinated by beetles. However, Dressler (1990) suggested that the pollinators are small bees; a view in keeping with the fact that most pseudopollen-forming flowers examined to date are either pollinated by melponini or halictid bees (Goss, 1977; Pettersson and Nilsson, 1993; Singer and Cocucci, 1999; Roubik, 2000; Singer, 2003; Singer and Koehler, 2004). This may well be the case since the labellar hairs of Eria spp. and certain species of Polystachya, a genus known to be pollinated by halictid bees, are very similar and stain identically when tested for protein and starch. Moreover, the presence of presumed wax deposits upon the labellar hairs would also tend to argue strongly in favour of bee pollination, although, at present, there is no unequivocal evidence to support this. Wasps, however, probably do not pollinate Eria spp. since these insects do not utilize pollen and are thus unlikely to be attracted by pseudopollen. Nevertheless, it is possible that the branched multicellular hairs that occur on the reverse of tepals and upon the pedicellate ovary may also be involved in attracting/rewarding insects. These hairs easily become detached and in the case of fluid-preserved flowers, form a dense layer at the base of the specimen tube. In life, it is possible that such hairs may be gathered by insects and used for nest-building but again, evidence for this is lacking.
Ecological considerations
Representatives of Eria section Mycaranthes are restricted to south-east Asia and pseudopollen is invariably formed in these species as unicellular, clavate trichomes become detached from the labellum. These hairs contain protein that is distributed throughout the cytoplasm and starch that occurs within amyloplasts. The most remarkable feature, however, is that the pseudopollen bears flaky, wax-like deposits upon its surface. Thus, the pseudopollen of Eria spp. is very different from that observed to date for other orchids such as Maxillaria (American tropics and subtropics) (Davies et al., 2000, 2003a; Davies and Turner, 2004a), Polystachya (tropical Africa and Madagascar with some species in southern Africa, Australia and central South America) (Porsch, 1906; Beck, 1914; Davies et al., 2002) and D. unicum (Laos and Northern Thailand) (Kjellson and Rasmussen, 1987; Davies and Turner, 2004b).
Pseudopollen of diverse morphology in unrelated taxa occurring on different continents would indicate that this character is not homologous and arose independently in response to similar pollinator pressures. Moreover, differences in pseudopollen structure and the foods it contains, as well as the possible presence of wax, may confer greater pollinator selection or even allow pollination by a larger number of insect species—a matter that can only be resolved by intensive field work. However, the occurrence of moniliform, pseudopollen-forming hairs in Maxillaria and in species assigned to section Polystachya such as P. concreta (Jacq.) Garay & H.R. Sweet which occurs both in Africa and tropical America would indicate a degree of convergence. Consequently, it would be useful to establish whether the same insect species pollinate P. concreta on both continents.
In the past, orchidologists generally viewed all insect visitors as potential pollinators, regardless of whether or not they were actually observed transferring pollinia. Similarly, although great strides have already been made with respect to identifying pseudopollen-foraging insects and relating them to named orchid taxa, large gaps in our knowledge still remain. For example, although meliponini have been seen gathering pseudopollen from a small number of species, it is still not known for certain whether it is actually ingested or indeed how it is used. Nor have the energy requirements of producing pseudopollen yet been considered in terms of the reproductive success of orchids or whether the energy source it contains is sufficient for the needs of the insect or the colony. Meliponini seemingly also gather wax from the labella of Maxillaria spp. (Flach et al., 2004), but whether insects gather the pseudopollen of Eria spp. for the presumed waxy deposits it bears is not known. It may simply be that the latter protect the pseudopollen from desiccation or, alternatively, by reducing the wettability of the pseudopollen, aid its dispersal. If eventually it can be proven unequivocally that insects gather wax from the labella of orchids then this, in turn, would pose yet other problems since it is known that meliponini and honey bees can make their own wax, whereas euglossine and halictid bees neither make wax nor use it for nest building (D. W. Roubik, pers. comm.). Thus, much work remains to be done, and until it is possible to relate the micromorphology and nutritional value of labellar hairs to the behaviour of potential pollinators it will not be possible to understand fully the significance of pseudopollen and the evolutionary advantage it confers.
Acknowledgments
The authors are grateful to the Stanley Smith (UK) Trust for partly funding the project and to E. Tredwell (Royal Botanic Gardens Kew) and J. Vermeulen (Singapore Botanic Gardens) for the specimens required for this study. The authors also acknowledge the help of P. O'Byrne, Y. P. Ng, the staff of the National Museum and Gallery of Wales (Cardiff, UK) and A. Gregg (Swansea Botanical Complex, UK).
LITERATURE CITED
- Arditti J. 1992.Fundamentals of orchid biology. New York: John Wiley & Sons. [Google Scholar]
- Bancroft JD. 1967.An introduction to histochemical technique. London: Butterworth & Co. [Google Scholar]
- Beck G. 1914. Die Pollennachahmung in den Blüten der Orchideen-Gattung Eria. Sitzungs Berichte Akadamie der Wissenschaften in Wien 123: 1033–1046. [Google Scholar]
- Brummitt RK, Powell CE. 1992.Authors of plant names. Kew: Royal Botanic Gardens. [Google Scholar]
- Coetzee J, van der Merwe CF. 1984. Extraction of substance during glutaraldehyde fixation of plant cells. Journal of Microscopy 135: 147–158. [Google Scholar]
- Davies KL, Turner MP. 2004. Morphology of floral papillae in Maxillaria Ruiz & Pav. (Orchidaceae). Annals of Botany 93: 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KL, Turner MP. 2004. Pseudopollen in Dendrobium unicum Seidenf. (Orchidaceae): reward or deception? Annals of Botany 94: 129–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KL, Winters C. 1998. Ultrastructure of the labellar epidermis in selected Maxillaria species (Orchidaceae). Botanical Journal of the Linnean Society 126: 349–361. [Google Scholar]
- Davies KL, Roberts DL, Turner MP. 2002. Pseudopollen and food-hair diversity in Polystachya Hook. (Orchidaceae). Annals of Botany 90: 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KL, Turner MP, Gregg A. 2003. Atypical pseudopollen-forming hairs in Maxillaria (Orchidaceae). Botanical Journal of the Linnean Society 143: 151–158. [Google Scholar]
- Davies KL, Turner MP, Gregg A. 2003. Lipoidal labellar secretions in Maxillaria (Orchidaceae). Annals of Botany 91: 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies KL, Winters C, Turner MP. 2000. Pseudopollen: its structure and development in Maxillaria (Orchidaceae). Annals of Botany 85: 887–895. [Google Scholar]
- Dodson CH. 1962. The importance of pollination in the evolution of the orchids of tropical America. American Orchid Society Bulletin 31: 525–534, 641,–649, 731–735. [Google Scholar]
- Dodson CH, Frymire GP. 1961. Natural pollination of orchids. Missouri Botanical Garden Bulletin 49: 133–139. [Google Scholar]
- Dressler RL. 1990.The orchids: natural history and classification. London: Harvard University Press. [Google Scholar]
- Dressler RL. 1993.Phylogeny and classification of the orchid family. Cambridge, MA, Dioscorides Press. [Google Scholar]
- Flach A, Dondon RC, Singer RB, Koehler S, Amaral M Do Carmo E, Marsaioli AJ. 2004. The Chemistry of pollination in selected Brazilian Maxillariinae orchids: floral rewards and fragrance. Journal of Chemical Ecology 30: 1045–1056. [DOI] [PubMed] [Google Scholar]
- Goss GJ. 1977. The reproductive biology of the epiphytic orchids of Florida 6. Polystachya flavescens (Lindley) J.J. Smith. American Orchid Society Bulletin. 46: 990–994. [Google Scholar]
- Hayat MA. 1981.Fixation for electron microscopy. New York: Academic Press. [Google Scholar]
- Janse JM. 1886. Imitirte pollenkörner bei Maxillaria sp. Deutsche Botanische Gesellschaft Berichte 4: 277–283. [Google Scholar]
- Johansen DA. 1940.Plant micro-technique. New York: McGraw-Hill Book Co. [Google Scholar]
- Kjellsson G, Rasmussen FN. 1987. Does the pollination of Dendrobium unicum Seidenf. involve pseudopollen? Die Orchidee 38: 183–187. [Google Scholar]
- Neiland MR, Wilcock CC. 1998. Fruit set, nectar reward and rarity in the Orchidaceae. American Journal of Botany 85: 1657–1671. [PubMed] [Google Scholar]
- Pettersson B, Nilsson LA. 1993. Floral variation and deceit pollination in Polystachya rosea (Orchidaceae) on an inselberg in Madagascar. Opera Botanica 121: 237–245. [Google Scholar]
- Porsch O. 1905. Beiträge zur ‘histologischen’ Blütenbiologie I. Österreichische Botanische Zeitschrift 55: 253–260. [Google Scholar]
- Porsch O. 1906. Beiträge zur ‘histologischen’ Blütenbiologie II. Oesterreichische Botanische Zeitschrift 56: 41–47, 83,–95, 125,–143, 176–185. [Google Scholar]
- Roubik DW. 2000. Deceptive orchids with Meliponini as pollinators. Plant Systematics and Evolution 222: 271–279. [Google Scholar]
- Seidenfaden G. 1982. Orchid Genera in Thailand. X. Trichotosia Bl. and Eria Lindl. Opera Botanica 62: 24–25, 67–72. [Google Scholar]
- Seidenfaden G, Wood JJ. 1992.Orchids of peninsular Malaysia and Singapore. Fredensborg, Denmark: Olsen & Olsen. [Google Scholar]
- Senghas K. 1993. Subtribus Maxillariinae. In: Breiger FG, Maatsch R, Senghas K. eds. Rudolph Schlechter: Die Orchideen, Vol. 28. Berlin: Blackwell Wissenschafts-Verlag, 1727–1776. [Google Scholar]
- Singer RB. 2003. Orchid pollination: recent developments from Brazil. Lankesteriana 7: 111–114. [Google Scholar]
- Singer RB, Cocucci AA. 1999. Pollination mechanisms in four sympatric southern Brazilian Epidendroideae orchids. Lindleyana 14: 47–56. [Google Scholar]
- Singer RB, Koehler S. 2004. Pollinarium morphology and floral rewards in Brazilian Maxillariinae (Orchidaceae). Annals of Botany 93: 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pijl L, Dodson CH. 1969.Orchid flowers: their pollination and evolution. Coral Gables, FL: University of Miami Press. [Google Scholar]
- Vogel S. 1979. Evolutionary shifts from reward to deception in pollen flowers. In: Richards AJ, ed. The pollination of flowers by insects. London: Academic Press, 89–96. [Google Scholar]
- von Kirchner O. 1925. Uber die sogenannten Pollenblumen und die Ausbeutestoffe der Blüten. Flora 118/119: 312–330. [Google Scholar]