Abstract
• Background and Aims Plants adjust the distribution of photosynthetic capacity and chlorophyll to canopy density. The importance of the gradient in the red : far-red ratio (R : FR) relative to the irradiance gradient was studied for its perception with respect to this partitioning of photosynthetic resources. Whether the relative importance of these two signals varied between six species of different growth habit (Phaseolus vulgaris, Lysimachia vulgaris, Hedera helix, Ficus benjamina, Carex acutiformis and Brachypodium pinnatum) was investigated further.
• Methods Single leaves of plants were shaded in daylight by a spectrally neutral filter or a leaf. In another experiment, leaves were treated with supplemental FR. In most cases, treatment effects were evaluated after 2 weeks.
• Key Results Nitrogen and photosynthetic capacity (Amax) per leaf area, parameters pertaining to between-leaf resource partitioning, were strongly reduced in neutral shade but not additionally by spectral leaf shade. Supplemental FR reduced these parameters also, except in Carex. Acceleration of induction of senescence was observed in spectral leaf shade in primary bean leaves. Amax per unit chlorophyll, a parameter pertaining to within-leaf resource partitioning, was reduced in neutral shade, but not in spectral leaf shade or supplemental FR.
• Conclusions Signalling mechanisms associated with perception of the R : FR gradient in canopies were less important than those associated with the irradiance gradient for between-leaf and within-leaf partitioning of photosynthetic resources. The relative importance of the signals differed between species because Carex was the only species for which no indications were found for an involvement of the spectral gradient in perception of canopy density.
Key words: Chlorophyll, herbaceous dicotyledonous species, irradiance, leaf canopies, light gradient, monocotyledonous species, photosynthetic capacity, photosynthetic resource partitioning, red : far-red ratio, spectral quality, shade acclimation, woody species
INTRODUCTION
The partitioning of photosynthetic resources between leaves is adjusted to canopy density in many plants in such a way that upper leaves in high irradiance have high leaf nitrogen content (NLA) and photosynthetic capacity (Amax) per unit area (Hirose et al., 1988; Schieving et al., 1992; Pons et al., 1993; Rousseaux et al., 1996; Anten et al., 1998; Pons and Jordi, 1998; Lötscher et al., 2003). Plants are also plastic in the partitioning of resources within their leaves between two main functions of the photosynthetic apparatus, capacity and photon absorption (Anderson et al., 1995; Hikosaka, 1996; Murchie and Horton, 1998). Adjustment to canopy density of between-leaf partitioning of resources for photosynthetic capacity and within-leaf partitioning of photosynthetic resources in chloroplasts can both contribute to efficient utilization of these resources for whole-plant photosynthesis (Evans, 1993b; Anten et al., 2000; Pons and Anten, 2004). The question addressed here is what environmental signals are involved in the perception of canopy density with respect to both forms of partitioning?
The light gradient in canopies has a quantitative and a qualitative component. The total irradiance and the red : far-red ratio (R : FR) decrease with canopy depth (Holmes and Smith, 1977; Barkman and Stoutjesdijk, 1992). Plants can potentially use both components as a signal for canopy density. Multiple signalling pathways may be involved in the response to a single environmental factor. A relevant example is the morphological shade avoidance response in canopies where several photoreceptors act as irradiance or spectral quality detectors (Ballaré, 1999; Gyula et al., 2003; Pierik et al., 2004). It is likely that there are also multiple signalling pathways involved in density effects on resource partitioning because these can be induced by spectrally neutral irradiance gradients and spectral quality gradients independent of each other (Barreiro et al., 1992; Pons et al., 1993; Hikosaka et al., 1994; Rousseaux et al., 1996; Vos and van der Putten, 2001; Frak et al., 2001).
Perception of variation in R : FR in leaf canopies occurs through phytochrome. The involvement of this family of photoreceptors in morphogenetic shade responses is well known (Smith and Whitelam, 1997; Ballaré, 1999), but it has received relatively little attention as a possible signal involved in resource partitioning in canopies. However, processes that are relevant in this study, allocation, photosynthetic protein synthesis and particularly leaf senescence can also be under phytochrome control (Stoddart and Thomas, 1982; Rousseaux et al., 1997; Kappers et al., 1998; Pons and Jordi, 1998). It has been shown for annual dicots that the R : FR in the canopy light gradient is indeed involved in between-leaf photosynthetic resource partitioning and in the induction of senescence in lower leaves (Guiamet et al., 1989; Barreiro, 1992; Rousseaux et al., 1996, 1997, 2000). However, this leaves the possibility open that the importance of spectral signal may be different for other growth forms. Most experiments dealing with R : FR effects on plants have been carried out not with light gradients, but with a homogeneous distribution of light over a plant. Several species showed a decrease in Amax and the chlorophyll a/b ratio in homogenously low R : FR, but others did not respond (Corré, 1983; Barreiro et al., 1992; Smith et al., 1993; Tinoco-Ojanguren and Pearcy, 1995; Walter and Horton, 1995; Murchie and Horton, 1998). These species-specific R : FR effects on photosynthetic parameters indicate that the spectral gradient in canopies may be used differently by species for density perception with respect to photosynthetic resource partitioning.
Further evidence of possible species-specific differences in the relative importance of the R : FR gradient in canopies was obtained from the comparison of NLA and Amax distributions in canopies and in experimental irradiance gradients. Lysimachia vulgaris, Phaseolus vulgaris and Carex acutiformis showed strong effects in both conditions (Hirose et al., 1988; Schieving et al., 1992; Pons et al., 1993; Pons and Bergkotte, 1996; Pons and Jordi, 1998; Pons et al., 2001), which suggests that the R : FR gradient would not be important in these species. However, canopy gradients in Brachypodium pinnatum and Ficus benjamina could not be satisfactorily explained from responses to spectrally neutral effects, which lead to the hypothesis that the R : FR component would be more important for density perception in these species (Pons et al., 1993; Pons and Jordi, 1998).
This study was an investigation into the relative importance of the R : FR gradient as a signal for the partitioning of photosynthetic resources in canopies compared with the irradiance gradient. The above-mentioned five species were included for an evaluation of species-specific variation. Two levels of resource partitioning were investigated – the between-leaf re-allocation from shaded parts and the within-leaf partitioning between photosynthetic functions. Fully expanded leaves were shaded with a tobacco leaf or with a spectrally neutral filter in daylight conditions in a glasshouse. Leaves were also irradiated with supplemental FR in a growth chamber. Between-leaf resource partitioning was evaluated on the basis of measurements of leaf nitrogen and Amax, and within-leaf partitioning between photosynthetic functions on the basis of Amax per unit chlorophyll and chlorophyll a/b ratio. The species that were included have widely different growth habit and life history: two herbaceous dicotyledons (Phaseolus and Lysimachia), two monocotyledons (Brachypodium and Carex) and the evergreen woody climber Hedera helix was added so that together with the hemi-epiphytic tree Ficus, there were two evergreen woody species.
MATERIALS AND METHODS
Plant material
A climbing bean cultivar Phaseolus vulgaris L. ‘Mechelse markt’ was grown from seed and used for the experiment at 2 weeks after planting. The other species were propagated vegetatively and used for the experiments after several months of growth in a glasshouse until a suitable vegetative growth stage was achieved (see below). Lysimachia vulgaris L. was grown from over-wintering rhizomes. The evergreen woody climber Hedera helix L. was grown from cuttings obtained from the Botanical Garden of Utrecht University. Small plants of the tropical rainforest hemi-epiphyte Ficus benjamina L. ‘Exotica’ were supplied by a commercial grower. The monocotyledonous Carex acutiformis Ehrh. and Brachypodium pinnatum L. were grown from cuttings taken from fens north of Utrecht and chalk grassland in South Limburg, respectively. The plants were grown in the glasshouse in 1-L pots with potting soil and 3 g of slow release complete fertilizer (Osmocote plus; release time of 3–4 months).
The experiments
The light gradient in canopies is best simulated in quantitative as well as qualitative terms with green leaves in daylight. Two experiments were carried out in a glasshouse in natural daylight. There was no supplemental lighting, light from other compartments of the glasshouse was kept out by black curtains and full sunlight was admitted. Air temperature was thermostatically regulated at 20 °C during the day and 16 °C at night. This was the actual temperature during cloudy days, but it increased during warm sunny days up to 26 °C. Relative humidity was kept at 70 % by means of water dispersers. About 40 % of diffuse daylight was transmitted to the plants, but peak irradiances in direct sunlight (up to 2000 µmol m−2 s−1) were about 85 % from outside. Attached tobacco leaves were used to shade single leaves of the experimental plants, and they were compared with the effect of a spectrally neutral filter of the same transmission of photosynthetically active radiation (PAR).
In the first experiment carried out in May and June 2001 effects of shading single leaves were compared in the six species. The entire leaf was shaded, except in the case of Carex, where shading was applied to the upper 25 cm of the 40–50 cm long leaf blade. All leaves were removed from the tobacco plants except the one used for the shade treatment. The tobacco leaves were placed on top of the horizontally held target leaf of the experimental plants. Occasionally damaged or senescing tobacco leaves were replaced by fresh ones. The spectrally neutral shading device consisted of two layers of white paper of which the lower one was printed a shade of grey that gave the combination the same transmission as the tobacco leaf (7 % of PAR). The target leaves of the experimental plants, the covering tobacco leaves and the neutral shading screens were supported by wires that kept them in a more or less horizontal orientation. The use of white reflective paper resulted in similar leaf temperatures in direct sunlight of the shaded and unshaded leaves, which was about 4 °C above air temperature. Leaf temperature under the tobacco leaf was also similar. Daylight in the glasshouse had an R : FR photon ratio (R=655–665 nm; FR=725–735 nm) of 1·05 under overcast conditions as measured with a spectroradiometer (Licor LI-1800). Light under the neutral filter was not significantly different and light transmitted through the tobacco leaves had a R : FR of about 0·08. Target leaves also received some reflected light of high R : FR from below, but that was not evaluated. Three similar branches, shoots or tillers per plant were selected for the three treatments. Each treatment was applied to a just fully expanded leaf on a shoot. This scheme was applied to all species except Phaseolus. One treatment was applied per plant on one of the pair of primary leaves of that species. The treatment duration was 1 week for Phaseolus and 2 weeks for the other species as in earlier experiments (Pons and Bergkotte, 1996; Pons and Jordi, 1998; Pons et al., 2001). There were six replications for all species. After the treatment, Amax, area, dry mass, nitrogen concentration and chlorophyll were measured.
The experiment on spectral leaf shading effects on induction of senescence of primary Phaseolus leaves was carried out 1 year later in a way similar to the previous one. A mosquito screen was fixed underneath the spectrally neutral and leaf-shade devices in a way that the target Phaseolus leaf could easily be removed for non-destructive chlorophyll measurements. The treatment was maintained until abscission and withering of the shaded leaves.
The experiment on the effect of supplemental FR was carried out in a growth cabinet at a PFD of 250 µmol m−2 s−1 provided by a mixture of fluorescent and incandescent lamps. Plants were grown in the glasshouse and taken to the cabinet at least 2 weeks in advance, except Phaseolus that was fully grown in the cabinet. FR was irradiated from below on the abaxial side of the horizontal leaf. The FR source was made of a halogen narrow beam spotlight with aluminium reflector (Philips; Halotone B15d, 20 W) with a cut-off filter transmitting λ > 715 nm (Schott, RG-715) above it. Increase in leaf temperature (<1 °C) was prevented by putting a 7-cm water filter between lamp and filter, which blocked the longer wavelengths. Target leaves, including controls, were clamped between two circular wires and the FR spot of about 3 cm diameter was focused on the target leaf from below. FR (725–735 nm) intensity was 75 µmol m−2 s−1 as measured with a spectroradiometer. The R : FR of growth cabinet light was 3·6. The combination of the two light sources resulted in an R : FR of 0·05 at the spot of the leaf, combining the white light from above and the FR from below. Treatment durations and number of replications were the same as in the leaf shading experiment. After the treatment, measurements were taken of Amax, dry mass, nitrogen concentration and chlorophyll of the irradiated part of the leaves and control leaves that were clamped but not irradiated.
Leaf analysis and gas exchange
The light and CO2 saturated rate of photosynthesis (Amax) was determined at 120 Pa partial pressure of CO2 in air at a PFD of 1200 µmol m−2 s−1. The leaves were clamped in a Parkinson leaf chamber with a circular window of 18 mm diameter. An infra-red gas analyser (Licor LI-6262) was used to measure the difference in CO2 and H2O partial pressure. Further measurement conditions were: leaf temperature 25 °C and a leaf-to-air vapour pressure difference of about 1 kPa. Other details of the set-up were described by Pons and Welschen (2002).
Chlorophyll was determined spectrophotometrically after extraction of fresh leaf material with dimethylformamide (Inskeep and Bloom, 1985). Non-destructive chlorophyll measurements were carried out with a chlorophyll meter (SPAD-502, Minolta) that was calibrated with destructive samplings. Dried and homogenized leaf material was analysed for nitrogen with an elemental analyser (Carlo Erba, Model EA NA 1110, Milan, Italy). Nitrate was analysed in the same material using salicylic acid as a reagent (Cataldo et al., 1975). Nitrate concentrations were subtracted from total nitrogen in the case of Phaseolus, Ficus and Carex that had up to 10 atom% nitrogen as nitrate. The other species had insignificant amounts of nitrate.
RESULTS
Leaf shade
The spectrally neutral shading of a leaf in the glasshouse reduced nitrogen per leaf area (NLA) and photosynthetic capacity (Amax) relative to unshaded control leaves by 33 % and 49 %, respectively, as an average across all species (Fig. 1). Shading other leaves on the same plant by a tobacco leaf with the same transmittance for PAR had the same effect as the neutral filter on these parameters in all species (Fig. 1). This suggests that the spectral quality of shade light is not important for between-leaf resource partitioning. The shading treatments also caused a reduction in leaf mass per area (LMA) of 26 %, which contributed substantially to the effect on NLA (Fig. 1). Nitrogen per leaf mass (NLM), the other factor contributing to variation in NLA was also reduced somewhat (average 9 %) by the shading treatments in most species except Hedera and Carex.
Fig. 1.
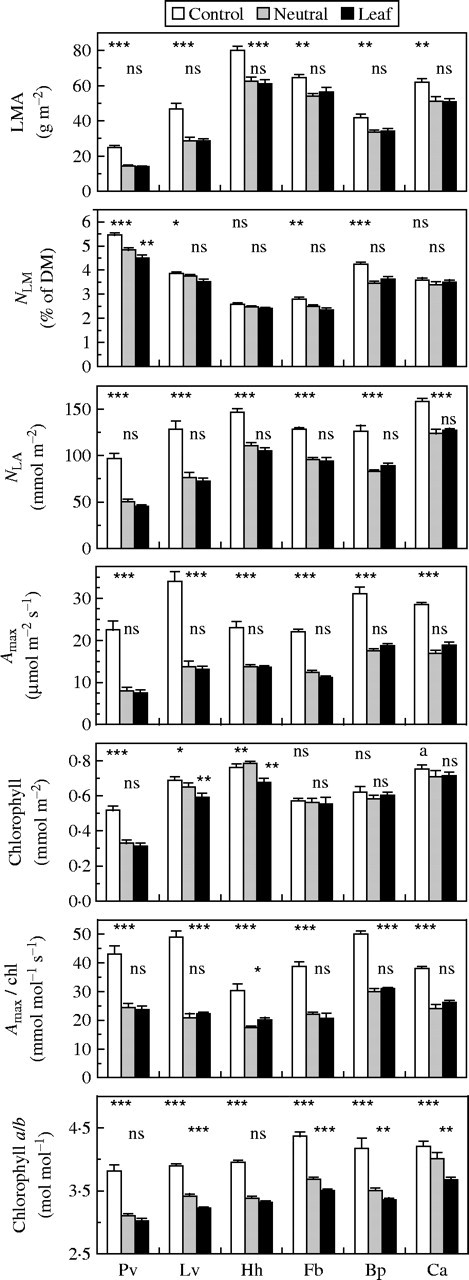
The effect of shading one leaf by a spectrally neutral filter or a tobacco leaf on parameters pertaining to between-leaf resource partitioning and within-leaf chloroplast organization. Both shading devices had the same PAR transmittance. Six species were compared: Phaseolus vulgaris (Pv), Lysimachia vulgaris (Lv), Hedera helix (Hh), Ficus benjamina (Fb), Brachypodium pinnatum (Bp) and Carex acutiformis (Ca). Parameters (means ± s.e.) are: leaf mass per area (LMA), nitrogen per leaf mass (NLM), nitrogen per leaf area (NLA), photosynthetic capacity per area (Amax) and per chlorophyll (Amax/chl), total chlorophyll per area and chlorophyll a/b ratio. The upper row of statistical notations refers to the results of a one-way ANOVA on the three treatment means per species. The lower row refers to the significance of the differences between the two shading treatments based on a Student's t-test performed on data expressed relative to unshaded control leaves on the same plant. *P < 0·05; **P < 0·01; ***P < 0·001; n.s., not significant.
Chlorophyll per leaf area was not reduced by neutral shading in all species except Phaseolus, and leaf shading caused small additional reductions in Lysimachia and Hedera only. The strong effects on Amax combined with small or absent effects on chlorophyll resulted in strong effects of shading on Amax per unit chlorophyll (Amax/chl) in all species, but spectral effects were also absent (Fig. 1). This was different for the chlorophyll a/b ratio, because a shift in favour of chlorophyll b occurred in response to the spectral shade of a tobacco leaf, in addition to the decreased in chlorophyll a/b observed in neutral shade in four of the six species (Fig. 1). There were thus spectral leaf shade effects on one of the two within-leaf partitioning parameters.
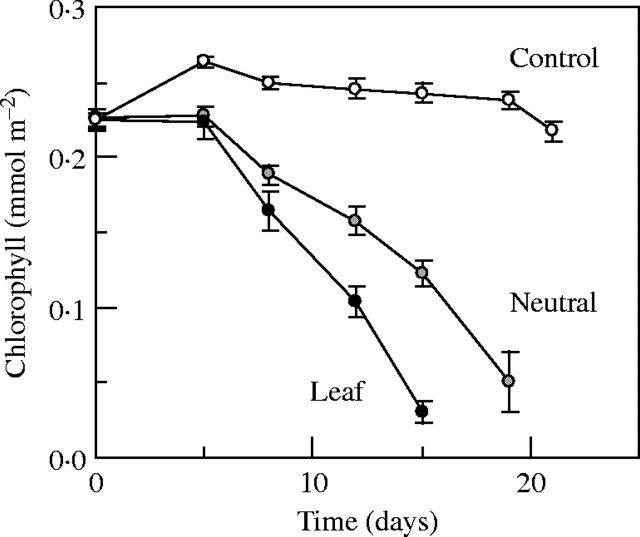
Phaseolus showed a tendency of a spectral effect on between-leaf partitioning in the treatment period of 1 week, but that was not statistically significant (Fig. 1). A period of 2 weeks, as in the other herbaceous species, would have resulted in senescence of the primary leaves and thus have prohibited the estimation of all partitioning parameters. However, the sensitivity of these leaves for induction of senescence by shade makes them suitable for investigating spectral effects on that process. The shading devices were kept over one of the primary leaves until full senescence. Abscission of the leaves took on average 22 d in the case of neutral shade, which was significantly accelerated to 17 d by spectral leaf shade (Table 1). The spectral effect only became evident after 1 week when chlorophyll declined in the senescence phase and went more rapidly under leaf shade (Fig. 2). Ultimate re-allocation of resources from senescing leaves was not differently affected as LMA, NLM and NLA of senesced leaves were the same for the two shading treatments (Table 1).
Table 1.
Effect of shading one leaf of the pair of primary Phaseolus vulgaris leaves by a tobacco leaf and a spectrally neutral filter with the same PAR transmittance
| Initial |
No shade |
Neutral shade |
Leaf shade |
Spectral effect |
|
|---|---|---|---|---|---|
| Days until abscission | – | – | 22 (1) | 17 (1) | * |
| LMA (g m−2) | 14·3 (0·3) | 23·8 (0·4) | 10·2 (0·4) | 9·9 (0·2) | n.s. |
| NLM (% of DM) | 6·13 (0·05) | 3·20 (0·12) | 1·90 (0·08) | 1·95 (0·09) | n.s. |
| NLA (mmol m−2) | 62·8 (1·3) | 54·3 (2·4) | 13·8 (0·8) | 13·8 (0·8) | n.s. |
| Leaf area (cm2) | 74·4 (6·3) | 96·0 (6·0) | 74·2 (4·1) | 72·4 (3·4) | n.s. |
Leaf dry mass per area (LMA) and nitrogen per leaf dry mass (NLM) of shaded leaves were determined after abscission.
Leaf area was measured at 12 d after the start of the treatments and used for the calculation of nitrogen per leaf area (NLA). Control leaves on separate plants were harvested at 21 d after start of treatment.
Differences between means of the two shading treatments were tested with a t-test, for statistical notation see legend to Fig. 1.
Fig. 2.
Effect of shading one leaf of the pair of primary Phaseolus vulgaris leaves by a tobacco leaf and a spectrally neutral screen with the same PAR transmittance on the time course of chlorophyll per area (means ± s.e.). Chlorophyll was measured non-destructively on the same leaves until abscission.
Supplemental FR
The set-up of the leaf shade experiment implies a large effect of reduction in irradiance, which may have masked a possible separate effect of low R : FR. It may thus have resulted in underestimation of the importance of the spectral component of the canopy light gradient for resource partitioning. A strong beam of supplemental FR in the growth light conditions incident on the abaxial side of the leaves over a sufficiently large area that allowed photosynthesis measurement was used to reduce R : FR independent of PAR. This treatment reduced NLA and/or Amax in all species except Carex by an average of 15 % and 21 %, respectively (Fig. 3). Chlorophyll was similarly reduced, hence, Amax/chl remained constant (Fig. 3). The chlorophyll a/b ratio was also reduced under low R : FR from on average 4·2 to 3·7 (Fig. 3). This indicates that at constant PAR between-leaf resource partitioning was altered by a low R : FR in most species but not in Carex. Aspects of chloroplast organization had changed in all species including Carex as is evident from chlorophyll a/b, but not the within-leaf partitioning between light harvesting and photosynthetic capacity.
Fig. 3.
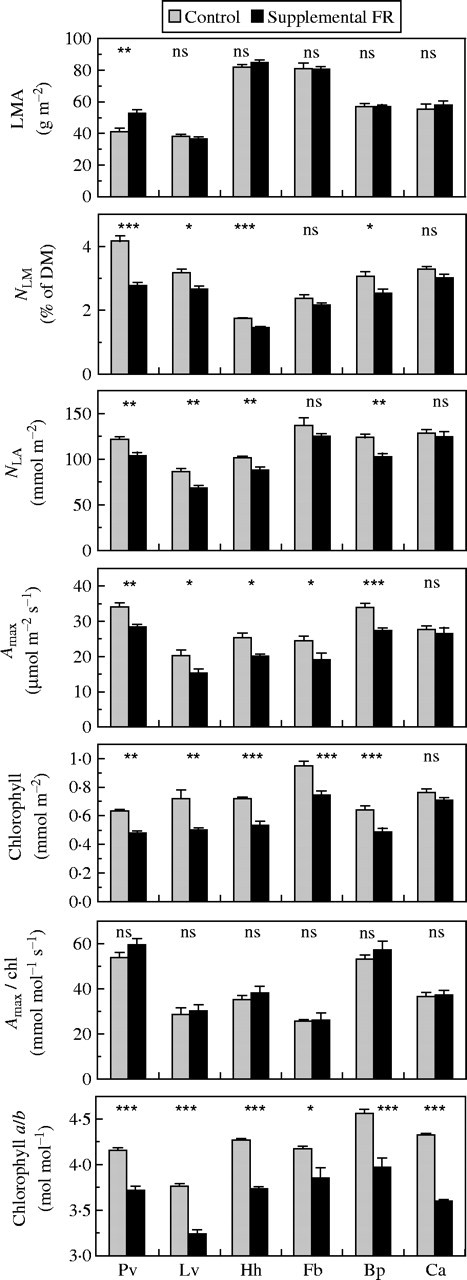
The effect of supplemental FR applied on a 3-cm-diameter spot of a leaf on parameters pertaining to between-leaf resource partitioning and within-leaf chloroplast organization. Six species were compared: Phaseolus vulgaris (Pv), Lysimachia vulgaris (Lv), Hedera helix (Hh), Ficus benjamina (Fb), Brachypodium pinnatum (Bp) and Carex acutiformis (Ca). Parameters (means ± s.e.) are: leaf mass per area (LMA), nitrogen per leaf mass (NLM), nitrogen per leaf area (NLA), photosynthetic capacity per area (Amax) and per chlorophyll (Amax/chl), total chlorophyll per area and chlorophyll a/b ratio. Differences between means were tested for significance with a t-test, for notation see legend to Fig. 1.
DISCUSSION
Between-leaf resource partitioning
The large effect of a reduction of irradiance incident on one leaf of a plant irrespective of the use of either a spectrally neutral screen or a leaf as a shading device (Fig. 1) suggests that the irradiance gradient in canopies is the dominant environmental signal used by all six species for neighbour detection with respect to between-leaf resource partitioning. That is consistent with the many experiments in which canopy light gradients were experimentally simulated in a spectrally neutral manner (Pons et al., 1993; Hikosaka et al., 1994; Pons and Pearcy, 1994; Pons and Jordi, 1998). However, the way the experiment on spectral leaf shading was carried out includes inevitably that irradiance is the main experimental factor and that the spectral effect is added as a secondary factor. This may have precluded the expression of a spectral effect superimposed on the already large irradiance effect, because these two factors may drive parallel signalling pathways and one may evoke the full response on its own. To further investigate the possible involvement of the spectral component as a separate signal perceived by phytochrome in canopy density responses, the R : FR was reduced with supplemental FR at moderate irradiance. This treatment affects only phytochrome, and not other possible signalling pathways that are associated with irradiance such as UV and blue light photoreceptors, transpiration rate and photosynthetic activity (Ono et al., 2001; Pons et al., 2001). The possible temperature effect of the increased total irradiance was ruled out. Supplemental FR reduced NLA and Amax in most species (Fig. 3), but the effect was smaller compared with the effect of irradiance reduction alone (Fig. 1). Hence, it is concluded that in five out of the six species, there are at least two signalling pathways involved in the perception of canopy density with respect to between-leaf resource partitioning, but that the spectral component of the light gradient is less important than the irradiance component.
The above conclusion pertains to the first week (Phaseolus) or 2 weeks (other species) of shading. However, a clear effect of spectral leaf shade in between-leaf resource partitioning appeared in addition to the irradiance effect in the experiment that was taken to full senescence of primary Phaseolus leaves. Although spectral effects were not yet clear after 1 week, later on induction of senescence was accelerated under a leaf compared with a neutral filter (Fig. 2 and Table 1). Spectral leaf shade effects might thus also have emerged in the other species when the duration of the treatment would have been taken to the senescence phase. However, that would take much longer than with Phaseolus, particularly in the evergreens. Effects of manipulative changes of R : FR incident on single leaves were also most evident towards senescence in Glycine max (Guiamét et al., 1989) and Helianthus annuus (Rousseaux et al., 1996, 2000). Results of the many studies on irradiance and spectral treatments of whole plants may be less relevant with respect to whole-plant resource partitioning in canopy gradients because source–sink relationships are differently affected. Leaf senescence was indeed not induced in whole-plant shading, whereas it was induced in single-leaf shading or darkening (Pons and Pearcy, 1994; Weaver and Amasino, 2001). The data on Glycine and Helianthus of Guiamét et al. (1989) and Rousseaux et al. (1996, 2000), together with the present results for Phaseolus, suggest that, at least in annual dicots, a spectral effect on phytochrome is not so much involved in early responses of resource reallocation to canopy density, but more towards the later stages when leaf senescence is induced. This contrasts eith the role of phytochrome in early neighbour detection with respect to morphological shade avoidance in herbaceous plants (Morgan et al., 1980; Ballaré et al., 1990; Ballaré, 1999). The situation may be somewhat different in woody plants, because senescence was not induced even after almost 3 months of exposure to a low R : FR in Juglans trees (Frak et al., 2001). Nevertheless, spectrally modified daylight reduced NLA and Amax after that treatment period in Juglans, whereas this was not the case with the woody Hedera and Ficus in our experiments of shorter duration (Fig. 1). This suggests that phytochrome may also become progressively more important for neighbour detection after canopy closure with respect to between-leaf resource partitioning in woody plants without the induction of leaf senescence.
In contrast to the other species, Carex acutiformis has erect leaves that span the whole depth of the canopy in the vegetative phase. The term ‘between-leaf’ partitioning may thus be confusing, because the partitioning of resources for photosynthetic capacity is between parts of leaves in different canopy positions rather than between leaves. Still that term is maintained for this form of partitioning. Carex did not show a reduction in NLA, Amax or chlorophyll in the experiment with supplemental FR (Fig. 3) and also not in a preliminary experiment (data not shown). The lower part in low light of the erect leaves that span the whole light gradient supports the upper part (Hirose et al., 1989; Schieving et al., 1992; Pons et al., 1993). That lower part has thus not only a photosynthetic function, but also a support function for the upper part that may be jeopardized when senescence is induced there. It is therefore possible that plants of this growth form lack a low R : FR-induced senescence altogether.
Within-leaf partitioning between photosynthetic functions
The chlorophyll a/b ratio was clearly reduced as a result of a lower irradiance in all species (Fig. 1). This phenomenon was consistently found in many other studies and is ascribed to the relative increase of the chlorophyll b-containing light-harvesting complex II (LHCII), which is associated with the decrease in Amax/chl (Anderson et al., 1995). A low R : FR further reduced the chlorophyll a/b ratio, although not significantly in all species in response to spectral leaf shade (Fig. 1), but clearly in all species under supplemental FR (Fig. 3). This phenomenon is found in many studies and is ascribed to an increase in the PSII : PSI ratio and thus in relative abundance of LCHII (Chow et al., 1990a; Anderson et al., 1995; Tinoco-Ojanguren and Pearcy, 1995; Walters and Horton, 1995; Murchie and Horton, 1998). The imbalance in excitation of the two photosystems as a result of the FR absorption by PSI is re-established by this altered stoichiometry of the photosystems, which maximizes quantum yield (Chow et al., 1990b). The low chlorophyll a/b ratio in the lower ranges of leaf canopies will thus be the combined result of the reduced irradiance and the lower R : FR.
The strong effect of reduced irradiance incident on a leaf on Amax/chl (Fig. 1) indicates that the partitioning between the capacity and light-harvesting functions within the photosynthetic apparatus acclimated to the local light conditions. This was true for all species and is consistent with the results of many earlier studies (Terashima and Evans, 1988; Evans, 1989; Anderson et al., 1995; Hikosaka, 1996; Murchie and Horton, 1998). In most of these studies, the light treatment was applied to whole plants instead of parts of their foliage as in the present experiments, but there are no arguments to suppose different effects on chloroplast organization (Pons and Pearcy, 1994). The acclimation of stoichiometry between PSI and PSII to a low R : FR that caused the decrease in the chlorophyll a/b ratio is, however, not necessarily accompanied by an altered Amax/chl. Spectral leaf shade and supplemental FR had, indeed, contrary to irradiance, no significant effects on Amax/chl in all species except Hedera, where it caused a small increase in the leaf shade treatment only (Figs 1 and 3). Absence of an effect of R : FR on this parameter was also found in three tropical trees (Tinoco-Ojanguren and Pearcy, 1995), increases and decreases in Amax/chl were found in four wild herbaceous species (Murchie and Horton, 1998) and it also increased under low R : FR in Pisum sativum (Chow et al., 1990a). Effects of R : FR were thus absent or small in these studies. Similar ranges in Amax/chl were found in canopies compared with spectrally neutral gradients (Pearcy and Seemann, 1990; Burkey and Wells, 1991; Evans, 1993a; Pons and Jordi, 1998). On the basis of these literature data and the present results, it is concluded that the partitioning between photosynthetic functions within leaves acclimates mainly to the local irradiance and that the spectral component is either not involved or insignificant.
Acknowledgments
The authors thank Fred Siesling for growing plants in the glasshouse. Preliminary experiments for this study were carried out by Servé van Rijt, Marc Bergkotte and Wim Huibers.
LITERATURE CITED
- Anderson JM, Chow WS, Park YI. 1995. The grand design of photosynthesis: acclimation of the photosynthetic apparatus to environmental cues. Photosynthesis Research 46: 129–139. [DOI] [PubMed] [Google Scholar]
- Anten NPR, Miyazawa K, Hikosaka K, Nagashima H, Hirose T. 1998. Leaf nitrogen distribution in relation to leaf age and photon flux density in dominant and subordinate plants in dense stands of a dicotyledonous herb. Oecologia 113: 314–324. [DOI] [PubMed] [Google Scholar]
- Anten NPR, Hikosaka K, Hirose T. 2000. Nitrogen utilisation and the photosynthetic system. In: Marshall B, Roberts JA, eds. Leaf development and canopy growth. Sheffield: Sheffield Academic Press, 171–203. [Google Scholar]
- Ballaré CL. 1999. Keeping up with neighbours: phytochrome sensing and other signalling mechanisms. Trends in Plant Science 4: 97–102. [DOI] [PubMed] [Google Scholar]
- Ballaré CL, Scopel AL, Sánchez RA. 1990. Far-red radiation reflected from adjacent leaves – an ealy signal of competition in plant canopies. Science 247: 329–332. [DOI] [PubMed] [Google Scholar]
- Barkman JJ, Stoutjesdijk P. 1992.Microclimate, vegetation and fauna. Uppsala: Opulus Press. [Google Scholar]
- Barreiro R, Guiamét JJ, Beltrano J, Montaldi ER. 1992. Regulation of the photosynthetic capacity of primary bean leaves by red:far-red ratio and photosynthetic photon flux density of incident light. Physiologia Plantarum 85: 97–101. [Google Scholar]
- Burkey KO, Wells R. 1991. Response of soybean photosynthesis and chloroplast membrane function to canopy development and mutual shading. Plant Physiology 97: 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo DA, Haroon M, Schrader LE, Youngs VL. 1975. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Communications in Soil Science and Plant Analysis 6: 71–80. [Google Scholar]
- Chow WS, Goodchild DJ, Miller C, Anderson JM. 1990. The influence of high levels of brief or prolonged supplementary far-red illumination during growth on the photosynthetic characteristics, composition and morphology of Pisum sativum chloroplasts. Plant, Cell & Environment 13: 135–145. [Google Scholar]
- Chow WS, Melis A, Anderson JM. 1990. Adjustment of photosystem stoichiometry in chloroplasts improve the quantum efficiency of photosynthesis. Proceedings of the National Academy of Sciences of the USA 87: 7502–7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corré WJ. 1983. Growth and morphogenesis of sun and shade plants. II.The influence of light quality. Acta Botanica Neerlandica 32: 185–202. [Google Scholar]
- Evans JR. 1989. Partitioning of nitrogen between and within leaves grown under different irradiances. Australian Journal of Plant Physiology 16: 533–548. [Google Scholar]
- Evans JR. 1993. Photosynthetic acclimation and nitrogen partitioning within a Lucerne canopy. I. Canopy characteristics. Australian Journal of Plant Physiology 20: 55–67. [Google Scholar]
- Evans JR. 1993. Photosynthetic acclimation and nitrogen partitioning within a Lucerne canopy. II. Stability through time and comparison with a theoretical optimum. Australian Journal of Plant Physiology 20: 69–82. [Google Scholar]
- Frak E, Le Roux X, Millard P, Dreyer E, Jaouen G, Saint-Joanis B, Wendler R. 2001. Changes in total leaf nitrogen and partitioning of leaf nitrogen drive photosynthetic acclimation to light in fully developed walnut leaves. Plant, Cell & Environment 24: 1279–1288. [Google Scholar]
- Guiamét JJ, Willemoes JG, Montaldi ER. 1989. Modulation of progressive leaf senescence by the red:far-red ratio of incident light. Botanical Gazette 150: 148–151. [Google Scholar]
- Gyula P, Schäfer E, Nagy F. 2003. Light perception and signalling in higher plants. Current Opinion in Plant Biology 6: 446–452. [DOI] [PubMed] [Google Scholar]
- Hikosaka K. 1996. Effects of leaf age, nitrogen nutrition and photon flux density on the organization of the photosynthetic apparatus in leaves of a vine (Ipomoea tricolor Cav.) grown horizontally to avoid mutual shading of leaves. Planta 198: 144–150. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Terashima I, Katoh S. 1994. Effects of leaf age, nitrogen nutrition and photon flux density on the distribution of nitrogen among leaves of a vine (Ipomoea tricolor Cav.) grown horizontally to avoid mutual shading of leaves. Oecologia 97: 451–457. [DOI] [PubMed] [Google Scholar]
- Hirose T, Werger MJA, Pons TL, van Rheenen JWA. 1988. Canopy structure and leaf nitrogen distribution in a stand of Lysimachia vulgaris L. as influenced by stand density. Oecologia 77: 145–150. [DOI] [PubMed] [Google Scholar]
- Hirose T, Werger MJA, Van Rheenen WA. 1989. Canopy development and leaf nitrogen distribution in a stand of Carex acutiformis Ecology 70: 1610–1618. [Google Scholar]
- Holmes MG, Smith H. 1977. The function of phytochrome in the natural environment. II. The influence of vegetation canopies on the spectral energy distribution of natural daylight. Photochemistry and Photobiology 25: 539–545. [Google Scholar]
- Inskeep WP, Bloom PR. 1985. Extinction coefficients of chlorophyll a and b in N,N-dimethylformamide and 80 % acetone. Plant Physiology 77: 483–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappers IF, Jordi W, Maas FM, Stoopen GM, van der Plas LHW. 1998. Gibberellin and phytochrome control senescence in Alstroemeria leaves independently. Physiologia Plantarum 103: 91–98. [Google Scholar]
- Lötscher M, Stroh K, Schnyder H. 2003. Vertical leaf nitrogen distribution in relation to nitrogen status in grassland plants. Annals of Botany 92: 679–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DC, Brien TO, Smith H. 1980. Rapid photomodulation of stem extension in light-grown Sinapis alba L. Planta 150: 95–101. [DOI] [PubMed] [Google Scholar]
- Murchie EH, Horton P. 1998. Contrasting patterns of photosynthetic acclimation to the light environment are dependent on the differential expression of the responses to altered irradiance and spectral quality. Plant, Cell & Environment 21: 139–148. [Google Scholar]
- Ono K, Nishi Y, Watanabe A, Terashima I. 2001. Possible mechanisms of adaptive leaf senescence. Plant Biology 3: 234–243. [Google Scholar]
- Pearcy RW, Seemann JR. 1990. Photosynthetic induction state of leaves in a soybean canopy in relation to light regulation of ribulose-1,5-bisphosphate carboxylase and stomatal conductance. Plant Physiology 94: 628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik R, Whitelam GC, Voesenek LACJ, de Kroon H, Visser EJW. 2004. Canopy studies on ethylene-insensitive tobacco identify ethylene as a novel element in blue light and plant-plant signalling. Plant Journal 38: 310–319. [DOI] [PubMed] [Google Scholar]
- Pons TL, Anten NPR. Is plasticity in partitioning of photosynthetic resources between and within leaves important for whole-plant carbon gain in canopies? Functional Ecology (in press). [Google Scholar]
- Pons TL, Bergkotte M. 1996. Nitrogen allocation in response to partial shading of a plant: possible mechanisms. Physiologia Plantarum 98: 571–577. [Google Scholar]
- Pons TL, Jordi W. 1998. Induction of leaf senescence and shade acclimation in leaf canopies – variation with leaf longevity. In: Lambers H, Poorter H, van Vuuren MMI, eds. Inherent variation in plant growth. physiological mechanisms and ecological consequences. Leiden: Backhuys Publishers, 121–137. [Google Scholar]
- Pons TL, Pearcy RW. 1994. Nitrogen reallocation and photosynthetic acclimation in response to partial shading in soybean plants. Physiologia Plantarum 92: 636–644. [Google Scholar]
- Pons TL, Welschen RAM. 2002. Overestimation of respiration rates in commercially available clamp-on leaf chambers. Complications with measurement of net photosynthesis. Plant, Cell & Environment 25: 1367–1372. [Google Scholar]
- Pons TL, Jordi W, Kuiper D. 2001. Acclimation of plants to light gradients in leaf canopies: evidence for a possible role for cytokinins transported in the transpiration stream. Journal of Experimental Botany 52: 1563–1574. [DOI] [PubMed] [Google Scholar]
- Pons TL, van Rijnberk H, Scheurwater I, van der Werf A. 1993. Importance of the gradient in photosynthetically active radiation in a vegetation stand for leaf nitrogen allocation in two monocotyledons. Oecologia 95: 416–424. [DOI] [PubMed] [Google Scholar]
- Rousseaux MC, Ballaré CL, Jordan ET, Vierstra RD. 1997. Directed overexpression of PHYA locally suppresses stem elongation and leaf senescence responses to far-red radiation. Plant, Cell & Environment 20: 1551–1558. [Google Scholar]
- Rousseaux MC, Hall AJ, Sánchez RA. 1996. Far-red enrichment and photosynthetically active radiation level influence leaf senescence in field-grown sunflower. Physiologia Plantarum 96: 217–224. [Google Scholar]
- Rousseaux MC, Hall AJ, Sánchez RA. 2000. Basal leaf senescence in a sunflower (Helianthus annuus) canopy: responses to increased R/FR ratio. Physiologia Plantarum 110: 477–482. [Google Scholar]
- Schieving F, Pons TL, Werger MJA, Hirose T. 1992. The vertical distribution of nitrogen and photosynthetic activity at different plant densities in Carex acutiformis Plant and Soil 14: 9–17. [Google Scholar]
- Smith H, Samson G, Fork DC. 1993. Photosynthetic acclimation to shade: probing the role of phytochromes using photomorphogenic mutants of tomato. Plant, Cell & Environment 16: 929–937. [Google Scholar]
- Smith H, Whitelam GC. 1997. The shade avoidance syndrome: multiple responses mediated by multiple phytochromes. Plant, Cell & Environment 20: 840–844. [Google Scholar]
- Stoddart JL, Thomas H. 1982. Leaf senescence. In: Boulter D, Parthier B, eds. Encyclopedia of Plant Physiology, New Series V. 14A. Berlin: Springer Verlag, 592–636. [Google Scholar]
- Terashima I, Evans JR. 1988. Effects of light and nitrogen nutrition on the organisation of the photosynthetic apparatus in spinach. Plant Cell Physiology 29: 143–155. [DOI] [PubMed] [Google Scholar]
- Tinoco-Ojanguren C, Pearcy RW. 1995. A comparison of light quality and quantity effects on the growth and steady state and dynamic photosynthetic characteristics of three tropical tree species. Functional Ecology 9: 222–230. [Google Scholar]
- Vos J, van der Putten PEL. 2001. Effects of partial shading of the potato plant on photosynthesis of treated leaves, leaf area expansion and allocation of nitrogen and dry matter in component plant parts. European Journal of Agronomy 14: 209–220. [Google Scholar]
- Walters RG, Horton P. 1995. Acclimation of Arabidopsis thaliana to the light environment: regulation of chloroplast composition. Planta 197: 475–481. [DOI] [PubMed] [Google Scholar]
- Weaver LM, Amasino RM. 2001. Senescence is induced in individually darkened Arabidopsis leaves but inhibited in whole darkened plants. Plant Physiology 127: 876–886. [PMC free article] [PubMed] [Google Scholar]