Abstract
• Background and Aims Colleters are secretory structures consisting of a parenchymatic middle axis surrounded by a layer of palisade-like epidermal cells. Colleters occur in a large number of rubiaceous species. Their function is to protect the developing shoot apex. They are also taxonomically useful in the Rubiaceae. This study characterized the structure of the colleters of Simira glaziovii, S. pikia and S. rubra and the biochemistry of secretions in S. glaziovii.
• Methods Stipules of the shoot apices of the three species studied were collected at Barragem de Saracuruna, in Rio de Janeiro state, Brazil. The samples were fixed according to the usual methods for light and electron microscopy. Secretion stipules of S. glaziovii were washed with 0·1 m Tris–HCl plus 0·1 %Triton X-100 to extract proteins and carbohydrates.
• Key Results Colleters in these species are located at the base of the stipule. Each species shows a different pattern of distribution. They form as emergentia from the stipules. Simira glaziovii was different from the other two species because it exhibited vascular traces. The epidermal cells of colleters have dense cytoplasm, nuclei, small vacuoles, endoplasmic reticulum, Golgi apparatus, mitochondria and extraplasmic spaces if they are secretory. The outer cell wall of the mature colleters differs from the outer cell wall of stipule cells and immature colleters. Both carbohydrates and proteins were found in secretions from the stipules of S. glaziovii.
• Conclusions Few ultrastructural differences were noted among the three species. These secretory structures not only protect the shoot apex, but also have taxonomic importance below the genus level.
Key words: Colleters, secretory structure, microscopy, plant anatomy, ultrastructure, development, biochemistry, Simira, Rubiaceae
INTRODUCTION
The colleter, an epidermal secretory structure, can be found mainly on the adaxial side of stipules and/or sepals among 60 families of the angiosperms (Thomas, 1991). These structures have been regarded as trichomes (Horner and Lersten, 1968), but develop from both protoderm and other ground elements as emergentia. Other names have been reported for these structures including ‘squamallae’ (Ramayya and Bahadur, 1968) and ‘stipular glands’ (Van Hove and Kagoyre, 1974).
Most of the functional aspects of these secretory structures are unknown. Several authors report that colleter secretions cover and protect the developing shoot apex (Williams et al., 1982; Thomas and Dave, 1989, 1990). In nodulated rubiaceous species, however, the secretions of colleters are surmised to play a vital role in the nutrition of symbiotic bacteria (Van Hove and Kagoyre, 1974; Lersten, 1975). They might also act as a pathway for bacterial entry into the leaves (Miller et al., 1983). In contrast, some secretory structures have evolved as defense against pathogens and insects (Farrell et al., 1991; Zalucki et al., 2001; Cruz et al., 2002). Insects and pathogens that attack secretion-producing plants are faced with a combination of both physical barriers and chemical defenses provided by exudate-secreting structures (Giordani and Lafon, 1993; Wititsuwannakul et al., 2002; Azarkan et al., 2003).
The anatomical structure of colleters can be described as a parenchymatic cellular axis surrounded by a layer of palisade-like epidermal cells (Thomas, 1991; Da Cunha and Vieira, 1997). The epidermal cells of colleters are known to be secretory. Occasionally, the middle axis develops vascular bundles (Thomas, 1991). Horner and Lersten (1968) and Lersten (1974a, b) described four types colleters in the Rubiaceae based on the appearance of their epidermis: standard, reduced, brush-like and dendroid. The standard type is composed of compact epidermal cells. It is the most common type and shows some variability among species in size and in constrictions at their bases (Robbrecht, 1988).
Colleters are located on the adaxial surfaces or margins of stipules in a large number of species of the Rubiaceae. These secretory structures not only protect the shoot apex, but also have taxonomic importance below the familial level (Robbrecht, 1988). Rubiaceous species are one of the most important components of the plant community of the Atlantic Rain Forest at Macaé de Cima in the state of Rio de Janeiro (Lima and Guedes-Bruni, 1997). The family is also important ecologically (Guedes-Bruni, 1998).
This study was performed to further the understanding of the anatomical structure and secretions of the colleters of Simira glaziovii, S. pikia and S. rubra.
MATERIALS AND METHODS
Botanical material
Stipules of the shoot apices of Simira glaziovii, S. pikia (K. Schum.) Steyerm and S. rubra (Mart.) Steyerm were collected in Barragem de Saracuruna, in the city of Duque de Caxias, in Rio de Janeiro state, Brazil, during the months of March, April and December in 2001. Stipules of Simira glaziovii were used for studies of development and secretory composition. For comparison with Simira glaziovii, only one stage of development of colleters in S. pikia and S. rubra was observed by microscopy.
Light microscopy
Whole stipules or stipular fragments were fixed for 2 h in a solution of 2·5 % glutaraldehyde and 4·0 % paraformaldehyde buffered with 0·05 m cacodylate buffer to pH 7·2. Subsequently, the samples were rinsed three times with buffer and post-fixed for 2 h at room temperature with 1·0 % osmium tetroxide in 0·05 m cacodylate buffer to pH 7·2. The post-fixed samples were dehydrated in a graded series of acetone solutions (30, 50, 70, 90 and 100 %; 1 h each). The material was infiltrated and embedded in the epoxy resin Epon (Polybed). Microtome sections (1·0 µm) were cut and stained with toluidine blue (0·05 % aqueous solution). The slides were sealed with Entellan® (Merk) and examined with an Axioplan ZEISS microscope.
Transmission electron microscopy
The stipule fragments were fixed, post-fixed, dehydrated and embedded as described above. Ultrathin sections were collected with 300-mesh grids, stained with 1·0 % uranyl acetate followed by 5·0 % lead citrate for routine observation. Three other stains were used to elucidate the cytochemistry of colleter cells: (1) imidazole-buffered osmium tetroxide to enhance and observe the preservation and contrast of lipids (Angermüller and Fahimi, 1982); (2) 1 % ruthenium red to detect negatively charged components of the colleter palisade cells (Luft, 1971); and (3) periodic acid-thiocarbohydrazide (THC)–silver proteinate (PATAg) to detect polysaccharides containing 1,2-glycol groups. For the last technique, sections were either treated with periodic acid or, to provide controls, solutions not containing periodic acid and THC (Thiéry, 1967). Sections were observed at 80 kV using a transmission electron microscope (ZEISS EM 900).
Scanning electron microscopy
The stipules were fixed, post-fixed and dehydrated as for light microscopy. The samples were subsequently critically point dried in CO2, sputter coated with 20 nm gold, and observed with a digital scanning electron microscope (ZEISS DSEM 962).
Biochemical assays
Stipules of Simira glaziovii were washed in the presence of 0·1 m Tris–HCl plus 0·1 % Triton X-100, pH 8·0, to extract proteins and carbohydrates. The material was filtered through 0·45-µm Millipore filters prior to biochemical analysis. Protein determinations were performed by the method of Bradford (1976) using bovine serum albumin as a standard. The absorbance at 595 nm was measured on a UV-visible spectrophotometer. The filtrate obtained was precipitated overnight with solid ammonium sulfate (90 % saturation). The resulting precipitate was dialysed against distilled water (48 h), and recovered by freeze-drying for electrophoresis. SDS–polyacrylamide gel electrophoresis (PAGE) was performed as described by Laemmli (1970). Proteins utilized as molecular mass standards for SDS–PAGE were bovine serum albumin (66 kDa), ovalbumin (45 kDa), glyceraldehyde-3-phosphate dehydrogenase (36 kDa), carbonic anhydrase (29 kDa), trypsinogen (24 kDa), trypsin inhibitor (20 kDa) and α-lactoglobulin (14 kDa).
The amount of carbohydrate in the secretory fraction was evaluated by the phenol–sulfuric acid method as described by Dubois et al. (1956) employing glucose as standard. The absorbance at 486 nm of each fraction was measured. The average of the three absorbance determinations from each sample was used to calculate the amount of carbohydrate present in the secretions.
RESULTS
External morphology
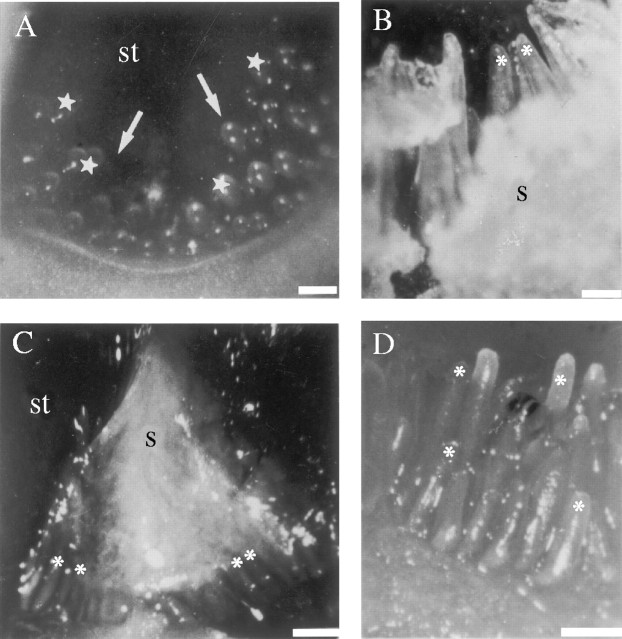
Colleters are found on the adaxial surface of stipules of Simira glaziovii, S. pikia and S. rubra. In S. glaziovii (Fig. 1A and B), the colleters form two triangular groups that contour the base of the leaf primordia at the shoot apex (Fig. 1A, arrows). The colleters are aligned in a single row in S. pikia (Fig. 1C) and several rows in S. rubra (Figs 1D and 2C). Developing colleters appear as rounded projections on the young stipule (as shown for S. glaziovii in Figs 1A and 2B). Fully developed colleters are cylindrical (Figs 1B–D and 2A and C). Secretions (Figs 1B and C and 2A) cover the interior of the entire shoot apex. The secretion is viscous and becomes sticky as it dries. Secretions are yellowish for S. glaziovii, and colourless for S. pikia and S. rubra.
Fig. 1.
Stipules detached from the shoot apex and observed with the aid of a stereomicroscope. (A and B) Colleters of Simira glaziovii at (A) initial stage and (B) fully developed. Note the triangular organization at the base of stipule (arrows in A). (C) Colleters of S. pikia and (D) colleters of S. rubra. Scale bars: A, C and D = 125 µm; B = 1·0 mm. Asterisks, colleters; s, secretion; st, stipule; stars, immature colleters.
Fig. 2.
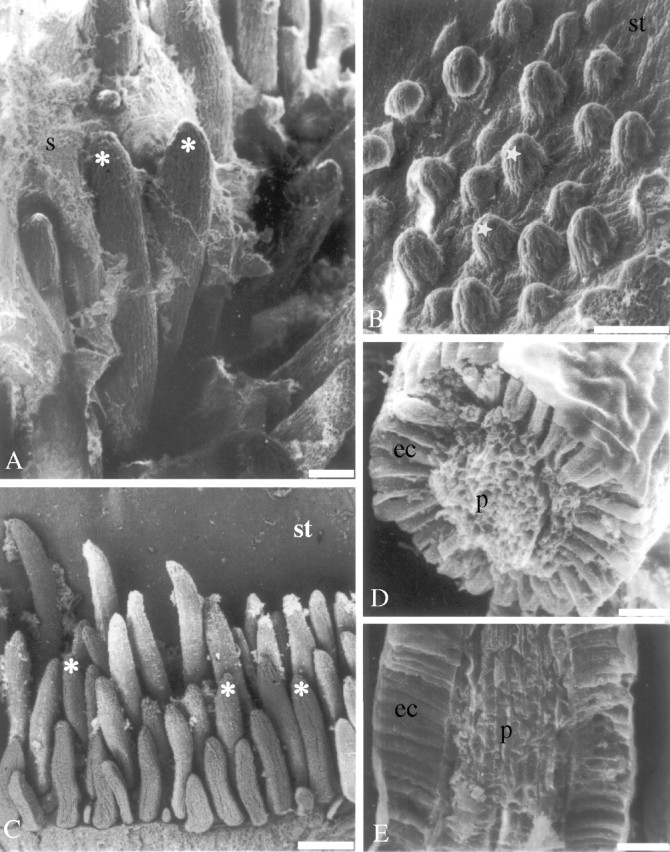
Scanning electron microscopy. (A and B) An overview of the colleters of Simira glaziovii at (A) entirely developed and (B) initial stage; (C) an overview of the colleters of S. rubra; (D) cross-section of a colleter of S. glaziovii; and (E) longitudinal section of a colleter of S. pikia. Scale bars: A and B = 100 µm; C = 250 µm; D and E = 25 µm. Asterisks, colleter; stars, immature colleters; ec, epidermal cells; p, parenchyma; s, secretion; st, stipule.
Internal morphology
Observation of thick sections of colleters showed that the colleters of the three species were the standard type (Figs 2D, 2E and 3B–D). Meristematic epidermal and subepidermal layers contribute to the morphology of these structures (Fig. 3A); as is characteristic of an emergentia. Before complete development of the colleters, the epidermal cells are difficult to differentiate from parenchyma cells (Fig. 3A). However, at maturity, the epidermal cells become more densely cytoplasmic and columnar (Fig. 3B–D).
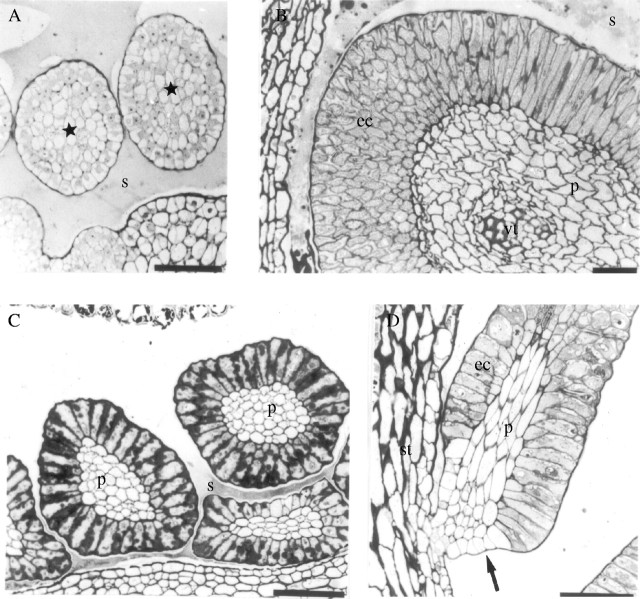
Fig. 3.
Light microscopy. (A and B) Cross-sections of the colleters of Simira glaziovii at (A) initial stage and (B) fully developed; (C) cross-section of the colleters of S. pikia; and (D) longitudinal section of a colleter of S. rubra. Scale bars: A, B and D = 50 µm; C = 60 µm. Stars, immature colleters; arrow, constriction; ec, epidermal cells; p, parenchyma; s, secretion; st, stipule; vc, vascular trace.
A constriction develops at the colleter base (Fig. 3D) in the three species studied. The epidermal cells in the region of constriction are parenchyma-like in appearance. Colleters of S. pikia and S. rubra have only parenchyma cells at their centres (Fig. 3C and D). Simira glaziovii may have a vascular trace within the parenchymatic core (Fig. 3B). Epidermal cells of S. pikia, in contrast to the other two species, have dark-staining materials adjacent to the cell walls (Fig. 3C).
Ultrastructure of colleters
During early development of the colleters of S. glaziovii (Fig. 2A) epidermal (Fig. 4A) and subepidermal cells are undifferentiated. Both epidermal and subepidermal cells at this early stage of development are isodiametric, and exhibit small vacuoles, endoplasmic reticulum, Golgi apparatus, mitochondria, the nucleus and numerous electron-dense lipid bodies (Fig. 4A). As colleters develop, epidermal cells become more elongate and rectangular. At an intermediate stage of maturity, colleters become densely cytoplasmic. Vacuoles, endoplasmic reticulum, Golgi apparatus, mitochondria and the nucleus are evident (Fig. 4B). At this stage, parenchymatic cells of the core are also rapidly differentiating but differ because they have a large vacuole.
Fig. 4.
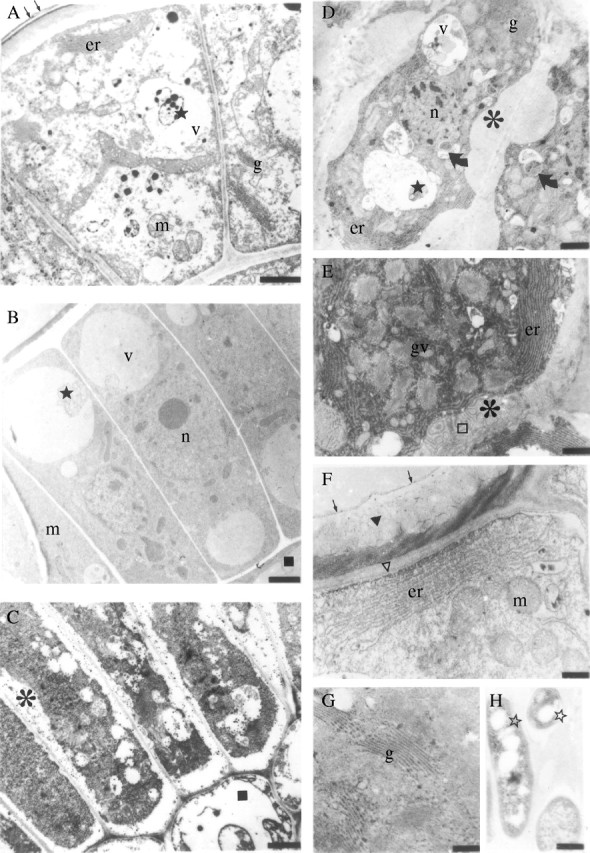
Transmission electron microscopy. (A–C) Secretory cells of Simira glaziovii in (A) immature stage, (B) intermediate stage and (C) mature stage of development; (D and E) secretory cells and (G) Golgi apparatus of S. pikia; (F) outer cell wall and organelles of the secretory cell of S. rubra; and (H) bacteria adjacent to the outer cell wall of the colleter of S. glaziovii. Scale bars: A = 1·0 µm; B and C = 2·5 µm; D = 1·7 µm; E = 1·1 µm; F = 0·4 µm; G and H = 0·25 µm. Asterisks, extraplasmic space; er, endoplasmic reticulum; g, Golgi stacks; gv, Golgi vesicles; m, mitochondria; open stars, microorganisms; n, nucleus; curved arrows, plastid; square parenchymatic cell; v, vacuole; arrows, cuticle proper; triangle, cuticular membrane; open triangle, polysaccharide layer; open square, fibrilar structures; stars, debris.
In the outer cell wall of epidermis at an intermediate stage of maturity, the cuticular layer has not fully developed a reticulated network of polysaccharides as observed by cytochemical staining. The polysaccharide portion of the cuticular layer reacts with both PATAg (specific for polysaccharides) and with ruthenium red (specific for pectins). In contrast, the lipid portion of the cuticular membrane, the cuticle proper and the matrix of the cuticular layer react with imidazole.
At maturity, the epidermal cells of the colleters in the three species studied become columnar. They have a dense cytoplasm with abundant ribosomes, a nucleus, small vacuoles, many mitochondria, endoplasmic reticulum and Golgi apparatus (Fig. 4C–G). In contrast, the parenchymatic cells have a large central vacuole. In the three species, the outer cell wall of the colleters has an lamellar layer, a cuticular membrane and cuticle proper (Fig. 4F). The cuticular membrane is reticulated (Fig. 4F), being different from the outer cell wall of the stipules that remains non-reticulated. The cuticle does not appear to rupture at maturity.
Extraplasmic spaces are found between the cell wall and the plasma membrane. Large quantities of endoplasmic reticulum are observed near the membrane (Fig. 4D–F). The Golgi apparatus is observed in all regions of these cells (Fig. 4D and E), and several vesicles are observed close to this organelle (Fig. 4G).
Several unique features were observed only in the epidermal cells of the three species. The mature epidermal cells of S. glaziovii showed lipidic bodies and large vesicles near the plasma membrane (Fig. 4C). In S. pikia, plastids are observed in the epidermal cells (Fig. 4D). In this species, the spaces between the plasma membrane and the cell wall are also filled with secretory material (Fig. 4D and E). Portions of this secretion have fibrillar structures in them (Fig. 4E). Simira rubra exhibits plasmodesmata between epidermal cells and the parenchymatic cells.
A few microorganisms were found embedded in the secretion closest to the outer cell wall of colleters of S. glaziovii (Fig. 4H) and S. rubra.
Biochemistry of secretion
Carbohydrates and proteins were detected in the secretion of S. glaziovii. Concentrations were 0·045 mg of protein mg−1 dry secretion and 0·2 mg of carbohydrate mg−1 dry secretion. Crude secretion preparations analysed by SDS–PAGE (Fig. 5) showed that the secretion from S. glaziovii is a mixture of proteins with molecular masses covering a range of approx. 45 to 14 kDa, with five major proteins of 45, 36, 29, 22 and 16 kDa (Fig. 5, column A). Inter- or intra-chain disulfide linkages occur in some of these proteins as demonstrated by treatment with reducing agents (Fig. 5, column B).
Fig. 5.
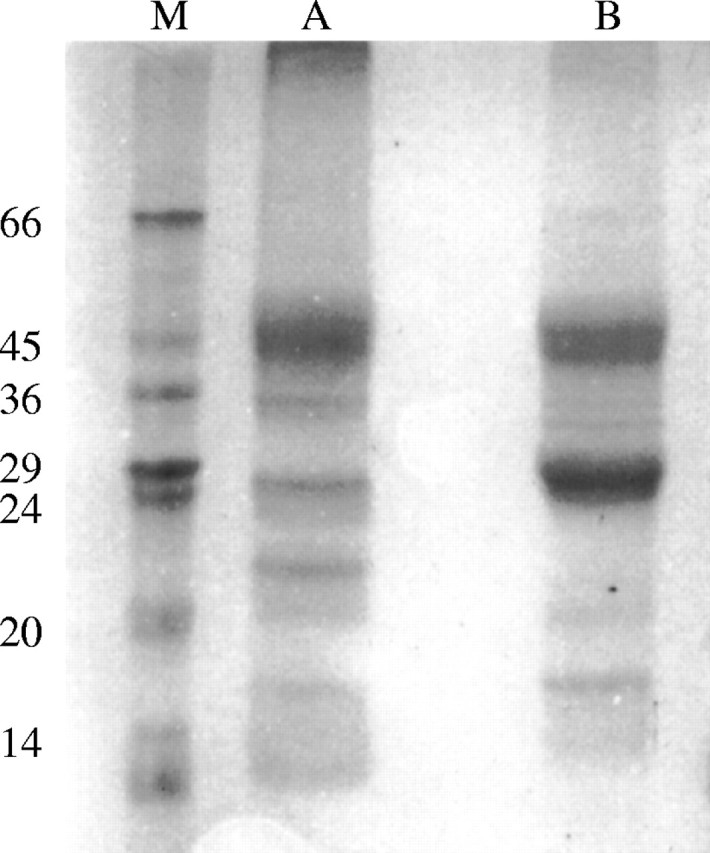
SDS–polyacrylamide gel electrophoresis of proteins from Simira glaziovii secretion. A, Ammonium sulfate fraction; B, ammonium sulfate fraction treated with β-mercaptoethanol; M, markers (kDa).
DISCUSSION
Simira glaziovii, S. pikia and S. rubra develop colleters at the bases of their stipules. However, the three species show a different distribution of colleters on their stipules, a triangular arrangements in S. glaziovii, a single row in S. pikia, and many rows in S. rubra. This arrangement of colleters on the stipule can be used as an aid in species identification. The species studied are woody taxa of the tribe Rondeletieae in the subfamily Cinchonoideae (Silva Neto, 2000).
Colleters of the three species are the standard type (Lersten, 1974a, b). The characters that describe the standard type, and separate it from other types, are that epidermal cells are columnar and not separated from each other. They also have a central parenchymatic axis (Lersten, 1974b).
The presence of a constriction or pedicel at the base of the colleters is poorly discussed in the literature. In species of Simira studied, colleters develop a constriction at their base. This character was observed in other rubiaceous species (Lersten, 1974a; Da Cunha and Vieira, 1997). In addition, the presence of chlorophylous pedicel cells was noted in the colleters of Allamanda (Apocynaceae; Ramayya and Bahadur, 1968).
Vascular traces, observed only in the parenchymatous core of the colleters of S. glaziovii, do not appear to have any importance in the activity of these secretory structures. Appezzato-da-Gloria and Estelita (2000) argued that the presence of a vascular trace is only dependent upon the proximity of these traces to the region of the colleter projection. More studies regarding the relationship between the colleters and vascularization are required, however, to establish its importance and its role in the transport of nutrients to secretory cells.
The knowledge of the colleter ultrastructure is restricted to a few species (Horner and Lersten, 1968; Dexheimer and Guenin, 1981; Miller et al., 1983; Durkee et al., 1984; Mohan and Inamdar, 1986). In all the species examined ultrastructurally, the endoplasmic reticulum occurred in perinuclear and peripheral locations in the cells. Secretory material was also observed between the cell membrane and the cell wall. In all cases, the endoplasmic reticulum and Golgi apparatus appeared to be involved in the production of mucilage (Fahn, 1988) as for Simira. Dexheimer and Guenin (1981) observed that, for the colleters of Psychotria bacteriophila (Rubiaceae), the production of mucilage protein occurred at the endoplasmic reticulum and polysaccharides on the Golgi apparatus. In contrast, mitochondria were also shown to be involved directly in mucilage production in the root hairs of Sorghum (Werker and Kislev, 1978). For Simira species, the presence of endoplasmic reticulum and Golgi apparatus in the periphery of the cell, next to the spaces between the cell wall and plasma membrane, suggests an involvement of these organelles in the production of secretions.
A few studies have described changes in secretory constituents during the development of secretory structures. In S. glaziovii, lipidic bodies were observed at matures stage of cell development. The absence of these bodies in S. pikia and S. rubra colleters may imply that observed colleters were less mature. Conversely, the presence of plastids in S. pikia cannot be explained in the same manner, considering that plastids may be involved in the production of secretions (Horner and Lersten, 1968; Kristen, 1976; Miller et al., 1983; Mohan and Inamdar, 1986). The presence of plasmodesmata in colleters of S. rubra was discovered for the first time for a colleter epidermal cell.
The three species studied exhibited a space between the plasma membrane and the epidermal cell wall. This space was identified as the extraplasmic space by Akers et al. (1978). These spaces were not found to be filled with secretory material in S. glaziovii and S. rubra. However, S. pikia was found to have these spaces filled with a dense secretion. These differences in secretion partitioning for different species can be found in other secretory structures (Rachmilevitz and Fahn, 1972; Zamski et al., 1987).
This study showed that the outer cell wall of the mature colleter developed a reticulated network of polysaccharides in its cuticular layer. In contrast, the immature cell wall does not develop this network. Although many studies report that secretions are released via cuticular rupture (Horner and Lersten, 1968; Thomas and Dave, 1989), the cuticle was not observed to rupture in colleters of Simira. This observation suggests an involvement of the outer cell wall in the secretion process of, for example, the trichomes of Cannabis (Cannabaceae). In Cannabis, the secretion must pass through the outer cell walls in a vesicle-like structure to exit the cell (Mahlberg and Kim, 1992; Kim and Mahlberg, 1995). In the colleters of Simira, these vesicle-like structures were not found in the outer cell walls.
The general properties of colleters as defensive structures have been described for several species (Ramayya and Bahadur, 1968; Williams et al., 1982, Thomas and Dave, 1990). The biological significance of the colleter secretion is enhancing protection of the meristematic tissues (Robbrecht, 1988). In addition, the main function of secretions in plants is thought to be a defense mechanism against herbivores and microorganisms (Zalucki et al., 2001; Cruz et al., 2002). Exudates, for example, are known to contain several proteins related to plant defense, including chitinases, polyphenol oxidases and β-1,3-glucanases (Subroto et al., 1996; Wititsuwannakul et al., 2002; Azarkan et al., 2003). Our present biochemical analysis shows the presence of proteins and carbohydrates in secretions from S. glaziovii. These proteins are possibly associated with defense mechanisms against microorganisms. Further studies are required to determine the role of these proteins in plant protection.
Acknowledgments
We thank the technicians of Laboratório de Biologia Celular e Tecidual/UENF and Botânica Estrutural/JBRJ for their assistance. We also thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Fundação de Amparo a Pesquisa do Rio de Janeiro (FAPERJ), the Fundação Estadual do Norte Fluminense, the Fundação Margareth Mee and the PETROBRÁS for their financial support. This study is a part of the MSc degree thesis of D.E.K. carried out at the Universidade Estadual do Norte Fluminense.
LITERATURE CITED
- Akers PC, Weybrew JA, Long RC. 1978. Ultrastructure of glandular trichomes of leaves of Nicotiana tabacum L., cv. xanthi American Journal of Botany 65: 282–292. [Google Scholar]
- Angermüller S, Fahimi DH. 1982. Imidazole-buffered osmium tetroxide: an excellent stain for visualization of lipids in transmition electron microscopy. Histochemical Journal 14: 823–825. [DOI] [PubMed] [Google Scholar]
- Apezzato-da-Gloria B, Estelita MEM. 2000. Development, structure and distribution of colleters in Mandevilla illustris and M. velutina (Apocynaceae). Revista Brasileira de Botânica 23: 113–120. [Google Scholar]
- Azarkan M, El Moussaoui A, van Wuytswinkel D, Dehon G, Looze Y. 2003. Fractionation and purification of the enzymes stored in the latex of Carica papaya Journal of Chromatography B – Analytical Technologies in the Biomedical and Life Sciences 790: 229–238. [DOI] [PubMed] [Google Scholar]
- Bradford MM. 1976. A rapid sensitive method for the quantification of microgram quantities of protein utilising the principle of dye binding. Biochemistry 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Cruz MAL, Gomes VM, Machado OLT, Fernandes KVS, Xavier Filho J. 2002. Defense proteins of carnauba tree (Copernicia cerifera) Wax. Identification and partial characterization of a chitinase and a β-1,3-glucanase. Plant Physiology and Biochemistry 40: 11–16. [Google Scholar]
- Da Cunha M, Vieira RC. 1997. Anatomia foliar de Psychotria velloziana Benth. (Rubiaceae). Rodriguésia 49: 39–50. [Google Scholar]
- Dexheimer J, Guenin F. 1981. Étude de la sécrétion de mucilage par le trichomes stipulaires de Psychotria bacteriophila (Rubiaceae). Cytologia 46: 731–747. [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. 1956. Colorimetric methods for determination of sugars and related substances. Analytical Chemistry 28: 350–356. [Google Scholar]
- Durkee LT, Baird CW, Cohen PF. 1984. Light and electron microscopy of the resin glands of Passiflora foetida (Passifloraceae). American. Journal of Botany 71: 596–602. [Google Scholar]
- Fahn, A. 1988. Secretory tissues in vascular plants. New Phytologist 108: 229–257. [DOI] [PubMed] [Google Scholar]
- Farrell BD, Dussourd DE, Mitter C. 1991. Escalation of plant defence: do latex and resin canals spur plant diversification? American Naturalist 138: 881–900. [Google Scholar]
- Giordani R, Lafon L. 1993. Action of Carica papaya latex on cell wall glycosidases from Lactuca sativa Phytochemistry 34: 1473–1475. [Google Scholar]
- Guedes-Bruni RR. 1998.Composição, estrutura e similaridade florística de dossel em seis unidades de Mata Atlântica no Rio de Janeiro. Tese de Doutorado, Universidade de São Paulo, Brasil. [Google Scholar]
- Horner HT, Lersten NR. 1968. Development, structure and function of secretory trichomes in Psychotria bacteriophila (Rubiaceae). American Journal of Botany 55: 1089–1099. [Google Scholar]
- Kim ES, Mahlberg PG. 1995. Glandular cuticle formation in Cannabis (Cannabaceae). American Journal of Botany 82: 1207–1214. [Google Scholar]
- Kristen U. 1976. Die morphologie der Schleimsekretion im fruchtnoten von Aptenia cordifolia Protoplasma 89: 221–233. [Google Scholar]
- Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4 Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- Lersten NR. 1974. Morphology and distribution of colleters and crystals in relation to the taxonomy and bacterial leaf nodules in Psychotria (Rubiaceae). American Journal of Botany 61: 973–981. [Google Scholar]
- Lersten NR. 1974. Colleter morphology in Pavetta, Neorosea and Tricalysia (Rubiaceae) and its relationship to the bacterial leaf nodule symbiosis. Botanical Journal of Linnean Society 69: 125–136. [Google Scholar]
- Lersten NR. 1975. Colleter types in Rubiaceae, especially in relation to the bacterial leaf nodule symbiosis. Botanical Journal of Linnean Society 71: 311–319. [Google Scholar]
- Lima HC, Guedes-Bruni RR. 1997. Plantas arbóreas da Reserva Ecológica de Macaé de Cima. In: Lima HC, Guedes-Bruni RR. Serra de Macaé de Cima: Diversidade Florística e Conservação em Mata Atlântica. Rio de Janeiro: Instituto de Pesquisa Jardim Botânico do Rio de Janeiro, 53–64. [Google Scholar]
- Luft JH. 1971. Ruthenium red and violet. II. Fine structural localization in animal tissues. Anatomical Record 171: 369–376. [DOI] [PubMed] [Google Scholar]
- Mahlberg PG, Kim ES. 1992. Secretory vesicle formation in glandular trichomes of Cannabis sativa (Cannabaceae). American Journal of Botany 79: 166–173. [Google Scholar]
- Miller IM, Scott A, Gardner IC. 1983. The development, structure and function of dendroid colleters in Psychotria Kirkii Hiern (Rubiaceae). Annals of Botany 51: 621–630. [Google Scholar]
- Mohan JSS, Inamdar JA. 1986. Ultrastructure and secretion of extrafloral nectaries of Plumeria rubra L. Annals of Botany 57: 389–401. [Google Scholar]
- Rachmilevitz T, Fahn A. 1972. Ultrastructure of nectaries of Vinca rosea L., Vinca major L. and Citrus sinensis Osbeck cv. Valencia and its relation to the mechanism of nectar secretion. Annals of Botany 37: 1–9. [Google Scholar]
- Ramayya N, Bahadur B. 1968. Morphology of the ‘squamellae’ in the light of their ontogeny. Current Science 37: 520–522. [Google Scholar]
- Robbrecht E. 1988. Tropical woody Rubiaceae. Characteristic features and progressions. Contributions to a new subfamilial classification. Opera Botanica Belgica 1: 1–271. [Google Scholar]
- Silva Neto SJ. 2000.O gênero Simira Aubl. (Rubiaceae, Rondeletieae) no Brasil extra-amazônico. Tese de Mestrado, Museu Nacional, Universidade Federal do Rio de Janeiro, Brasil. [Google Scholar]
- Subroto T, VanKoningsveld GA, Schreuder HA, Soedjanaatmadja UMS, Beintema JJ. 1996. Chitinase and beta-1,3-glucanase in the lutoid-body fraction of Hevea latex. Phytochemistry, 43: 29–37. [DOI] [PubMed] [Google Scholar]
- Thiéry JP. 1967. Mise en evidence des polysaccharides sur coupes fines en microscopie eletronique. Journal Microscopie 6: 987–1016. [Google Scholar]
- Thomas V. 1991. Structural, functional and phylogenetic aspects of the colleter. Annals of Botany 68: 287–305. [Google Scholar]
- Thomas V, Dave Y. 1989. Histochemistry and senescence of colleters of Allamanda cathartica (Apocynaceae). Annals of Botany 64: 201–203. [Google Scholar]
- Thomas V, Dave Y. 1990. Structure and necrosis of stipular colleter in Mitragyna parvifolia (Rubiaceae). Belgian Journal of Botany 123: 67–72. [Google Scholar]
- Van Hove C, Kagoyre K. 1974. A comparative study of stipular glands in nodulating and non-nodulating species of Rubiaceae. Annals of Botany 38: 989–991. [Google Scholar]
- Werker E, Kislev M. 1978. Mucilage on the root surface and root hairs of Sorghum: heterogeneity in structure, manner of productions and site of accumulation. Annals of Botany 42: 809–816. [Google Scholar]
- Williams RF, Metcalf RA, Gust LW. 1982. The genesis of form in oleander (Nerium oleander L.). Australian Journal of Botany 30: 677–687. [Google Scholar]
- Wititsuwannakul D, Chareonthiphakorn N, Pace M, Wititsuwannakul D. 2002. Polyphenol oxidases from Hevea brasiliensis: purification and characterization. Phytochemistry 61: 115–121. [DOI] [PubMed] [Google Scholar]
- Zalucki MP, Brower LP, Alonso A. 2001. Detrimental effects of latex and cardiac glycosides on survival and growth on first-instar monarch butterfly larvae Danaus plexippus feeding on the sandhill milkweed Asclepias humistrata Ecological Entomology 26: 212–224. [Google Scholar]
- Zamski E, Soham O, Palevitch D, Levy A. 1987. Ultrastructure of capsinoid-secreting cells in pungent and nonpungent red pepper (Capsicum annuum L.) cultivars. Botanical Gazette 148: 1–6. [Google Scholar]