Abstract
• Background and Aims Sub-arctic mountain birch Betula pubescens var. pumila communities in the North Atlantic region are of variable stature, ranging from prostrate scrubs to forests with trees up to 12 m high. Four hypotheses were tested, relating growth and population characteristics of sub-arctic birch woodland and scrub to tree stature; i.e. the variable stature of birch woods is due to differences in (1) the mean growth rate; (2) the age-related patterns of growth rate; (3) the life expectancy of stems; or (4) the tree form.
• Methods A stratified random sample of 300 birch trees was drawn from the total population of indigenous birch woodlands and scrub in Iceland, yielding 286 valid sample genets. The population was divided into three sub-populations with dominant trees 0–2, 2–4 and 4–12 m tall, referred to as birch scrub, birch scrub-woodland and birch forest, respectively.
• Key Results Trees in the scrub population were of more contorted growth form than birch in the scrub-woodland and forest populations. Mean growth rates, mean age and median life expectancies increased significantly with sub-population of greater tree stature. At the population level, annual increment and longevity of birch stems was apparently interrelated as the stems in vigorously growing birch sub-populations had a longer life expectancy than those of slower growth. However, no difference was observed between sub-populations in age-related patterns of extension growth rate.
• Conclusions The results were consistent with hypotheses (1), (3) and (4), but hypothesis (2) was rejected. Hence, mountain birch of more vigorous growth attains a greater stature than birch of lesser increment due to faster extension growth rate and a longer lifespan. In addition, the more contorted stem form of scrub populations contributes to their low stature.
Key words: Betula pubescens var. pumila, stature, height, growth, increment, population dynamics, life span, tree line, sub-arctic
INTRODUCTION
The mountain birch Betula pubescens var. pumila (Govaerts and Frodin, 1998) forms a gradient from prostrate shrubs to trees of up to 12 m high, 30 cm in diameter and more than 100 years of age, at the boundary between the boreal and arctic zones in the North Atlantic region.
Tree-size birch is reported from sheltered, edaphically favourable sites in South Greenland (Böcher, 1979; Kuivinen and Larson, 1982; Feilberg and Folving, 1990), Iceland (Bjarnason et al., 1977; Kristinsson 1995), Scandinavia and the Kola Peninsula of north Russia (Hämet-Ahti, 1963; Sjörs, 1963). Wide-ranging scrub-woods, with trees 3–5 m tall, cover dry and infertile sites in northern Scandinavia (Hämet-Ahti, 1963) and the Kola Peninsula of north-west Russia (Tseplyaev, 1965). There are considerable areas of birch scrub in Iceland (Kristinsson, 1995) and at exposed coastal sites in north Norway (Holmgren, 1912; Hämet-Ahti, 1963).
In Iceland, about 28 000 km2 (28 % of the land area) is within the climatic species limit of mountain birch, and at the time of human settlement in the 9th Century ad birch woods and scrub may have covered about 20 000 km2 (Jónsson, 1999). Most of this woodland cover has been lost due to clearing of the woods for pasture and hay fields, overgrazing and over-exploitation for firewood and charcoal (H Bjarnason, 1971, 1974; Thorarinsson, 1974; Guðbergsson 1975, 1996; Á Bjarnason, 1980). At present the natural birch woods and scrub cover approximately 1250 km2, and 80·8, 15·1, 2·4 and 1·7 % of that area is with dominant trees 0–2, 2–4, 4–8 and 8–12 m, respectively (Bjarnason et al., 1977). Hustich (1979) defined the tree line (i.e. the demarcation line between forests and tundra) as the upper- or outermost woody stem 2 m high or more. Hence, most of the birch in Iceland is beyond the tree line by this definition.
Mountain birch tree lines in Scandinavia receded in response to cooler summers and increasing oceanity during the latter part of the Holocene period (Kullman, 1995). However, this trend was reversed in the 20th Century (e.g. Aas, 1969; Kullman, 1979, 2000, 2001; Sonesson and Hoogesteger, 1983). The degree and rate at which the stature of mountain birch responds to changes in growing conditions will depend on the growth characteristics and the population dynamics of birch woods and patterns of recruitment.
Near the climatic tree limit, regeneration from seed is frequently found to be sporadic and the population structure of natural timberline forests may reflect the periodicity of regeneration and succession during climatically favourable periods (Payette and Gagnon, 1979; Packham et al., 1992). A number of studies have reported a period of increased growth and regeneration in both conifers and mountain birch close to the tree-line during the 1920s–1940s, followed by several decades of almost no regeneration and growth decline (Hytteborn et al., 1987; Kullman, 1996; Kullman and Engelmark, 1997).
A peak in the age distribution of tree-limit birches in Scandinavia coincided with the warm phase of the 1920s–1940s (Kullman, 1979), and an altitudinal age structure gradient, with a lower maximum age at the tree line and beyond than in favourable locations at lower levels (Sonesson and Hoogesteger, 1983) is persuasive evidence for the episodic regeneration of birch. However, an implied assumption in that interpretation of the age structure is that the age-related pattern of growth rate, the life expectancy of birch stems, and tree shape and posture are independent of tree vigour.
Tree size is the result of cumulative net increment; hence the size distribution in a tree population, and thus the general stature of the woodland, must be related to both growth rate and age structure of the tree population. However, conspecific trees at different sites may differ in the pattern of growth rate with age (Spurr and Barnes, 1980). Other things being equal, a difference in the age-related pattern of growth rate would result in a dissimilar general stature of the tree populations.
A negative correlation between growth rate and life span is well documented in the literature and may enable trees with different growth strategies to coexist and attain similar size at the same site (Enquist et al., 1999). Conversely, differences in life expectancy of tree populations would lead to divergence in the general tree size if growth rates are similar.
The objective of this study was to evaluate the degree to which the stature of sub-arctic mountain birch woods responds to changes in growth rate. In order to do so, four hypotheses were tested relating the stature of natural mountain birch populations to growth characteristics and population dynamics, i.e. that the variable stature of birch woods is due to a difference in (1) the mean extension growth rate, (2) the age-related pattern of extension growth rate, (3) the life expectancy of stems, or (4) the stem form (Fig. 1).
Fig. 1.

Four hypotheses relating stature of birch woodlands to growth characteristics and population dynamics. Variation in canopy height may be due to difference in (1) mean growth rate; (2) age-related pattern of increment; (3) life span; or (4) growth habit, i.e. (a) leaning, (b) decumbent, or (c) crooked stems.
The present study was a part of the Icelandic birch project, which started in 1987 (Jónsson, 2000). The project is in two parts: (1) an inventory and description of the native birch woodland resource, and (2) a study of the production and population ecology of the native birch woods. This paper presents results from the latter part of this project.
MATERIALS AND METHODS
A stratified random sample (Husch, 1971; Philip, 1983) of 300 trees of Betula pubescens Ehrh. var. pumila (L.) Govaerts was drawn from the total population of indigenous birch woodlands and scrub in Iceland, excluding all anthropogenic birch woods both seeded and planted (Fig. 2). One of three canopy height classes, 0–2, 2–4 or 4–12 m, was assigned to each natural birch woodland block in the country based on visually assessed general elevation of the birch canopy above ground level. The resultant grouping of the birch populations by these three canopy height classes are referred to (in ascending order of size) as scrub, scrub-woodland and forest sub-populations (Fig. 3). The demarcation of the population and canopy height classification was based on an inventory from 1972–1975 and subsequent amendments to woodland maps (Bjarnason et al., 1977; Aradóttir et al., 1995; Jónsson, 2000; Aradóttir et al., 2001).
Fig. 2.
Location within Iceland of 300 sample trees (black dots).
Fig. 3.
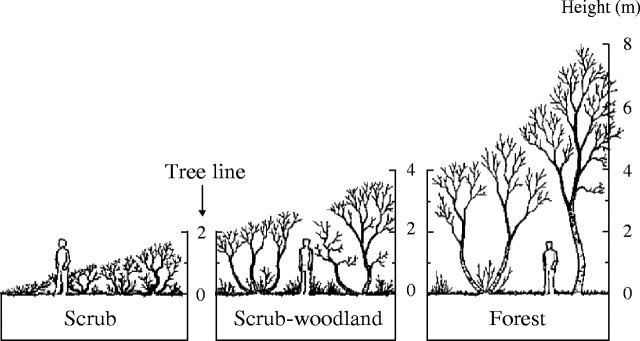
Classification of sub-arctic birch populations by canopy height into scrub (0–2 m), scrub-woodland (2–4 m) and forest (4–12 m). For clarity of presentation, the rare birch trees more than 8 m high are not indicated on the drawing.
A total of 60, 90 and 150 sampling units were selected from the birch scrub, scrub-woodland and forest sub-populations respectively, with equal probabilities within sub-populations.
Sampling units were randomly assigned to woodland blocks with probabilities in proportion to the contribution of the woodland block to the total sub-population area (Philip, 1983), and were located within a woodland block by randomly chosen co-ordinates on woodland maps. At the predetermined co-ordinates, a randomly chosen bearing and distance, in the range 0–30 m, defined the sampling point. The sampling tree was the closest tree to this point, defined as all stems in a cluster apparently originating from a single root system, irrespective of stem sizes. Hence, the sampling unit was the apparent genet.
Trees were sampled in the dormant season from September 1987 to April 1988. The number of stems per genet was recorded and their diameter measured at ground level and 50 cm from the ground. The thickest stem, at 50 cm above the ground, was defined as the dominant stem. The dominant stem was felled with a cut flush to the ground and its stem length, to the tip of the tree, was measured in two ways: (1) the straight line distance from ground level, and (2) stem length, measured with tape along the stem surface, from the root collar. The ratio of the straight-line length of ascending stem to total length of the dominant stem is a measure of tree shape used in this study. The occurrence of trees with decumbent stem form was also noted and leaning of the stem from a vertical posture was visually assessed.
Branches less than 5 cm in diameter were cut off the main stem. One of these branches was selected for sampling and all twigs were cut off that branch at the point where twig diameter = 1 cm. A sub-sample of three twigs was taken to the laboratory where the length of all long shoots was measured: the longest shoot per twig sample provides the estimate of current rate of extension growth used in this study.
Stem discs, approximately 2 cm thick, were cut at ground level and 50 cm above ground in all trees, and at 50 cm intervals up the bole to the point of 5 cm diameter in trees of sufficient stem thickness.
In the laboratory, annual rings were carefully counted on all discs with the aid of a binocular stereoscope. On each disc, two perpendicular straight lines were drawn through the pith, such that one traversed the greatest diameter. The distance along these lines was measured from the pith to (a) the fifth annual ring from the cambium, (b) the cambium, and (c) the bark surface.
The age of the tree was estimated as the number of annual rings at ground level. The average rate of radial growth, in this study, was taken as the mean width of annual rings, derived by dividing the lengths of the four radii to the cambium by the corresponding number of annual rings in the discs at ground level and 50 cm from ground. The current rate of radial growth was similarly estimated by the average width of the five most recent annual rings.
The initial growth rate of basal sprouts destined to become dominant stems was measured as the difference between the age of the tree at ground level and 50 cm from the root collar, and average extension growth rate is presented as stem length divided by stem age.
Life tables (e.g. Southwood, 1978) with median life expectancies for birch trees by ten-year age classes were constructed for each birch sub-population using the survival analysis package of Statistica™ software, based on the assumption that the cumulative age distribution curves of dominant stems per genet may be interpreted as static survivorship curves, i.e. that there is continuous regeneration in the population and that mortality in each age class may be assumed to be constant (cf. Packham et al., 1992).
RESULTS
A total of 286 valid samples of birch genets were obtained from 109 birch woodland blocks throughout the country, with 55, 81 and 149 sampling units from the birch scrub, scrub-woodland and forest sub-populations respectively (Table 1). Hence, there was a total of 14 missing sampling units.
Table 1.
Maximum, minimum and mean values with 95 % CL and number of observations (within brackets) of tree size and growth rate characteristics of birch scrub (0–2 m), scrub-woodlands (2–4 m) and forest (4–12 m) populations in Iceland, with F-statistics and P-values for comparison between sub-populations
| Unit |
Min |
Max |
Scrub |
Scrub-woodland |
Forest |
F statistic |
P-value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tree size | ||||||||||||||||
| Length of ascending stem segment | m | 0·15 | 8·50 | 1·03 ± 0·18 (54) | 1·82 ± 0·25 (75) | 2·36 ± 0·33 (79) | F2,205 = 20·91 | P < 0·0001 | ||||||||
| Total stem length | m | 0·50 | 9·13 | 1·75 ± 0·34 (37) | 2·71 ± 0·37 (71) | 4·22 ± 0·30 (140) | F2,245 = 41·52 | P < 0·0001 | ||||||||
| Diameter at ground level | cm | 1·01 | 38·07 | 4·21 ± 0·70 (54) | 6·47 ± 0·82 (80) | 9·00 ± 0·83 (148) | F2,279 = 26·59 | P < 0·0001 | ||||||||
| Growth rate | ||||||||||||||||
| Time to reach 50 cm | years | 1 | 36 | 10·6 ± 2·1 (50) | 8·6 ± 1·3 (79) | 7·2 ± 0·8 (147) | F2,273 = 6·72 | P < 0·0014 | ||||||||
| Increment in stem length | cm yr−1 | 1·09 | 22·97 | 4·82 ± 0·75 (34) | 6·30 ± 0·60 (70) | 7·30 ± 0·49 (137) | F2,238 = 12·53 | P < 0·0001 | ||||||||
| Mean annual ring width | mm yr−1 | 0·46 | 4·65 | 0·50 ± 0·04 (54) | 0·58 ± 0·04 (80) | 0·65 ± 0·04 (148) | F2,279 = 9·42 | P < 0·0001 | ||||||||
| Average width of five most recent annual rings | mm yr−1 | 0·14 | 2·78 | 0·48 ± 0·06 (55) | 0·63 ± 0·07 (81) | 0·80 ± 0·08 (149) | F2,282 = 12·92 | P < 0·0001 | ||||||||
| Longest shoot | cm | 1·5 | 29·5 | 9·54 ± 2·54 (23) | 9·73 ± 1·17 (66) | 11·53 ± 0·94 (134) | F2,220 = 3·23 | P < 0·0416 | ||||||||
A valid model explaining tree stature in birch woods must predict significantly different growth and population characteristics between the birch sub-populations of unlike stature, and the characteristics should change directionally with sub-populations of increasing stature.
Analysis of variance and the Kruskal–Wallis ANOVA, median test, were used to evaluate differences in tree size, growth form, growth rate and age among the three birch sub-populations. Linear contrast was used to test linear trends along the gradient of sub-populations of increasing stature.
The data were analysed using the Statistica™ software (Kernel release 5.5A, StatSoft, Inc.).
Tree size
The classification of the native birch population in Iceland into shrubs (0–2 m), scrub-woodland (2–4 m) and forest (4–12 m) sub-populations, based on the subjectively assessed general elevation of the canopy, not surprisingly closely reflects tree size. Average tree size, measured as length of ascending stem section, total stem length and diameter at ground level were highly significantly different between sub-populations (Table 1) and increased in the order scrub < scrub woodland < birch forest (linear trends: length of ascending stem section F1,191 = 23·35, P < 0·0001; total stem length F1,245 = 64·77, P < 0·0001; diameter at ground level F1,279 = 48·42, P < 0·0001).
Growth rate
Growth rate increased with birch sub-population of higher tree stature. A significant difference between sub-populations (Table 1) and linear trend with increasing tree stature was observed for average width of annual rings (linear trend F1,279 = 17·77, P < 0·0001), average width of five most recent annual rings (linear trend F1,282 = 23·28, P < 0·0001), number of years to reach 50 cm length from root collar (linear trend F1,273 = 12·93, P < 0·0004), and average extension growth rate (linear trend F1,238 = 23·26, P < 0·0001). The results are consistent with hypothesis (1), that stature of birch populations is affected by mean extension growth rate (Table 1).
To compare the populations for age-related differences in growth rate, tree size (stem length and diameter) and current growth rate (shoot length and 5-year mean radial growth rate) data were compared for three age classes: 1–30, 31–60 and 61–90 years. Very few trees were older than 90 years in the birch scrub and scrub-woodland populations; hence the analysis was limited to age classes up to 90 years. A statistically significant interaction between birch sub-population and age class in tree size, and main effect differences in current growth rate by age classes, were the tests of difference in age-related growth rate between birch populations.
The average width of the five most recent annual rings was not statistically different between 30-year age classes up to the age of 90 years (F2,257 = 0·372, P = 0·689) and no significant interaction was observed between age class and birch sub-population for average 5-year radial increment prior to sampling (F4,257 = 0·170, P = 0·954).
The population means of shoot lengths for the summer of 1987, measured as the longest shoot in a sample of three twigs, were 9·5, 9·7 and 11·5 for the scrub, scrub-woodland and forest sub-populations, respectively (Table 1). The shoot lengths were highly variable within birch sub-populations and only a marginally significant difference was observed between sub-populations (Table 1). Maximum shoot lengths were not significantly different between age classes (F2,200 = 0·70, P = 0·4955).
All measures of tree size (length of ascending stem section, total stem length and diameter at ground level) increase with 30-year age classes up to the age of 90 years (Fig. 4), with no indication of interaction between birch sub-population and age class in any measures of size (length of ascending stem section, F4,191 = 1·576, P = 0·1823; total stem length, F4,221 = 1·062, P = 0·3763; diameter at ground level, F4,257 = 0·944, P = 0·4392). Hence there is no evidence, in the present study, for a difference in age-related pattern of growth between the three birch sub-populations. Based on these results hypothesis (2) is rejected.
Fig. 4.
Mean length (± s.e.) of dominant birch stems per genet by 30-year age classes, for three populations of increasing stature: scrub (0–2 m), scrub-woodland (2–4 m) and forest (4–12 m).
Age
The oldest tree in the present study had 135 annual rings at the stem base. Only six trees in the birch forest sub-population were more than 100 years of age and the oldest trees in the sample from the birch scrub and birch scrub-woodland populations were 100 and 99 years, respectively.
The mean age was 36·1 ± 5·7, 47·3 ± 5·0 and 58·9 ± 3·9 years (±95 % confidence limits, CL) in birch scrub, birch scrub-woodland and birch forest populations, respectively. Age differences between birch sub-populations are highly significant (F2,279 = 21·32, P < 0·0001, Kruskal–Wallis H = 38·16, d.f. = 2, N = 282, P < 0·0001; median test χ2 = 27·22, d.f. = 2, P < 0·0001). Furthermore, a positive linear trend of mean age with populations of increasing stature was highly significant (linear trend, F1,279 = 39·38, P < 0·0001). It may be concluded that birch trees in populations of high stature attain a greater mean age than birch in populations of low stature.
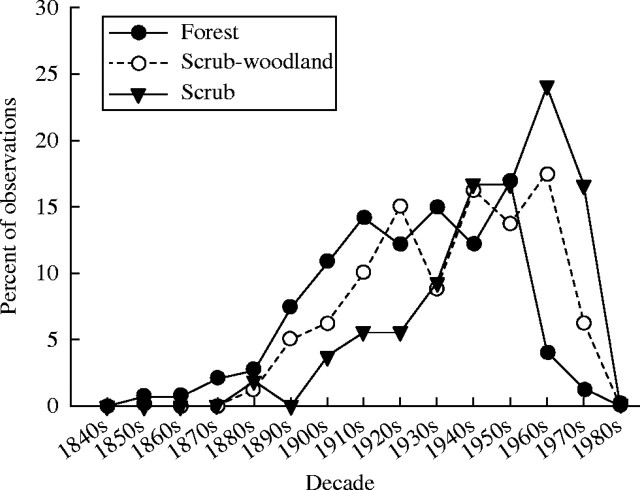
The age distribution of mountain birch is frequently presented by decade of stem initiation (e.g. Kullman, 1979, 1991a, b, 1993; Hofgaard, 1993), based on the assumption that the age distribution may reflect periodicity of regeneration. In the present study, the age distributions of dominant stems per genet by decade of origin from all three sub-populations are of similar shape, but are apparently not in phase, and with no stems in the most recent time period (1980–1987) (Fig. 5).
Fig. 5.
Age distributions of dominant birch stems per genet by decade of stem origin, for three populations of increasing stature: scrub (0–2 m), scrub-woodland (2–4 m) and forest (4–12 m).
The age distribution of the scrub sub-population has a distinct peak during the relatively cold 1960s. However, stems of 2 m in length or more were predominantly initiated during the 1920s–1940s (Fig. 6).
Fig. 6.
Age distribution of dominant birch stems per genet in birch scrub (0–2 m) by decade of stem origin, classified by mean stem length classes 0–1, 1–2 and >2 m.
Longevity
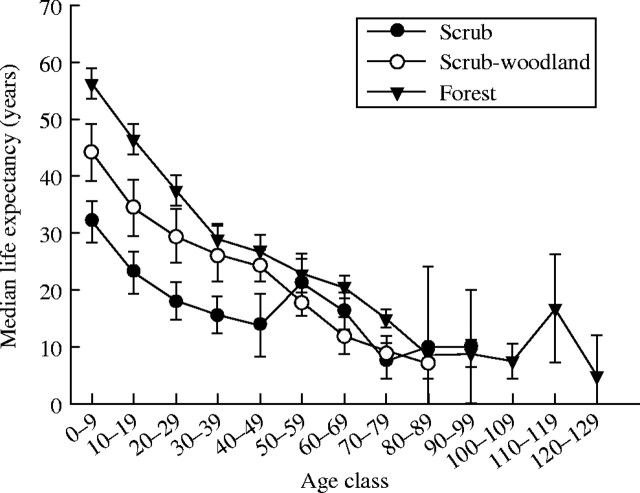
The median life expectancies of dominant birch stems per genet in scrub, scrub-woodland and forest sub-populations, respectively, are 32·0 ± 3·7, 44·2 ± 5·0 and 56·2 ± 2·8 years (± s.e.) at the time of stem initiation, but only 7·5 ± 3·1, 9·0 ± 2·4 and 15·0 ± 1·7 years for trees surviving at 80 years of age (Fig. 7). The results are consistent with hypothesis (3), i.e. the difference in stature between birch sub-populations of unlike canopy height is partly due to the different life expectancy of dominant stems.
Fig. 7.
Median life expectancy (±s.e.) of dominant birch stems per genet by 10-year time intervals, for three sub-populations of increasing stature: scrub (0–2 m), scrub-woodland (2–4 m) and forest (4–12 m).
Life expectancy by growth rate
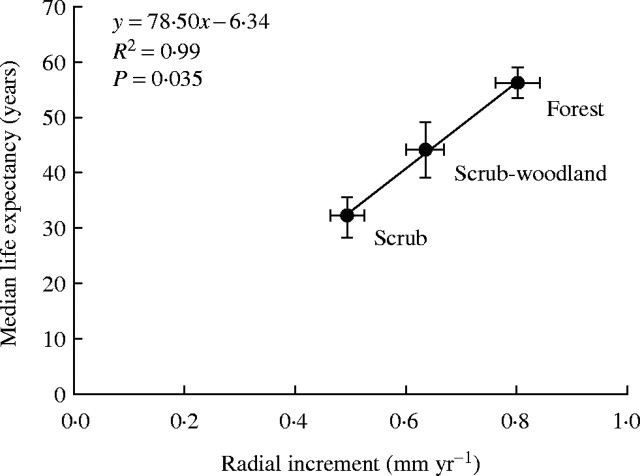
Both growth rate and median life expectancy of birch increase in a near-linear fashion between the three birch populations of progressively higher tree stature. The 5-year mean radial growth rate prior to sampling is the most precise measure of growth rate in the present study (derived from measurements on eight radii from each tree) and it is computationally independent of age, as opposed to mean growth rate derived from cumulative growth divided by age. Hence, median life expectancy of stems was plotted against 5-year radial increment to assess whether life expectancy may be related to growth rate.
There is an almost perfect linear fit (adjusted r2 = 0·994, P = 0·035) between median life expectancy at the time of stem initiation and 5-year mean radial growth rate, indicating that trees in birch populations of high growth rate tend to survive to a greater age than trees in populations of lesser growth rate (Fig. 8).
Fig. 8.
Relationship between median life expectancy (± s.e.) at the time of stem origination and 5-year mean radial increment (± s.e., mean for the period 1982–1987) of dominant birch stems per genet, for three populations of increasing stature: scrub (0–2 m), scrub-woodland (2–4 m) and forest (4–12 m).
Growth habit
Overall, there were three stems per genet (median value) and the median number of stems was not significantly different between sub-populations (Kruskal–Wallis H = 5·04, d.f. = 2, N = 189, P = 0·0806; median test χ2 = 5·06, d.f. = 2, P = 0·0795).
Birch in the scrub sub-populations had a significantly more contorted stem form compared with trees in the scrub-woodland and forest sub-populations (Kruskal–Wallis H = 13·63, d.f. = 2, N = 172, P = 0·0011; median test χ2 = 9·01, d.f. = 2, P = 0·0111). The median value of straight-line length of the ascending stem section was only 56 % of total stem length in the birch scrub population, compared with 73 and 75 % in the scrub-woodland and forest populations respectively. The results, therefore, are consistent with hypothesis (4) that the stature of birch populations is affected by growth habit. However, as only the birch scrub has a more contorted stem form, stem form has not been conclusively shown to change directionally with populations of increasing stature or growth rate.
A third of the trees in this study (33 %) were noted to have a decumbent stem form and this growth habit was apparently prevalent on sloping ground and sites of heavy snow accumulation. Very few trees had their stems in a vertical posture and many trees leaned 30–50° from vertical. Hence the vertical height of the birch is even lower than indicated by the straight-line length of ascending stems. Analysis of the frequency of decumbent stem form or stem posture by sub-populations was not warranted due to missing observations for these characteristics.
DISCUSSION
The present study was based on a stratified random sample of individual genets from the total population of natural birch in Iceland. This approach has the advantage of highlighting general growth and population characteristics of birch in relation to tree size and woodland stature, as opposed to features related to spatial scale or the temporal stage of stand development.
The results are consistent with three out of the four hypotheses put forward in the Introduction, i.e. the difference in stature between birch populations is partly due to (1) different mean extension growth rates, (3) different life expectancy of dominant stems, and (4) different tree shapes between birch populations. Hypothesis (2), that the difference in canopy height arises because of differences in age-related patterns of extension growth rate is, however, rejected. Therefore factors resulting in a tree-size ceiling, such as climatic pruning to a fixed height (e.g. Holmgren, 1912; Hämet-Ahti, 1963; Anderson et al., 1966), are not important in limiting the stature of mountain birch populations in general, although climatic pruning may be locally important. Nevertheless, shoot die-back may contribute to slower mean extension growth rate and to more contorted stem forms of birch in the scrub sub-population (T. H. Jónsson, unpublished data).
There is, of course, a fifth hypothesis that is not explicitly addressed in the present study and which would explain the smaller size and younger age of birch populations of lesser stature compared with those of greater tree size: this is that the birch population is expanding and the birch scrub represents a recent front of advance. However, this hypothesis was not considered valid as there is sufficient historical evidence (e.g. Magnússon and Vídalín, 1913–1943; Kofoed-Hansen, 1925) to show that the present birch scrub is not of recent origin and most of the scrublands have remained of similar stature for centuries. In fact, the natural birch woodland area declined considerably during the 18th and 19th Centuries (H Bjarnason, 1971, 1974; A Bjarnason, 1980; Guðbergsson, 1996) and has not expanded to any appreciable degree from the early 20th Century, except in a few areas protected from sheep grazing.
Human impact
More than 85 % of the natural birch population is open to grazing by sheep (Bragason, 1995) and the birch woods have been grazed for centuries (Bjarnason, 1974). The grazing pressure has until recently been sufficient throughout the country to prevent birch colonization of treeless sites. However, grazing has not hindered regeneration of the birch woodland remnants, as evidenced by their continuous existence, their regeneration in almost all decades during the 20th century (Fig. 5), and by the abundance of stem sprouts observed in birch woodlands and scrub throughout the country (Aradóttir et al., 1995, 2001).
Birch woods were an important source of charcoal, firewood and construction poles until the 1870s (Thorarinsson, 1974). The total annual cut was 800–2000 t from the 1880s to 1940s (Anonymous, 1997), and has since then been about 200 t annually (Hallanaro and Pylvänäinen, 2001) and limited to a few managed woods. This represents a very low level of cutting (Jónsson, 1999) for over a century, and it is unlikely to have markedly affected the age or size distribution at the population level.
Growth habit
Sub-arctic birch trees are highly variable and irregular structures with one-to-several crooked and leaning stems of variable size (Elkington and Jones, 1974; Verwijst, 1988). Therefore tree height (i.e. the vertical elevation of the treetop above the root collar) may be much lower than stem length (Elkington and Jones, 1974).
The polycormic, crooked and procumbent growth habit of the mountain birch has been attributed to stress factors such as snow load (Elkington and Jones, 1974; Kullman, 1979, 1989) and to soil conditions (Verwijst, 1988), as well as to genetic make-up (Sveinbjörnsson et al., 1993), for example gene flow between Betula pubescens and B. nana, which is reported from Greenland (Sulkinoja, 1990; Fredskild, 1991), Iceland (Elkington, 1968; Anamthawat-Jónsson et al., 1993; Anamthawat-Jónsson, 1994) and Scandinavia (Vaarama and Valanne, 1973; Kallio et al., 1983; Mäkelä, 1999).
In the present study, the number of stems per genet or stem form was not conclusively shown to change with woodland stature. Hence, without further evidence, growth habit cannot be assumed to respond to changes in growth rate.
Regeneration
The age distributions for the three birch sub-populations in this study (Figs 5, 6) are strikingly similar to previously published age distributions of mountain birch of similar stature in the sub-alpine (sub-arctic) birch woods (e.g. Kullman, 1979, 1991a, b, 1993) and natural stands of mountain coniferous forests (e.g. Hytteborn et al., 1987; Hofgaard, 1993) in Scandinavia. A further similarity is that stems 2 m or more in length originate both in the scrub sub-population in this study (Fig. 6) and at the tree line in Scandinavia (Kullman, 1979) before the cold 1960s, and mostly during the relatively warm 1920s–1940s, which may seem to indicate a regeneration pulse at that time.
If regeneration of birch were periodic in response to climatic variation, and the age distribution retains a regeneration signal, we would expect the demographic profile of the three birch sub-populations to be in phase and to reflect major climatic variations during the 20th Century. This signal should be particularly noticeable in the tree-line ecotone, as a number of studies have shown tree-line trees to respond strongly to changes in climate (e.g. Tranquillini, 1979; Kullman, 1993; Wardle, 1993). The birch scrub and scrub-woodland sub-populations in the present study are analogous to a tree-line ecotone (i.e. 2 m height classes on both sides of a tree line of 2 m).
The period 1920–1950 was relatively warm in Iceland, while the 1960s and 1970s were cold with sea ice at the north coast in some years (Bergthórsson et al., 1987). Hence a peak in the age distribution coinciding with regeneration in the 1920s–1950s and a nadir during the cold 1960s–1970s would be expected. This is not so; the age distributions are not in phase (Fig. 5) and, contrary to expectations, the highest proportion of dominant stems in the scrub population originate in the unusually cold 1960s (Fig. 6). The observed age structures could possibly originate from high mortality of older and bigger stems during the cold 1960s–1970s in sub-populations of low stature. However, a mortality event was not noticed at the time, but it would not have passed detection bearing in mind that there was a national inventory of natural birch woods in 1972–1975.
The age distributions for the three sub-populations show a progressive shift towards higher age with populations of greater tree stature (Fig. 5), and the frequency of stems in the scrub population, by 1-m stem length classes (Fig. 6), apparently reflects cohorts of stems attaining progressively greater size with increasing age.
The mountain birch is a slow-growing tree. The average length of top shoots of mountain birch from different parts of Scandinavia is reported to be in the range 4–11 cm (Kullman, 1979) with shoots up to 20–30 cm in favourable years (Kullman, 2001). The results of the present study fall within that range (Table 1).
Based on the mean net increments in stem length (Table 1) and median stem shape (56, 73 and 75 %, respectively) it would take an upright tree on average 74, 44 and 37 years to attain 2 m height in the scrub, scrub-woodland and forest populations, respectively. Furthermore, stems in the birch scrub will not take less than 20 years to attain 2-m height based on maximum growth rate and stem form observed in the present study. Considering that the birch is invariably leaning, the time needed to attain 2-m height is actually even longer. Hence most of the trees of 2-m height existing during the last quarter of the 20th Century in scrub or scrub-woodland populations would be expected from growth rate alone to have originated before the middle of that century.
Mountain birch woods regenerate almost exclusively by basal stem sprouts (Verwijst, 1988) and seedlings are rarely found within undisturbed birch woods (e.g. Aradóttir, 1991; Aradóttir et al., 1995, 2001).
Kullman (1991a) observed that basal sprouts are formed in abundance on genets right to the tree line, and birch genets may be much older than the oldest surviving stem (Kullman, 1993). The results of the present study show that, in all three sub-populations, new stems, which subsequently attained dominant position within the genet, were initiated in all decades during the 20th Century up to the 1980s (Fig. 5). The relatively poor representation of the youngest age classes observed in all three populations in the present study indicates the minimum time needed for cohorts of stem sprouts to grow to a dominant position in the genet. The peak in the age distribution reflects the age of highest replacement of declining dominants by subordinate stems.
Hence, at the population level, we may have profuse and continuous introduction of new stems. The results of the present study do not support intermittent regeneration of mountain birch in response to periods of variable climate.
Mortality
Verwijst (1988) observed that dominant stems in polycormous mountain birch trees were less vigorous compared with subordinant stems, having a sparse living crown with few long shoots and many dead short shoots. Sparse crowns of dominant stems and openings among trees would allow sufficient penetration of light for stems of lesser size to develop within the genet (Verwijst, 1988).
The largest stem in a polycormic birch should be relatively free from crown competition imposed by smaller-size stems on the same root system or neighbouring genets. Furthermore, Verwijst (1988) argued that density-dependent mortality by self-thinning is not important in the dynamics of polycormous mountain birch stands. Hence, the age structure of dominant stems per genet should reflect density-independent mortality, i.e. age- or size-related mortality.
Mountain birch stems may survive up to 200 years, as shown by Sonneson and Hogesteger (1983). However, birch trees over 100 years old are a small proportion of any mountain birch population reported in the literature (e.g. Kullman, 1979, 1991a, b, 1993), as would be expected from the short median life expectancies for trees of 80 years or more in the present study.
Verwijst (1988) proposed that the height of birch trees is limited by the increasing cost of tree size in accordance with the pipeline theory. He argued that the cost of tree size will limit height growth to a larger extent in an environment with low growth resources.
The ability of birch stems to resist both biotic and abiotic stress may be increasingly constrained as growth resources decline. This restriction is likely to increase as the stems grow bigger and time of exposure increases (i.e. the probability of experiencing stressful events). Hence, at the population level, both tree size and life expectancy may ultimately depend on growth resources.
Response of woodland stature to changes in growth rate
The alpine, arctic and antarctic distribution limit of tree species may be set by availability of viable seed, germination and survival of seedlings at the site (Weih and Karlsson, 1999; Cuevas, 2000; Karlsson and Weih, 2001). However, it should be borne in mind that the tree-line (tree-limit, timberline) refers to tree height (Hustich, 1979) and is not necessarily related to the distribution limit, as evidenced by wide-ranging birch scrublands in Iceland.
Kullman (1993) proposed that birch genets near the tree line are old relicts responding to climate variability by temporal fluctuations in vegetative effort, rather than by sexual population change. The results of the present study indicate that this statement may apply to mountain birch woods in general. Continuous rejuvenation of genets by stem sprouts, succession of cohorts of subordinate stems to dominant position, slow extension growth rate and age-related mortality of stems may adequately explain the characteristic age and size distributions of mountain birch woods.
Growth rate and life expectancy of dominant birch stems were positively correlated in the present study, hence relatively steep age and size structure gradients are expected along both temporal as well as spatial gradients of growth conditions. Birch woods may thus quickly attain greater canopy height in response to improved conditions, but the canopy height will recede with a lag time of several decades in response to deteriorating conditions, due to the life span of the existing canopy stems (cf. Kullman, 1993). It is, however, uncertain if, and then to what degree, growth habit will respond to changes in growth rate.
Acknowledgments
The author is indebted to Jón Gunnar Ottósson, director of the Icelandic Institute of Natural History, for his enduring commitment to the Icelandic birch project. Thanks are expressed to the staff at the Icelandic Forestry Research Station at Mógilsá for their high quality field and laboratory work. The government of Iceland supported the Icelandic birch project with a special project funding from 1987–1991. Additional funding from the Icelandic Research Council and the Icelandic Institute of Natural History is acknowledged. The author is grateful to Ævar Petersen, Guðmundur Halldórsson, Kristín Svavarsdóttir, Sveinn Aðalsteinsson, Thorleifur Jónsson and two referees for corrections and valuable comments on the manuscript, and to Hólmgeir Björnsson for discussion on statistical aspects of the present work.
LITERATURE CITED
- Aas B. 1969. Climatically raised birch lines in Southeastern Norway 1918–1968. Norsk geografisk Tidsskrift 23: 119–130. [Google Scholar]
- Anamthawat-Jónsson K. 1994. Genetic variation in Icelandic birch. Norwegian Journal of Agricultural Sciences. Supplement No 18: 9–14. [Google Scholar]
- Anamthawat-Jónsson K, Heslop-Harrison JS, Tómasson T. 1993. Genetics, cytogenetics, and molecular genetics of Icelandic birch: Implications for breeding and reforestation. In: Alden J, Mastrantonio JL, Ødum S, eds. Forest development in cold climates. New York: Plenum Press, 357–367. [Google Scholar]
- Anderson DJ, Cooke RC, Elkington TT, Read DJ. 1966. Studies on structure in plant communities. II. The structure of some dwarf-heath and birch-copse communities in Skjaldfannardalur, North-West Iceland. Journal of Ecology 54: 781–793. [Google Scholar]
- Anonymous. 1997.Hagskinna, Icelandic Historical Statistics. Reykjavík: Statistics Iceland, Iceland [In Icelandic and English]. [Google Scholar]
- Aradóttir AL. 1991.Population biology and stand development of birch (Betula pubescens Ehrh.) on disturbed sites in Iceland. PhD Thesis, Texas A and M University, USA. [Google Scholar]
- Aradóttir AL, Thorsteinsson I, Sigurdsson S. 1995.Survey of Icelandic birch woodlands 1987–1991. I. Overview, methods and results for Laugardalshreppur district in South-Iceland and Hálshreppur district in North Iceland. Reykjavik: Icelandic Forest Research Station Report 11. [In Icelandic, English summary]. [Google Scholar]
- Aradóttir AL, Thorsteinsson I, Sigurdsson S. 2001. Distribution and characteristics of birch woodlands in North Iceland. In: Wielgolaski FE, ed. Nordic mountain birch ecosystems. Paris: Unesco and The Parthenon Publishing Group, 51–61. [Google Scholar]
- Bergthórsson P, Björnsson H, Dýrmundsson Ó, Gudmundsson B, Helgadóttir Á, Jónmundsson JV. 1987. The effect of climatic variations on agriculture in Iceland. The impact of climatic variations on agriculture. In: Parry ML, Carter RR, Konijn NT, eds. Assessment in cool temperate and cold regions, volume 1. Dordrecht: Reidel, [Google Scholar]
- Bjarnason ÁH. 1980. The history of woodland in Fnjóskadalur. Acta Phytogeographica Suecica 68: 31–42. [Google Scholar]
- Bjarnason H. 1971. Um friðun lands og frjósemi jarðvegs [Protection and soil fertility]. Ársrit Skógræktarfélags Íslands 1971: 4–19. [In Icelandic, English summary]. [Google Scholar]
- Bjarnason H. 1974. Athugasemdir við sögu Íslendinga í sambandi við eyðingu skóglendis [Notes on the history of Iceland in relation to deforestation]. Ársrit Skógræktarfélags Íslands 1974: 30–43. [In Icelandic, English summary]. [Google Scholar]
- Bjarnason H, Sigurðsson S, Jörundarson H. 1977.Skóglendi Íslands. Athuganir á stærð þess ogástandi [Woodlands in Iceland, A survey of its extent and condition]. Reykjavík: Skógrækt ríkisins og Skógræktarfélag Íslands. [In Icelandic]. [Google Scholar]
- Böcher TW. 1979. Birch woodlands and tree growth in southern Greenland. Holarctic Ecology 2: 218–221. [Google Scholar]
- Bragason Á. 1995. Exotic trees in Iceland. Icelandic Agricultural Sciences 9: 37–45. [Google Scholar]
- Cuevas JG. 2000. Tree recruitment at the Nothofagus pumilio alpine timberline in Tierra del Fuego, Chile. Journal of Ecology 88: 840–855. [Google Scholar]
- Elkington TT. 1968. Introgressive hybridisation between Betula nana L. and B. pubescens Ehrh. in north-west Iceland. New Phytologist 67: 109–118. [Google Scholar]
- Elkington TT, Jones BMG. 1974. Biomass and primary productivity of birch (Betula pubescens s. lat.) in South-West Greenland. Journal of Ecology 56: 821–830. [Google Scholar]
- Enquist BJ, West GB, Charnov EL, Brown JH. 1999. Allometric scaling of production and life-history variation in vascular plants. Nature 401: 907–911. [Google Scholar]
- Feilberg J, Folving S. 1990. Mapping and monitoring of woodlands and scrub vegetation in Qingua-dalen, South Greenland. Meddelelser on Grønland, Bioscience 33: 9–20. [Google Scholar]
- Fredskild B. 1991. The genus Betula in Greenland – Holocene history, present distribution and synecology. Nordic Journal of Botany 11: 393–412. [Google Scholar]
- Govaerts R, Frodin DG. 1998.World checklist and bibliography of Fagales (Betulaceae, Corylaceae, Fagaceae and Ticodendraceae). London: The Royal Botanical Gardens, Kew. [Google Scholar]
- Guðbergsson G. 1975. Myndun móajarðvegs í Skagafirði [Soil formation in Skagafjörður, northern Iceland]. Journal of Agricultural Research in Icelandic 7: 20–45. [In Icelandic, English summary]. [Google Scholar]
- Guðbergsson G. 1996. Í norðlenskri vist. Um gróður, jarðveg, búskaparlög og sögu [The influence of human habitation on soil and vegetation in three counties in North Iceland]. Icelandic Agricultural Sciences 10: 31–89. [In Icelandic, English summary]. [Google Scholar]
- Hallanaro E, Pylvänäinen M. 2001.Nature in Northern Europe. Biodiversity in a changing environment. Helsinki: Nordic Council of Ministers, Nord 2001:13. [Google Scholar]
- Hämet-Ahti L. 1963. Zonation of the mountain birch forests in northernmost Fennoscandia. Annales Botanici Societatis Zoologicæ Botanicæ Fennicæ ‘Vanamo’ 34: 127p. [Google Scholar]
- Hofgaard A. 1993. Structure and regeneration patterns in a virgin Picea abies forest in northern Sweden. Journal of Vegetation Science 4: 601–608. [Google Scholar]
- Holmgren A. 1912.Studier öfver nordligaste skandinaviens björkskogar. Stockholm: P.A. Norstedt and Söner. [In Swedish]. [Google Scholar]
- Husch B. 1971.Planning a forest inventory. FAO Forestry and Forest Products Studies No. 17. Rome: Food and Agriculture Organisation of the United Nations. [Google Scholar]
- Hustich I. 1979. Ecological concepts and biographical zonation in the North: the need for a generally accepted terminology. Holarctic Ecology 2: 208–217. [Google Scholar]
- Hytteborn H, Packham JR, Verwijst T. 1987. Tree population dynamics, stand structure and species composition in the montane virgin forest of Vallibäcken, northern Sweden. Vegetatio 72: 3–19. [Google Scholar]
- Jónsson TH. 1999. Sustainable use of northern timberline forests in Iceland. In: Kankaanpää S, Tasanen T, Sutinen M-L eds. Sustainable development in northern timberline forests. Proceedings of the Timberline workshop, May 10–11, 1998 in Whitehorse, Canada. Helsinki: Finnish Forest Research Institute, Research Papers 734, 143–148. [Google Scholar]
- Jónsson TH. 2000. Rannsóknir á íslensku birki [Studies into Icelandic birch]. Reykjavík: Náttúrufræðistofnun Íslands, Ársrit 1998 [Icelandic Institute of Natural History, Annual report for 1998], 13–18. [In Icelandic]. [Google Scholar]
- Kallio P, Niemi S, Sulkinoja M. 1983. The Fennoscandian birch and its evolution in the marginal forest zone. Collection Nordicana 47: 101–110. [Google Scholar]
- Karlsson PS, Weih M. 2001. Soil temperatures near the distribution limit of the mountain birch (Betula pubescens ssp. czerepanovii): Implications for seedling nitrogen economy and survival. Arctic, Antarctic, and Alpine Research 33: 88–92. [Google Scholar]
- Kofoed-Hansen AF. 1925.Skógfræðileg lýsing Íslands [Sylvicultural description of Iceland]. Reykjavík: Bókaverslun Sigfúsar Eymundssonar. [In Icelandic]. [Google Scholar]
- Kristinsson H. 1995. Post-settlement history of Icelandic forests. Icelandic Agricultural Sciences 9: 31–35. [Google Scholar]
- Kullman L. 1979. Change and stability in the altitude of birch tree-limit in the southern Swedish Scandes 1915–1975. Acta Phytogeographica Suecica 65. [Google Scholar]
- Kullman L. 1989. Recent retrogression of the forest-alpine tundra ecotone (Betula pubescens Ehrh. ssp. tortuosa (Ledeb.) Nyman) in the Scandes Mountains, Sweden. Journal of Biogeography 16: 83–90. [Google Scholar]
- Kullman L. 1991. Structural change in a sub-alpine birch woodland in North Sweden during the past century. Journal of Biogeography 18: 53–62. [Google Scholar]
- Kullman L. 1991. Pattern and process of present tree-limits in the Tärna region southern Swedish Lapland. Fennia 169: 25–38. [Google Scholar]
- Kullman L. 1993. Tree limit dynamics of Betula pubescens ssp. tortuosa in relation to climate variability: evidence from central Sweden. Journal of Vegetation Science 4: 765–772. [Google Scholar]
- Kullman L. 1995. Holocene tree-limit and climate history from the Scandes Mountains, Sweden. Ecology 76: 2490–2502. [Google Scholar]
- Kullman L. 1996. Recent cooling and recession of Norway spruce (Picea abies (L.) Karst.) in the forest-alpine tundra ecotone of the Swedish Scandes. Journal of Biogeography 23: 843–854. [Google Scholar]
- Kullman L. 2000. Tree-limit rise and recent warming: a geoecological case study from the Swedish Scandes. Norwegian Journal of Geography 54: 49–59. [Google Scholar]
- Kullman L. 2001. 20th century climate warming and tree-limit rise in the southern Scandes of Sweden. Ambio 30: 72–80. [DOI] [PubMed] [Google Scholar]
- Kullman L, Engelmark 1997. Neoglacial climate control of subarctic Picea abies stand dynamics and range limit in northern Sweden. Arctic and Alpine Research 29: 315–326. [Google Scholar]
- Kuivinen L, Lawson MP. 1982. Dendroclimatic analysis of birch in south Greenland. Arctic and Alpine Research 14: 243–250. [Google Scholar]
- Magnússon Á, Vídalín P. 1913–1943.Jarðabók Árna Magnússonar og Páls Vídalíns [An inventory of farms conducted 1703–1714]. Copenhagen: Hið íslenska fræðafélag. [11 volumes, in Icelandic]. [Google Scholar]
- Mäkelä E. 1999.The Holocene history of birch in northeastern Fennoscandia—an interpretation based on fossil birch pollen measurements. PhD Thesis, University of Helsinki, Finland. [Google Scholar]
- Packham JR, Harding DJL, Hilton GM, Stuttard RA. 1992.Functional ecology of woodlands and forests. London: Chapman and Hall. [Google Scholar]
- Payette S, Gagnon, R. 1979. Tree-line dynamics in Ungava peninsula, northern Quebec. Holarctic Ecology 2: 239–248. [Google Scholar]
- Philip MS. 1983.Measuring trees and forests. A textbook written for students in Africa. Dar es Salaam: University of Dar es Salaam. [Google Scholar]
- Sjörs H. 1963. Amphi-atlantic zonation, nemoral to arctic. In: A. Löve A, Löve D. North Atlantic biota and their history. Oxford: Pergamon Press, 109–125. [Google Scholar]
- Sonesson M, Hoogesteger J. 1983. Recent tree-line dynamics (Betula pubescens Ehrh. ssp. tortuosa [Ledeb.] Nyman) in Northern Sweden. Collection Nordicana 47: 47–54. [Google Scholar]
- Southwood TRE. 1978.Ecological methods with particular reference to the study of insect populations. London: Chapman and Hall. [Google Scholar]
- Spurr SH, Barnes BV. 1980.Forest ecology. New York: John Wiley and Sons. [Google Scholar]
- Sulkinoja M. 1990. Hybridization, introgression and taxonomy of the mountain birch in SW Greenland compared with related results from Iceland and Finnish Lapland. Meddelelser om Grønland, Bioscience 33: 21–29. [Google Scholar]
- Sveinbjörnsson B, Sonesson M, Nordell OK, Karlsson SP. 1993. Performance of mountain birch in different environments in Sweden and Iceland: Implications for afforestation. In: Alden J, Mastrantonio JL, Ødum S, eds. Forest development in cold climates. New York: Plenum Press, 79–88. [Google Scholar]
- Thorarinsson Th. 1974. þjóðin lifði en skógurinn dó [The people survived but the woods perished]. Ársrit Skógræktarfélags Íslands, 1974, 16–29. [In Icelandic, English summary]. [Google Scholar]
- Tranquillini W. 1979.Physiologocal ecology of the alpine timberline. Berlin: Springer Verlag. [Google Scholar]
- Tseplyaev VP. 1965.The forests of the U.S.S.R. Translated from Russian by Prof. A. Gourevitch. Jerusalem: Israel Program for Scientific Translations. [Google Scholar]
- Vaarama A, Valanne T. 1973. On the taxonomy, biology and origin of Betula tortuosa Ledeb. Report of the Kevo Subarctic Research Station 10: 70–84. [Google Scholar]
- Verwijst T. 1988. Environmental correlates of multiple-stem formation in Betula pubescens ssp. tortuosa Vegetatio 76: 29–36. [Google Scholar]
- Wardle W. 1993. Causes of alpine timberline: a review of the hypothesis. In: Alden J, Mastrantonio JL, Ødum S, eds. Forest development in cold climates. New York: Plenum Press, 89–104. [Google Scholar]
- Weih M, Karlsson PS. 1999. The nitrogen economy of mountain birch seedlings: implications for winter survival. Journal of Ecology 87: 211–219. [Google Scholar]