Abstract
• Background and aims Bamboo culms have excellent physical and mechanical properties, which mainly depend on their fibre content and anatomical structure. One of the features which is known to contribute to the high tensile strength in bamboo is the multilayered structure of the fibre cell wall. The aim of this study was to characterize the development of the layered structure in fibre cell walls of developing and maturing culms of Dendrocalamus asper.
• Methods Cell wall development patterns were investigated in phloem fibre caps of vascular bundles in the inner culm wall areas of Dendrocalamus asper of three different age classes (<6 months old, 1 year old, 3 years old). A combination of light microscopy and image analysis techniques were employed to measure cell wall thickness and to determine number of cell wall layers, as well as to describe the layering structure of fibre walls. Two-dimensional maps showing the distribution pattern of fibres according to the number of cell wall layers were produced.
• Key results The cell walls of fibres in phloem fibre caps located in the inner part of the culm wall of D. asper developed rapidly during the first year of growth. Six different fibre types could be distinguished based upon their cell wall layering and all were already present in the young, 1-year-old culm. In the mature stage (3 years of age) the multilayering was independent of the cell wall thickness and even the thinner-walled fibres could have a large number of wall layers. The multilayered nature of cell wall structure varied considerably between individual cells and was not exclusively related to the cell wall thickness. Nevertheless, fibres at the periphery of the fibre bundles and immediately adjacent to the phloem elements exhibited a consistent and high degree of layering in their cell walls.
• Conclusions The multilayered structure of fibre cell walls was formed mainly during the first year of growth by the deposition of new wall layers of variable thickness, resulting in a high degree of heterogeneity in the layering patterns amongst individual fibres. A degree of ‘order’ in the distribution of multilayered fibres within the caps does exist, however, with multilayered cell walls common in fibres adjacent to phloem elements and around the edge of the fibre cap. These findings confirm the observations, primarily in Phyllostachys viridi-glaucescens. The layering structure was not found to be specifically related to the thickness of the cell wall.
Key words: Bamboo, cell wall, Dendrocalamus asper, fibre, layering, phloem
INTRODUCTION
The structural properties of bamboo combined with a fast growth rate, widespread distribution and great diversity of uses make it a suitable choice of woody material that can, under appropriate conditions, be managed sustainably. As in wood, the anatomy of the bamboo culm is known to determine its physical and mechanical properties. A cross-section of the culm reveals the typical monocotyledon arrangement of scattered vascular bundles within ground tissue composed of parenchyma cells. The fibres are characteristically thick-walled at maturity and the high tensile strength of bamboo tissue is attributed mainly to their multilayered cell wall structure, which is also typical of parenchyma cells.
The secondary wall of lignified wood fibres and tracheids is normally characterized by a two- to three-layered structure (Harada and Côté, 1985). In contrast, bamboo fibre and parenchyma cells possess typically multilayered or ‘polylamellated’ cell walls (Tono and Ono, 1962) with alternating transverse and oblique cellulose microfibril angles (Parameswaran and Liese, 1980). The term ‘lamella’ has been generally defined as a ‘concentric agglomeration of cellulose microfibrils within a hemicellulose–lignin matrix present within a cell wall layer’ (Brändström, 2001). However, in the literature concerning bamboos, the terms ‘layer’ and ‘lamella’ have been used synonymously to describe cell wall subdivisions. In order to avoid confusion, only the term layer will be used in this paper.
Fibres are often described as cells having no living protoplasts at maturity. However, many exceptions have been documented. Examples include septate and non-septate fibres, libriform fibres and fibre tracheids, some of which may take on functions such as storage (Fahn and Arnon, 1962; Fahn and Leshem, 1963; Esau, 1965). Living fibres have also been reported in 5-year-old rattan palms (Rhapis excelsa, Calamus axillaris) (Weiner et al., 1996). Similarly, bamboo fibres have been shown to retain their living protoplasts over several growing seasons as they are needed for the cell walls to continue to thicken, acquire multiple layers and lignify (Murphy and Alvin, 1997a, b; Liese, 1998).
Fibre characteristics such as diameter, cell wall thickness and number of cell wall layers have been shown to vary according to their location in the culm and within vascular bundles, as well as with state of maturation of the culm (Parameswaran and Liese, 1976; Murphy and Alvin, 1992, 1997b; Latif, 2001; Bhat, 2003). However, few studies have attempted to characterize accurately the layering patterns in fibre sheaths. One exception is the work of Murphy and Alvin (1992) with Phyllostachys viridi-glaucescens, which showed that the degree of layering in fibre cell walls varied according to the fibre position within the vascular bundle, the position of the vascular bundle in the culm wall, and also with state of maturity. The highest degree of layering was found to be in the fibres adjacent either to vascular elements or at the periphery of the fibre caps next to ground tissue parenchyma. These observations were particularly pronounced in the phloem fibre caps.
The main objective of this study was to characterize the development of the layering structure in cell walls during fibre maturation in the bamboo Dendrocalamus asper and to assess their distribution by mapping them according to the number of cell wall layers in the vascular bundles of culms at different developmental stages. The emphasis was placed on fibres from the phloem cap, as they appear to have a distinct layering distribution pattern across the phloem fibre sheath. The layering pattern was analysed within the individual fibre cell wall and in relation to cell wall thickness and culm development.
MATERIALS AND METHODS
Plant material
Culm samples of Dendrocalamus asper (Schultes f.) Backer ex Heyne were collected from a 10-year-old plantation in co-operation with the ERDB Experimental Station (Laguna, Mount Makiling) in the Philippines. Based on visual appearance (colour, presence/absence of foliage, culm sheaths and surface lichens), the culms were estimated to be: less than 6 months old (elongating), 1 year old (young, fully elongated) and 3 years old (mature). The culms were obtained from the same clump and are likely to be the same genetic individual.
Specimen preparation
Specimen blocks from the inner third of the culm wall measuring approximately 0·5 × 0·5 × 1·0 cm3 were prepared. This part of the culm wall was chosen because its developmental sequence takes place at a slower rate than the rest of the wall; thus cell wall changes can be more systematically studied at the selected ages. The tissue blocks were taken from the middle of internode 7 (counted from the base) of specimens previously fixed in FAA (formalin–acetic acid–alcohol) in the field. Transverse sections were cut to 15 mm thickness with a Reichert sliding microtome, stained and dehydrated for 2 min in each of the following solutions: 1 % alcoholic Safranin O (Sigma S-2255) (in 50 % ethanol), distilled water, and/or 1 % aqueous Alcian Blue (Agar R1702), and dehydrated in a graded series of ethanol. The sections were immersed in Histo-Clear (Agar Scientific R1345) prior to permanent mounting on glass slides with DPX medium (Agar Scientific R1340). Observations were carried out using a Leitz Diaplan Photomicroscope and micrographs were taken on 100 ASA Provia Colour Photo Reversal film.
Image analysis and scoring protocols
Three vascular bundles containing phloem fibre caps of similar shape and size located close to each other and 2–3 bundles away from the inner culm cavity were selected from each age category. Cell wall thickness and number of cell wall layers for each fibre cell in the phloem fibre cap were measured and two-dimensional fibre maps produced (Gritsch, 2003).
Cell wall thickness
Images of all the fibres in the phloem caps as seen in cross-section were captured via a video camera linked to a light microscope (Nikon Eclipse E600, ×40 objective) and an Apple Macintosh computer. Three to four images of each fibre cap were taken so that all fibres could be included. The cell wall thickness of each fibre cell in the phloem fibre cap was measured using the public domain NIH Image programme (developed at the US National Institutes of Health and available on the Internet at http://rsb-info.nih.gov/nih-image). Each measured cell was automatically numbered so that each cell wall thickness measurement could be later correlated with its corresponding number of layers. The images containing the numbered cells were printed out and used to record the number of layers on each cell observed under the microscope. Fibre tips were excluded from the analysis. As the cytoplasm of fibres extends very close to the fibre ends, the tips were identified by their much reduced size and flattened shape. Data were graphically represented using Microsoft Excel 7.0. An analysis of variance (ANOVA) followed by a Tukey's test to verify which sets of data were significantly different from each other were performed using XLSTAT 7.1.
Number of cell wall layers
Cell wall layers for each numbered cell were counted as observed under the microscope, mainly under bright field illumination using the ×100 oil-immersion objective. Polarized light was also used to help confirm the number of layers seen under bright light. Both narrow and broad layers were counted. Due to frequent lack of clarity between the primary wall and the first secondary wall layer, all visible layers were counted, including the first birefringent layer (in some cases this may have been the primary wall). The innermost layer adjacent to the lumen was occasionally difficult to distinguish. For the above reasons the number of layers for each cell should be interpreted with a margin of error of ± 2 layers. As before, fibre tips were excluded from the analysis. The data were analysed and graphically represented using Microsoft Excel 7.0.
Two-dimensional fibre maps (Gritsch 2003)
A digital photograph of each phloem fibre cap was taken under bright field illumination using the ×10 objective. A printed enlargement was used to trace each cell in the phloem fibre cap onto tracing paper. The traced drawings of the fibre caps were then scanned and overlaid onto the original digital photograph using Adobe Photoshop 5.0. Fibres were categorised according to the number of cell wall layers (1–2, 3–4, 5–6, 7–8, 9 or more) and a colour was assigned to each category with the darkest representing the highest number. For one mature sample, a similar map showing the distribution of fibres according to their cell wall thickness was also produced for one of the three bundles observed.
RESULTS
Cell wall thickness
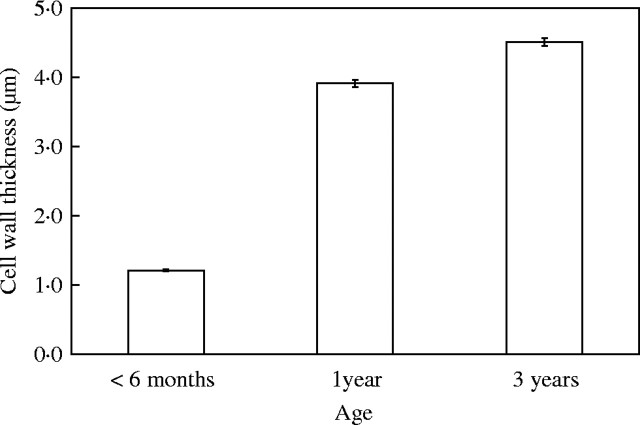
Figure 1 shows representative vascular bundles from culms of the three ages analysed. Within the fibre caps, the typical pattern of wall thickening was observed, with fibres close to xylem and phloem elements maturing first. The walls of fibres immediately adjacent to the phloem completed their thickening at the same time as large-diameter fibres at the periphery of the phloem cap (Fig. 1B, C). Occasional observations indicated that the metaxylem fibres undergo a similar pattern of development. The main period of secondary wall deposition and thickening in phloem fibres occurred during the first year of growth, between 6 months and 1 year (Figs 1 and 2, Table 1). The fibre cell wall thickness increased significantly from <6 months to 1 year old and from 1 year to 3 years of age (Fig. 2, Table 1). In total, an average increase of over 3 µm in cell wall thickness occurred between 6 months and 3 years. Although the average thickness of the fibre cell wall in the mature culm was 4·5 µm (Table 1), the maximum wall thickness measured was up to 12 µm or more in some individual cells.
Fig. 1.
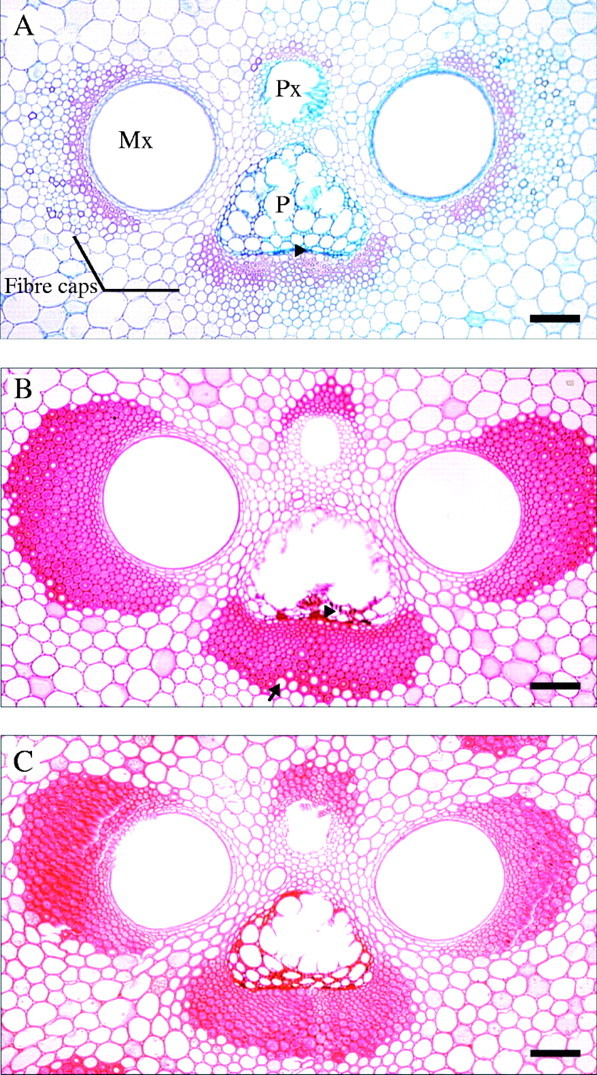
Vascular bundles from the inner culm wall of D. asper. (A) Elongating culm (<6 months). Fibres with the thickest cell walls are found close to the vascular elements, but cells adjacent to phloem remain relatively thin-walled (arrowhead). (B) Young culm (1 year old). The majority of fibre cell walls are close to their maximum thickness. Fibres immediately adjacent to the phloem (arrowhead) and those in the outer part of the xylem and phloem caps (arrow) still have the potential for further cell wall thickening. (C) Mature culm (3 years old). Fibre cell walls are close to or have reached their maximum thickness/layering potential. Note the difference in fibre wall development of fibres in the outer part of the fibre caps between (B) and (C). Mx: metaxylem, P: phloem, Px: protoxylem. Scale bars = 100 μm.
Fig. 2.
Average increase in cell wall thickness in phloem fibres in elongating, 1-year-old (young) and 3-year-old (mature) culms. Bars are standard errors; n = 925 (<6 months), 956 (1 year), 1009 (3 years). Means are all significantly different from one another (P < 0·0001).
Table 1.
Average cell wall thickness (CWT) in μm and average number of layers in fibres from each phloem fibre cap of three replicate vascular bundles
| Elongating (<6months) (n = 925) |
Young (1 year) (n = 956) |
Mature (3 years) (n = 1009) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Bundle |
CWT |
Layers |
CWT |
Layers |
CWT |
Layers |
|||
| 1 | 1·29 | 2 | 3·53 | 5 | 4·38 | 6 | |||
| 2 | 1·22 | 2 | 4·09 | 6 | 4·67 | 6 | |||
| 3 | 1·12 | 2 | 4·11 | 5 | 4·11 | 5 | |||
| Average | 1·21 (0·40) | 2·0 (1·05) | 3·91 (1·53) | 5·0 (1·40) | 4·51 (1·80) | 5·0 (1·80) | |||
Values in brackets are the SD of the mean.
Layering structure within the individual fibre cell wall
The layering pattern between individual fibres varied in terms of number of layers present and thickness of individual layers (mainly evident in broad layers). Narrow layers usually appeared as dark thin lines under bright field illumination, were highly birefringent under polarized light, and appeared to have a more or less constant thickness (Fig. 3). At maturity, several different cell wall layering patterns could be observed. In order to establish the degree of variation in cell wall patterning amongst fibres, a classification system of the most commonly observed patterns was devised based on light microscopy observations in phloem caps of mature culms (Fig. 4). Type V and VI fibres were concentrated at the edges of the fibre cap. Fully developed (i.e. with small lumina) fibres immediately adjacent to the phloem had highly multilayered walls with regularly spaced layers of type VI (Fig. 3D). Similarly, most of the fibres with the largest diameters in the outer part of the fibre cap tended to have type V or VI layering at maturity (Fig. 3C). In other parts of the fibre cap, the distribution of types was more variable and tended to include types I to IV.
Fig. 3.
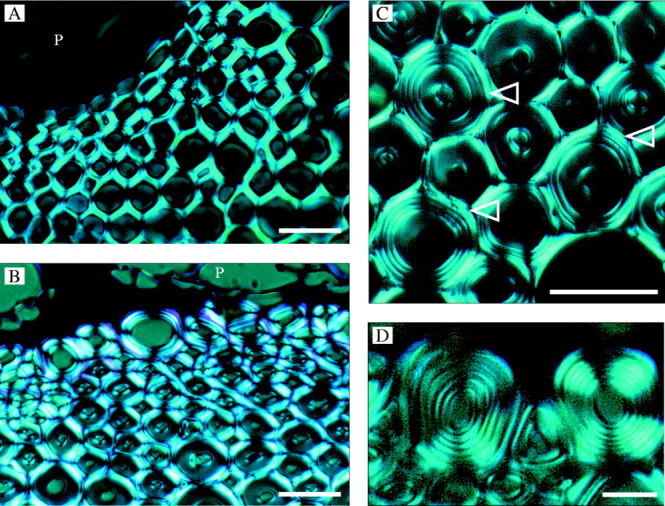
Layering patterns in the phloem fibres (under polarized light). (A) Elongating culm. Fibres close to the phloem (P). The majority of fibres have two to three cell wall layers. (B) Young 1-year-old culm. Fibres close to the phloem. Note the large lumina of fibres adjacent to the phloem. (C, D) Mature 3-year-old culm. (C) Large-diameter fibres on the periphery of the phloem cap showing several cells with layering pattern Type V (arrows). (D) Mature culm. Fibres adjacent to the phloem showing regular cell wall layering (type VI). P: phloem. Scale bars: (A–C) 25 μm, and (D) 5 μm. See Fig. 4 for classification of fibre types.
Fig. 4.
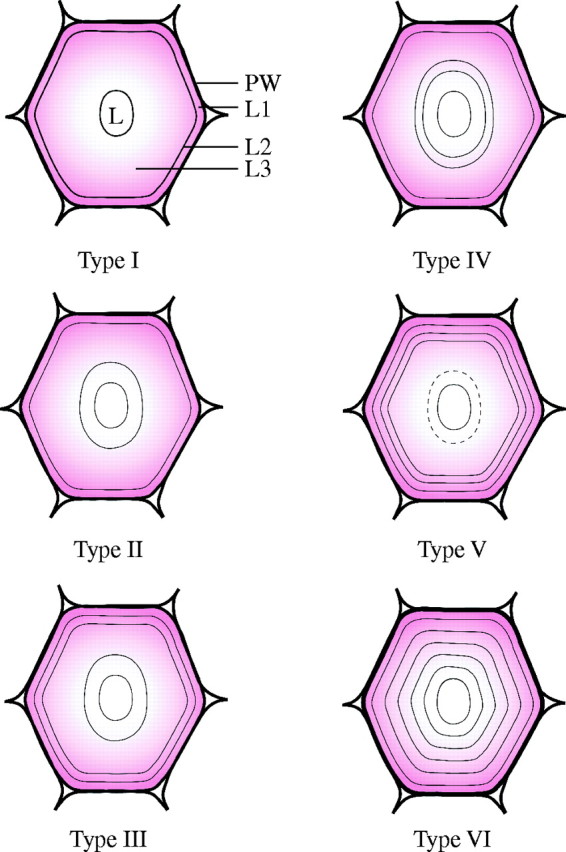
Distribution of thin and broad layers in secondary walls of mature fibres from the phloem cap in D. asper. ‘Thin’ layers are those which are birefringent under polarized light and are depicted as black lines (as also seen under bright field), whereas the ‘broad’ layers are those which are not birefringent (coloured pink). A number has been assigned to the different types of layering for convenience. The thickness of the broad layers is variable, whereas the thin layers appear to have a more constant thickness. The dotted line in Type V indicates that this layer is not always present. PW: primary cell wall; L1, L2, L3: secondary cell wall layers, L: lumen.
Cell wall layering and development
During culm elongation the majority of the fibres had a low number of layers, with an average of two. However, a distinct multilayered wall structure with up to 5–6 layers was discernible in individual fibres close to the phloem elements (Table 1, Fig. 5A). After 1 year, 55 % of fibres had an average of 5–6 layers (Table 1, Fig. 5C) and the percentage of fibres with 7–8 layers had increased from 1 % to 26 %. The main change in the 3-year-old culm was seen in the further increase in the number of fibres with seven and more layers (Fig. 5E). The average number of layers, however, remained the same (Table 1).
Fig. 5.
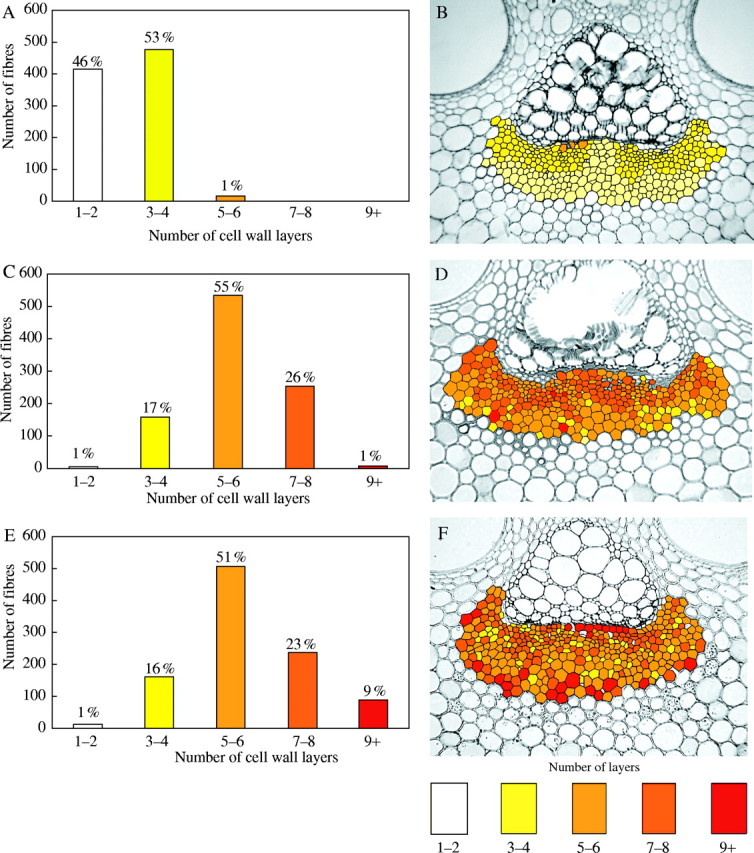
Data regarding the layering of fibre cell walls (A, C and E) and two-dimensional maps (B, D and F) of the distribution of fibres in phloem caps according to the number of cell wall layers. (A, B) Elongating culm showing a high percentage of fibres with a low number of layers. (C, D) Young 1-year-old culm showing a substantial increase in the number of layers (most cells have around five layers). (E, F) Mature 3-year-old culm showing increased number of cells with nine or more layers.
Two-dimensional pattern maps of phloem fibres
Two-dimensional maps of representative phloem fibre caps for each age, showing the distribution pattern of fibres according to their number of wall layers, are presented in Fig. 5 (B, D and F). In the young, elongating culm the colours used illustrate that fibres with the highest number of layers are located close to the phloem elements (Fig. 5B). The middle and outer parts of the fibre cap are composed of cells with only one or two layers (pale yellow colour). In the 1-year-old culm the distribution is more heterogeneous. Nevertheless, there is a strong tendency for fibres with the highest number of layers to be near the phloem (Fig. 5D). In the mature culm, the main change in the pattern was the development of highly layered walls in some centrally located fibres immediately adjacent to the phloem and in fibres at the periphery of the fibre cap (Fig. 5F). The latter characteristically had the largest diameters within the cap. Fibres adjacent to the phloem were generally narrower.
Cell wall thickness and number of cell wall layers
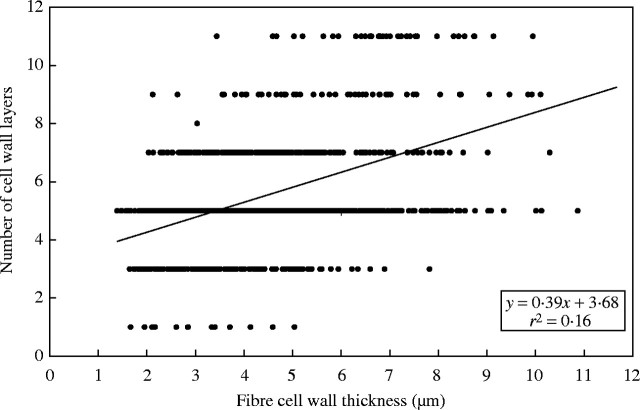
According to the distribution pattern within the mature phloem cap (Fig. 6) the cell wall thickness seems to increase from the inner areas of the cap towards the outer parts. Nevertheless, a poor correlation between cell wall thickness and the number of cell wall layers was observed in the three ages analysed. Only 16 % (r2 = 0·16) of the variation in cell wall thickness can be accounted for by the variation in the number of cell wall layers (Fig. 7). The correlation between both parameters is therefore not significant (P > 0·05). In the elongating and young culms no association between cell wall thickness and number of wall layers could be determined either. These results indicate that the number of cell wall layers is more closely associated with the location of a fibre within the bundle than with overall cell diameter or wall thickness. For instance, the fibres with the largest number of layers are generally located at the inner and outer edges of the bundle. Equally, there are many cells in the centre of the bundle that have relatively small diameters and thin walls, but which are also multilayered.
Fig. 6.
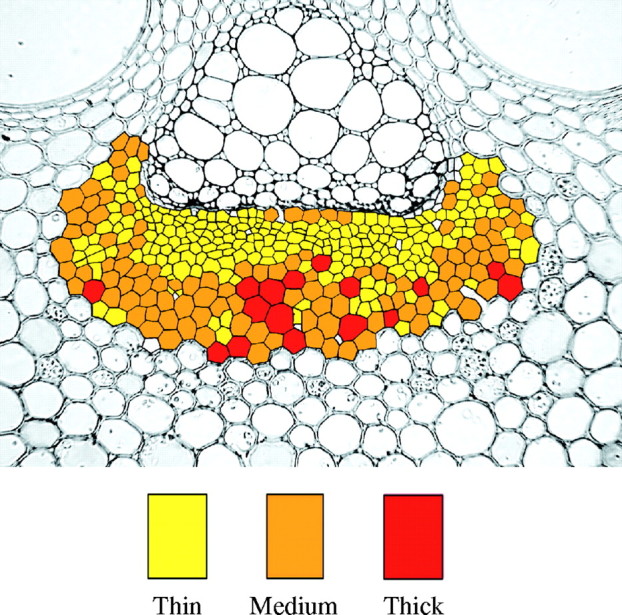
Two-dimensional map of the distribution of fibres in phloem caps according to the thickness of their cell walls. Thin: 1 to <5 μm, Medium: 5 to <9 μm, Thick: 9 μm and thicker.
Fig. 7.
Scatter graph of cell wall thickness vs. number of layers for three bundles of the 3-year-old culm (n = 1009).
DISCUSSION
In the present study the development of fibre cell walls located in the phloem fibre cap was investigated, as previous work has shown that changes in cell wall layering across the phloem fibre cap are similar but somewhat more pronounced than in the xylem caps (Murphy and Alvin, 1992). The development in xylem and phloem fibres, however, is similar, and the sequence of development for phloem fibres can be considered as a reasonable representation for bamboo fibres in general.
Cell wall thickness
The cell walls of fibres in the phloem cap begin to thicken actively during the late stages of culm elongation and it takes up to 1 year for a large part of secondary wall deposition to be almost completed. The majority of fibres in this area thus achieve most of their full cell wall thickness potential during the first growing season, but some, usually of larger diameter, remain relatively thin-walled and substantial further thickening occurs in subsequent growing season(s). These are usually located at the periphery of the fibre cap close to parenchyma cells but some are in direct contact with the phloem elements. Murphy and Alvin (1992, 1997b) reported similar observations, suggesting that such a pattern of fibre distribution is typical of bamboos. Bhat (2003) reported that fibre thickening in the inner culm wall bundles of Bambusa bambos and Dendrocalamus strictus starts in the vicinity of the phloem and xylem tissues and proceeds towards the outer parts of the bundle. Our present observations in D. asper confirm the same general pattern. However, several fibres immediately adjacent to the phloem did not mature first; instead, they completed their thickening at the same time as the large diameter fibres in the periphery of the bundle. Such a developmental pattern might be linked to a parenchymatous origin of the cells. Evidence that some fibres at the periphery of the vascular bundle may be derived from ground tissue parenchyma cells has been shown in Phyllostachys viridi-glaucescens (Crow, 2000). This could, in turn, confer them a certain degree of intergradation between fibres and parenchyma cell types and result in a developmental pattern with aspects similar to true parenchyma cells. Cells with intermediate characteristics between parenchyma and fibres have been described in bamboos. For example, Murphy and Alvin (1992) mention the presence of cells with some degree of intergradation between fibre and parenchyma cells. These cells have square rather than tapered ends, have highly lamellated walls and are located at the periphery of the fibre sheaths.
In contrast to the phloem fibre cap, in the free fibre cap or sheath (i.e. bundle of fibres not associated with vascular tissue) the pattern of wall development has been shown to be somewhat different, as illustrated by results for Gigantochloa scortechinii (Murphy and Alvin, 1997a). In this case, no significant increase in wall thickening occurred in fibres in the free fibre cap from the first to the second year of growth. However, an increase of about 190 % was recorded in the third year of growth in fibre cell walls of the free fibre caps located in the inner culm region of G. scortechinii. In general agreement with the present findings, the same authors pointed out that fibres of the vascular caps are smaller and undergo early maturation, therefore they have less potential for continued development over several years. Previously, Fujii (1985) and Kawase et al. (1986) also found that secondary wall deposition continued during the second and third years of growth in larger fibres of bundle sheaths of Pleioblastus chino and Sasa kurilensis, respectively. Since secondary thickening (present in dicotyledons) is absent in bamboos, it has been suggested that the capacity for further cell wall thickening provides a mechanism for strengthening of primary tissues as the culm ages (Murphy and Alvin, 1997b).
Layering structure of phloem fibre cell walls
There was a great degree of heterogeneity in the layering structure of fibre cell walls as represented by the fact that we were able to identify six main types in D. asper (intermediate types were also observed). Much variation in wall structure has also been reported for Phyllostachys viridi-glaucescens by Murphy and Alvin (1992), who categorized fibres into four major types according to their layering structure. Kawase et al. (1986) reported that in the bamboo Sasa kurilensis the fibre cell walls at less than 1 year old are thin and composed of one or two layers, at 2 years they are composed of 3–4 layers and after 4 years of 7–8 layers. In the present study, the average number of wall layers was two in the young culm and increased to five in the fully elongated older culms. This average, however, masks the increase in the number of fibres with more than five layers as in the case of the mature culm, where a large number of fibres had nine or more layers compared with the 1 year old.
Multilayered cell walls are not an exclusive feature of bamboo fibre and parenchyma cells and appear to have evolved in a variety of taxa. They have been described in root fibres of several woody monocotyledons (Mueller and Beckman, 1979). They also occur in cell walls of a number of plants. For example, the sclerotic bark fibres of Douglas fir (Pseudotsuga menziesii) have thick cell walls with numerous individual layers irregularly arranged; vascular bundles of coconut (Cocos nucifera) stem fibres possess up to ten regularly organized lamellae (Parameswaran and Liese, 1981); vascular bundle fibres in rattan stems (Calamus manan) have an average of seven lamellae (Parameswaran and Liese 1985); and phloem fibres of flax fibres are also polylamellated (Linum usitatissimum) (Esau 1965). In a tropical hardwood species (Homalium foetidum), Daniel and Nilsson (1996) also described a ‘concentric polylaminate’ layering of the secondary wall of some fibres. As in bamboo, layered and non-layered fibres developed adjacent to each other and the layers alternated between narrow and thin.
Cell wall thickness and number of wall layers
Parameswaran and Liese (1976) described the presence of two kinds of fibres, namely thick-walled ‘polylamellated’ fibres with up to 18 ‘lamellae’ and non-lamellated thin-walled fibres in several bamboo species. In contrast, and similar to the observations reported by Murphy and Alvin (1992), the present data show that the number of layers within any one wall does not depend entirely on its thickness, since very thick walls can posses anything from one or two layers up to more than nine. In fibres of Raphis excelsa (rattan palm), the thickness and the formation of new cell wall layers were positively correlated in the top region of the stem. In contrast, less correlation was found in the middle of the stem. In this case, the thickening of the wall was attributed to the thickening of existing layers (Weiner et al., 1996). Moreover, the same authors observed that in comparison with Calamus axillaris, there was more correlation between cell wall thickness and number of cell wall lamellae in Raphis excelsa. The explanation given for this was that in Calamus the broad lamellae have a more homogeneous thickness (1·5–1·8 µm) than those of Raphis (1·0–3·0 µm).
The two-dimensional map of cell wall thickness demonstrated that the thickness of the cell wall is related not only to the location of the fibre within the bundle but also (although this is only a visual observation) to the diameter of the cell.
Liese and Weiner (1996) have shown that the development of bamboo fibres occurs in two main phases. The main one happens during the first 2 years and leads to the development of fibres with lignified cell walls. A second phase follows for several years after during which additional lamellae are formed. Such lamellae are initially unlignified and are laid down onto an already lignified secondary wall. Eventually these new lamellae also become lignified themselves. In the present investigation it was not possible to verify a second phase of wall development due to the range of culm ages available. However, given the small lumina in phloem fibres in culms 3 years of age, it seems unlikely that significant wall deposition in later years (i.e. beyond 3 years) would have been possible. Compared with fibres of free fibre sheaths or large protoxylem caps, phloem fibres mature at a faster rate.
Two-dimensional maps of phloem fibre caps
At maturity, fibres with the highest number of wall layers were located immediately adjacent to the phloem and in the peripheral region of the cap. Large-diameter fibres exhibited type VI layering. This type is comparable to type IV fibres described in Phyllostachys viridi-glaucescens (Murphy and Alvin, 1992), which were also found in areas spatially associated with parenchyma or the vascular elements. Liese and Weiner (1996) also reported similar results based on visual observations for P. viridi-glaucescens.
Although studies such as those of Murphy and Alvin (1992) using single transects can show to an extent how the fibres are distributed according to their number of lamellae within the bundles, a much more detailed picture has been revealed from the two-dimensional maps showing that fibre layering can vary within the whole sheath. It appears that the distribution of cells with multilayered cell walls has not been systematically studied in most plant taxa. Much research has been devoted to the structure and dimensions of the layers and sub-layers, or ‘lamellae’, and the mechanisms of cellulose microfibril deposition, such as illustrated by work on conifers (e.g. Abe et al., 1994, 1995, 1997; see also review by Brändström, 2001). It seems that further analysis not only of the structure of the walls but also of their distribution and development will be needed before a complete picture emerges of their functional anatomy in bamboos.
CONCLUSIONS
Within the phloem fibre caps of D. asper, six different fibre types could be distinguished and were all already present in the young, elongated culm. In the mature stage, the layering structure was independent of the cell wall thickness, i.e. thinner-walled fibres could also have a large number of wall layers. Nevertheless, the number of wall layers rose in fibres at the periphery of the fibre bundles and in those close to the phloem. In both areas, the same high degree of layering in individual fibres was observed. These findings demonstrate not only a great heterogeneity in the fibre wall structure but also a degree of ‘order’ in the distribution of multilayered fibres within the caps. They confirm the observations by Murphy and Alvin (1992) on Phyllostachys and Sinarundinaria species.
Further histo- and topochemical work with D. asper is under way, focusing on the lignification of the multilayered fibre cell walls during maturation.
Acknowledgments
We thank the European Commission for funding the INCO-DC project (ERBIC18CT970176) under which this research was undertaken. We are also most grateful to Dr A. Palijón and Prof. E. Fernandez (University of the Philippines) for their assistance with collection of specimens in the field, and Mr D. Bacon (Imperial College London) for his help with the preparation of the two-dimensional maps on the computer.
LITERATURE CITED
- Abe H, Funada R, Imaizumi H, Ohtani J, Fukazawa K. 1995. Changes in the arrangement of microtubules and microfibrils in conifer tracheids during the expansion of cells. Annals of Botany 75: 305–310. [Google Scholar]
- Abe H, Funada R, Ohtani J, Fukazawa K. 1997. Changes in the arrangement of cellulose microfibrils associated with the cessation of cell expansion in tracheids Trees Structure and Function 11: 328–332. [Google Scholar]
- Abe H, Ohtani J, Fukazawa K. 1994. A scanning electron microscopic study of changes in microtubule distributions during secondary wall formation in tracheids. IAWA Journal 15: 185–189. [Google Scholar]
- Bhat KV. 2003. Anatomical changes during culm maturation in Bambusa bambos (L.) Voss and Dendrocalamus strictus Nees. Journal of Bamboo and Rattan 2: 153–166. [Google Scholar]
- Brändström J. 2001. Micro- and ultrastructural aspects of Norway spruce tracheids: a review. International Association of Wood Anatomists Journal 22: 333–353. [Google Scholar]
- Crow E. 2000.Development of fibre and parenchyma cells in the bamboo Phyllostachys viridi-glaucescens (Carr.) Riv. & Riv. PhD Thesis, Imperial College, University of London, UK. [Google Scholar]
- Daniel G, Nilsson T. 1996. Polylaminate concentric cell wall layering in fibres of Homalium foetidum and its effect on degradation by microfungi. In: Donaldson LA, Singh AP, Butterfield BG, Whitehouse LJ, eds, Recent advances in wood anatomy. New Zealand Forest Research Institute, 369–372. [Google Scholar]
- Esau K. 1965.Plant anatomy, 2nd edn. New York: John Wiley & Sons. [Google Scholar]
- Fahn A, Arnon N. 1962. The living wood fibres of Tamarix aphylla and the changes occurring in them in transition from sapwood to heartwood. New Phytologist, 62: 99–104. [Google Scholar]
- Fahn A, Leshem B. 1963. Wood fibres with living protoplasts. New Phytologist, 62: 91–98. [Google Scholar]
- Fujii T. 1985. Cell wall structure of the culm of Azumanezasa (Pleioblastus chino Max.). Mokuzai Gakkaishi 31: 865–872. [Google Scholar]
- Gritsch CS. 2003.Aspects of cell wall formation during culm development in the Bamboo Dendrocalamus asper (Schultes F.) Backer ex Heyne. PhD thesis, Imperial College, University of London, UK. [Google Scholar]
- Harada H, Côté WA. 1985. Structure of wood. In: Higuchi T, ed., Biosynthesis and biodegradation of wood components: Orlando: Academic Press, 1–42. [Google Scholar]
- Kawase K, Sato K, Imagawa H, Ujiie U. 1986. Studies on utilisation of Sasa-bamboos as forest resources. 4. Pulping of young culms and histological change of cell structure of the culms in growing process. Research Bulletin of the College Experiment Forests, Hokkaido University 43: 73–96. [Google Scholar]
- Latif MA. 2001. Anatomical features of Bambusa vulgaris and Gigantochloa scortechinii from four harvesting sites in Peninsular Malaysia. Journal of Tropical Forest Products 7: 10–28. [Google Scholar]
- Liese W. 1998.The anatomy of bamboo culms. Technical Report, INBAR, Beijing, China. [Google Scholar]
- Liese W, Weiner G. 1996. Ageing of bamboo culms. A review. Wood Science and Technology 30: 77–89. [Google Scholar]
- Mueller WC, Beckman CH. 1979. Isotropic layers in the secondary cell walls of fibres in the roots of banana and other monocotyledons. Canadian Journal of Botany 57: 2776–2781. [Google Scholar]
- Murphy RJ, Alvin KL. 1992. Variation in fibre wall structure in bamboo. IAWA Bulletin 13: 403–410. [Google Scholar]
- Murphy RJ, Alvin KL. 1997. Fibre maturation in the bamboo Gigantochloa scortechinii International Association of Wood Anatomists Journal 18: 147–156. [Google Scholar]
- Murphy RJ, Alvin KL. 1997. Fibre maturation in bamboos. In: Chapman PG, ed., The Bamboos Linnaean Society Symposium Series 19. London, UK: Academic Press, 93–303 [Google Scholar]
- Parameswaran N, Liese W. 1976. On the fine structure of bamboo fibres. Wood Science and Technology 10: 231–246. [Google Scholar]
- Parameswaran N, Liese W. 1980. Ultrastructural aspects of bamboo cells. Cellulose Chemistry and Technology 14: 587–609. [Google Scholar]
- Parameswaran N, Liese W. 1981. Occurrence of polylamellate walls in some lignified cells. In: Robinson DG, Quadar H (eds.), Proceedings of the Second Cell Wall Meeting. Göttingen, Germany: 171–187. [Google Scholar]
- Parameswaran N, Liese W. 1985. Fibre cell wall architecture in the stem of rotan manau (Calamus manan). In Wong KM, Manokaran N eds, Proceedings of the Rattan Seminar. The Rattan Information Centre. Kuala Lumpur, Malaysia, 123–129. [Google Scholar]
- Tono T, Ono K. 1962. The layered structure and its morphological transformation by acid treatment. Journal of Japanese Wood Research Society 8: 245–249. [Google Scholar]
- Weiner G, Liese W, Schmitt U. 1996. Cell wall thickness in fibres of the palms Rhaphis excelsa (Thunb.) Henry and Calamus axillaris Becc. In: Donaldson LA, Singh AP, Butterfield BG, Whitehouse LJ, eds, Recent advances in wood anatomy. New Zealand Forest Research Institute, NZ, 191–197. [Google Scholar]