Abstract
• Background and Aims In the last decade, the number of young plants of Ulmus pumila in the Hunshandak Sandland has decreased sharply because of severe sand burial, and their ecological protective function has been weakened. In order to develop an understanding of the tolerance of U. pumila to sand burial and to suggest reasonable measures to protect the sparse-elm–grassland ecosystem, the effects of burial on the survival, growth, photosynthesis and biomass allocation in U. pumila were studied.
• Methods Seedlings were buried at five different depths in pot experiments: no burial (control), partial burial (33 % and 67 % stem height), and complete burial (100 % and 133 % stem height). Growth analyses and measurements of photosynthesis were carried after the plants had been uncovered.
• Key Results All the plants survived partial burial, but about 30 % and 80 % of the seedlings died as a result of the 100 % and 133 % sand burial treatments, respectively. The numbers of newly produced leaves and branches, and the height of the stems of the seedlings in the 33 % and 67 % burial treatments during the period of the experiment were significantly greater than those in the control. Furthermore, net photosynthetic rate, transpiration rate and water use efficiency were also elevated by the partial burial, but not affected by burial time. This might be attributed to the increased root length, which improved water acquisition. The biomass and biomass allocation of the seedlings were significantly changed by the burial treatments and burial times. The biomass was enhanced by partial burial but was reduced by complete burial at each burial time. However, the biomass allocation was not significantly changed by the 33 % and 67 % sand burial treatments 2 or 4 weeks following the burial.
• Conclusions Ulmus pumila was shown to be tolerant to partial sand burial, but must be protected from complete burial.
Key words: Biomass allocation, Ulmus pumila, gas exchange, growth, Hunshandak Sandland, sand burial
INTRODUCTION
Ulmus pumila (Ulmaceae) is a widespread tree species in North China, especially in the semi-arid sandland (Liu, 1989; Yan et al., 1997). Many trees of this species grow naturally on the dunes of the Hunshandak Sandland in the Inner Mongolia Autonomous Region of China, and the region of Zhenglan Banner (an administrative division in Inner Mongolia, equivalent to a county) is well-known for these trees, which are referred to as Lanqi elm (Chen and Guo, 1960). In the past ten years, the number of young plants of U. pumila in the Hunshandak Sandland has decreased sharply, and their ecological protective function has been weakened (Li et al., 2003). Considering the increase in sand storms in recent years, sand burial may be considered as one of the important factors contributing to the decrease of U. pumila (Li et al., 2003). After emergence, seedlings may be buried by sand to various depths during their establishment in late spring and early summer. In order to find a reasonable approach to help protect the sparse-elm–grassland ecosystem, understanding the effects of burial on U. pumila seedlings is necessary.
The tolerance of some specific species and the general growth responses of plants to varying depth of burial have been extensively studied in coastal and lake-shore dune systems (Zhang, 1996; Voesenek et al., 1998). It has generally been concluded that the growth of some coastal plant species could be stimulated by sand burial below a certain threshold level of burial, but the plants could not survive if buried to a depth above the threshold (Maun et al., 1996; Brown, 1997). Moreover, sand burial may also lead to a shift of biomass and nutrients from the roots to above-ground components (Harris and Davy, 1988; Zhang and Maun, 1992), while photosynthetic capacity is maintained (Yuan et al., 1993; Brown, 1997). However, contradictory results have also been reported. Perumal (1994) reported similar increases in below-ground and above-ground biomass, while Sykes and Wilson (1990) reported decreased shoot/root ratios with burial depth. The lack of overall agreement in these findings indicates a need for further studies of plant responses to burial.
Much of our knowledge of burial effects comes from direct observations rather than from controlled replicated experiments. Only recently have a few such studies been attempted by Sykes and Wilson (1990) in New Zealand, Hesp (1991) in Australia, Brown (1997) in California, and Maun et al. (1996) in Canada. Compared with species in coastal sand dunes, studies on the adaptation of seedlings of inland dune plants to sand burial are few, and direct evidence for this adaptation is limited (Sykes and Wilson, 1990; Brown, 1997; Singh and Rathod, 2002). In order to develop understanding of the tolerance of U. pumila to sand burial in the Hunshandak Sandland, experiments were conducted in which seedlings were buried experimentally and measurements were made of survival, growth, photosynthesis and biomass allocation patterns.
MATERIALS AND METHODS
Preparation of plants and burial treatments
Ulmus pumila is a woody species with monopodial branching and alternate leaves. One-year-old seedlings of U. pumila were randomly selected from the Grass Cultivation Station in Zhenglan Banner, Inner Mongolia, China (42°16′N, 115°57′E), where the experiments were conducted. Seedlings of similar size were planted individually in plastic pots of 0·2 m height and 0·22 m diameter in June 2002. The pots were filled with sand taken from the nearby dunes and sieved to remove debris and seeds. Two weeks later, the potted seedlings were randomly divided into five groups. The height of the stem and number of leaves of each seedling were measured and counted, respectively. Then, burial treatments at depths of 0 % (control), 33 %, 67 %, 100 % and 133 % of the seedling height were made. For burial treatments, extensions of PVC pipes were taped onto the pots to contain the covering sand to the designated depth in an upright position. Seedlings were kept vertical while being buried. In both 100 % and 133 % burial treatments, the individuals were completely buried except for a few seedlings in the 100 % burial treatment where some green leaves originating from the top of the plants were exposed. On the day of burial, ten other randomly selected seedlings were harvested for determination of the initial measurements for growth analysis.
Each treatment had 24 replicates. In total, there were 120 samples. All the pots in each treatment were randomly divided into three subgroups, which were randomly assigned to harvests at 2-week intervals. Seedlings among the treatments and among the subgroups within each treatment were not significantly different in number of leaves and height of the stems at the beginning of the experiment, according to one-way ANOVA. The pots were placed outdoors and artificially watered as necessary to keep at approximate field capacity. No fertilizer was applied. All the plants except the proportion buried in sand were subject to the normal solar radiation and natural wind. During the experiment, the mean temperature near the plants during daytime was 29·1 °C (ranging from 21·4–38·3 °C) and at night was 16·8 °C (ranging from 25·0–11·9 °C).
Measurements and data analysis
Only plants that survived the experiment were measured. One and two plants in the 100 % and 133 % sand burial treatments, respectively, were dead at 4 weeks following burial, and two and six plants in the 100 % and 133 % sand burial treatments, respectively, were dead at 6 weeks following burial. Thus the harvest at 4 weeks after burial was made up of 37 surviving plants, and that at 6 weeks was made up of 32 surviving plants.
Growth measurements
At 2-week intervals following the initial burial treatment, the stem height and number of leaves and branches above the new sand surface, and the survival of the seedlings were recorded. The leaves, stems (all the above-ground plant parts excluding leaves) and roots of each seedling in each subgroup of each treatment were separately harvested. The ‘leaf’ and ‘stem’ in this paper refer to both buried and unburied portions of the plant. The enhancement ratio was calculated as [(value at specified time after burial – value before burial) / value before burial]. The root length was the sum of the total length of roots with the same diameter. Six weeks after the sand burial, the experiment was terminated and the seedlings were carefully washed out of the pots and separated into leaves, shoots and roots. The plant samples were then dried at 80 ± 1 °C to a constant weight.
Gas exchange measurements
Because the 133 % burial treatment did not have enough functional leaves, gas exchange and leaf water content were only measured in the other four treatments. Net photosynthetic rate (PN) and transpiration rate (E) were measured using a LCA-4 Portable Photosynthesis System (ADC, Hoddesdon, England) at 2-week intervals. The measurements started from 1000 h when photosynthetic photon flux density (PPFD) was above the saturation point. The conditions for measurements were as follows: ambient CO2 concentration (Ca) 350 mol mol−1, vapour pressure deficit (VPD) 2·0 ± 0·4 kPa, leaf temperature 35 ± 0·26 °C and PPFD 1800 ± 42 µmol m−2 s−1. Water use efficiency (WUE) was calculated as PN/E. Fully expanded functional leaves in upper shoots were used for measurements, with the leaf kept horizontal so that the effect of leaf angle on incident photon flux was minimized. The gas exchange of leaves in 133 % treatment was not measured because there were almost no leaves above the soil surface. In the 100 % sand burial treatment, leaves were measured on surviving seedlings. Leaf areas for calculation of gas exchange were measured using an area meter (AM100, ADC, Hoddesdon, England).
Data analysis
The effects of sand burial depth, burial time and their interaction on growth-related traits (enhancement ratio for stem height, branch number, leaf number and branch length, as well as plant biomass), and physiological traits (net photosynthetic rate, transpiration rate, water use efficiency and leaf water content) were analysed using two-way analysis of variance (ANOVA). A significant time-by-burial effect would indicate different response patterns to sand burial between the three burial times. When there was a significant interaction between time and burial treatment, one-way analysis of variance (ANOVA) was carried out to analyse the difference between the treatments at separate times. The height of newly produced stems, the numbers of newly produced lateral branches and leaves, as well as the length of roots (only measured 6 weeks following burial) were also analysed using one-way analyses of variance (ANOVA). The least significant differences (Duncan's method) between the means were estimated at the 95 % confidence level. All the statistical analyses were performed using the SPSS 10.0 package (SPSS 10.0 Inc., Chicago, USA).
RESULTS
Survival of seedlings
All the individual seedlings in the control and partial burial treatments (i.e. 33 % and 67 % sand burial) survived during the whole experiment period. The death rates were about 12·5 % and 30 %, respectively, in the 100 % and 133 % burial treatments 4 weeks following burial, and rose to 30 % and 80 %, respectively, 6 weeks following burial. All the seedlings in the complete burial treatment survived 2 weeks following burial (Table 1).
Table 1.
Percentage of plants that had emerged from the sand surface after burial
| Burial treatment |
2 weeks |
4 weeks |
6 weeks |
|---|---|---|---|
| 0 | 100 | 100 | 100 |
| 33 % | 100 | 100 | 100 |
| 67 % | 100 | 100 | 100 |
| 100 % | 100 | 87·5 | 70 |
| 133 % | 100 | 70 | 20 |
Growth response to sand burial
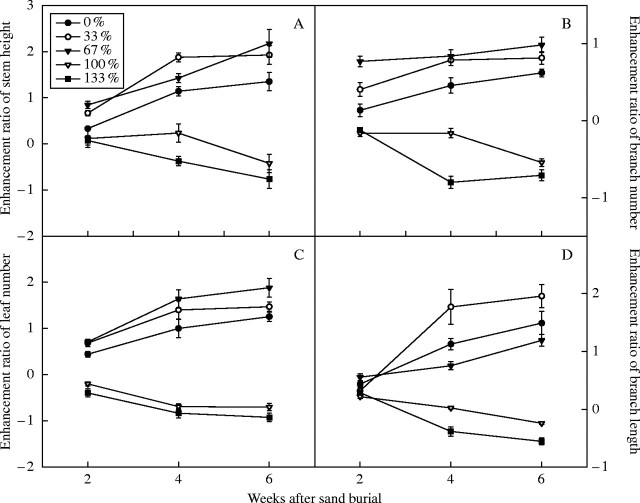
Both the control and partial-burial seedlings displayed a positive enhancement ratio in stem height, numbers of branches and leaves during the experiment, while the enhancement ratio of these characters in the 100 % and 133 % burial treatments were negative after burial (Fig. 1). There were significant effects of burial treatment, burial time and their interaction on the height of newly produced stem, and on the numbers of newly produced lateral branches and leaves (Table 2). Values of these traits always increased (for partial burial and control) or decreased (for complete burial) with prolonged burial time (Fig. 1). Compared with the control, the 33 % and 67 % burial treatments had significantly higher values for the above-mentioned traits at each of the three burial times (P < 0·001, Fig. 1; the statistic here and subsequently refers to one-way ANOVA, unless stated otherwise).
Fig. 1.
Growth enhancement ratios of Ulmus pumila seedlings with different sand burial treatments. Enhancement ratios are given for (A) stem height, (B) branch number (C) leaf number and (D) branch length. Values are means ± s.e., n = 8.
Table 2.
F-statistics and P-values for burial time, burial depth and their interaction relating to the traits of experimental plants as determined by ANOVA, together with corresponding degrees of freedom
| Burial time |
Burial depth (treatment) |
Time × treatment |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Character |
d.f. |
F |
P |
d.f. |
F |
P |
d.f. |
F |
P |
|||||||||
| Enhancement ratio | ||||||||||||||||||
| Shoot height | 2, 94 | 43·96 | <0·001 | 4, 94 | 74·52 | <0·001 | 8, 94 | 7·63 | <0·001 | |||||||||
| Branch number | 2, 94 | 0·27 | 0·762 | 4, 94 | 199·96 | <0·001 | 8, 94 | 17·06 | <0·001 | |||||||||
| Leaf number | 2, 94 | 10·84 | <0·001 | 4, 94 | 170·23 | <0·001 | 8,94 | 11·69 | <0·001 | |||||||||
| Branch length | 2, 94 | 18·04 | <0·001 | 4, 94 | 76·33 | <0·001 | 8, 94 | 18·26 | <0·001 | |||||||||
| Photosynthetic rate | 2, 81 | 0·46 | 0·634 | 3, 81 | 60·39 | <0·001 | 6, 81 | 2·33 | 0·04 | |||||||||
| Transpiration rate | 2, 81 | 0·03 | 0·968 | 3, 81 | 19·48 | <0·001 | 6, 81 | 0·49 | 0·81 | |||||||||
| Water use efficiency | 2, 81 | 2·37 | 0·100 | 3, 81 | 7·84 | <0·001 | 6, 81 | 1·33 | 0·25 | |||||||||
| Leaf water content | 2, 81 | 4·96 | 0·009 | 3, 81 | 5·46 | 0·002 | 6, 81 | 6·14 | 0·001 | |||||||||
| Biomass | ||||||||||||||||||
| Leaf | 2, 94 | 101·73 | <0·001 | 4, 94 | 93·62 | <0·001 | 8, 94 | 34·27 | <0·001 | |||||||||
| Shoot | 2, 94 | 76·74 | <0·001 | 4, 94 | 78·08 | <0·001 | 8, 94 | 23·40 | <0·001 | |||||||||
| Root | 2, 94 | 135·90 | <0·001 | 4, 94 | 90·84 | <0·001 | 8, 94 | 35·58 | <0·001 | |||||||||
| Biomass allocation | ||||||||||||||||||
| Leaf | 2, 94 | 4·03 | 0·021 | 4, 94 | 10·14 | <0·001 | 8, 94 | 12·43 | <0·001 | |||||||||
| Shoot | 2, 94 | 8·61 | <0·001 | 4, 94 | 108·53 | <0·001 | 8, 94 | 40·38 | <0·001 | |||||||||
| Root | 2, 94 | 15·26 | <0·001 | 4, 94 | 79·026 | <0·001 | 8, 94 | 10·55 | <0·001 | |||||||||
| Newly produced | ||||||||||||||||||
| Shoot height | 3, 26 | 20·13 | <0·001 | |||||||||||||||
| Lateral branch | 3, 26 | 30·76 | <0·001 | |||||||||||||||
| Leaves | 3, 26 | 19·98 | <0·001 | |||||||||||||||
| Root length | ||||||||||||||||||
| >0·2 cm | 4, 27 | 33·98 | <0·001 | |||||||||||||||
| 0·1–0·2 cm | 4, 27 | 24·08 | <0·001 | |||||||||||||||
| <0·1 cm | 4, 27 | 298·72 | <0·001 | |||||||||||||||
The number of newly produced leaves on the seedlings 6 weeks after burial was 20 % and 73 % more in the 33 % and 67 % sand burial treatments, respectively, than those of the control, but 54 % less in the surviving seedlings of the 100 % burial treatment (Fig. 2C). Other growth characters, such as the number of newly produced lateral branches (Fig. 2B) and the height of newly produced stem (Fig. 2A) were also significantly increased by partial burial, whereas they were decreased by complete burial (Table 2).
Fig. 2.
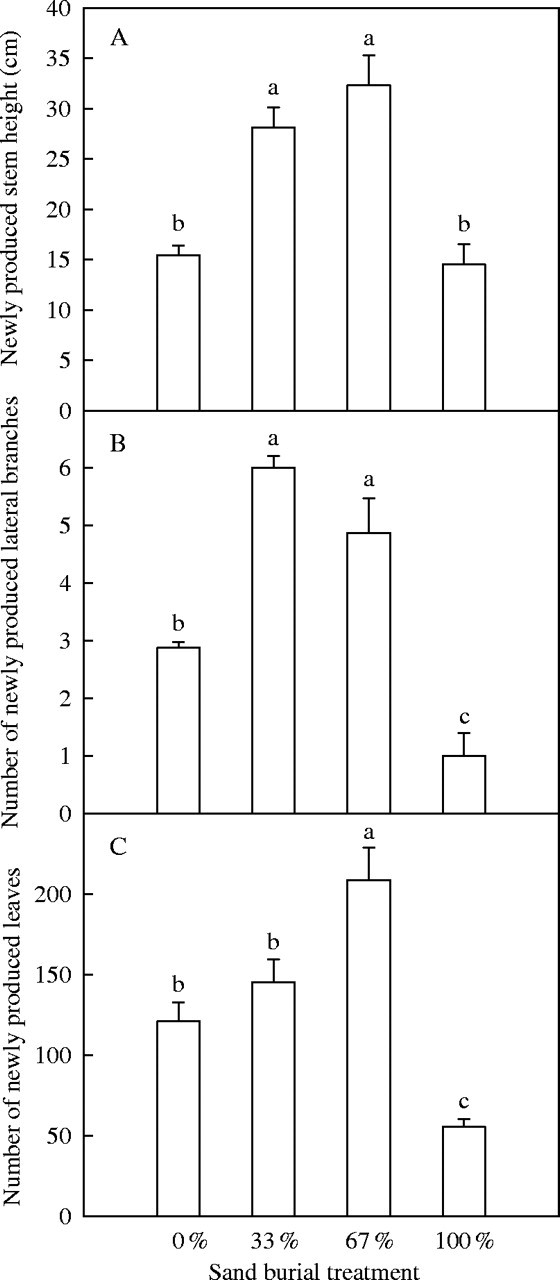
Stem height (A), and the number of newly produced branches (B) and leaves (C) of Ulmus pumila seedlings 6 weeks following sand burial. Values are means ± s.e., n = 8. Different letters indicate that the differences between the treatments are significant at P < 0·05.
Root length
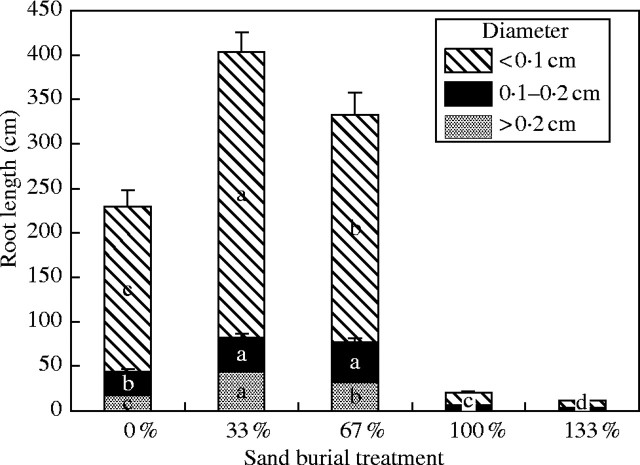
The length of roots with different diameters increased significantly with partial burial of sand 6 weeks after burial (Fig. 3, Table 2). In the 33 % and 67 % burial treatments, respectively, it was increased by 73·5 % and 37·8 % in roots with diameters less than 0·1 cm, by 41·8 % and 67·9 % in roots with diameters of 0·1–0·2 cm, and by 152·2 % and 81·8 % in roots with diameters above 0·2 cm. However, complete burial led to a decrease in root length.
Fig. 3.
Root length for roots of different diameters in Ulmus pumila seedlings 6 weeks following sand burial. Values are means ± s.e., n = 8. Different letters indicate that the differences between the treatments are significant at P < 0·05.
Gas exchange and leaf water content
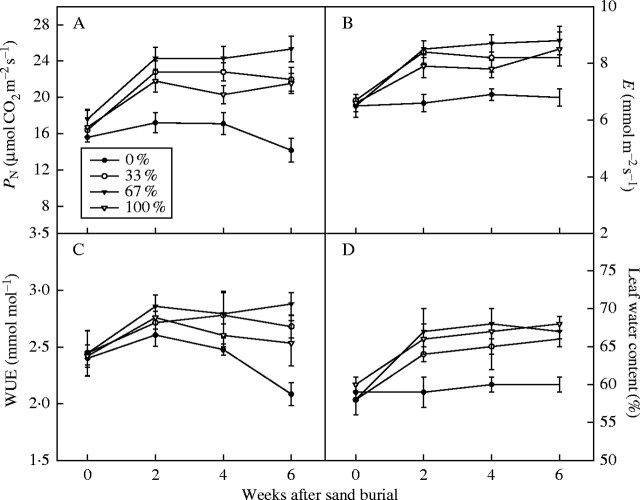
There were significant effects of burial treatments on photosynthetic rate, transpiration rate and water use efficiency (Table 2). For example, compared with the control, photosynthetic rate was increased by 55 %, 79 % and 52 % in plants exposed to 33 %, 67 % and 100 % sand burial treatments, respectively, 6 weeks following burial (P < 0·001; Fig. 4A). However, the time effects on gas exchange were not significant (Table 2). Leaf water content was significantly affected by both the burial depth and time (Table 2). It was similarly higher (about 12 %) in the partial burial and 100 % burial treatments compared with the control. The three sampling times had different response patterns to burial treatment in terms of photosynthetic rate and leaf water content (Table 2).
Fig. 4.
Net photosynthetic rate (A), transpiration rate (B), water use efficiency (C) and leaf water content (D) in Ulmus pumila seedlings at different times after sand burial. Values are means ± s.e., n = 8.
Biomass and allocation patterns
There were significant effects of time, treatment and their interaction on biomass and biomass allocation patterns (Table 2). The seedlings in the 33 % and 67 % burial treatments accumulated significantly higher biomass of shoots, roots and leaves than those in the control and in the complete burial treatment at each of the three burial times (P < 0·001; Fig. 5A–C). Whole-plant biomass at the time of last measurement, ranked in treatment order from the largest to the smallest, was 33 %, 67 %, control, 100 % and 133 % burial treatment (P < 0·001; Fig. 5C).
Fig. 5.
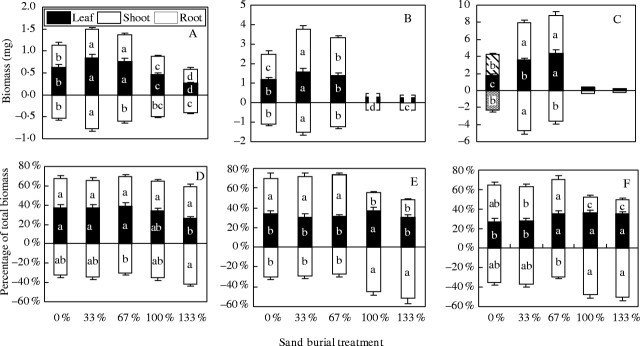
Biomass and biomass allocation of Ulmus pumila seedlings 2 weeks (A, D), 4 weeks (B, E) and 6 weeks (C, F) following sand burial. Values are means ± s.e., n = 8. Different letters indicate that the differences between the treatments are significant at P < 0·05.
The biomass allocation of the seedlings between the control, 33 % and 67 % sand burial treatments was not significantly different from each other when measured 2 or 4 weeks following burial (P > 0·05; Fig. 5D, E). In the complete burial treatments, the biomass allocation to roots was significantly increased after 4 weeks of burial (P < 0·001; Fig. 5E, F), which resulted in the higher below-ground/above-ground biomass ratio in the deeper sand burial treatment.
DISCUSSION
In an environment with moving sand, tolerance to partial burial seems to be a requisite for the dominant plant species. Ulmus pumila is one of the major species in the vegetation of the dune fields in the Hunshandak Sandland. Burial with sand (caused by frequent sandstorms) is a recurrent event in the sandland ecosystem and has strong effects on plant fitness (Yu et al., 2002). Tolerance to sand burial is a survival and growth strategy for plants to adapt to sand deposition (Disraeli, 1984). The ability to survive sand burial aids the species in populating the dune-field. In our experiment, complete sand burial (burial to 100 % and 133 % stem height) resulted in a high mortality of seedlings, with increased mortality with longer burial time (Table 1). This indicates that some adequate artificial sand binding measures should be taken to protect the seedlings of this species from being completely buried. On the other hand, U. pumila displayed enhanced growth with partial burial (i.e. 33 % and 67 % burial) and growth was stimulated in terms of biomass accumulation and leaf production. Thus, seedlings of the species have an ability to withstand partial sand burial.
The threshold of sand burial for plant survival or growth is different for different species. Most, and sometimes all, Hedysarum laeva seedlings were found to be killed by complete burial (Zhang et al., 2002). According to the reports of Zhang and Maun (1991, 1992) and Maun et al. (1996), seedlings of Agropyron psamophilum, Panicum virgatum and Cirsium pitcheri were unable to emerge from complete burial of sand. However, in two dune annuals, Cakile edentula and Strophostyles helvola, some or all of the seedlings emerged from the new sand surface following 100 % or greater burial depth (Maun, 1994; Yanful and Maun, 1996). Compared with these species, U. pumila was much more tolerant to sand burial, as evidenced by the 70 % and 20 % survival ratio in the 100 % and 133 % burial treatments, respectively.
The morphological responses of plants to sand burial are to some extent similar to the responses to neutral shading or competition for light (Brown, 1997; Maun, 1998). Under shading or competition for light, some vertical structures such as stem and lateral branches tend to elongate (Fig. 1D). Similar results have been reported for some other species (Stuefer and Huber, 1998). In order to increase the capture of light under shading or competition, petiole elongation may shift the leaves to a higher and brighter position in the canopy (Huber, 1996; Price and Hutchings, 1996). Increased number of branches (Fig. 2B) and leaves (Fig. 2C), and increased stem height (Fig. 2A) under burial potentially enable the seedling to emerge from the sand cover to get more light.
Buried plants of U. pumila generally had a higher photosynthetic rate than unburied ones during the experiment (Fig. 4A), which might be a kind of compensatory mechanism commonly found in plants. Increased biomass and fitness of plants in response to moderate defoliation are typical examples of compensatory growth (Belskey, 1987; Oba, 1994). Sand burial also reduces the total amount of photosynthetic area and thus initiates a process of compensatory responses in the affected plants, i.e. higher photosynthetic rate per unit leaf area (Fig. 4A), to make up for the reduced photosynthetic area and to balance the carbon and resource requirements of the plants.
The majority of the evidence showing improved growth of buried plants is based on biomass and final fitness, while far fewer studies have examined the physiological responses, especially the photosynthesis response, of plants to sand burial. Yuan et al. (1993) attributed the increased photosynthetic rate of buried plants to thicker leaves and a greater total area of mesophyll cells exposed to intercellular spaces per unit leaf area. This hypothesis is relevant because Yuan et al. (1993) used established adult plants in their study. The well-developed root system of established plants may minimize the limiting effect of soil nutrients and moisture on photosynthesis. According to Zhang (1996), buried plants tend to have a lower, rather than higher photosynthetic rate than unburied ones when the root system of a plant reaches a depth of 20 cm. Other plants have responded to experimental burial with an increase in leaf components. For example, the burial of Ammophila breviligulata resulted in increased levels of chlorophyll (Disraeli, 1984) and the burial of Elymus farctus increased leaf nitrogen levels (Harris and Davy, 1988). It follows, then, that sand burial alone is not likely to enhance the photosynthetic capacity of plants. For young seedlings or annual plants, however, the nutrition and moisture status of the microhabitat may be crucial to their photosynthetic abilities. In the Hunshandak Sandland, dry soil conditions and high temperature are important factors limiting plant growth at midday during the summer (Niu et al., 2003). When a plant was buried, the amount of moisture in the root zone increased and soil temperature decreased with the increase in soil depth, which lessened the negative effects of the stress conditions. In addition, the length of the roots, especially of the fibrous roots, increased with partial burial (Fig. 3), which benefited water absorption and resulted in higher leaf water content (Fig. 4D). So the photosynthesis and growth of seedlings were stimulated by the 33 % and 67 % burial treatments (Fig. 4A, Figs 1, 2). However, oxygen is essential for root growth, and if they are deprived of oxygen they will soon perish (Kurz, 1939). Because of this, total burial of the seedlings led to their death.
The allocation and utilization of resources is a fundamental and vital activity of plants (Bazzaz and Grace, 1997). When buried, individuals of some dune species shift resources such as biomass and nutrients from roots to above-ground components in an effort to maintain their photosynthetic capacity (Harris and Davy, 1987; Brown, 1997). In contrast, other dune species do not change, or even increase their root/shoot ratio (Sykes and Wilson, 1990; Brown, 1997) in the same situation. In our study, U. pumila seedlings did not significantly shift their biomass allocation pattern between below- and above-ground components when buried at levels of 33 % and 67 % of their plant height (Fig. 5), although biomass among the five treatments and three measuring times were significantly different (Table 2). On the one hand, the plants maintained root growth to maintain water absorption in the arid sandland environment; on the other hand, they maintained leaf growth in order to continue photosynthesis. Both the above- and below-ground parts were developed under partial burial. The seemingly higher root biomass allocation in complete sand burial treatments 4 and 6 weeks after burial (Fig. 5 EF) was largely due to wilting of the above-ground plant parts.
CONCLUSIONS
This study indicates that U. pumila had greater tolerance to sand burial compared with some other plants in similar environments. But complete sand burial led to partial death of the seedlings. Photosynthesis and growth conditions were stimulated by partial sand burial, due to a better sand environment, i.e. improved soil water conditions and decreased soil temperature. These responses perhaps have significant ecological and evolutionary values, and other responses should receive more attention in future, such as tolerance to darkness, and the redistribution of chemical compounds within the seedlings.
Supplementary Material
Acknowledgments
This work was supported by Key Innovation Project of the Chinese Academy of Science (KSCX1-08-02) and Combting Desertification Research Project of the Ministry of Science and Technology (FS2000-009). We thank Dr Xiaobai Jin for improving the English, and David Causton, Patrick Hesp and Steven Franks for their thoughtful comments.
LITERATURE CITED
- Bazzaz FA, Grace J. 1997.Plant resource allocation. San Diego: Academic Press. [Google Scholar]
- Belskey AJ. 1987. The effect of grazing: confounding of ecosystem: community and organism scales. The American Naturalist 129: 777–783. [Google Scholar]
- Brown JF. 1997. Effects of experimental burial on survival, growth, and resource allocation of three species of dune plants. Journal of Ecology 85: 151–158. [Google Scholar]
- Chen JS, Guo CM. 1960. Landscape of Little Tenggeli sandy land in Inner Mongolia. Acta Geoerophica Sinica 26: 23–33. [Google Scholar]
- Disraeli DJ. 1984. The effect of sand deposits on the growth and morphology of Ammophila breviligulata Journal of Ecology 72: 145–154. [Google Scholar]
- Harris D, Davy AJ. 1987. Seedling growth in Elymus fractus after episodes of burial with sand. Annals of Botany 60: 587–593. [Google Scholar]
- Harris D, Davy AJ. 1988. Carbon and nutrient allocation in Elymus fractus seedlings after burial with sand. Annals of Botany 61: 147–157. [Google Scholar]
- Hesp PA. 1991. Ecological processes and plant adaptations on coastal dunes. Journal of Arid Environment 1: 165–191. [Google Scholar]
- Huber H. 1996. Plasticity of internodes and petioles of prostrate and erect Potentilla species. Functional Ecology 10: 401–409. [Google Scholar]
- Kurz H. 1939. The reaction of magnolia, scrub live-oak, slash-pine, palmetto and other plants to dune activity on the western Florida coast. Proceedings of the Florida Academy of Science 4: 195–203. [Google Scholar]
- Li YG, Jiang GM, Gao LM, Niu SL, Liu MZ,Yu SL, Peng Y. 2003. Impacts of human disturbance on Elms-motte-veldt in Hunshandak Sandland. Acta Phytoecologica Sinica 27: 829–834. [Google Scholar]
- Liu QJ. 1989. Drought tolerance of some tree species in Buxin, Liaoxi, North-East China. Journal of North-East China Forestry University 17: 93–99. [Google Scholar]
- Maun MA. 1994. Adaptations enhancing survival and establishment of seedlings on coastal dune systems. Vegetation 111: 59–70. [Google Scholar]
- Maun MA. 1998. Adaptations of plants to burial in coastal sand dunes. Canadian Journal of Botany 76: 713–738. [Google Scholar]
- Maun MA, Elberling H, D'Ulisse A, 1996. The effects of burial by sand on survival and growth of Pitcher's thistle (Cirsium pitcheri) along Lake Huron. Journal of Coastal Conservation 2: 3–12. [Google Scholar]
- Niu SL, Jiang, GM, Li YG, Gao LM. Liu MZ, Peng Y, Ding L. 2003. Comparison of photosynthetic traits between two typical shrubs: legume and non-legume in Hunshandak Sandland. Photosynthetica 41: 237–242. [Google Scholar]
- Oba G. 1994. Responses of Indigofera spinosa to simulated herbivory in a semidesert of North-West Kenya. Acta Oecologica 15: 105–117. [Google Scholar]
- Perumal J. 1994.Effects of burial in sand on dune plant communities and ecophysiology of component species. PhD thesis, University of Western Ontario, London, Ontario. [Google Scholar]
- Price EAC, Hutchings MJ. 1996. The effects of competition on growth and form in Glechoma hederacea Oikos 75: 279–290. [Google Scholar]
- Singh G, Rathod TR. 2002. Plant growth, biomass production and soil water dynamics in a shifting dune of Indian desert. Forest Ecology and Management 171: 309–320. [Google Scholar]
- Stuefer JF, Huber H. 1998. Differential effects of light quantity and spectral light quality on growth, morphology and development of two stoloniferous Potentialla species. Oecologia 117: 1–8. [DOI] [PubMed] [Google Scholar]
- Sykes MT, Wilson JB. 1990. An experimental investigation into the response of New Zealand sand dune species to different depths of burial by sand. Acta Botanica Neerl 39: 171–181. [Google Scholar]
- Voesenek LACJ, Van der Putten WH, Maun MA, Blom CWPM. 1998. The role of ethylene and darkness in accelerated shoot elongation of Ammophila breviligulata upon sand burial. Oecologia 115: 359–365. [DOI] [PubMed] [Google Scholar]
- Yan W, Li WB, Wu QW. 1997. Lanqi Elms (Ulmus pumila) is suitable for plantation in arid grassland. Journal of Inner Mongolia Forestry College (Nature Science) 19: 32–35. [Google Scholar]
- Yanful M, Maun MA. 1996. Effects of burial of seeds and seedlings from different seed sizes on the emergence and growth of Strophostyles helvola Canadian Journal of Botany 74: 1322–1330. [Google Scholar]
- Yu FH, Chen YF, Dong M. 2002. Clonal integration enhances survival and performance of Potentilla anserina, suffering from partial sand burial on Ordos plateau, China. Evolutionary Ecology 15: 303–318. [Google Scholar]
- Yuan T, Maun MA, Hopkins WG. 1993. Effects of sand accretion on photosynthesis, leaf-water potential and morphology of two dune grasses. Functional Ecology 7: 676–682. [Google Scholar]
- Zhang CY, Yu FH, Dong M. 2002. Effects of sand burial on the survival, growth, and biomass allocation in semi-shrub Hedysarum laeve seedlings. Acta Botanica Sinica 44: 337–343. [Google Scholar]
- Zhang J, Maun MA. 1991. Establishment and growth of Panicum virgatum L. seedlings on a Lake Erie sand dune. Bull. Torrey Botanical Club 118: 141–153. [Google Scholar]
- Zhang J, Maun MA. 1992. Effects of burial in sand on the growth and reproduction of Cakile edentula Ecography 15: 296–302. [Google Scholar]
- Zhang JH. 1996. Interactive effects of soil nutrients, moisture and sand burial on the development, physiology, biomass and fitness of Cakile edentula Annals of Botany 78: 591–598. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.