Abstract
• Background and Aims Ozone effects on peatland vegetation are poorly understood. Since stress responses are often first visible in cell ultrastructure, electron microscopy was used to assess the sensitivity of common peatland plants to elevated ozone concentrations.
• Methods Three moss species (Sphagnum angustifolium, S. magellanicum and S. papillosum), a graminoid (Eriophorum vaginatum) and two dwarf shrubs (Vaccinium oxycoccus and Andromeda polifolia), all growing within an intact canopy on peat monoliths, were exposed to a concentration of 0, 50, 100 or 150 ppb ozone in two separate growth chamber experiments simulating either summer or autumn conditions in central Finland. After a 4- or 5-week-long exposure, samples were photographed in a transmission electron microscope and analysed quantitatively using image processing software.
• Key Results In the chlorophyllose cells of the Sphagnum moss leaves from the capitulum, ozone exposure led to a decrease in chloroplast area and in granum stack thickness and various changes in plastoglobuli and cell wall thickness, depending on the species and the experiment. In E. vaginatum, ozone exposure significantly reduced chloroplast cross-sectional areas and the amount of starch, whereas there were no clear changes in the plastoglobuli. In the dwarf shrubs, ozone induced thickening of the cell wall and an increase in the size of plastoglobuli under summer conditions. In contrast, under autumn conditions the cell wall thickness remained unchanged but ozone exposure led to a transient increase in the chloroplast and starch areas, and in the number and size of plastoglobuli.
• Conclusions Ozone responses in the Sphagnum mosses were comparable to typical ozone stress symptoms of higher plants, and indicated sensitivity especially in S. angustifolium. The responses in the dwarf shrubs suggest stimulation of photosynthesis by low ozone concentrations and ozone sensitivity only under cool autumn conditions.
Key words: Andromeda polifolia, electron microscopy, Eriophorum vaginatum, ozone, peatland, Sphagnum, ultrastructure, TEM, Vaccinium oxycoccus
INTRODUCTION
Ozone responses of natural vegetation have been mainly investigated by measuring growth or assessing visible injury (Reiling and Davison, 1992; Bergmann et al., 1995; Pleijel and Danielsson, 1997; Davison and Barnes, 1998; Franzaring et al., 2000; VanderHeyden et al., 2001; Power and Ashmore, 2002). Results of these studies are rather variable, indicating different ozone responses between and within species and depending on exposure time (Davison and Barnes, 1998; Bergmann et al., 1999). Microscope studies into ozone responses of wild plants are, except for forest trees, non-existent, even though microscopy could serve as an additional and sensitive tool in the assessment of plant responses to ozone. By means of electron microscopy, early stress responses can be observed in the cell structure before the first visible symptoms occur (Holopainen et al., 1992).
Peatland plants have received little attention in ozone research, although it is well established that the vascular plants growing in moist habitats may have high ozone uptake because they can continuously maintain open stomata (Franzaring et al., 2000; Power and Ashmore, 2002). As far as is known, this is the first report on ultrastructural responses of peatland plants to ozone. Peatlands are important ecosystems with regard to atmospheric greenhouse gas composition. They are long-term sinks of carbon dioxide (Gorham, 1991; Turunen et al., 2002) and significant sources of methane (Bartlett and Harriss, 1993). Plants play a key role in the exchange of both of these gases; balance between photosynthesis and decomposition determines carbon sink strength, and plant physiology (e.g. root exudates as substrates for methane production) and anatomy (plant-mediated methane conduction through enlarged intercellular spaces, i.e. aerenchyma) strongly affect methane emission. Therefore, the adverse effects of ozone on peatland plants could indirectly influence greenhouse gas exchange in peatlands with possible feedbacks on atmospheric gas composition.
Previous results from a growth chamber experiment indicated that elevated ozone concentrations can increase ecosystem respiration and methane emission in peatland microcosms (Niemi et al., 2002a). Sphagnum moss species, which often dominate the moss layer in boreal and subarctic peatlands and tundra, differ in ozone tolerance. Significant changes of chlorophyll content, membrane leakage, photosynthesis and growth have been observed, for example in S. magellanicum, S. rubellum, and in the species belonging to the Sphagnum recurvum complex (S. flexuosum, S. recurvum and S. angustifolium) (Gagnon and Karnosky, 1992; Potter et al., 1996a, b; Niemi et al., 2002a), whereas S. capillifolium, S. cuspidatum and S. papillosum have been reported to tolerate experimental ozone exposures with little injury (Potter et al., 1996b). There are virtually no published data on the responses of vascular peatland plants to elevated ozone concentrations.
The main aim of this study was to assess measurable ozone responses in the ultrastructure of six common peatland plant species, and to compare the potential responses with those reported for other species. The chosen species represent functionally and structurally different plant groups: mosses (Sphagnum angustifolium, S. magellanicum and S. papillosum), dwarf shrubs (Vaccinium oxycoccus and Andromeda polifolia) and graminoids (Eriophorum vaginatum). The Sphagnum mosses have leaves that are one cell layer thick and lack both cuticle and stomata. Living chlorophyllose cells are located in between porose hyaline cells, which are often filled with water (van Breemen, 1995). The leaves of the dwarf shrubs are evergreen, and have a thick cuticle and wax layer on the adaxial side and stomata only on the abaxial side (Jacquemart, 1997, 1998). The cross-section of the leaves shows a clear differentiation into upper palisade and lower spongy mesophyll layers. In deciduous E. vaginatum the leaves are triangular in cross-section, possess stomata on two of the sides of the triangle, and contain extensive aerenchyma (see Niemi et al., 2002b). Evans et al. (1996) have suggested that high stomatal densities and high percentage of intercellular space among palisade mesophyll cells are associated with ozone sensitivity.
The following hypotheses were tested on vascular plants: (1) E. vaginatum is more sensitive to ozone than the dwarf shrubs; (2) in the hypostomatous leaves of the dwarf shrubs, ozone effects are more severe close to the stomata in the spongy mesophyll than in the palisade mesophyll, because open stomata are the main gateway for ozone into the leaf (Kerstiens and Lendzian, 1989). Furthermore, the ozone responses were assessed in simulated summer and autumn conditions of central Finland in order to elucidate whether the time of occurrence of ozone episodes can affect the ozone responses. Special emphasis was put on chloroplast ultrastructure because alterations in the chloroplast could lead to changes in carbon assimilation and biomass accumulation. Furthermore, it is well established that chloroplast structure is altered by ozone, usually before other cell organelles (Sutinen et al., 1990; Holopainen et al., 1996). In addition, the thickness of the cell wall, which is an early target of ozone, was measured (Günthardt-Goerg et al., 1997). This paper follows a fully quantitative approach using the guidelines of morphometry (Toth, 1982).
MATERIALS AND METHODS
Experimental design
Peatland vegetation originating from an oligotrophic fen in Eastern Finland (62°47′N, 30°56′E) was grown intact within a natural canopy on top of peat monoliths in two separate ozone exposure experiments. There were altogether 40 peat monoliths (diameter 10·5 cm), ten in each treatment. The moss layer consisted of Sphagnum angustifolium (Russow) C. Jens, Sphagnum papillosum Lindb. and Sphagnum magellanicum Brid. The vascular plants in the study were Eriophorum vaginatum L., Andromeda polifolia L. and Vaccinium oxycoccus L.
Ozone exposure was applied in growth chambers simulating either June (henceforth ‘summer experiment’, for 5–6 weeks) or September (henceforth ‘autumn experiment’, for 4 weeks) conditions in central Finland. In the summer experiment the light/dark cycle was 22 h/2 h, air temperature 19 °C (day) to 12 °C (night) and humidity 52–80 %. In the autumn experiment, the light/dark cycle was 12 h/12 h, air temperature 11 °C (day) to 7 °C (night) and humidity 71–92 %. Daytime photosynthetic photon flux density of 480–500 µE m−2 s−1 at the level of the plants was provided by two rows of three Osram HQI-T 250W/D lamps, and a middle row of two Osram HQI-T 400W/DH lamps. In both the experiments, there were four treatments corresponding to ozone concentrations of 0, 50, 100 and 150 nL L−1 (ppb) 9 h per day. The AOT40 (accumulated ozone exposure over a threshold of 40 nL L−1) indexes for these treatments were 0, 7·1, 12·4 and 28·5 µL L−1 h in the summer experiment, and 0, 2·5, 13·8 and 25·3 µL L−1 h in the autumn experiment. Treatments were rotated between four growth chambers to eliminate chamber effects (for details, see Niemi et al., 2002a).
Sample preparation for electron microscopy
After 36 and 27 d of exposure in the summer and autumn experiments, respectively, Sphagnum mosses (S. angustifolium, S. papillosum and S. magellanicum), E. vaginatum, V. oxycoccus and A. polifolia were sampled for electron microscopy. Ten samples per species and treatment were randomly taken from all the peat monoliths and pooled for each treatment. Sphagnum samples were taken from the medium-long or outer branches of the capitulum (Flatberg, 2002). Those of V. oxycoccus and A. polifolia were from the youngest fully expanded leaves, and those of E. vaginatum were pieces cut 3 cm from the apex of leaves that had developed during the same year.
Samples were immediately immersed in 2·5 % glutaraldehyde fixative in phosphate buffer (0·1 m, pH 7·0). In the laboratory, 1–1·5-mm-long pieces were cut one-third from the tip of the branch/leaf with a razor blade and left in 2·5 % glutaraldehyde at 4 °C over night. The samples were post-fixed in 1 % OsO4 solution at 4 °C for 5 h, dehydrated in a graded ethanol series followed by propylene oxide, and finally embedded in LX 122 Epon.
Five samples per species, treatment and experiment (240 samples in total) in which the leaf or branch segment was in a perpendicular position towards the cutting edge were chosen for electron microscopy. Thin sections (40–70 nm) were cut with an Ultracut E (Reichert-Jung), mounted on copper grids and stained with lead citrate and uranylacetate.
Analyses of ultrastructure
Thin sections were studied with a transmission electron microscope (TEM; Jeol JEM-1200 EX, Tokyo, Japan) operating at 80 kV. Three chlorophyllose cells, preferably from different leaf cross-sections and excluding the cells at the edges of the leaves, of each Sphagnum moss branch cross-section were photographed with a digital camera attached to the microscope. Cells of S. angustifolium were photographed at ×20 000 magnification, those of S. magellanicum at ×12 000 or ×15 000, and those of S. papillosum at ×7500 or ×10 000. Of V. oxycoccus and A. polifolia, three digital TEM micrographs at ×15 000 magnification were taken from random cells both in spongy and palisade mesophyll, avoiding cells surrounding vascular bundles. Of E. vaginatum, three digital TEM micrographs at ×15 000 magnification were taken from mesophyll cells close to the stomata. These magnifications were chosen to discern clearly the structure of the chloroplasts, which were the main target organelles in this study.
In Sphagnum mosses, a cross-section of a whole chlorophyllose cell fitted into a micrograph. Numbers of chloroplasts, thickness of the cell wall and areas of the chloroplasts and lipid bodies were measured. The number of plastoglobuli was counted and areas of the plastoglobuli and the starch grains, and the thickness of thickest granum stack were measured in each chloroplast.
In vascular plants, each micrograph showed parts of one or two cells. Thickness of the cell wall (at two or three places) and areas of the two largest chloroplasts were measured. In these two chloroplasts, the number of the plastoglobuli was counted and the areas of the plastoglobuli and starch grains were measured. In addition, the occurrence of lipid bodies in the micrograph was recorded by scoring 0 for absence and 1 for presence of lipid bodies in the cytoplasm. In E. vaginatum and A. polifolia, the total area of the plastoglobuli was measured, and the area of one plastoglobulus was obtained by dividing the total area with the number of plastoglobuli. In V. oxycoccus, only the area of the largest plastoglobulus was measured, because some chloroplasts contained so many plastoglobuli that measuring the total area of the plastoglobuli would have been very laborious.
With E. vaginatum, photographing at a lower magnification and thereby measuring a greater number of chloroplasts was tested to see if it would affect the variables measured. If micrographs at ×7500 magnification were used instead of ×15 000, results were the same but the significance levels were higher. To obtain higher accuracy in the measurements, it was decided to use the greater magnifications (as described above).
All the measurements were done using Adobe Photoshop 5.0 or Adobe Photoshop Elements 2.0 (Adobe Systems, San Jose, CA, USA).
Statistical analyses
All the statistical analyses were run in the SPSS 11.0 package (SPSS Inc., Chicago, IL, USA). To test whether elevated ozone concentrations affected Sphagnum species and whether these differed in response, the data were subjected to multivariate analysis of variance (MANOVA) with ozone treatment and Sphagnum species as factors. The vascular plants were analysed, species separately, for ozone effects and differences in spongy and palisade mesophyll (except E. vaginatum only for ozone effects) using MANOVA. The parameter describing the experiment could not be included in the models because of high heteroscedasticity. Instead, the differences between the summer and autumn experiments were tested using the unequal-variance t-test. Since the MANOVAs yielded significant effects for the combination of the variables, they were followed by univariate analysis of variance (ANOVA) for each measured variable separately. Polynomial contrasts following ANOVAs for each species and tissue separately were used to analyse if measured parameters responded to increasing ozone concentration according to a linear, cubic or quadratic trend (Scheiner, 2001). Whenever several trends were significant, only the most significant was chosen. In the autumn experiment, the number of E. vaginatum samples was too low for statistical analyses, and therefore this data set was analysed using each TEM micrograph as a replicate, and the results are interpreted with caution.
Before analyses the data were tested for normality and homoscedasticity, and log- or square-root-transformed when necessary. Non-transformed values are presented. Parameters that could not be transformed to meet the assumptions of MANOVA were not included in the analysis but tested separately for ozone effects using non-parametric Jonckheere–Terpstra test (Pirie, 1983). It tests whether the response variable differs according to a linear trend across the ozone levels.
The occurrence of cytoplasmic lipid bodies in vascular plants was cross-tabulated with ozone treatment levels, and subjected to χ2 test. No significant differences were observed in any species, and therefore the data are not shown.
RESULTS
Sphagnum mosses
The Sphagnum species that were studied differed significantly in cell anatomies and ozone responses in most parameters. The cell walls of the chlorophyllose cells were thicker in the summer (Table 1) than in the autumn experiment (Table 2) for all species (P < 0·02, t-test). In both the experiments, the cell wall thickness of S. angustifolium was not clearly affected by ozone. In contrast, the cell walls of S. magellanicum got thinner with increasing ozone concentration in the summer experiment (Table 1) and thicker, especially at the 100-ppb ozone level, in the autumn experiment (Table 2). In S. papillosum, the cell walls became thinner with the increase in the ozone concentration in the autumn experiment (Table 2).
Table 1.
Effects of four ozone concentrations (0, 50, 100 or 150 ppb) on the ultrastructure of the chlorophyllose cells in Sphagnum angustifolium, S. magellanicum and S. papillosum in the summer experiment
| Cell wall (µm) |
Lipid area (% of cell) |
Chloroplast area (% of cell) |
Chloroplast (µm2) |
Plastoglobuli (no. µm−2 stroma) |
Plastoglobulus (×10−3 µm2) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. angustifolium | ||||||||||||
| 0 ppb | 0·40 ± 0·04 | 5·3 ± 0·7 | 17·1 ± 2·6 | 2·0 ± 0·4 | 4·1 ± 0·7 | 16 ± 2 | ||||||
| 50 ppb | 0·35 ± 0·02 | 3·9 ± 1·1 | 11·7 ± 2·4 | 1·0 ± 0·2 | 7·3 ± 1·1 | 15 ± 1 | ||||||
| 100 ppb | 0·36 ± 0·01 | 3·3 ± 0·5 | 13·1 ± 2·1 | 1·4 ± 0·2 | 9·6 ± 1·8 | 17 ± 2 | ||||||
| 150 ppb | 0·42 ± 0·01 | 4·3 ± 0·6 | 14·3 ± 0·5 | 1·8 ± 0·1 | 7·2 ± 0·9 | 19 ± 1 | ||||||
| *Quadratic | **Quadratic | *Quadratic | ||||||||||
| S. magellanicum | ||||||||||||
| 0 ppb | 0·67 ± 0·05 | 4·9 ± 0·8 | 16·4 ± 1·1 | 2·3 ± 0·2 | 4·4 ± 0·6 | 14 ± 2 | ||||||
| 50 ppb | 0·60 ± 0·04 | 6·7 ± 1·2 | 15·2 ± 2·0 | 2·4 ± 0·2 | 4·3 ± 1·1 | 13 ± 1 | ||||||
| 100 ppb | 0·56 ± 0·02 | 10·0 ± 2·7 | 9·4 ± 1·2 | 1·8 ± 0·1 | 6·0 ± 1·4 | 18 ± 1 | ||||||
| 150 ppb | 0·48 ± 0·02 | 6·9 ± 1·3 | 10·5 ± 2·3 | 2·2 ± 0·2 | 4·6 ± 1·0 | 13 ± 1 | ||||||
| ***Linear | **Linear | *Cubic | *Cubic | |||||||||
| S. papillosum | ||||||||||||
| 0 ppb | 0·61 ± 0·09 | 7·5 ± 1·7 | 18·5 ± 2·3 | 3·6 ± 0·7 | 3·5 ± 0·5 | 15 ± 2 | ||||||
| 50 ppb | 0·73 ± 0·08 | 16·7 ± 4·6 | 19·9 ± 5·6 | 3·0 ± 0·5 | 3·4 ± 0·5 | 15 ± 1 | ||||||
| 100 ppb | 0·61 ± 0·07 | 9·7 ± 2·2 | 11·4 ± 1·6 | 1·8 ± 0·2 | 10·3 ± 2·0 | 16 ± 3 | ||||||
| 150 ppb | 0·55 ± 0·05 | 11·9 ± 3·5 | 16·0 ± 1·3 | 2·4 ± 0·5 | 3·2 ± 0·5 | 15 ± 2 | ||||||
| ***Cubic | ||||||||||||
| ANOVA | ||||||||||||
| Species | <0·001 | <0·001 | 0·109 | <0·001 | 0·016 | 0·183 | ||||||
| Ozone | 0·180 | 0·341 | 0·022 | 0·016 | <0·001 | 0·238 | ||||||
| Species × ozone | 0·106 | 0·151 | 0·469 | 0·181 | 0·070 | 0·462 | ||||||
Cell wall thickness (µm), cytoplasmic lipid area (% of cell), chloroplast area (% of cell, µm2), the number of plastoglobuli per µm2 chloroplast stroma, and the mean area of a plastoglobulus are shown.
Values are mean ± s.e., n = 5.
Statistical significance levels of univariate analysis of variance (ANOVA) are shown for each parameter. Significant polynomial trends across ozone levelsare presented within each species as significance levels *P < 0·05, **P < 0·01, ***P < 0·001 and as a fit of the significant contrast (linear, cubic or quadratic).
Table 2.
Effects of four ozone concentrations (0, 50, 100 or 150 ppb) on the ultrastructure of the chlorophyllose cells in Sphagnum angustifolium, S. magellanicum and S. papillosum in the autumn experiment
| Cell wall (µm) |
Lipid area (% of cell) |
Chloroplast area (% of cell) |
Chloroplast (µm2) |
Granum stack (µm) |
Plastoglobuli (number µm−2 stroma) |
Plastoglobulus (×10−3 µm2) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. angustifolium | ||||||||||||||
| 0 ppb | 0·35 ± 0·01 | 6·2 ± 2·7 | 24·1 ± 3·6 | 1·3 ± 0·2 | 0·19 ± 0·02 | 5·2 ± 0·9 | 12 ± 1 | |||||||
| 50 ppb | 0·32 ± 0·02 | 6·8 ± 1·5 | 17·3 ± 2·2 | 1·3 ± 0·2 | 0·14 ± 0·01 | 9·0 ± 1·9 | 11 ± 2 | |||||||
| 100 ppb | 0·36 ± 0·03 | 8·4 ± 1·4 | 12·7 ± 3·1 | 1·3 ± 0·1 | 0·09 ± 0·01 | 6·0 ± 0·3 | 14 ± 3 | |||||||
| 150 ppb | 0·33 ± 0·01 | 4·7 ± 0·3 | 13·4 ± 1·4 | 1·2 ± 0·1 | 0·13 ± 0·03 | 6·1 ± 1·1 | 12 ± 1 | |||||||
| **Linear | *Linear | |||||||||||||
| S. magellanicum | ||||||||||||||
| 0 ppb | 0·38 ± 0·01 | 12·3 ± 2·8 | 17·3 ± 2·1 | 1·6 ± 0·1 | 0·21 ± 0·01 | 4·6 ± 0·4 | 15 ± 1 | |||||||
| 50 ppb | 0·39 ± 0·01 | 8·4 ± 0·6 | 15·2 ± 2·2 | 1·6 ± 0·1 | 0·20 ± 0·02 | 5·8 ± 0·7 | 15 ± 3 | |||||||
| 100 ppb | 0·50 ± 0·02 | 7·9 ± 1·2 | 14·6 ± 2·6 | 2·9 ± 0·5 | 0·21 ± 0·01 | 4·1 ± 0·7 | 14 ± 1 | |||||||
| 150 ppb | 0·43 ± 0·03 | 9·5 ± 1·8 | 12·1 ± 1·9 | 1·3 ± 0·1 | 0·23 ± 0·02 | 5·0 ± 0·8 | 15 ± 3 | |||||||
| P = 0·026† | **Cubic | |||||||||||||
| S. papillosum | ||||||||||||||
| 0 ppb | 0·54 ± 0·08 | 9·0 ± 1·9 | 11·5 ± 1·7 | 2·7 ± 0·5 | 0·21 ± 0·02 | 4·2 ± 1·0 | 24 ± 2 | |||||||
| 50 ppb | 0·56 ± 0·06 | 8·5 ± 0·7 | 14·3 ± 2·9 | 2·3 ± 0·3 | 0·21 ± 0·03 | 5·5 ± 1·4 | 14 ± 2 | |||||||
| 100 ppb | 0·46 ± 0·03 | 3·2 ± 0·7 | 17·5 ± 5·3 | 1·8 ± 0·3 | 0·17 ± 0·01 | 4·6 ± 1·3 | 16 ± 1 | |||||||
| 150 ppb | 0·37 ± 0·03 | 4·4 ± 1·0 | 12·8 ± 4·7 | 1·2 ± 0·2 | 0·15 ± 0·01 | 4·4 ± 0·8 | 16 ± 3 | |||||||
| P = 0·022† | **Linear | **Linear | **Linear | *Linear | ||||||||||
| ANOVA | ||||||||||||||
| Species | NI | 0·009 | 0·393 | <0·001 | <0·001 | 0·021 | 0·002 | |||||||
| Ozone | NI | 0·094 | 0·281 | 0·006 | 0·011 | 0·063 | 0·089 | |||||||
| Species × ozone | NI | 0·120 | 0·219 | 0·001 | 0·038 | 0·849 | 0·075 | |||||||
Cell wall thickness (µm), cytoplasmic lipid area (% of cell), chloroplast area (% of cell, µm2), granum stack thickness (µm), the number of plastoglobuli perµm2 chloroplast stroma, and the mean area of a plastoglobulus are shown.
NI, not included in MANOVA.
Jonckheere–Terpstra test. For other symbols and statistics, see Table 1.
In the summer experiment, cytoplasmic lipid bodies were most abundant in S. papillosum and fewest in S. angustifolium, and unaffected by ozone treatments in all species (Table 1). In the autumn experiment, species differed in ozone response (Table 2). In S. angustifolium, the relative lipid area tended to increase towards the 100-ppb ozone level and dropped at the 150-ppb level. In S. papillosum, this area decreased linearly with increasing ozone concentration, whereas in S. magellanicum it was lower in all the ozone treatments than at the 0-ppb ozone level.
In the summer experiment, the cell cross-sectional area occupied by chloroplasts was significantly decreased by elevated ozone concentrations in all species. In S. magellanicum the decrease was linear with increasing ozone concentration (Table 1). These changes in the proportional area were due to reductions of the chloroplast size, and not affected by the number of chloroplasts per cell because there were no differences between the treatments in this parameter (data not shown). In the autumn experiment, ozone treatments caused no distinct effects on the chloroplast area per cell cross-section (Table 2) or on the number of chloroplasts per cell cross-section (data not shown).
The average area of a chloroplast cross-section was significantly altered by ozone, and the species differed greatly in their responses (Tables 1 and 2). In the summer experiment, the chloroplast cross-section of S. angustifolium was smallest at the 50-ppb ozone level (half of the 100-ppb level), whereas in the other two species it was smallest at the 100-ppb ozone level (Table 1). In the autumn experiment, the chloroplast size of S. angustifolium was unaffected by ozone. In S. papillosum, the chloroplast size decreased linearly with increasing ozone concentration being, at the 150-ppb ozone level, less than half of the size at the 0-ppb ozone level (Table 2). In S. magellanicum, the chloroplast size was 80 % higher at the 100-ppb ozone level and about 20 % lower at the 150-ppb level compared with the 0- and 50-ppb levels (Table 2).
In the autumn experiment, most chloroplasts lacked starch and there were no treatment effects (data not shown; Fig. 1A and C). In the summer experiment, the relative area of starch per chloroplast cross-section was significantly altered by ozone in S. angustifolium, indicating the least amount of starch at the 100-ppb ozone level and the most at the 150-ppb ozone level (Figs 1B and 2A). The granum stack thickness decreased significantly by ozone treatments in S. angustifolium and S. papillosum in both the experiments, whereas it was not significantly affected in S. magellanicum (Fig. 2B and Table 2).
Fig. 1.

Electron micrographs showing cell ultrastructure of peatland plants in the summer (B, D–F) or in the autumn (A, C) experiment. (A) Sphagnum angustifolium chlorophyllose cell, 0-ppb ozone level; (B) S. angustifolium chlorophyllose cell, 150 ppb-ozone level; (C) Sphagnum magellanicum chlorophyllose cell, 0-ppb ozone level; (D) part of an Eriophorum vaginatum mesophyll cell, 50-ppb ozone level; (E) part of a Vaccinium oxycoccus palisade parenchyma cell, 50-ppb ozone level; (F) part of a V. oxycoccus palisade parenchyma cell, 100-ppb ozone level. Arrows point to plastoglobuli. Cw, cell wall; L, lipid body; s, starch; g, granum stack; m, mitochondrion; v, vacuole; t, tannin. Scale bars = 1 μm.
Fig. 2.

Area of starch as a percentage of chloroplast cross-section (A) and granum stack thickness (B) of Sphagnum angustifolium, S. magellanicum and S. papillosum after 36 d of exposure to a concentration of 0, 50, 100 or 150 ppb ozone in the summer experiment. Mean ± s.e., n = 5. Statistical significances of Jonckheere–Terpstra test are shown for each species separately.
The number of plastoglobuli per µm2 of chloroplast stroma was highest at the 100-ppb ozone level in all the species in the summer experiment (Table 1) and at the 50-ppb ozone level in the autumn experiment (Table 2). The average plastoglobulus size was affected by ozone only in S. papillosum under autumn conditions; it decreased significantly across increasing ozone concentrations (Table 2). The relative area of plastoglobuli cover in a chloroplast followed trends observed in the number of plastoglobuli in both the experiments (data not shown).
Vascular plants
Ultrastructure of the vascular plants that were studied differed in some parameters between the summer and autumn experiments. Especially in V. oxycoccus, the cell walls were thicker in the summer experiment than in the autumn experiment (P < 0·01, t-test). Furthermore, in both the dwarf shrubs chloroplasts were clearly larger and contained more starch in the summer than in the autumn experiment (P < 0·001, t-test).
Vaccinium oxycoccus. In the summer experiment, the cell walls of the palisade mesophyll were thicker the higher the ozone concentration, whereas in the spongy mesophyll there were no significant trends (Table 3). In the autumn experiment, the cell wall thickness was unaffected by ozone treatments (Table 3).
Table 3.
Effects of four ozone concentrations (0, 50, 100 or 150 ppb) on the ultrastructure of spongy and palisade mesophyll of Vaccinium oxycoccus in the summer and autumn experiments
| Summer experiment |
Autumn experiment |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell wall (µm) |
Chloroplast (µm2) |
Plastoglobuli (no. µm−2 stroma) |
Plastoglobulus (×10−3 µm2) |
Cell wall (µm) |
Chloroplast (µm2) |
Plastoglobuli (no. µm−2 stroma) |
Plastoglobulus (×10−3 µm2) |
|||||||||
| Spongy | ||||||||||||||||
| 0 ppb | 1·06 ± 0·14 | 13·0 ± 0·6 | 2·5 ± 0·5 | 24 ± 5 | 0·94 ± 0·09 | 5·9 ± 0·3 | 2·6 ± 0·3 | 25 ± 3 | ||||||||
| 50 ppb | 1·16 ± 0·07 | 10·6 ± 1·5 | 3·3 ± 0·7 | 29 ± 4 | 1·02 ± 0·08 | 7·4 ± 1·4 | 3·7 ± 0·2 | 54 ± 16 | ||||||||
| 100 ppb | 1·34 ± 0·21 | 15·6 ± 1·7 | 2·2 ± 0·4 | 90 ± 17 | 0·97 ± 0·09 | 9·6 ± 1·5 | 3·5 ± 0·2 | 33 ± 77 | ||||||||
| 150 ppb | 1·09 ± 0·12 | 11·3 ± 2·5 | 2·0 ± 0·5 | 34 ± 3 | 0·92 ± 0·02 | 8·2 ± 0·9 | 2·8 ± 0·5 | 26 ± 3 | ||||||||
| *Cubic | ***Cubic | *Linear | *Quadratic | *Quadratic | ||||||||||||
| Palisade | ||||||||||||||||
| 0 ppb | 0·61 ± 0·06 | 16·2 ± 1·2 | 2·8 ± 0·4 | 35 ± 9 | 0·61 ± 0·03 | 7·3 ± 0·5 | 2·0 ± 0·1 | 38 ± 5 | ||||||||
| 50 ppb | 0·64 ± 0·05 | 15·8 ± 1·8 | 3·6 ± 0·7 | 46 ± 6 | 0·59 ± 0·04 | 10·9 ± 1·5 | 2·9 ± 0·8 | 69 ± 20 | ||||||||
| 100 ppb | 0·71 ± 0·05 | 17·6 ± 1·1 | 2·6 ± 0·4 | 108 ± 26 | 0·55 ± 0·03 | 10·8 ± 1·4 | 4·2 ± 0·5 | 48 ± 15 | ||||||||
| 150 ppb | 0·78 ± 0·03 | 13·2 ± 3·0 | 2·1 ± 0·4 | 39 ± 6 | 0·55 ± 0·03 | 11·1 ± 0·9 | 2·8 ± 0·4 | 43 ± 2 | ||||||||
| *Linear | *Cubic | *Linear | *Quadratic | |||||||||||||
| ANOVA | ||||||||||||||||
| Tissue | <0·001 | 0·023 | 0·467 | 0·147 | <0·001 | 0·006 | 0·471 | 0·024 | ||||||||
| Ozone | 0·391 | 0·113 | 0·069 | <0·001 | 0·696 | 0·005 | 0·002 | 0·018 | ||||||||
| Tissue × ozone | 0·517 | 0·774 | 0·990 | 0·941 | 0·809 | 0·644 | 0·203 | 0·995 | ||||||||
The cell wall thickness (µm), chloroplast area (µm2), number of plastoglobuli per µm2 chloroplast stroma, and the area of a plastoglobulus are shown.
The values are mean ± s.e., n = 5.
Statistical significance levels of univariate analysis of variance (ANOVA), performed for experiments separately, are shown for each parameter. Significantpolynomial trends across ozone levels are presented within each mesophyll tissue as significance levels *P < 0·05, **P < 0·01, ***P < 0·001 and as a fit of thesignificant contrast (linear, cubic or quadratic).
Ozone altered chloroplast ultrastructure in a similar direction both in the spongy and palisade mesophyll. In the summer experiment, the average chloroplast cross-sectional area was highest at the 100-ppb ozone level and slightly lower at the 50- and 150-ppb levels than at the 0-ppb ozone level, while in the autumn experiment it increased linearly across increasing ozone concentrations (Table 3). Starch covered on average 55 % of a chloroplast cross-section in the summer experiment, and the amount was not significantly affected by ozone (data not shown). In the autumn experiment, starch covered only about 26 % of a chloroplast cross-section, and the relative area of starch showed a non-significant trend of increase due to ozone exposure (data not shown). The number of plastoglobuli was relatively unaffected in the summer experiment, whereas it was increased significantly by ozone in the autumn experiment; it was highest at the 50-ppb or 100-ppb ozone level (Table 3). The size of the plastoglobuli was altered differently in the summer and autumn experiments. In the summer experiment, the plastoglobuli were nearly three times larger at the 100-ppb ozone level compared with the other treatment levels (Fig. 1E and F), whereas in the autumn experiment they were about double the size at the 50-ppb ozone level compared with the 0-ppb ozone level, and smaller at the 100- and 150-ppb levels (Table 3).
Andromeda polifolia. Cell walls were significantly thicker in the spongy than in the palisade mesophyll, while in other parameters there were no significant differences between the mesophyll tissues. In the summer experiment, the cell walls of the spongy mesophyll got significantly thicker with increasing ozone concentration (Table 4). In the palisade mesophyll, they were thickest at the 50-ppb ozone level. In the autumn experiment, the cell walls became thinner towards the 100-ppb ozone level and were approximately equal at 150-ppb and 0-ppb ozone levels both in the spongy and palisade mesophyll (Table 4).
Table 4.
Effects of four ozone concentrations (0, 50, 100 or 150 ppb) on the ultrastructure of spongy and palisade mesophyll of Andromeda polifolia in the summer and autumn experiments
| Summer experiment |
Autumn experiment |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell wall (µm) |
Chloroplast (µm2) |
Plastoglobuli (no. µm−2 stroma) |
Plastoglobulus (×10−3 µm2) |
Starch (% of chloroplast area) |
Cell wall (µm) |
Chloroplast (µm2) |
Plastoglobuli (no. µm−2 stroma) |
Plastoglobulus (×10−3 µm2) |
Starch (% of chloroplast area) |
|||||||||||
| Spongy | ||||||||||||||||||||
| 0 ppb | 0·75 ± 0·05 | 13·6 ± 1·3 | 2·4 ± 0·6 | 18 ± 3 | 66·7 ± 2·3 | 1·11 ± 0·08 | 7·7 ± 0·7 | 2·3 ± 0·9 | 10 ± 2 | 36·8 ± 5·5 | ||||||||||
| 50 ppb | 0·99 ± 0·07 | 16·0 ± 0·8 | 2·7 ± 0·5 | 20 ± 4 | 70·0 ± 1·8 | 0·96 ± 0·06 | 9·1 ± 0·3 | 2·5 ± 0·4 | 19 ± 1 | 42·6 ± 5·5 | ||||||||||
| 100 ppb | 0·96 ± 0·03 | 16·1 ± 2·1 | 2·2 ± 0·2 | 17 ± 2 | 72·3 ± 2·7 | 0·79 ± 0·06 | 9·4 ± 1·6 | 2·2 ± 0·4 | 19 ± 2 | 47·0 ± 2·9 | ||||||||||
| 150 ppb | 1·13 ± 0·12 | 13·5 ± 1·5 | 2·3 ± 0·4 | 26 ± 9 | 63·5 ± 3·2 | 1·00 ± 0·11 | 7·8 ± 0·7 | 3·0 ± 0·3 | 15 ± 1 | 40·2 ± 4·5 | ||||||||||
| **Linear | *Quadratic | *Quadratic | *Quadratic | |||||||||||||||||
| Palisade | ||||||||||||||||||||
| 0 ppb | 0·64 ± 0·03 | 17·2 ± 1·9 | 3·6 ± 0·8 | 17 ± 2 | 71·1 ± 2·6 | 0·68 ± 0·06 | 9·1 ± 0·8 | 1·9 ± 0·2 | 13 ± 2 | 44·7 ± 3·8 | ||||||||||
| 50 ppb | 0·74 ± 0·01 | 21·6 ± 2·3 | 4·2 ± 0·9 | 18 ± 2 | 73·5 ± 4·4 | 0·56 ± 0·03 | 10·5 ± 0·3 | 3·1 ± 0·7 | 20 ± 1 | 51·4 ± 2·8 | ||||||||||
| 100 ppb | 0·64 ± 0·05 | 19·4 ± 2·8 | 2·1 ± 0·3 | 14 ± 1 | 73·1 ± 2·2 | 0·56 ± 0·05 | 9·3 ± 0·6 | 2·1 ± 0·4 | 18 ± 3 | 49·6 ± 3·4 | ||||||||||
| 150 ppb | 0·70 ± 0·04 | 17·0 ± 2·4 | 2·8 ± 0·5 | 38 ± 17 | 59·1 ± 5·3 | 0·66 ± 0·06 | 6·4 ± 0·6 | 4·9 ± 1·3 | 12 ± 1 | 20·4 ± 7·0 | ||||||||||
| *Linear | **Linear | *Linear | **Quadratic | **Linear | ||||||||||||||||
| ANOVA | ||||||||||||||||||||
| Tissue | <0·001 | 0·010 | 0·093 | 0·942 | 0·648 | <0·001 | 0·520 | 0·283 | 0·848 | 0·966 | ||||||||||
| Ozone | 0·005 | 0·263 | 0·272 | 0·268 | 0·006 | 0·023 | 0·010 | 0·010 | 0·002 | 0·003 | ||||||||||
| Tissue × ozone | 0·122 | 0·945 | 0·581 | 0·812 | 0·531 | 0·703 | 0·239 | 0·718 | 0·376 | 0·016 | ||||||||||
The cell wall thickness (µm), chloroplast area (µm2), number of plastoglobuli per μm2 chloroplast stroma, the mean area of a plastoglobulus, and area of starch (% of chloroplast area) are shown.
For statistics, see Table 3.
The average chloroplast cross-sectional area and the number of plastoglobuli were not clearly affected by ozone in the summer experiment (Table 4). In contrast, both parameters were significantly altered by ozone in the autumn experiment: in the spongy mesophyll, the average chloroplast cross-sectional area increased with ozone concentration until the 100-ppb ozone level and was about the same at the 150-ppb and 0-ppb ozone levels (Table 4). However, in the palisade parenchyma the chloroplast cross-sectional area was highest at the 50-ppb ozone level, and thereafter it decreased linearly with increasing ozone concentration (Table 4). The number of plastoglobuli was highest at the 150-ppb ozone level in both the mesophyll tissues (Table 4). Ozone treatments affected the area of starch per chloroplast cross-section in a similar way in both experiments. The relative area of starch was higher at the 50- and 100-ppb ozone levels and lower at the 150-ppb ozone level than at the 0-ppb level in both the mesophyll tissues in the summer experiment and in the palisade mesophyll in the autumn experiment (Table 4). The plastoglobulus size was in general increased by ozone exposure. In the summer experiment, there were no statistically significant differences due to high variance in data (Table 4). In the autumn experiment, the plastoglobulus size was greater in all the ozone treatments compared with the 0-ppb ozone level in the spongy mesophyll. In the palisade mesophyll, it was largest at the 50- and 100-ppb ozone levels, and equal at the 0-ppb and 150-ppb ozone levels (Table 4).
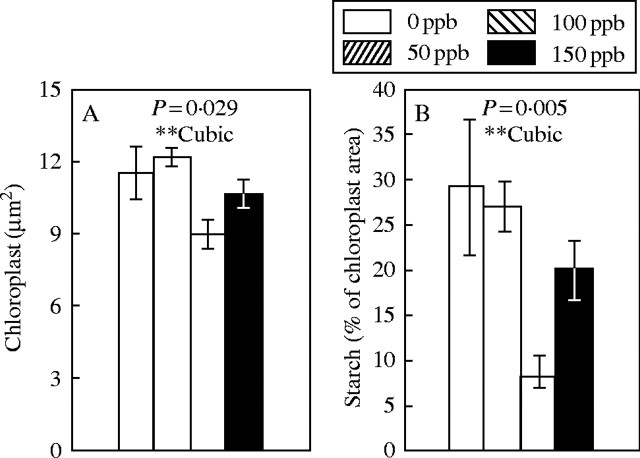
Eriophorum vaginatum. In the summer experiment, ozone treatments had no significant effects on the cell wall thickness of E. vaginatum (data not shown; see Fig. 1D for an example of the cell structure). Cell walls were on average 0·34 µm thick. The average chloroplast cross-sectional area and the area of starch per chloroplast cross-section were significantly decreased by ozone (Fig. 3). Both parameters had lowest values at the 100-ppb ozone level, about 20 % and 70 % lower than at the 0-ppb level, respectively. The number and size of plastoglobuli were not significantly affected by ozone treatments (data not shown). In the autumn experiment, ozone responses followed similar trends to the summer experiment, except for the cell wall thickness, which increased linearly with increasing ozone concentration (P < 0·001, analysed using TEM micrograph as a replicate).
Fig. 3.
Chloroplast cross-sectional area (A) and percentage area of starch in a chloroplast cross-section (B) of Eriophorum vaginatum mesophyll after 36 d of exposure to a concentration of 0, 50, 100 or 150 ppb ozone in the summer experiment. Mean ± s.e., n = 5. P-values for ANOVA, significance levels for polynomial contrasts (**P < 0·01) and fit of the significant contrast (linear, cubic or quadratic) are shown.
The responses of all the plant species are summarized in Table 5.
Table 5.
The summary of ozone responses in Sphagnum angustifolium, S. magellanicum, S. papillosum, Vaccinium oxycoccus, Andromeda polifolia and Eriophorum vaginatum combined from the summer and autumn experiments
|
S. angustifolium |
S. magellanicum |
S. papillosum |
V. oxycoccus |
A. polifolia |
E. vaginatum |
|
|---|---|---|---|---|---|---|
| Cell wall thickness | 0 | +/− | − | + | +/− | + |
| Amount of lipid bodies | 0 | − | − | 0 | 0 | 0 |
| Chloroplast size | − | − | − | + | 0 | − |
| Granum stack thickness | − | 0 | − | n.d. | n.d. | n.d. |
| Starch area | + | 0 | 0 | 0 | +/− | − |
| No. of plastoglobuli | + | + | + | + | + | 0 |
| Plastoglobulus size | 0 | 0 | − | + | + | 0 |
0, no effects; +, increase due to ozone; −, decrease due to ozone; +/−, different response in the two experiments; n.d., not determined.
DISCUSSION
Ozone effects on the ultrastructure of vascular plants
This study shows that exposure to ozone concentrations of 50–150 ppb in a growth chamber changes the ultrastructure of the cells of the dwarf shrubs V. oxycoccus and A. polifolia, and a graminoid E. vaginatum. As in many earlier studies with other plant species (Holopainen et al., 1992; Kivimäenpää et al., 2003), measurable changes in the chloroplast ultrastructure were observed (Table 5).
In V. oxycoccus, the chloroplast size was increased by ozone exposure, whereas in A. polifolia the size was increased when the plants were exposed to 50–100 ppb of ozone and decreased by an exposure to 150 ppb of ozone. Ozone-induced transient increase in chloroplast area has been suggested for Norway spruce (Picea abies) (Sutinen et al., 1998; Kivimäenpää et al., 2003), although higher ozone concentrations or prolonged exposures more commonly induce shrinkage of chloroplasts (Miyake et al., 1989; Sutinen et al., 1990; Holopainen et al., 1996). Similar to observations by Kivimäenpää et al. (2003), the changes in chloroplast size followed changes in the area of starch, and therefore the chloroplast size itself may not be affected by ozone. The transient stimulation of starch formation in response to ozone exposure can indicate impaired translocation of assimilates (Dizengremel, 2001). This may be typical for the life form of woody evergreen species and/or represent ozone resistance of conifers and other evergreens. These plants may possess high resistance thanks to slow metabolism, low stomatal uptake of gases and effective oxidative defence.
In the graminoid species E. vaginatum, both chloroplast size and the relative area of starch were decreased by ozone, especially at the 100-ppb ozone level, showing a negative ozone impact in the photosynthetic cells close to the stomata. This observation supports the hypothesis that E. vaginatum would be an ozone-sensitive species. However, the limited data on E. vaginatum from the autumn experiment restricts this conclusion.
In the dwarf shrubs, the cell wall increased in thickness under elevated ozone concentrations in the summer experiment, whereas there were no changes or a slight decrease in the autumn experiment. The cell wall is an early target of ozone (Günthardt-Goerg et al., 1997). In birch and poplar, cell walls have been reported to thicken in response to ozone and this has been associated with an increase in pectinaceous exudates in the cell wall (Günthardt-Goerg et al., 1997). Thickening of cell walls may be related to avoiding ozone exposure. Thicker cell walls could have more detoxification capacity since the aqueous matrix of the cell wall contains an abundance of compounds scavenging ozone and the reactive oxygen species formed from ozone, including ascorbate, polyamines, glutathione and various enzymes (Long and Naidu, 2002).
Plastoglobuli, which consist mainly of triacylglycerols, plastohydroquinone and a-tocopherol, and may function as storage pools of thylakoid constituents (Steinmüller and Tevini, 1985; Murphy, 2001), were markedly affected by ozone. In the dwarf shrub species that were studied, ozone increased the size but not the number of plastoglobuli under summer conditions. Under autumn conditions, both the number and the size increased in response to ozone – although not linearly with the ozone concentration increase. These findings are consistent with many observations on the increase in the number and/or size of the plastoglobuli in response to ozone exposure or to ageing of plant cells (Miyake et al., 1989; Ojanperä et al., 1992; Pääkkönen et al., 1996; Günthardt-Goerg et al., 2000; Britvec et al., 2001; Oksanen et al., 2001).
The finding that the ozone-induced changes in the chloroplast ultrastructure did not always follow a linearly progressive response to increasing ozone exposure is in contrast with some other studies. Ojanperä et al. (1992) reported that the senescence-like symptoms, including smaller size of the chloroplasts and more plastoglobuli, in the flag leaf cells of spring wheat (Triticum aestivum ‘Drabant’) following an open-top exposure to up to 56 ppb of ozone (7 h seasonal mean), progressed at a faster speed the higher the ozone concentration. Britvec et al. (2001) suggest that low ozone concentrations accelerate senescence in grapevine (Vitis vinifera) leaves, whilst higher concentrations (about 80–100 ppb) or prolonged exposures damage the thylakoid membranes.
Initially ozone damages cells that are located close to the stomata (Leipner et al., 2001). On the other hand, light-exposed cells have been reported to suffer greater ozone injury than cells exposed to less light (Vollenweider et al., 2003). In the present work, there were no significant differences in the ultrastructural changes between the spongy mesophyll (close to the stomata) and the palisade mesophyll (exposed to more light) of the dwarf shrubs. This indicates either that the ozone exposure has been severe enough for the oxidative stress to advance from spongy to palisade mesophyll or that light has indeed intensified the ozone effects.
Experimental conditions had a clear effect on the responses to ozone. Ozone effects in the dwarf shrubs were typically more pronounced when the same exposure protocol was conducted under simulated autumn conditions of central Finland (day temperature 11 °C, light/dark cycle 12/12 h) instead of summer conditions (day temperature 19 °C, light/dark cycle 22/2 h). This finding is well in line with an observation that long-term ozone exposure only at temperatures of 5 °C or below leads to significant responses in another shrub Calluna vulgaris (Foot et al., 1996), and suggests that dwarf shrubs are most sensitive to ozone during cool and humid conditions. In the present study, the absence of ozone-induced thickening of cell walls in the autumn experiment may have led to lower protection against oxidative stress. Indeed, ozone induced more responses in the chloroplast ultrastructure in the autumn conditions. The lack of thickening in cell walls under cooler conditions may have resulted from lowered growth rate since most active shoot growth, at least in A. polifolia, occurs during late May and early June (Lindholm, 1982). Another explanation for this difference could be that the samples from the autumn experiment were exposed 9 d less to ozone than those from the summer experiment.
Especially in the summer experiment, ultrastructural changes were most apparent at the 100-ppb ozone level. This finding is in agreement with previously published results from the summer experiment; ecosystem gross photosynthesis and ecosystem respiration were lower at the 100-ppb treatment level compared with the 50-ppb or 150-ppb levels (Niemi et al., 2002a). Eriophorum vaginatum is the dominant vascular plant and controls CO2 exchange in the ecosystem studied (Rinnan et al., 2003) and thus the observed lower chloroplast and starch areas in E. vaginatum can impact on the ecosystem processes more than changes in the dwarf shrubs which are a minor component of the plant cover.
Ozone effects on the ultrastructure of Sphagnum mosses
The Sphagnum mosses (S. angustifolium, S. magellanicum and S. papillosum) showed different signs of ozone stress in their cells (Table 5), some of which support the earlier reported physiological responses from the same experiments (Niemi et al., 2002a). Transmission electron microscope studies into the ozone responses of mosses are scarce, which limits the discussion.
Lack of starch in the chloroplasts in the autumn experiment suggests little photosynthetic activity. The observed ozone-induced decreases in the area of cytoplasmic lipids of S. magellanicum and S. papillosum under autumn conditions thus indicate that energy stored in the lipids was possibly used to maintain cell functioning during ozone stress. In normal senescence, the amount of total lipids increases in the Sphagnum capitulum during autumn (Karunen and Salin, 1982).
In the summer experiment, species showed different responses in the amount of starch. Starch accumulated in the chloroplasts of S. angustifolium and S. magellanicum when they were exposed to a concentration of 150 ppb of ozone. In contrast to this, ozone exposure decreased the amount of starch in the S. papillosum chloroplasts. This indicates that the photosynthetic activity of S. papillosum had decreased and that of S. angustifolium and S. magellanicum increased as a result of ozone exposure. Moreover, the granum stack thickness of S. papillosum decreased significantly with increasing ozone concentration.
Despite the differing metabolic states of the Sphagnum mosses between the summer and autumn experiment, ozone effects on the chloroplast ultrastructure were rather similar and comparable to effects observed in higher plants. The chloroplast area and granum stack thickness of the chlorophyllose cells of the Sphagnum mosses in general decreased due to ozone exposure, which are common ozone stress symptoms in higher plants (Miyake et al., 1989; Holopainen et al., 1996). The number of plastoglobuli increased slightly due to ozone exposure in the autumn experiment, but only in S. angustifolium in the summer experiment, while it followed a cubic response curve in S. papillosum and remained unaffected in S. magellanicum. The plastoglobulus size rather decreased than increased. The alterations in the chloroplast ultrastructure were not accompanied by changes in chlorophyll or carotenoid concentrations (Niemi et al., 2002a). However, the chloroplast area per cell cross-section correlated with the leakage of magnesium from the tissue of S. angustifolium; leakage of magnesium increased and chloroplast area decreased with increasing ozone concentration (Niemi et al., 2002a).
The thinning of the cell walls of S. magellanicum in the summer and of S. papillosum in the autumn experiment is in contrast to the more common thickening in response to ozone exposure (Günthardt-Goerg et al., 1997). Thinner cell walls under ozone exposure may result from a delay in cell wall differentiation. Cell wall measurements are also prone to inaccuracies because the cell wall thickness varies considerably between and within cells.
The Sphagnum species studied showed significantly different ozone responses in the chlorophyllose cells. Sphagnum angustifolium appeared ozone sensitive in both the experiments, whereas S. papillosum showed more significant responses in the autumn experiment. The measured changes in S. magellanicum were not as clear as those of the other two species. The sensitivity of S. angustifolium is in accordance with previous reports on ozone responses of the Sphagnum recurvum complex species (Gagnon and Karnosky, 1992; Potter et al., 1996a, b; Niemi et al., 2002a). One species of this group, Sphagnum fallax, has also shown irreversible damage in chlorophyllose cells due to prolonged drought in contrast to the more tolerant S. magellanicum and S. capillifolium (Gerdol et al., 1996), stressing the sensitivity of this group.
The different ozone tolerances may be due to anatomical characteristics of the species; the chlorophyllose cells in S. angustifolium are located close to the convex leaf surface and separated from the atmosphere only with a cell wall of the chlorophyllose cell, whereas those in S. papillosum and S. magellanicum are completely enclosed within the hyaline cells. Thus, the thinner cell wall in S. angustifolium may provide lower detoxification efficiency. However, there is some evidence that air pollution reduces peroxidase and superoxide dismutase activities in Sphagnum mosses in contrast to vascular plants (Lee and Studholme, 1992), which suggests that enzymatic detoxification may not be important for Sphagnum.
CONCLUSIONS
This study reports, for the first time, ultrastructural responses of different peatland plant species to a gradient of ozone concentrations. The results were obtained from experiments in a controlled environment, and the changes may not be as pronounced in the field situation. Nevertheless, the observed responses indicate that ozone exposure leads to metabolic changes in the plants studied. In the dwarf shrubs, symptoms that may be related to initial ozone-induced photosynthetic stimulation (larger chloroplasts and more starch) are comparable to responses of conifers, and may indicate ozone tolerance. In contrast, the negative responses of the graminoid E. vaginatum and the Sphagnum mosses suggest that these species may be more sensitive to ozone than the dwarf shrubs.
Acknowledgments
We thank Minna Kivimäenpää, Siegfried Fink and Michael Tausz for valuable comments on this manuscript. Laboratory assistance by Raimo Pesonen and the personnel of the Department of Electron Microscopy at the University of Kuopio are gratefully acknowledged. The research was financially supported by the Academy of Finland (project no. 39465) and the Kone Foundation.
LITERATURE CITED
- Bartlett KB, Harriss RC. 1993. Review and assessment of methane emissions from wetlands. Chemosphere 26: 261–320. [Google Scholar]
- Bergmann E, Bender J, Weigel HJ. 1995. Growth responses and foliar sensitivities of native herbaceous species to ozone exposures. Water Air and Soil Pollution 85: 1437–1442. [Google Scholar]
- Bergmann E, Bender J, Weigel HJ. 1999. Ozone threshold doses and exposure-response relationships for the development of ozone injury symptoms in wild plant species. New Phytologist 144: 423–435. [DOI] [PubMed] [Google Scholar]
- Britvec M, Reichenauer T, Soja G, Ljubešić N, Eid M, Pecina M. 2001. Ultrastructure changes in grapevine chloroplasts caused by increased tropospheric ozone concentrations. Biologia, Bratislava 56: 417–424. [Google Scholar]
- Davison AW, Barnes JD. 1998. Effects of ozone on wild plants. New Phytologist 139: 135–151. [Google Scholar]
- Dizengremel P. 2001. Effects of ozone on the carbon metabolism of forest trees. Plant Physiology and Biochemistry 39: 729–742. [Google Scholar]
- Evans LS, Albury K, Jennings N. 1996. Relationships between anatomical characteristics and ozone sensitivity of leaves of several herbaceous dicotyledonous plant species at Great Smoky Mountains National Park. Environmental and Experimental Botany 36: 413–420. [Google Scholar]
- Flatberg KI. 2002. The Norwegian sphagna: a field colour guide. NTNU Vitenskapsmuseet Rapport botanisk serie 2002–1: 1–44. [Google Scholar]
- Foot JP, Caporn SJM, Lee JA, Ashenden TW. 1996. The effect of long-term ozone fumigation on the growth, physiology and frost sensitivity of Calluna vulgaris New Phytologist 133: 503–511. [Google Scholar]
- Franzaring J, Tonneijck AEG, Kooijman AWN, Dueck T. 2000. Growth responses to ozone in plant species from wetlands. Environmental and Experimental Botany 44: 39–48. [DOI] [PubMed] [Google Scholar]
- Gagnon ZE, Karnosky DF. 1992. Physiological response of three species of Sphagnum to ozone exposure. Journal of Bryology 17: 81–91. [Google Scholar]
- Gerdol R, Bonora A, Gualandri R, Pancaldi S. 1996. CO2 exchange, photosynthetic pigment composition, and cell ultrastructure of Sphagnum mosses during dehydration and subsequent rehydration. Canadian Journal of Botany 74: 726–734. [Google Scholar]
- Gorham E. 1991. Northern peatlands: role in the carbon cycle and probable responses to climatic warming. Ecological Applications 1: 182–195. [DOI] [PubMed] [Google Scholar]
- Günthardt-Goerg MS, McQuattie CJ, Maurer S, Frey B. 2000. Visible and microscopic injury in leaves of five deciduous tree species related to current critical ozone levels. Environmental Pollution 109: 489–500. [DOI] [PubMed] [Google Scholar]
- Günthardt-Goerg MS, McQuattie CJ, Scheidegger C, Rhiner C, Matyssek R. 1997. Ozone-induced cytochemical and ultrastructural changes in leaf mesophyll cell walls. Canadian Journal of Forest Research 27: 453–463. [Google Scholar]
- Holopainen T, Anttonen S, Palomäki V, Kainulainen P, Holopainen JK. 1996. Needle ultrastructure and starch content in Scots pine and Norway spruce after ozone fumigation. Canadian Journal of Botany 74: 67–76. [Google Scholar]
- Holopainen T, Anttonen S, Wulff A, Palomäki V, Kärenlampi L. 1992. Comparative evaluation of the effects of gaseous pollutants, acidic deposition and mineral deficiencies: structural changes in the cells of forest plants. Agriculture, Ecosystems and Environment 42: 365–398. [Google Scholar]
- Jacquemart A. 1997.Vaccinium oxycoccos L. (Oxycoccus palustris Pers.) and Vaccinium microcarpum (Turcz. ex Rupr.) Schmalh. (Oxycoccus microcarpus Turcz. ex Rupr.). Journal of Ecology 85: 381–396. [Google Scholar]
- Jacquemart A. 1998.Andromeda polifolia L. Journal of Ecology 86: 527–541. [Google Scholar]
- Karunen P, Salin M. 1982. Seasonal changes in lipids of photosynthetically active and senescent parts of Sphagnum fuscum Lindbergia 8: 35–44. [Google Scholar]
- Kerstiens G, Lendzian KJ. 1989. Interactions between ozone and plant cuticles. 1. Ozone deposition and permeability. New Phytologist 112: 13–19. [Google Scholar]
- Kivimäenpää M, Sutinen S, Karlsson PE, Selldén G. 2003. Cell structural changes in the needles of Norway spruce exposed to long-term ozone and drought. Annals of Botany 92: 779–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JA, Studholme CJ. 1992. Responses of Sphagnum species to polluted environments. In: Bates JW, Farmer AM, eds. Bryophytes and lichens in a changing environment. Oxford: Oxford University Press, 314–332. [Google Scholar]
- Leipner J, Oxborough K, Baker NR. 2001. Primary sites of ozone-induced perturbations of photosynthesis in leaves: identification and characterization in Phaseolus vulgaris using high resolution chlorophyll fluorescence imaging. Journal of Experimental Botany 52: 1689–1696. [PubMed] [Google Scholar]
- Lindholm T. 1982. Growth dynamics and the effect of frost in Andromeda polifolia on a raised bog. Annales Botanici Fennici 19: 193–201. [Google Scholar]
- Long SP, Naidu SL. 2002. Effects of oxidants at the biochemical, cell and physiological levels, with particular reference to ozone. In: Bell JNB, Treshow M, eds. Air pollution and plant life. Chichester: John Wiley & Sons, 69–88. [Google Scholar]
- Miyake H, Matsumura H, Fujinuma Y, Totsuka T. 1989. Effects of low concentrations of ozone on the fine structure of radish leaves. New Phytologist 111: 187–195. [DOI] [PubMed] [Google Scholar]
- Murphy DJ. 2001. The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Progress in Lipid Research 40: 325–438. [DOI] [PubMed] [Google Scholar]
- Niemi R, Martikainen PJ, Silvola J, Holopainen T. 2002. Ozone effects on Sphagnum mosses, carbon dioxide exchange and methane emission in boreal peatland microcosms. The Science of the Total Environment 289: 1–12. [DOI] [PubMed] [Google Scholar]
- Niemi R, Martikainen PJ, Silvola J, Sonninen E, Wulff A, Holopainen T. 2002. Responses of two Sphagnum moss species and Eriophorum vaginatum to enhanced UV-B in a summer of low UV intensity. New Phytologist 156: 509–515. [DOI] [PubMed] [Google Scholar]
- Ojanperä K, Sutinen S, Pleijel H, Selldén G. 1992. Exposure of spring wheat, Triticum aestivum L. cv. Drabant, to different concentrations of ozone in open-top chambers: effects on the ultrastructure of flag leaf cells. New Phytologist 120: 39–48. [Google Scholar]
- Oksanen E, Sober J, Karnosky DF. 2001. Impacts of elevated CO2 and/or O3 on leaf ultrastructure of aspen (Populus tremuloides) and birch (Betula papyrifera) in the Aspen FACE experiment. Environmental Pollution 115: 437–446. [DOI] [PubMed] [Google Scholar]
- Pääkkönen E, Vahala J, Holopainen T, Karjalainen R, Kärenlampi L. 1996. Growth responses and related biochemical and ultrastructural changes of the photosynthetic apparatus in birch (Betula pendula) saplings exposed to low concentrations of ozone. Tree Physiology 16: 597–605. [DOI] [PubMed] [Google Scholar]
- Pirie W. 1983. Jonckheere tests for ordered alternatives. In: Kotz S, Johnson NL, eds. Encyclopedia of statistical sciences. New York: John Wiley & Sons, 315–318. [Google Scholar]
- Pleijel H, Danielsson H. 1997. Growth of 27 herbs and grasses in relation to ozone exposure and plant strategy. New Phytologist 135: 361–367. [Google Scholar]
- Potter L, Foot JP, Caporn SJM, Lee JA. 1996. The effects of long-term elevated ozone concentrations on the growth and photosynthesis of Sphagnum recurvum and Polytrichum commune New Phytologist 134: 649–656. [DOI] [PubMed] [Google Scholar]
- Potter L, Foot JP, Caporn SJM, Lee JA. 1996. Responses of four Sphagnum species to acute ozone fumigation. Journal of Bryology 19: 19–32. [Google Scholar]
- Power SA, Ashmore MR. 2002. Responses of fen and fen-meadow communities to ozone. New Phytologist 156: 399–408. [DOI] [PubMed] [Google Scholar]
- Reiling K, Davison AW. 1992. The response of native, herbaceous species to ozone: growth and fluorescence screening. New Phytologist 120: 29–37. [Google Scholar]
- Rinnan R, Impiö M, Silvola J, Holopainen T, Martikainen PJ. 2003. Carbon dioxide and methane fluxes in boreal peatland microcosms with different vegetation cover – effects of ozone or ultraviolet-B exposure. Oecologia 137: 475–483. [DOI] [PubMed] [Google Scholar]
- Scheiner SM. 2001. MANOVA: multiple response variables and multispecies interactions. In: Scheiner SM, Gurevitch J, eds. Design and analysis of ecological experiments. New York: Oxford University Press, 99–115. [Google Scholar]
- Steinmüller D, Tevini M. 1985. Composition and function of plastoglobuli. Planta 163: 201–207. [DOI] [PubMed] [Google Scholar]
- Sutinen S, Skärby L, Wallin G, Selldén G. 1990. Long-term exposure of Norway spruce, Picea abies (L.) Karst., to ozone in open-top chambers. II. Effects on the ultrastructure of needles. New Phytologist 115: 345–355. [DOI] [PubMed] [Google Scholar]
- Sutinen S, Wallin G, Karlsson PE, Skärby L, Selldén G. 1998. Cell ultrastructure of needles from saplings of Norway spruce, Picea abies (L) Karst., exposed to ozone and low phosphorus supply in open-top chambers. Chemosphere 36: 691–696. [Google Scholar]
- Toth R. 1982. An introduction to morphometric cytology and its applications to botanical research. American Journal of Botany 69: 1694–1706. [Google Scholar]
- Turunen J, Tomppo E, Tolonen K, Reinikainen A. 2002. Estimating carbon accumulation rates of undrained mires in Finland – application to boreal and subarctic regions. The Holocene 12: 69–80. [Google Scholar]
- van Breemen N. 1995. How Sphagnum bogs down other plants. Trends in Ecology & Evolution 10: 270–275. [DOI] [PubMed] [Google Scholar]
- VanderHeyden D, Skelly J, Innes J, Hug C, Zhang J, Landolt W, Bleuler P. 2001. Ozone exposure thresholds and foliar injury on forest plants in Switzerland. Environmental Pollution 111: 321–331. [DOI] [PubMed] [Google Scholar]
- Vollenweider P, Ottiger M, Günthardt-Goerg MS. 2003. Validation of leaf ozone symptoms in natural vegetation using microscopical methods. Environmental Pollution 124: 101–118. [DOI] [PubMed] [Google Scholar]