Abstract
• Background and Aims Hylocereus and Selenicereus are native to tropical and sub-tropical America. Based on its taxonomic status and crossability relations it was postulated that H. megalanthus (syn. S. megalanthus) is an allotetraploid (2n = 4x = 44) derived from natural hybridization between two closely related diploid taxa. The present work aimed at elucidating the genetic relationships between species of the two genera.
• Methods Crosses were performed and the putative hybrids were analysed by chromosome counts and morphological traits. The ploidy level of hybrids was confirmed by fluorescent in situ hybridization (FISH) of rDNA sites. Genomic in situ hybridization (GISH) was used in an attempt to identify the putative diploid genome donors of H. megalanthus and an artificial interploid hybrid.
• Key Results Reciprocal crosses among four diploid Hylocereus species (H. costaricensis, H. monacanthus (syn. H. polyrhizus), H. undatus and Hylocereus sp.) yielded viable diploid hybrids, with regular chromosome pairing. Reciprocal crosses between these Hylocereus spp. and H. megalanthus yielded viable triploid, pentaploid, hexaploid and aneuploid hybrids. Morphological and phenological traits confirm the hybrid origin. In situ detection of rDNA sites was in accord with the ploidy status of the species and hybrid studied. GISH results indicated that overall sequence composition of H. megalanthus is similar to that of H. ocamponis and S. grandiflorus. High sequence similarity was also found between the parental genomes of H. monacanthus and H. megalanthus in one triploid hybrid.
• Conclusions The ease of obtaining partially fertile F1 hybrids and the relative sequence similarity (in GISH study) suggest close genetic relationships among the taxa analysed.
Key words: Cactaceae, fluorescent in situ hybridization (FISH), genetic relationships, genomic in situ hybridization (GISH), Hylocereus, pitaya, polyploidy, Selenicereus, vine cacti
INTRODUCTION
The vine cacti of the genera Hylocereus (Berger) Br. and R. and Selenicereus (Berger) Br. and R. from tropical and sub-tropical America are a group of potential new fruit crops (Mizrahi and Nerd, 1999). Hylocereus species are characterized by elongated, normally three-angled stems, branches with aerial roots, and large flowers, mostly white (rarely red), with the ovary and tube bearing large foliaceous scales but not spines. The fruits are spineless but with several or many foliaceous scales. They are mostly large and edible (Fig. 1; see also Britton and Rose, 1963). Selenicereus species are distinguished by ribbed or angled stems, irregularly giving off aerial roots. The flowers are often large and the scales of the ovary and flower-tube are small, usually with long felt hairs and bristles in their axils. The large reddish fruits are covered with clusters of deciduous spines, bristles and hairs (Britton and Rose, 1963) (Fig. 1). According to Barthlott and Hunt (1993) the genus Hylocereus comprises 16 species, and the genus Selenicereus, 20 species. Recently, however, the taxonomy of this group was revised (Bauer, 2003). Here Bauer's nomenclature is used, giving synonyms (Barthlott and Hunt, 1993) in parentheses to allow comparisons to be made with published studies (Lichtenzveig et al., 2000; Tel-Zur et al., 2003, 2004).
Fig. 1.
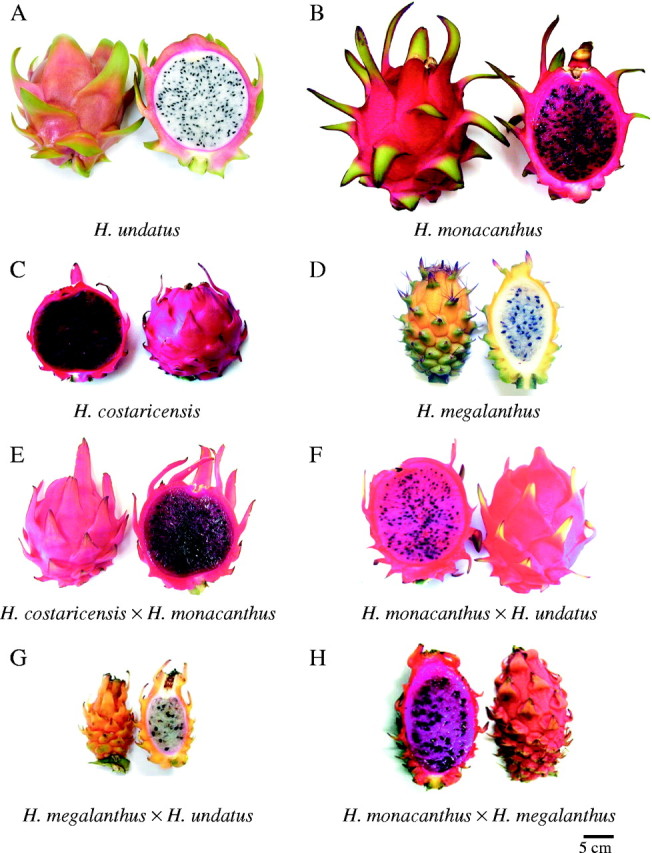
Fruits of three diploid Hylocereus spp., H. megalanthus, two homoploid and two interploid hybrids, all photographed at the same magnification.
Cytological observations show that H. cubensis (syn. H. triangularis), H. guatemalensis, H. monacanthus (including H. polyrhizus), H. ocamponis (syn. H. purpusii), H. triangularis (including H. cubensis), H. trigonus, H. undatus, S. grandiflorus, S. grandiflorus, subsp. hondurensis (syn. S. hondurensis), S. pteranthus and S. spinulosus are diploid, whereas H. megalanthus is tetraploid (Beard, 1937; Spencer, 1955; Lichtenzveig et al., 2000). Britton and Rose (1963) provided a detailed description of the morphology of H. megalanthus which has a triangular stem like that of Hylocereus, and spiny fruits like those of Selenicereus. Accordingly, they classified it into a separate genus named Mediocactus, thereby implying both an intermediate morphology and an intermediate taxonomic status (Britton and Rose, 1963). Bauer's placement of this species in Hylocereus reflects the close affinity between the tetraploid taxon and the diploid Hylocereus species (Bauer, 2003). The fact that a tetraploid taxon shares morphological features with two diploid taxa, which are also cross compatible (Lichtenzveig et al., 2000), might imply an allotetraploid origin. Moreover, recent molecular data support an allopolyploid origin of this species (Tel-Zur et al., 2004).
In this work an attempt was made to test the above hypothesis regarding the origin of H. megalanthus by trying to identify its possible diploid ancestors. Identification of the putative diploid genome donors of H. megalanthus and better understanding of the evolutionary relationships among the diploid and tetraploid taxa in vine cacti are of both basic and applied importance. In this framework, the genetic relationships among Hylocereus and Selenicereus species were investigated. Hybrid plants were analysed in terms of chromosome counts and morphological traits. The ploidy of H. megalanthus and of the hybrids was confirmed by fluorescent in situ hybridization (FISH). Genomic in situ hybridization (GISH) was used in an attempt to identify the possible ancestors of H. megalanthus and the chromosome complements of a triploid hybrid from the cross H. monacanthus × H. megalanthus. The limitations of GISH as a diagnostic tool in this group of plants are discussed.
MATERIALS AND METHODS
Plant material and growth conditions
The taxa used in this study (including their origins) and the hybrids are listed in Table 1. Living specimens of all species, clones and hybrids involved are kept at the cactus gene-bank at Ben-Gurion University, Beer-Sheva, Israel. In addition, and in light of the recent taxonomic revision of this group (Bauer, 2003), scaled photographs of all materials involved will be deposited on the Mizrahi web site (http://www.bgu.ac.il/life/Faculty/Mizrahi/index.html) to allow future researchers to inspect the identity of our plant materials independently.
Table 1.
Clone number, ploidy, fruit characteristics and phenological traits of the species and hybrids used in the study
| Fruit characteristics |
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant material |
Chromosome no. |
Weight (g) ± s.d. |
Length/width ratio ± s.d. |
Spines |
Max. scale length (cm) ± s.d. |
Peel colour |
Flesh colour |
Flowering time |
Ripening duration (d) |
|||||||||||
| Parental species | Clonea | |||||||||||||||||||
| H. undatus | 87-6011 | 22 | 568 ± 12 | 1·45 ± 0·03 | – | 5·1 ± 0·2 | Red | White | Aug–Oct | 29 ± 3 | ||||||||||
| (Harworth) Br. & R. | 88-0272 | 22 | 274 ± 4 | 2·08 ± 0·13 | – | 4·2 ± 0·1 | Red | Red | June + Oct | 29 ± 3 | ||||||||||
| 89-0263 | 22 | 177 ± 2 | 1·37 ± 0·04 | – | 3·8 ± 0·2 | Red | White | July–Sept | 29 ± 3 | |||||||||||
| 89-0243,* | 22 | 525 ± 7 | 1·38 ± 0·02 | – | 5·2 ± 0·3 | Red | White | July–Oct | 29 ± 3 | |||||||||||
| H. monacanthus Bauer | 89-0283,* | 22 | 536 ± 7 | 1·43 ± 0·05 | – | 6·5 ± 0·9 | Purple | Purple | June–Oct | 29 ± 3 | ||||||||||
| Hylocereus sp. | 10-4873 | 22 | 320 ± 5 | 1·33 ± 0·05 | – | 4·9 ± 0·1 | Red | Red | May–Oct | 29 ± 3 | ||||||||||
| H. costaricensis (Weber) Br. & R. | 89-0233 | 22 | 294 ± 4 | 0·93 ± 0·03 | – | 3·0 ± 0·4 | Red | Purple | June–Oct | 29 ± 3 | ||||||||||
| H. megalanthus | 88-0234,* | |||||||||||||||||||
| Bauer | 90-0015,* | 44 | 218 ± 1 | 1·62 ± 0·05 | + | 0·7 ± 0·2 | Yellow | White | Oct−Jan | 100−160 ± 15b | ||||||||||
| 90-0025,* | ||||||||||||||||||||
| 90-0035,* | ||||||||||||||||||||
| Homoploid (2n × 2n) crosses ♀ × ♂ | ||||||||||||||||||||
| H. monacanthus × (89-028) | H. undatus (87-601) | 22 | 542 ± 10 | 1·35 ± 0·03 | – | 5·0 ± 0·5 | Red–purple | Light purple | June–Oct | 29 ± 3 | ||||||||||
| Hylocereus sp. × (10-487) and the reciprocal | H. undatus (88-027) cross | 22 | 318 ± 5 | ND | – | ND | Red | Red | May–Oct | 29 ± 3 | ||||||||||
| H. undatus × (89-026) | Hylocereus sp. (10-487) | 22 | 304 ± 3 | 1·28 ± 0·02 | – | 5·0 ± 0·6 | Red | Light red | May–Oct | 29 ± 3 | ||||||||||
| Hylocereus sp. × (10-487) and the reciprocal cross | H. monacanthus (89-028) | 22 | 390 ± 2 | 1·3 ± 0·06 | – | 4·1 ± 0·5 | Red–purple | Light purple | May–Oct | 29 ± 3 | ||||||||||
| H. monacanthus × (89-028) and the reciprocal cross | H. costaricensis (89-023) | 22 | 326 ± 3 | 1·12 ± 0·03 | – | 3·3 ± 0·4 | Red–purple | Purple | June–Oct | 29 ± 3 | ||||||||||
| Interploid crosses ♀ × ♂ | ||||||||||||||||||||
| H. megalanthus × (88-023) | H. undatus (87-601) | 58, 64-67†, 66 (3 plants) | 55 ± 2 | 2·3 ± 0·09 | + | 0·7 ± 0·2 | Yellow | White | Sept–Oct | 60−90 ± 10b | ||||||||||
| H. megalanthus × (90-003) | H. undatus (89-024) | 51-59†, 51-56† (2 plants) | 80 ± 5 | 1·8 ± 0·10 | + | 1·4 ± 0·4 | Yellow, red inner surface | White | Sept–Oct | 60−90 ± 10b | ||||||||||
| H. megalanthus × (90-001) | H. monacanthus (89-028) | No hybrids were obtained | ||||||||||||||||||
| H. megalanthus × (90-003) | H. monacanthus (89-028) | 55 (1 plant) | 96 ± 7 | 1·7 ± 0·6 | + | 1·1 ± 0·1 | Purple | Light purple | Sept–Nov | 60−90 ± 10b | ||||||||||
| H. monacanthus × (89-028) | H. megalanthus (90-mix)c | 32, 34, 35 (1 plant each), 33 (4 plants) | 220 ± 2 | 1·6 ± 0·2 | + | 1·1 ± 0·1 | Purple | Light purple | Sept–Nov | 60−90 ± 10b | ||||||||||
| H. megalanthus × (88-023) | Hylocereus sp. (10-487) | No hybrids were obtained | ||||||||||||||||||
| Hylocereus sp. × (10-487) | H. megalanthus (88-023) | No hybrids were obtained | ||||||||||||||||||
ND, Not detemined.
Clone origin: 1Private garden, Israel; 2wild, Colombia; 3Huntington Botanical Gardens, USA; 4wild, Ecuador; 5commercial plantation, Colombia.
Ripening duration of H. megalanthus clones and their hybrids is influenced by low temperatures (see Results).
Pollination was performed with a mixture of pollen taken from the clones 90-001, 90-002 and 90-003.
Chromosome number was reported by Lichtenzveig et al. (2000).
Range of chromosomes counted.
The study was carried out in Beer-Sheva and at the Habesor Research Station (Israel, 31°N, 34°E) during 1997–2001. Parental plants, 6–9 years old, and hybrid plants, 3–7 years old, were grown in net houses under 80 % shade.
Artificial pollination was performed manually with a brush as the flowers opened. Each stigma was then covered with a paper cup to prevent self-pollination. However, this measure was not fully effective, and chromosome counting and characterization of fruit morphology were required to confirm the hybrid nature of the progeny.
Cytology
Chromosome number was determined in pollen mother cells (PMCs) excised from flower buds (5–6 cm long) as previously described by Lichtenzveig et al. (2000). In some polyploid hybrids, chromosome counting was difficult, and hence a range of numbers is given.
In situ DNA hybridization
Chromosome preparations were performed as previously described by Tel-Zur et al. (2003). The ploidy of the hybrids was confirmed by FISH analysis with the entire wheat rDNA (pTa71) repeat unit (Gerlach and Bedbrook, 1979). The putative ancestors of H. megalanthus and the parental accessions of one triploid hybrid were tested by GISH analysis.
FISH and GISH analyses were performed according to Reader et al. (1994). DNA was labelled with fluorescent nucleotides after Simpson et al. (1988). For FISH analysis, the entire wheat rDNA probe was isolated with a NucleoSpin Plasmid Kit (Macherey-Nagel, #740588·50) and labelled with fluorescein–deoxyuridine triphosphate (fluorescein-11-dUTP). For GISH analysis, total genomic DNA was extracted from fresh roots of the particular species under study with a PUROGENE DNA Isolation Kit (Gentra, D-5000) and labelled with rhodamine-11-dUTP. To increase the probe specificity, the hybridization mixture was allowed to pre-anneal for 20 min at 65 °C before it was mounted onto a slide. After hybridization, the slides were washed twice in 2× saline sodium citrate (SSC) at 65 °C for 15 min, twice in 0·1× SSC at 42 °C for 15 min, and once in 4× SSC at room temperature. Total genomic DNA or salmon sperm DNA (Lim et al., 2000; Taketa et al., 2000) was used as the unlabelled DNA block after autoclave fragmentation to a range of 100–300 bp (121 °C for 5 min).
The slides were examined under a Zeiss AxiosKop 2 fluorescence microscope, and photomicrographs were taken using Fujicolor (800 ASA) film with 4-s exposure. The images obtained were scanned and manipulated with an HP image Editor by changing the brightness and contrast uniformly across the image. At least 20 cells were examined in each genotype in each experiment.
RESULTS
Cytology
All the 20 homoploid hybrids among diploid Hylocereus spp. investigated were found to be diploid, like their parents (Table 1), showing normal meiosis with regular pairing (11 bivalents) in the PMCs analysed. Seed germination in the F2 was close to 90 %, which is another good indication of normal gametogenesis in the F1 hybrids. Chromosome counts of 32 putative hybrids originating from crosses between diploid Hylocereus spp. and tetraploid H. megalanthus confirmed the hybrid nature of 13 plants, among which triploid, pentaploid, hexaploid and 3x–6x aneuploid plants were found (Table 1). The remaining progeny were diploid or tetraploid, showing morphological similarity to, and the same ploidy as, the mother plant.
Morphological and phenological traits
Morphological traits of fruits of the parent species and the F1 hybrids from crosses among them, as well as phenological traits, are presented in Table 1. The wide variation in fruit form (Fig. 1), from round to elongated ellipse, was manifested in the variations in the ratio of the maximal length to the maximal width of the fruit. Comparison between the parents and their hybrids facilitated deduction of the dominance relations between the alternative alleles controlling some of these traits (Table 2). In interploid hybrids, fruit peel colour and flesh colour were the most indicative traits for the hybrid nature of the plants. The fruits of the hybrids were intermediate between the fruits of their parents with respect to these characteristics, indicating partial dominance or co-dominance. Co-dominance was also implied for fruit size and fruit form, which were more or less intermediate between those of the parents.
Table 2.
Dominance relations between different alleles controlling the morphological and phenological traits, as deduced from Table 1
| Trait |
Dominance relations |
Relevant cross |
|---|---|---|
| Fruit weight | Co-dominance | All homoploid crosses, H. monacanthus × H. megalanthus |
| Length : width fruit ratio | Co-dominance | All hybrids |
| Spines | Spines dominant over spineless | All interploid crosses |
| Max. scale length (cm) | Co-dominance | All hybrids |
| Peel colour | Red dominant over purple (or co-dominant) | H. monacanthus × H. undatus, Hylocereus sp. × H. monacanthus*, H. monacanthus × H. costaricensis* |
| Yellow dominant over red (or co-dominant) | H. megalanthus (88-023) × H. undatus*(87-601), H. megalanthus (90-003) × H. undatus (89-024) | |
| Purple dominant over yellow | H. megalanthus × H. monacanthus* | |
| Flesh colour | Purple dominant over white (or co-dominant) | H. monacanthus × H. undatus, H. megalanthus × H. monacanthus* |
| Red dominant over white (or co-dominant) | H. undatus × Hylocereus sp. | |
| Purple dominant over red (or co-dominant) | Hylocereus sp. × H. monacanthus* | |
| Flowering time | Prolonged flowering period (Hylocereus sp.) dominant over short flowering period | Hylocereus sp. × H. undatus*, H. undatus × Hylocereus sp., Hylocereus sp. × H. monacanthus* |
| Co-dominance | All interploid crosses | |
| Ripening duration (months) | Co-dominance | All interploid crosses |
And the reciprocal cross.
The species and hybrids were evaluated over a number of seasons. The results showed little variation within genotypes and significant differences between genotypes. The prolonged flowering season (from May to October) of diploid Hylocereus sp. (clone 10-487) was found to be dominant in all the crosses studied. Clones of H. megalanthus flowered from October to January. Fruit from flowers pollinated during the hot season (October) took 100 d to ripen (Table 1), whereas fruit from flowers pollinated during November–January ripened slowly (160 d).
In interploid hybrids (confirmed by chromosome count) fruit weight was rather an indicative trait for the hybrid nature of the plants. The triploids and 3×-aneuploid hybrids bore larger fruits than their tetraploid male parents. The fruit weight of the hybrids declined as the ploidy level increased. Spiny peel, which is characteristic of H. megalanthus, proved to be dominant in all its hybrids. Fruit form, flowering time and ripening duration displayed partial dominance or co-dominance.
The hybridization rate in the interploid crosses was calculated as the number of hybrid plants among the total progeny planted. The values given in Table 3 are only indicative of the success of the crosses and should not be considered as definitive, since selfing, and probably also apomixis, took place in some of the cases. In this respect, it is worth mentioning that H. megalanthus and Hylocereus sp. are self compatible, whereas in the other species selfing occurs occasionally, especially at the beginning and the end of the flowering season (J. Mouyal, pers. comm.). As judged by morphological traits, the rate varied markedly (Table 3), being 92 % for H. monacanthus × H. megalanthus vs. 5 % for the reciprocal cross and 14 % for H. megalanthus × H. undatus vs. zero for the reciprocal cross. In the cross H. megalanthus × Hylocereus sp. the two reciprocal combinations failed totally.
Table 3.
Percentage of confirmed hybrids in interploid crosses as judged from morphological traits
| Cross combination ♀ × ♂ |
No. of progeny planted |
Percentage of confirmed hybrids |
|---|---|---|
| H. megalanthus × H. undatus | 77 | 14 |
| H. undatus × H. megalanthus | 13 | 0 |
| H. megalanthus × H. monacanthus | 84 | 5 |
| H. monacanthus × H. megalanthus | 48 | 92 |
| H. megalanthus × Hylocereus sp. | 8 | 0 |
| Hylocereus sp. × H. megalanthus | 43 | 0 |
In situ DNA hybridization
The haploid Hylocereus complement has one detectable rDNA site, as indicated by FISH analysis with the wheat rDNA probe. The number of rDNA sites of H. megalanthus was in accordance with its ploidy, with one strongly labelled site and one minor labelled site at each pole of the anaphase I spindle of the PMCs studied (Fig. 2). The diploid (cloneS-89), the triploid (clone 12–31) and the pentaploid (clone S-41) hybrids tested had the expected number of two, three and five rDNA hybridization sites, respectively (Fig. 3).
Fig. 2.
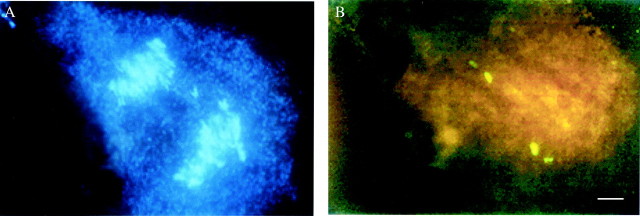
FISH analysis on a PMC at anaphase I of the tetraploid H. megalanthus. (A) Chromosomes stained with DAPI (4′,6-diamidino-2-phenylindole). (B) Following rDNA hybridization two hybridization sites of the pTa71 probe can be seen at each pole of the spindle. Bar = 10 μm.
Fig. 3.
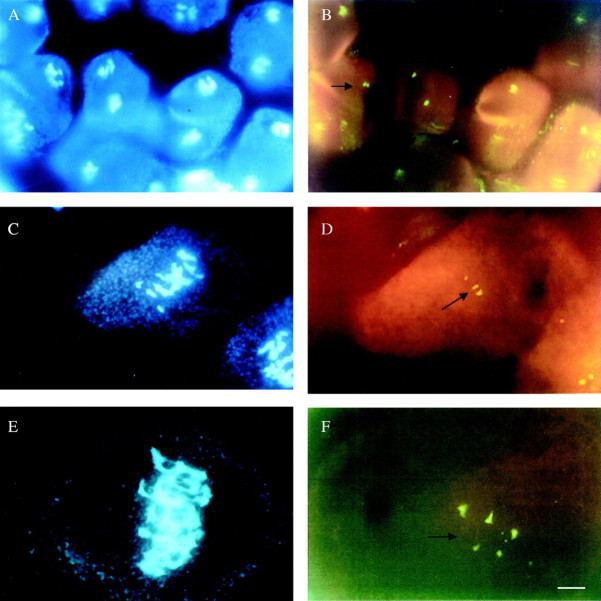
FISH analysis on PMCs of homoploid and interploid hybrids. (A) DAPI-stained chromosomes of a PMC at metaphase II of the diploid S-89 from the cross H. undatus × Hylocereus sp. (B) Following hybridization with pTa71 probe, two hybridization sites (arrow) can be seen. (C) DAPI-stained chromosomes of a PMC at metaphase I of the triploid 12–31 from the cross H. monacanthus × H. megalanthus. (D) Following hybridization with pTa71 probe, three hybridization sites (arrow) can be seen. (E) DAPI-stained chromosomes of a PMC at metaphase I of the pentaploid S-41 from the cross H. megalanthus × H. monacanthus. (F) Following hybridization with pTa71 probe, five hybridization sites (arrow) can be seen. Bar = 10 μm.
In an attempt to determine the genetic relationship between the diploid species (in our collection) and the tetraploid H. megalanthus, GISH analysis was carried out on chromosome preparations of this species. Labelled total DNA extracted from several species was used as tester DNA in different DNA tester : block ratios (Table 4). Hybridization experiments with tester DNA of either H. ocamponis or H. undatus and without block DNA resulted in uniform nonspecific strong red fluorescent signals on all H. megalanthus chromosomes. The signals decreased uniformly on all chromosomes with the decrease of the ratio of tester DNA : block DNA. Strong uniform signals on all H. megalanthus chromosomes were also found when total genomic DNA from H. ocamponis or S. grandiflorus was used as tester DNA, which did not decline with the decrease of tester DNA : block DNA ratio, even when salmon sperm DNA was used as the block DNA at a ratio of 1 : 200 (Table 4).
Table 4.
Fluorescence signal intensity*on meiotic chromosomes of the tetraploid H. megalanthus, with different ratios of tester DNA (labelled DNA from Hylocereus and Selenicereus species) to block DNA, unlabelled DNA from H. monacanthus or salmon sperm DNA in GISH analysis
| Tester : block ratio |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
|
H. monacanthus |
Salmon sperm DNA |
||||||||
| Source of tester DNA |
No block DNA |
1 : 5 |
1 : 10 |
1 : 10 |
1 : 100 |
1 : 200 |
|||
| H. ocamponis (Salm-Dyck) Br. & R. (94-031)1 | +++ | ND | ND | +++ | +++ | +++ | |||
| H. monacanthus (89-028) | ND | ND | ND | +++ | + | ND | |||
| H. ocamponis Bauer (89-025)2 | +++ | ND | ND | 0 | 0 | ND | |||
| H. undatus (87-601) | +++ | +++ | ++ | ++ | + | ND | |||
| S. grandiflorus subsp. grandiflorus Bauer (98-321)3 | ND | ND | ND | 0 | ND | ND | |||
| S. grandiflorus (L) Br. & R. (94-032)1 | +++ | ND | ND | ND | +++ | +++ | |||
ND, Not determined.
+, ++, +++, Arbitrary grades; 0, no signal detected.
Botanical Garden, Palermo, Italy.
Huntington Botanical Gardens, USA.
Rainbow Gardens Nursery, Vista, CA, USA.
The possibility of identifying the donor of a chromosome complement by GISH analysis in an artificial interploid hybrid was tested in the hybrid H. monacanthus × H. megalanthus, clone 12–28 (2n = 3× + 1 = 34). Here too, as in GISH analysis for the natural polyploid, H. megalanthus, no specificity was obtained when labelled total genomic DNA from either parent was used as tester DNA with unlabelled total genomic DNA from the other parent or when salmon sperm was used as block DNA (Table 5). At a low tester : block ratio, strong uniform fluorescent signals without any specificity were observed on all chromosomes of the hybrid plant. Furthermore, with an excess of block DNA (1 : 200 probe : block ratio) no fluorescent signal was obtained at all.
Table 5.
Fluorescence signal intensity*on meiotic chromosome of the triploid hybrid (clone 12-28) from the H. monacanthus and H. megalanthus cross, with different ratios of tester DNA (labelled DNA probe) to block DNA (unlabelled DNA) in GISH analysis
| Tester : block ratio |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source of tester DNA |
Source of block DNA |
1 : 2 |
1 : 10 |
1 : 16 |
1 : 50 |
1 : 100 |
1 : 200 |
|||||
| H. monacanthus | H. megalanthus | +++ | +++ | ND | + | ND | ND | |||||
| H. megalanthus | H. monacanthus | ND | ND | + | ND | ND | ND | |||||
| H. monacanthus | Salmon sperm DNA | ND | +++ | ND | ++ | + | 0 | |||||
| H. megalanthus | Salmon sperm DNA | ND | +++ | ND | ND | + | ND | |||||
ND, Not determined.
+, ++, +++, Arbitrary grades; 0, no signal detected.
DISCUSSION
The crossability among different taxa may provide valuable information about their genetic relationships. The Hylocereus spp. studied in this work cross readily. The regular meiotic chromosomal pairing in all Hylocereus hybrids studied also suggests that the species involved in the crosses differ little in their chromosomal linear order, thus indicating close relationships among them. Interploid crosses among diploid Hylocereus spp. and H. megalanthus were also obtained in various rates, depending on the particular diploid species of Hylocereus used (Table 3).
Chromosome numbers and fruit morphology indicated that no hybrids were obtained from crosses between the female H. undatus and the male H. megalanthus or from reciprocal crosses between Hylocereus sp. and H. megalanthus. The most successful cross was H. monacanthus × H. megalanthus, suggesting that H. megalanthus is closer to H. monacanthus than to H. undatus or Hylocereus sp.
Crosses between tetraploid and diploid parents are expected to produce triploid progeny. However, in this work interploid crosses also yielded pentaploids, hexaploids and aneuploids in addition to triploids. The origin of the pentaploids was attributed to unreduced female gametes from the tetraploid H. megalanthus and normal haploid male gametes from the diploid H. monacanthus, whereas the origin of the hexaploids was attributed to post-zygotic chromosome doubling (Tel-Zur et al., 2003).
Chromosome counting in polyploids can be difficult and time consuming. In such cases, FISH analysis with rDNA fluorescent probes may provide a reasonable estimate of the ploidy of the plants tested (Weiss and Maluszynska, 2001). In this work, the number of labelled rDNA sites found on the chromosomes was in line with the ploidy determined by chromosome counts in the hybrids. The two pairs of rDNA hybridization sites in H. megalanthus are in accordance with the tetraploid status of this taxon (Lichtenzveig et al., 2000). The differential intensity of the two pairs of rDNA loci is consistent with ‘differential amphyplasty’ following interspecific hybridization (Navashin, 1934; Pikaard, 1999). This observation and molecular data (Tel-Zur et al., 2004), in turn, strongly supports our hypothesis regarding the allotetraploid origin of H. megalanthus.
GISH analysis, which uses total genomic DNA as a probe, is a valuable method for testing the homology of genomes of plants and may help in the identification of the origin of whole parental genomes (Schwarzacher et al., 1989). However, even with the strict precautions of post-hybridization, high stringency washing and a low ratio of tester DNA : block DNA, as suggested by Schwarzacher and Heslop-Harrison (2000), no differential genome staining was observed in H. megalanthus. Therefore, at this stage no inference can be made regarding the diploid genome donors of H. megalanthus. The GISH results indicated that the overall molecular genome composition of tetraploid H. megalanthus is similar to that of diploid H. ocamponis and S. grandiflorus. Consequently, the morphological differences (and hence the taxonomic distinction) between these species may be a result of a small number of morphological genes and thus need not necessarily represent a large genetic distance. GISH experiments provided evidence for high sequence homology between the parental genomes, H. monacanthus and H. megalanthus, in the artificial triploid hybrid (clone 12–28), providing supporting evidence for the close genetic relationship between the tetraploid taxon and the diploid members of Hylocereus.
The information derived from the phenotype of F1 plants regarding the dominance relations between the alleles controlling the different morphological and phenological traits may be useful in future breeding programmes. Several traits, such as fruit size and prolonged flowering season, have important agricultural and economic value. It is considered that the inferior fruit size of plants with a high ploidy is the consequence of irregular chromosomal behaviour at meiosis, as previously suggested for H. megalanthus (Lichtenzveig et al., 2000), rather than the result of polyploidy per se or of the genetic distance between the parental taxa. Irregular chromosome pairing would have detrimental consequences in annual natural (or artificial) allo- or autopolyploids, because reduced gamete fertility is likely to reduce (or even eliminate) seed set. However, in the presence of genetic systems with the capacity for pairing regulation, like the wheat Ph system (Riley, 1960), fertility could be restored in such an individual (e.g. Milo et al., 1986, 1988). Due to the perennial habit of vine cacti, there is no strong selection pressure favouring regular meiotic pairing because clones with irregular meiosis are not lost after a single flowering season and can reproduce vegetatively.
The prolonged flowering season characteristic of Hylocereus sp. (clone 10–487) has important economic implications and could be exploited in crosses. It would also be possible to produce new cultivars with different peel–flesh colour combinations. At present, no conclusive information is available regarding the mode of inheritance of fruit taste, one of the most important horticultural traits. However, in this respect the triploid H. monacanthus × H. megalanthus hybrids, the fruits of which appear to combine the taste quality of H. megalanthus and the attractive appearance of H. monacanthus are especially valuable. The marketability of the triploid fruits is further enhanced by their acceptable size (200–300 g). Hence, these hybrids are the most promising candidates for cultivation.
Novel germplasm can be utilized as new crops to diversify our dietary basis and provide niche products for food, industrial and horticulture uses (Heslop-Harrison, 2002). However, the agronomic challenges require that the understanding of reproductive biology and taxonomy be enhanced. To this end, classical and molecular breeding tools need to be developed. For instance, relying on fruit morphology to confirm the hybrid nature of cross progeny is not economically feasible due to the relatively long juvenile stage of these species. Molecular markers can provide an adequate answer to this problem (Tel-Zur et al., 2004), and are much needed with perennial crops like vine cacti and fruit trees.
Acknowledgments
We thank the reviewers Dr J. M. Leggett and Dr N. P. Taylor for their valuable comments, Ms Hadassa van Oss (The Hebrew University of Jerusalem) and Mr Joseph Mouyal (Ben-Gurion University of the Negev) for technical assistance, and Ms Dorot Imber for editing the manuscript. This study was supported in part by the UCLA-BGU Program of Academic Cooperation.
LITERATURE CITED
- Barthlott W, Hunt DR. 1993. Cactaceae. In: Kubitzki K, ed. The families and genera of vascular plants, Vol. II. New York: Springer-Verlag, 161–197. [Google Scholar]
- Bauer R. 2003. A synopsis of the tribe Hylocereeae F. Buxb. Cactaceae Systematics Initiatives 17: 3–63. [Google Scholar]
- Beard CE. 1937. Some chromosome complements in the Cactaceae and a study of meiosis in Echinocereus papillosus. Botanical Gazette 99: 1–21. [Google Scholar]
- Britton NL, Rose JN. 1963. Hylocereanae. The Cactaceae, Vol. II. New York: Dover, 183–212. [Google Scholar]
- Gerlach WL, Bedbrook JR. 1979. Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Research 7: 1869–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heslop-Harrison JS. 2002. Exploiting novel germplasm. Australian Journal of Agricultural Research 53: 873–879. [Google Scholar]
- Lichtenzveig J, Abbo S, Nerd A, Tel-Zur N, Mizrahi Y. 2000. Cytology and mating systems in the climbing cacti Hylocereus and Selenicereus American Journal of Botany 87: 1058–1065. [PubMed] [Google Scholar]
- Lim KB, Chumg JD, Van Kronenburg BCE, Ramanna MS, De Jong JH, Van Tuyl JM. 2000. Introgression of Lilium rubellum Baker chromosome into L. longiflorum Thunb.: a genome painting study of the F1 hybrid, BC1 and BC2 progenies. Chromosome Research 8: 119–125. [DOI] [PubMed] [Google Scholar]
- Milo J, Levy A, Ladizinsky G, Palevitch D. 1986. Phylogenetic studies in Papaver section Oxytona: cytogenetics of the species and interspecific hybrids. Theoretical and Applied Genetics 72: 524–529. [DOI] [PubMed] [Google Scholar]
- Milo J, Levy A, Ladizinsky G, Palevitch D. 1988. Phylogenetic and genetic studies in Papaver section Oxytona: cytogenetics, isozyme analysis and chloroplast DNA variation. Theoretical and Applied Genetics 75: 795–802. [DOI] [PubMed] [Google Scholar]
- Mizrahi Y, Nerd A. 1999. Climbing and columnar cacti. New arid land fruit crops. In: Janick J, ed. Perspective in new crops and new uses. Alexandria, USA: ASHS Press, 358–366. [Google Scholar]
- Navashin M. 1934. Chromosomal alterations caused by hybridization and their bearing upon certain general genetic problems. Cytologia 5: 169–203. [Google Scholar]
- Pikaard CS. 1999. Nucleolar dominance and silencing of transcription. Trends in Plant Science 4: 478–483. [DOI] [PubMed] [Google Scholar]
- Reader S, Abbo S, Purdie KA, King IP, Miller TE. 1994. Direct labeling of plant chromosomes by rapid in situ hybridization. Trends in Genetics 10: 265–266. [DOI] [PubMed] [Google Scholar]
- Riley R. 1960. The diploidization of polyploidy wheat. Heredity 15: 407–429. [Google Scholar]
- Schwarzacher T, Heslop-Harrison JS. 2000.Practical in situ hybridization. Oxford: Bio Scientific Publishers. [Google Scholar]
- Schwarzacher T, Leitch AR, Bennett MD, Heslop-Harrison JS. 1989.In situ localization of parental genomes in a wide hybrid. Annals of Botany 64: 315–324. [Google Scholar]
- Simpson PR, Newman MA, Davis DR. 1988. Detection of legumin gene DNA sequences in pea by in situ hybridization. Chromosoma 96: 454–458. [Google Scholar]
- Spencer JL. 1955. A cytological study of the Cactaceae of Puerto Rico. Botanical Gazette 117: 33–37. [Google Scholar]
- Taketa, S, Ando H, Takeda K, Harrison GE, Heslop-Harrison JS. 2000. The distribution, organization and evolution of two abundant and widespread repetitive DNA sequences in the genus Hordeum Theoretical and Applied Genetics 100: 169–176. [Google Scholar]
- Tel-Zur N, Abbo S, Bar-Zvi D, Mizrahi Y. 2003. Chromosome doubling in vine cacti hybrids. Journal of Heredity 94: 329–333. [DOI] [PubMed] [Google Scholar]
- Tel-Zur N, Abbo S, Bar-Zvi D, Mizrahi Y. 2004. Clone identification and genetic relationship among vine cacti from the genera Hylocereus and Selenicereus based on RAPD analysis. Scientia Horticulturae 100: 279–289. [Google Scholar]
- Weiss H, Maluszynska J. 2001. Molecular cytogenetic analysis of polyploidization in the anther tapetum of diploid and autotetraploid Arabidopsis thaliana plants. Annals of Botany 87: 729–735. [Google Scholar]