Characterization of FcμR deficiency in lupus-prone mice
Keywords: auto-antibody, FcμR, IgM, marginal zone B cells, Mott cells
Abstract
The IgM-Fc receptor (FcμR) is involved in IgM homeostasis as evidenced by increased pre-immune serum IgM and natural auto-antibodies of both IgM and IgG isotypes in Fcmr-deficient C57BL/6 (B6) mice. To determine the impact of Fcmr-ablation on autoimmunity, we introduced the Fcmr null mutation onto the Fas-deficient autoimmune-prone B6.MRL Fas lpr/lpr mouse background (B6/lpr). Both IgM and IgG auto-antibodies against dsDNA or chromatin appeared earlier in FcμR(−) B6/lpr than FcμR(+) B6/lpr mice, but this difference became less pronounced with age. Splenic B2 cells, which were 2-fold elevated in FcμR(+) B6/lpr mice, were reduced to normal B6 levels in FcμR(−) B6/lpr mice, whereas splenic B1 cells were comparable in both groups of B6/lpr mice. By contrast, marginal zone (MZ) B cells were markedly reduced in FcμR(−) B6/lpr mice compared with either FcμR(+) B6/lpr or wild type (WT) B6 mice. This reduction appeared to result from rapid differentiation of MZ B cells into plasma cells in the absence of FcμR, as IgM antibody to a Smith (Sm) antigen, to which MZ B cells are known to preferentially respond, was greatly increased in both groups (B6/lpr and B6) of FcμR(−) mice compared with FcμR(+) B6/lpr or B6 mice. Mott cells, aberrant plasma cells with intra-cytoplasmic inclusions, were also increased in the absence of FcμR. Despite these abnormalities, the severity of renal pathology and function and survival were all indistinguishable between FcμR(−) and FcμR(+) B6/lpr mice. Collectively, these findings suggest that FcμR plays important roles in the regulation of auto-antibody production, Mott cell formation and the differentiation of MZ B cells into plasma cells in B6.MRL Fas lpr/lpr mice.
Introduction
Antibody has dual binding activities, to antigen via its amino terminal variable regions and to effector molecules such as Fc receptors (FcRs) via its carboxy terminal constant regions. Among the five different antibody isotypes, IgM appears first during phylogeny, ontogeny and immune responses and is the first line of defense against pathogens (1). IgM exists as a membrane-bound, monomeric form on the surface of B cells and as a secreted, mostly pentameric form with a mushroom-like shape (2). Both pre-immune ‘natural’ and antigen-induced “immune” IgM antibodies are important for protection against infection and autoimmunity by recognizing pathogens as well as self-antigens associated with host cell corpses (1). FcRs for switched immunoglobulin classes (i.e. FcγRs, FcεRs and FcαR) have been extensively characterized at both protein and genetic levels (3). Although there was biochemical evidence of an FcR for IgM (FcμR) for decades (4–6), the gene encoding this receptor (FCMR) defied identification until its recent functional cloning by our group (7). FcμR is a transmembrane sialoglycoprotein of ~60 kD with a single immunoglobulin-like domain that confers exclusive binding specificity for the Fc region of IgM. FcμR binds IgM pentamers with a strikingly high avidity of ~10nM (7). Upon IgM binding, FcμR is rapidly internalized into the lysosomal compartment (8). Unlike other FcRs, FcμR is expressed by lymphocytes: B, T and NK cells in humans (7, 9) and B cells only in mice (10–12), though there are conflicting data concerning the faint expression of FcμR by mouse granulocytes and macrophages (13–15). FCMR was originally designated Toso or Fas apoptotic inhibitory molecule 3 (FAIM3) (16). However, the original apoptotic assay leading this designation was performed with an agonistic anti-Fas mAb with an IgM isotype (16). The results from subsequent analyses by us and others clearly demonstrated that the Toso/FAIM3 designation is incorrect and that this gene instead encodes an authentic IgM Fc-binding receptor (7–9, 17). FCMR is a single copy gene located on chromosome 1q32.2, adjacent to two other IgM-binding receptor genes: polymeric Ig receptor (PIGR) and FcR for IgA and IgM (FCAMR) (7).
Fcmr-deficient [knockout (KO)] mice have been independently generated by three laboratories (K. H. Lee, Leibniz Center for Medicine and Biosciences, Borstel, Germany; H. Ohno, RIKEN, Yokohama, Japan; and T. W. Mak, Ontario Cancer Institute, Toronto, Canada) and have recently been characterized by five different groups, and there are clear differences in the reported phenotypes (11–13, 18, 19). Nevertheless, the abnormal phenotypes commonly observed in Fcmr KO mice are (i) alterations in B-cell subpopulations, (ii) dysregulation of humoral immune responses, (iii) impairment of B-cell proliferation upon ligation of BCR in vitro and (iv) predisposition to auto-antibody production (11, 12, 19). Notably, many abnormalities in FcμR KO mice mirror those observed in μs exon-targeted mice (μs−/−), which are able to express surface IgM and other immunoglobulin isotypes on B cells and to secrete all other classes of immunoglobulin except for IgM. Together, these observations emphasize the critical role in normal B cell functions both for secreted IgM and for its interaction with FcμR (1). Interestingly, pre-immune serum IgM and IgG3 are significantly elevated in Fcmr KO mice (11, 12). By contrast, serum IgM levels are unaffected in naive mice with null mutations of two other IgM-binding receptors, the pIgR on mucosal epithelial cells and the Fcα/μR on follicular dendritic cells (FDCs) (20, 21). Thus, FcμR appears to be the sole receptor in this family that is involved in IgM homeostasis. Fcmr KO mice also develop high levels of natural auto-antibodies of both IgM and IgG isotypes at 13–18 weeks of age (11, 12).
Autoreactive B cells play a critical role in the pathogenesis of systemic lupus erythematosus (SLE), which is characterized by circulating auto-antibodies and deposition of the resulting immune complexes in various tissues, particularly the kidneys, leading to glomerulonephritis. The importance of FcRs, especially the inhibitory FcγRIIb, in influencing the development of autoimmunity is suggested in mouse model systems and also seems to be the case for humans, as shown by analyses of large cohorts of autoimmune patients (22). For example, memory B cells in SLE patients fail to up-regulate cell surface FcγRIIb, and this is correlated with a reduced threshold for B-cell activation (23, 24). MRL/MpJlpr/lpr mice spontaneously develop an autoimmune disorder resembling human SLE and the molecular defect underlying this phenotype is a mutation in the Fas gene, which encodes a cell surface receptor of the TNF receptor superfamily that is important in apoptosis of lymphocytes (25, 26). We hypothesized that the introduction of the Fcmr null mutation onto the autoimmune-prone Fas lpr/lpr background would affect the autoimmune process depending on the balance of protective IgM versus pathologic IgG auto-antibodies. Our results indicate that Fcmr deficiency affects the kinetics and magnitude of auto-antibody production, but has no obvious impact on the B6.MRL Fas lpr/lpr-lupus-like nephritis.
Methods
Mice
The generation and characterization of Fcmr −/− (Fcmr KO) mice on a C57BL/6 (B6) background has been described previously (11). B6.MRL Fas lpr/lpr (B6/lpr) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). Fcmr KO mice were crossed with B6/lpr mice and the resultant F1 offspring were then intercrossed to generate F2 offspring. F2 siblings with appropriate genotype (i.e. Fcmr +/− /Fas lpr/lpr or Fcmr −/− /Fas +/lpr) were then mated to generate Fcmr −/− /Fas lpr/lpr and Fcmr +/+ /Fas lpr/lpr mice, hereafter designated, respectively, as FcμR(−) and FcμR(+) B6/lpr mice. The Fcmr and Fas genotypes were determined by genomic PCR of tail DNA using a diagnostic set of primers: (i) 5′-ctgtagggctgaggctgggctggtgacagg-3′ (forward), 5′-cgatggctaatatggcaatagtatgggatg-3′ (reverse) and 5′-cttctctcccatagtgtgggccatggtggc-3′ (reverse) corresponding to the 5′-flanking and 3′-flanking Fcmr exons 2 and 5, respectively (11), and (ii) 5′-gtaaataattgtgcttcgtcag-3′ (forward), 5′-caaatctaggcattaacagtg-3′ (reverse) and 5′-tagaaaggtgcacgggtgtg-3′ (reverse) corresponding to the 5′ and 3′ Fas intron 2 and the inserted early transposable element (Fas lpr), respectively, according to the vendor’s protocol. FcμR(−) and FcμR(+) B6/lpr mice were maintained along with wild type (WT) control mice in filter-topped isolator cages at our animal facility and only female mice were used in the present studies. Genomic PCR analysis with microsatellite markers of chromosome 1 [D1Mit419 (120.7Mb from the centromere) and D1Mit268 (157.4Mb)] was performed to determine the genotype of the Fcmr (130.9Mb), major lupus susceptibility locus (168.4–177.7Mb) and Fcgr2b (171.0Mb). All studies involving animals were conducted with approval of the University of Alabama at Birmingham institutional animal care and use committee.
RT–PCR
To confirm the lack of full-length FcμR transcripts in FcμR(−) B6/lpr mice, RT–PCR was performed as previously described (11). In brief, total RNA isolated from spleen was converted to first-strand cDNA before PCR amplification using a set of primers corresponding to the translation start and termination sites of the FcμR cDNA (forward, 5′-cctgtggagctcacagtctcag-3′ and reverse, 5′-cccagtgtagaacattgaagatg-3′). Amplification of the activation isoform of the paired immunoglobulin-like receptors (PIR-A) with a set of primers (forward, 5′-cctgtggagctcacagtctcag-3′ and reverse, 5′-cccagtgtagaacattgaagatg-3′) was performed as a control.
ELISA
Blood was withdrawn via the submandibular vein of naive animals at 10, 20 and 50 weeks of age. Sera were collected and stored at −80°C until assays were performed. Serum titers of IgM and IgG auto-antibodies against dsDNA, chromatin or Smith (Sm)/ribonuclear protein (RNP) antigen (ImmunoVision, Springdale, AR, USA) as well as serum concentrations of total IgM, IgG and IgA were assessed by ELISA as previously described (11).
Flow cytometry
Spleen, cervical lymph nodes (LNs) and femurs were removed from 20-week-old naive mice; spleen and LNs were weighed and a piece of known weight was excised and single cell suspensions were prepared from the piece of the excised tissues as previously described (11). The total cell numbers were assessed using the following formula: total cell numbers = [(cell number in the cell suspension)/(weight of the piece of organ for cell preparation)] × (weight of the entire organ). For flow cytometric analysis, cells were first incubated with a mixture of anti-FcγRII/III (Ab93) (27) and anti-FcγRIV (9E9) (28) or aggregated human IgG for blocking all FcγRs, and then with a combination of the following fluorochrome-labeled mAbs: FITC-labeled, anti-CD3 (145-2C11) and anti-CD19 (1D3) mAbs, PE/Cy7-labeled, anti-CD23 (B3B4) and anti-CD11b (M1/70) mAbs, allophycocyanin (APC)-labeled, anti-CD4 (RM4-5) and anti-CD5 (53–7.3) mAbs purchased from eBioscience (San Diego, CA, USA); PE-anti-CD8 mAb (53–6.7), PerCP-anti-B220 mAb (RA3-6B2), APC-labeled, anti-CD138 (281–2) and anti-CD21/CD35 (7G6) mAbs from BD Biosciences (San Jose, CA, USA), PE-labeled, anti-CD49d/integrin α4 (9C10), anti-CD29/integrin β1 (HMβ1-1), anti-CD11a/integrin αL (2D7) and anti-CD18/integrin β2 (M18/2) mAbs from BioLegend (San Diego, CA, USA). In some experiments, cells were incubated with biotinylated, anti-FcµR (MM3) or isotype-matched control mAb, washed and the bound biotinylated mAbs were detected by addition of PE-streptavidin (Southern Biotechnology Associates, Birmingham, AL, USA) as described previously (11). Stained cells with the light characteristics of lymphocytes and macrophages were analyzed using an Accuri C6 flow cytometer (BD Biosciences). The flow cytometric data were analyzed with FlowJo software (TreeStar, Ashland, OR, USA).
Histopathological and immunohistological analyses
A portion of each organ (kidneys, liver, spleen, LNs and lungs) removed at 20 and 50 weeks of age was fixed in 10% neutral-buffered formalin, dehydrated, treated with xylene, embedded in paraffin, sectioned and stained with H&E or periodic acid-Schiff (PAS) for histopathological analysis. Another portion of kidney and spleen tissues was embedded in optimum cutting temperature compound, snap-frozen in dry ice-acetone, cut in 4–6 μm thickness with a cryostat, fixed in acetone and rehydrated in PBS. Sections were incubated with appropriate concentrations of fluorochrome-labeled antibodies specific for mouse IgM (TI-2020; Vector Laboratories, Burlingame, CA, USA), IgG (A-11001; Life Technologies Co., Carlsbad, CA, USA), IgA or C3 (sc-3695 or 11H9; Santa Cruz Biotechnology). The immunostained tissue sections were examined by Olympus FV 1000 confocal fluorescence microscopy or Leica/Leitz DMRB microscope. Images of 10 randomly selected glomeruli from each mouse were acquired with the same detector sensitivity and the deposition of immunoglobulins and C3 in the selected areas was quantitatively assessed as the fluorescence intensity by using the KS400 image analysis system (version 4.0, Carl Zeiss Vision) as previously described (29). Two to three different investigators performed histopathological and immunohistological analyses of these tissue sections in a blind fashion.
Blood urea nitrogen assay
Blood urea nitrogen (BUN) was measured in duplicate using a urea assay kit (Synchron LX Systems, Beckman Coulter Reference 442750) according to the manufacturer’s recommendation except for the following modifications: 190 μl of reagent A, 10 μl of reagent B and 2 μl of serum or standard. The reaction was monitored at 340nm after 20–30min of incubation.
Statistical analysis
All data were analyzed with the Mann–Whitney test using GraphPad Prism 5 (version 5.04, La Jolla, CA, USA), and a P value of <0.05 was defined as statistically significant. Differences in group survival were analyzed using Mantel–Cox Log-rank P test.
Results
A rapid increase in serum auto-antibody titers in FcμR(−) B6/lpr mice
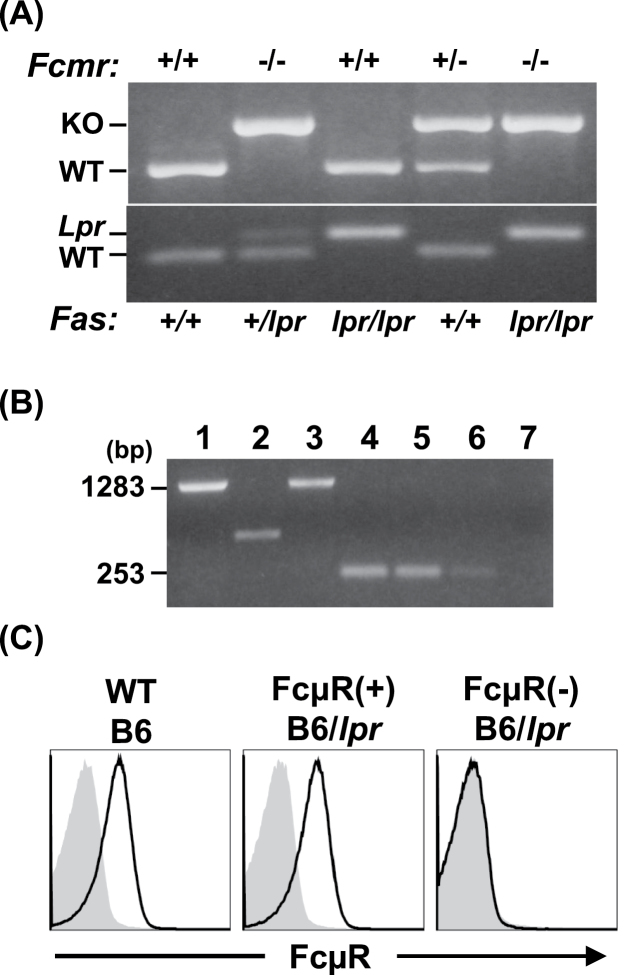
It has recently been shown from studies of Fcmr KO mice by us and others that FcμR plays an important regulatory role in IgM homeostasis, as evidenced by elevation of serum IgM levels and natural auto-antibody titers of both IgM and IgG isotypes in naive mutant animals (11, 12, 19). To determine whether the FcμR deficiency affects autoimmune processes, FcμR(−) or FcμR(+) B6/lpr mice were generated. The ablation of Fcmr in FcμR(−) B6/lpr mice was confirmed by genomic PCR and the absence of full-length FcμR transcripts and proteins (Fig. 1). Genomic PCR analysis of FcμR(−) B6/lpr mice with microsatellite DNA markers revealed that D1Mit419 (120.7Mb), which is close to the Fcmr (130.9Mb), was of 129 origin, while D1Mit268 (157.4Mb) and the Fcgr2b (171.0Mb) were of B6 origin, indicating the B6 origin of the major lupus susceptibility locus, Sle16 identified in distal chromosome 1 of the 129 strain (30, 31).
Fig. 1.
Lack of FcμR expression in Fcmr −/− B6/lpr mice. (A) Genotyping of offspring of Fcmr −/− C57BL/6 and B6.MRL Fas lpr/lpr mice. Tail DNA was subjected to genomic PCR using diagnostic sets of primers to determine the genotype of Fcmr and Fas in individual mice. Representative genotyping data are shown. The normal (WT) and targeted (KO) alleles of Fcmr give rise to 570 and 730-bp PCR products, respectively. The WT and mutant (Lpr) alleles of Fas are 179 and 217bp, respectively. (B) RT–PCR analysis. Total RNAs isolated from spleen of Fcmr +/+ (lanes 1 and 4) or Fcmr −/− (lanes 2 and 5) B6/lpr mice and control WT mice (lanes 3 and 6) were subjected to RT–PCR and amplified products of FcμR (lanes 1–3) and control PIR-A (lanes 4–6) were electrophoresed in 0.7% agarose and stained with ethidium bromide. Lane 7 is a PCR control without a first-strand cDNA template. Note the lack of full-length FcμR transcripts (1283bp) in Fcmr −/− B6/lpr mice and presence of PIR-A transcripts (253bp). (C) Flow cytometric analysis of cell surface FcμR. Splenocytes from the indicated mice were first incubated with FcγR-blocking mAbs and then with biotinylated, anti-FcμR (lines) or isotype-matched control mAb (shaded) and FITC-anti-CD19 mAb. The bound biotinylated mAbs were detected by addition of PE-streptavidin. CD19+ B cells were gated and examined for their expression of FcμR.
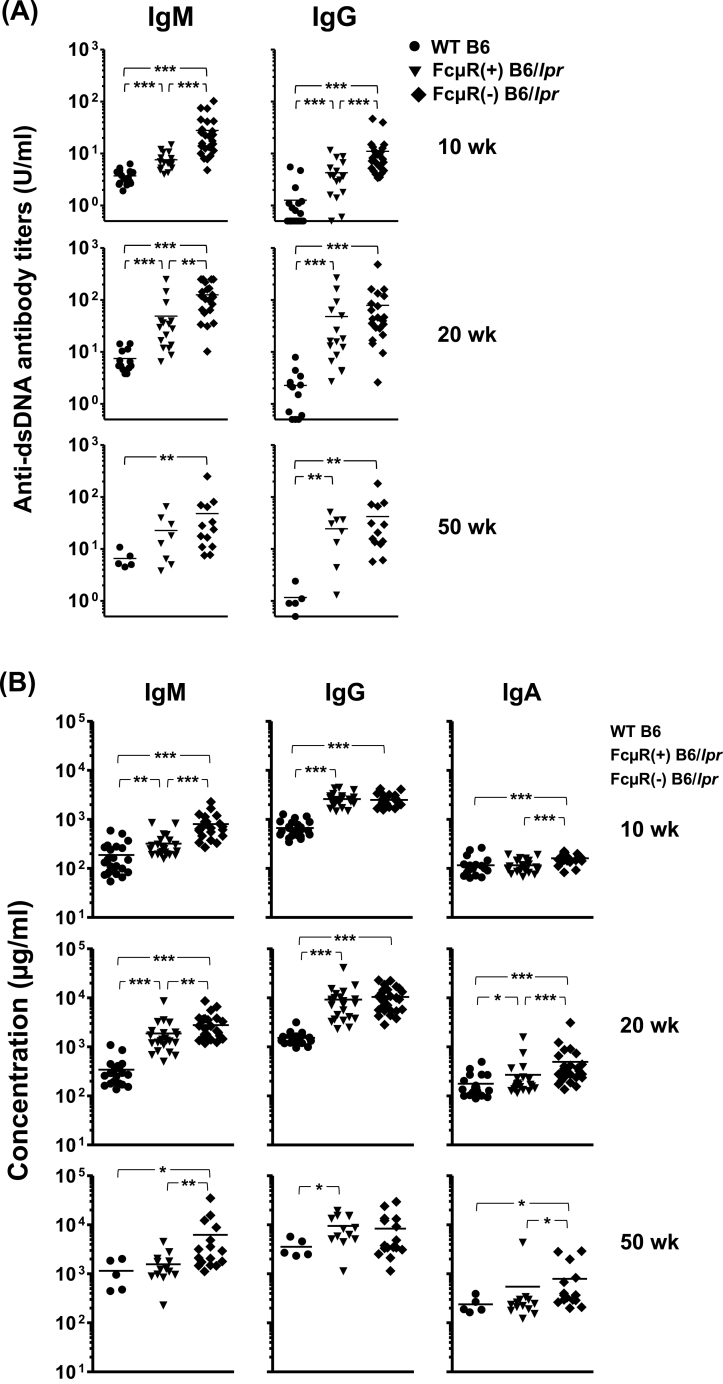
We then assessed serum auto-antibody titers in FcμR(−) and FcμR(+) B6/lpr as well as in WT B6 female mice at three different ages by ELISA using two representative self-antigens, dsDNA (Fig. 2A) and chromatin (see Supplementary Figure S1, available at International Immunology Online). At 10 weeks of age, serum IgM and IgG auto-antibody titers to both auto-antigens were clearly elevated in FcμR(−) B6/lpr mice as compared with the other groups of mice. At 20 weeks of age, a similar trend was still observed with IgM auto-antibodies to both antigens, but not with IgG auto-antibodies, although IgG antibodies in FcμR(−) and FcμR(+) B6/lpr mice were elevated as compared with WT mice. At 50 weeks of age, the trend became less clear, but FcμR(−) B6/lpr mice still had higher titers of anti-chromatin antibodies of both isotypes compared with other groups of mice. A similar, but less clear, trend was also observed with IgG2b, IgG2c and IgA auto-antibodies against dsDNA (see Supplementary Figure S1B, available at International Immunology Online). In addition to the above auto-antibody titers, the serum levels of total IgM and IgA in FcμR(−) B6/lpr mice were also higher than those in the other mice and were increased with age (Fig. 2B). By contrast, the serum level of total IgG was comparable in both groups of B6/lpr mice during the entire period of analysis, suggesting that IgG auto-antibodies elevated at an early age in FcμR(−) B6/lpr mice constitute a minor fraction of the total serum IgG. Collectively, these findings suggest that the ablation of Fcmr in B6/lpr mice leads to a rapid increase in serum auto-antibodies of IgM and, to a lesser extent, IgG isotypes at a relatively early stage in life as compared with control B6/lpr mice.
Fig. 2.
Serum levels of IgM and IgG auto-antibodies and of total IgM, IgG and IgA. Sera were collected from WT B6 (n = 5–24; closed circles), FcμR(+) B6/lpr (n = 13–23; closed inverted triangles) and FcμR(−) B6/lpr (n = 16–28; closed diamonds) female mice at the age of 10 weeks (top), 20 weeks (middle) and 50 weeks (bottom), and were subjected to ELISA for assessment of titers of antibody against dsDNA (A). Concentrations (μg/ml) of total serum IgM, IgG and IgA are also depicted (B). Each symbol represents data from an individual mouse. *P < 0.05, **P < 0.01 and ***P < 0.001, respectively.
Altered B-cell subsets in FcμR(−) B6/lpr mice
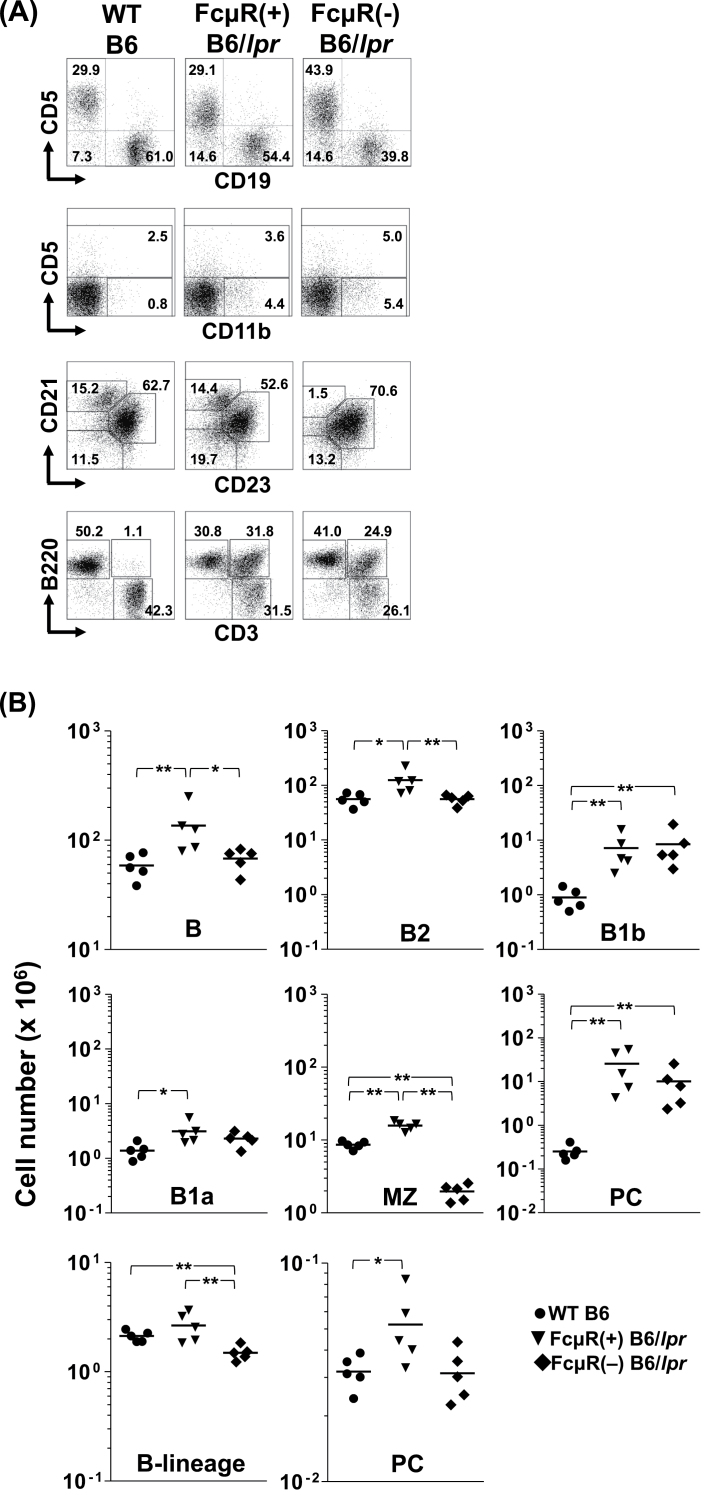
In our previous studies, the expression of mouse FcμR was shown to be restricted to B-lineage cells, from immature B to plasmablasts, except for a transient down-modulation during the germinal center (GC) reaction and that Fcmr ablation had no significant effect on overall B- and T-lymphocyte development except for a 4-fold reduction in marginal zone (MZ) B cells and a 2-fold increase in splenic B1 cells (11). To determine if the FcμR deficiency leads to alterations in lymphocyte development in B6/lpr mice and to gain insight to the mechanism of altered auto-antibody production in the absence of FcμR, we compared various cell populations in ~20-week-old mice by flow cytometry. There was significant splenomegaly in both FcμR(+) [255±129mg spleen weight (mean ± 1 SD)] and FcμR(−) (218±56mg) B6/lpr mice as compared with WT mice (78±12mg). This was also the case for the total cell numbers recovered from these tissues (Table 1). Representative immunofluorescent profiles and cell numbers of each subset are shown in Fig. 3A and Table 1, respectively.
Table 1.
Cell numbers of various subsets in lymphoid tissues from WT, FcμR(+) and FcμR(−) B6/lpr mice
| WT | FcμR(+) B6/lpr | FcμR(−) B6/lpr | Mann–Whitney U-test (P) | Fold increase | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n = 5 | n = 5 | n = 5 | WT versus FcμR(+)lB6/lpr | WT versus FcμR(−) B6/lpr | FcμR(+) versus FcμR(−) B6/lpr | FcμR(+) B6/lpr versus WT | FcμR(−) B6/lpr versus WT | ||||
| Mean | 1 SD | Mean | 1 SD | Mean | 1 SD | ||||||
| Spleen | |||||||||||
| Total cell number (×106) | 108.6 | 23.4 | 308.4 | 186.7 | 194.5 | 50.4 | <0.01 | <0.05 | NS | 2.8 | 1.8 |
| B cells (CD19+) | 58.8 | 15.3 | 135.9 | 69.0 | 68.1 | 15.6 | <0.01 | NS | <0.05 | 2.3 | 1.2 |
| B2 (CD19+/CD5−/CD11b−) | 56.1 | 14.4 | 124.3 | 61.8 | 56.2 | 11.1 | <0.05 | NS | <0.01 | 2.2 | 1.0 |
| B1a (CD19+/CD5+/CD11b+) | 1.4 | 0.5 | 3.1 | 1.5 | 2.3 | 0.7 | <0.05 | NS | NS | 2.3 | 1.7 |
| B1b (CD19+/CD5−/CD11b+) | 0.9 | 0.4 | 7.2 | 5.3 | 8.4 | 6.6 | <0.01 | <0.01 | NS | 8.0 | 9.5 |
| FO (CD21+/CD23+) | 44.6 | 11.3 | 94.4 | 54.6 | 44.6 | 8.8 | <0.05 | NS | <0.05 | 2.1 | 1.0 |
| MZ (CD21hi/CD23−) | 8.6 | 1.0 | 15.8 | 2.2 | 2.0 | 0.5 | <0.01 | <0.01 | <0.01 | 1.8 | 0.2 |
| NF (CD21−/CD23−) | 4.6 | 1.0 | 25.0 | 19.6 | 17.9 | 13.0 | <0.01 | <0.05 | NS | 5.4 | 3.9 |
| Plasma cells (CD138+/CD19−) | 0.25 | 0.1 | 25.6 | 23.3 | 10.1 | 9.4 | <0.01 | <0.01 | NS | 102.3 | 40.4 |
| T cells (CD3+) | 27.6 | 7.5 | 97.3 | 73.8 | 71.3 | 24.0 | <0.01 | <0.01 | NS | 3.5 | 2.6 |
| Normal T (CD3+/B220−) | 26.3 | 7.3 | 46.7 | 34.1 | 45.3 | 9.8 | NS | <0.05 | NS | 1.8 | 1.7 |
| CD4+ T | 13.2 | 4.8 | 28.8 | 28.5 | 24.7 | 14.3 | NS | NS | NS | 2.2 | 1.9 |
| CD8+ T | 11.2 | 2.7 | 11.6 | 5.6 | 16.2 | 9.6 | NS | NS | NS | 1.0 | 1.4 |
| Atypical T (CD3+/B220+) | 1.0 | 0.4 | 47.4 | 38.3 | 24.0 | 15.5 | <0.05 | <0.05 | NS | 45.2 | 22.9 |
| DN (CD3+/CD4−/CD8−) | 0.3 | 0.1 | 38.8 | 29.6 | 20.2 | 13.1 | <0.01 | <0.01 | NS | 117.1 | 60.9 |
| Lymph nodes | |||||||||||
| Total cell number (×106) | 10.3 | 5.5 | 786.4 | 937.3 | 295.3 | 233.8 | <0.01 | <0.01 | NS | 76.6 | 28.8 |
| B cells (CD19+) | 3.8 | 2.4 | 96.2 | 58.1 | 76.2 | 61.1 | <0.01 | <0.01 | NS | 25.4 | 20.1 |
| B2 (CD19+/CD5−/CD11b−) | 3.6 | 2.2 | 87.5 | 51.8 | 67.9 | 54.3 | <0.01 | <0.01 | NS | 24.5 | 19.0 |
| B1a (CD19+/CD5+/CD11b+) | 0.1 | 0.1 | 3.3 | 1.5 | 3.3 | 2.4 | <0.01 | <0.01 | NS | 23.8 | 23.7 |
| B1b (CD19+/CD5−/CD11b+) | 0.04 | 0.03 | 4.4 | 4.6 | 3.9 | 4.0 | <0.01 | <0.01 | NS | 101.1 | 89.5 |
| Plasma cells (CD138+/CD19−) | 0.03 | 0.01 | 108.4 | 147.9 | 39.2 | 54.2 | <0.01 | <0.01 | NS | 3838.9 | 1388.6 |
| T cells (CD3+) | 5.4 | 3.0 | 585.3 | 785.6 | 161.0 | 130.8 | <0.01 | <0.01 | NS | 107.8 | 29.7 |
| Normal T (CD3+/B220−) | 5.3 | 3.0 | 109.0 | 45.1 | 68.1 | 36.5 | <0.01 | <0.01 | NS | 20.6 | 12.9 |
| CD4+ T | 2.5 | 1.5 | 61.0 | 40.9 | 30.9 | 17.8 | <0.01 | <0.01 | NS | 23.9 | 12.1 |
| CD8+ T | 2.6 | 1.4 | 33.8 | 9.1 | 27.6 | 13.7 | <0.01 | <0.01 | NS | 13.1 | 10.7 |
| Atypical T (CD3+/B220+) | 0.09 | 0.07 | 413.0 | 634.2 | 89.5 | 95.8 | <0.01 | <0.01 | NS | 4437.3 | 961.3 |
| DN (CD3+/CD4−/CD8−) | 0.03 | 0.01 | 375.6 | 587.6 | 79.0 | 84.1 | <0.01 | <0.01 | NS | 14472.3 | 3044.7 |
| Bone marrow | |||||||||||
| Total cell number (×106) | 15.3 | 2.4 | 17.1 | 3.3 | 14.3 | 1.7 | NS | NS | NS | 1.1 | 0.9 |
| B-lineage cells (CD19+) | 2.1 | 0.2 | 2.7 | 0.8 | 1.5 | 0.2 | NS | <0.01 | <0.01 | 1.3 | 0.7 |
| Plasma cells (CD138+/CD19−) | 0.03 | 0.01 | 0.05 | 0.02 | 0.03 | 0.01 | <0.05 | NS | NS | 1.6 | 1.0 |
NS, not significant; SD, standard deviation.
Fig. 3.
Immunofluorescent assessment of cellular compartments in Fcmr −/− and control B6/lpr mice. (A) Representative immunofluorescence profiles. Splenocytes (top three rows) and LN cells (bottom row) isolated from WT B6 (left column), FcμR(+) B6/lpr (middle column) and FcμR(−) B6/lpr (right column) female mice at 20 weeks of age were first incubated with FcγR-blocking reagents and then with fluorochrome-labeled mAbs specific for the indicated surface markers. Stained cells with the light scatter characteristics of lymphocytes/macrophages were analyzed using an Accuri C6 flow cytometer. In the second and third rows, the CD19+ B cells were gated and examined for their expression of the indicated surface markers. Numbers indicate percentages of cells. Note (i) a sizable population of CD11b+/CD5− B cells, (ii) marked expansion of CD3+/B220+ atypical T cells in both FcμR(+) and FcμR(−) B6/lpr mice and (iii) a clear reduction of CD21+/CD23− MZ B cells in FcμR(−) B6/lpr mice. (B) Total cell numbers in each population in spleen (top two rows) and BM (bottom row) from WT B6 (closed circles), FcμR(+) B6/lpr (closed inverted triangles) and FcμR(−) B6/lpr (closed diamonds) mice (five in each group). Each symbol represents data from an individual mouse. The horizontal bar indicates the arithmetic mean. *P < 0.05 and **P < 0.01, respectively. Note (i) normal levels of splenic B and B2 cells and marked reduction of MZ B cells in FcμR(−) B6/lpr mice, (ii) comparable levels of B1b and B1a cells as well as plasma cells in both FcμR(+) and FcμR(−) B6/lpr mice and (iii) significant reduction of BM mature B cells and normal levels of BM plasma cells in FcμR(−) B6/lpr mice.
In spleen, the numbers of total B- and B2-cell populations were elevated by ~2-fold in FcμR(+) B6/lpr mice but were comparable between FcμR(−) B6/lpr and WT mice (Fig. 3B, top), suggesting normalization of the splenic B2-cell compartment by introduction of FcμR deficiency into B6/lpr mice. By contrast, the number of B cells with the unique surface phenotype of CD5−/CD11b+, equivalent to the B1b cells in peritoneal cavity, was markedly (8- to ~10-fold) increased in both FcμR(−) and FcμR(+) B6/lpr mice as compared with the WT mice. Likewise, B1a cells were also expanded in both groups of B6/lpr mice, though the difference between FcμR(−) B6/lpr and WT mice was statistically insignificant. Interestingly, we noted that the B1a/B1b ratio was inverted in B6/lpr mice irrespective of FcμR expression, as compared with WT mice (Table 1). [Similar hierarchies were also observed in subsets classified by other markers (i.e. follicular and newly formed B-cell subsets; see Supplementary Figure S2, available at International Immunology Online).] Notably, the number of MZ B cells in FcμR(−) B6/lpr mice was clearly lower than that of either FcμR(+) B6/lpr or WT mice, suggesting that the reduced MZ B-cell compartment in the absence of FcμR (11) is not normalized on the B6/lpr background (see Fig. 3A and B, middle). The number of mature plasma cells was markedly elevated by ~100-fold in FcμR(+) B6/lpr mice and by ~40-fold in FcμR(−) B6/lpr mice, compared with the WT mice, but the difference in both genotypes of B6/lpr mice was statistically insignificant due to the large individual variability (Fig. 3B, middle). The numbers of conventional (i.e. CD3+/B220−) T cells were also comparable between FcμR(+) and FcμR(−) B6/lpr mice, but were significantly elevated as compared with WT mice (see Supplementary Figure S2, available at International Immunology Online).
Essentially, similar results were also observed with remarkably enlarged LN tissues in both genotypes of B6/lpr mice. These included marked increases in the numbers of plasma cells, B1b, B1a and B2 cells as well as in the atypical T cells, defined as CD3+/B220+/CD4−/CD8− and known to be unique for the Fas lpr/lpr mutation, and in conventional CD4 T and CD8 T cells (see Supplementary Figure S3, available at International Immunology Online). These results indicate that lack of FcμR expression on B-lineage cells does not affect the development of atypical T cells in B6/lpr mice. In bone marrow (BM), the CD19+ B-lineage cells in FcμR(−) B6/lpr mice were significantly reduced as compared with FcμR(+) B6/lpr and WT mice and this reduction was more evident in the CD19hi recirculating mature B cells than the CD19lo pro-B/pre-B and immature B-cell compartments (Fig. 3B, bottom). Unlike the plasma cells in peripheral lymphoid tissues, the number of mature plasma cells in the BM from FcμR(−) B6/lpr mice was reduced to the normal level of WT mice. Collectively, these findings suggest that the ablation of Fcmr in B6/lpr mice leads to a reduction in splenic B cells including the B2 and MZ B-cell subsets and BM mature B and plasma cells, but does not significantly affect the markedly expanded plasma cell compartments in spleen and LNs or the B1-cell subsets, when compared with FcμR(+) B6/lpr mice.
Increase in anti-Sm antibody titers and Mott cell formation in Fcmr-deficient B6/lpr mice
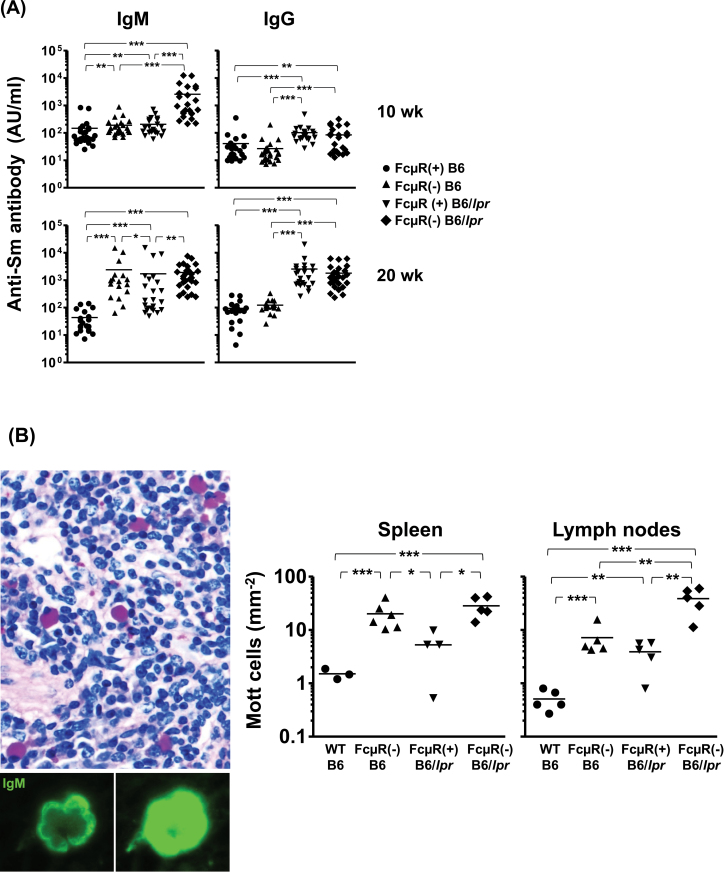
As described above, FcμR(−) B6/lpr mice had significantly higher titers of serum auto-antibodies of IgM and, to a lesser extent, IgG isotype, but equivalent numbers of B1 cells and markedly reduced numbers of MZ B cells, when compared with FcμR(+) B6/lpr mice. To test the possibility that MZ B cells rapidly differentiate into plasma cells in the absence of FcμR, we assessed the antibody titer to another self-antigen Sm/RNP, including FcμR(−) B6 mice as another control. We chose the antigen because MZ B cells preferentially respond to first encountering Sm/RNP-containing apoptotic cells in their unique anatomical site (32, 33). Among four groups of mice [FcμR(+) or (−) B6 and FcμR(+) or (−) B6/lpr mice], IgM anti-Sm antibody titers were the highest in FcμR(−) B6/lpr mice at 10 weeks of age, but later were clearly elevated in three groups of mice in the order: FcμR(−) B6/lpr = FcμR(−) B6 > FcμR(+) B6/lpr (Fig. 4A). By contrast, IgG anti-Sm antibody titers were more slowly increased in both genotypes of B6/lpr mice only. Thus, an early increase in IgM anti-Sm antibodies appears to result from synergy of both Fcmr-ablation and Fas-deficiency, but the Fcmr-ablation alone clearly contributes to an increase in IgM anti-Sm antibody production. The surface expression of adhesion molecules [leukocyte function- associated antigen 1 (LFA1) (αLβ2) and VLA4 (α4β1)], which are known to be critically involved in the localization and retention of MZ B cells in the marginal sinuses (34), was comparable regardless of the FcμR expression (data not shown), ruling out the possibility of a homing impairment of MZ B-cell precursors in FcμR-deficient mice. Collectively, these findings suggest that because MZ B cells may recognize blood-borne antigens by cross-linkage of multiple receptors such as the BCR and TLRs, FcμR regulates their rapid differentiation into plasma cells in red pulp.
Fig. 4.
High titer of anti-Sm antibody and enhanced Mott cell formation in the absence of FcμR. (A) Assessment of anti-Sm antibody. Serum levels of anti-Sm antibodies in FcμR (+) B6 (closed circles), FcμR(−) B6 (n = 17–27; closed triangles), FcμR(+) B6/lpr (closed inverted triangles) and FcμR(−) B6/lpr (closed diamonds) female mice were similarly assessed by ELISA as described in the Fig. 2 legend. (B) Mott cells. Left: Representative PAS-stained spleen sections from FcμR(−) B6/lpr mice of 20 weeks of age depicting strongly PAS+ Mott cells (upper) and representative immunofluorescent staining of Mott cells with FITC anti-μ antibodies with short (lower left) and long (lower right) exposure. Right: Number of Mott cells per mm2 of spleen and LNs from WT B6 (closed circles), FcμR(−) B6 (closed triangles), FcμR(+) B6/lpr (closed inverted triangles) and FcμR(−) B6/lpr (closed diamonds) mice (n = 3–6 in each group). *P < 0.05, **P < 0.01 and ***P < 0.001.
Next, when formalin-fixed, paraffin-embedded tissue sections of spleen and LNs were stained with PAS, we found an intriguing difference. As PAS, unlike H&E, does not stain erythrocytes, cellular changes in the red pulp of spleen are more easily detectable. Indeed, strongly PAS+ plasma-like cells were much more frequently observed in such areas of spleen as well as in the medulla and extrafollicular areas of LNs in both groups (B6/lpr and B6) of FcμR(−) mice as compared with FcμR(+) B6/lpr or B6 mice (Fig. 4B). These were Mott cells, an aberrant form of plasma cells in which large amounts of immunoglobulin accumulate in rough endoplasmic reticulum (ER)-bound vesicles as intra-cytoplasmic inclusions called Russell bodies. The majority of the Mott cells in FcμR(−) mice were IgM+, but a few were IgG+, indicating no isotype restriction of Mott cell formation in the mutant mice. Collectively, these findings suggest that the FcμR-deficiency is clearly involved in the augmented anti-Sm antibody production and Mott cell generation.
FcμR deficiency has no effect on the lupus-like nephritis associated with B6/lpr mice
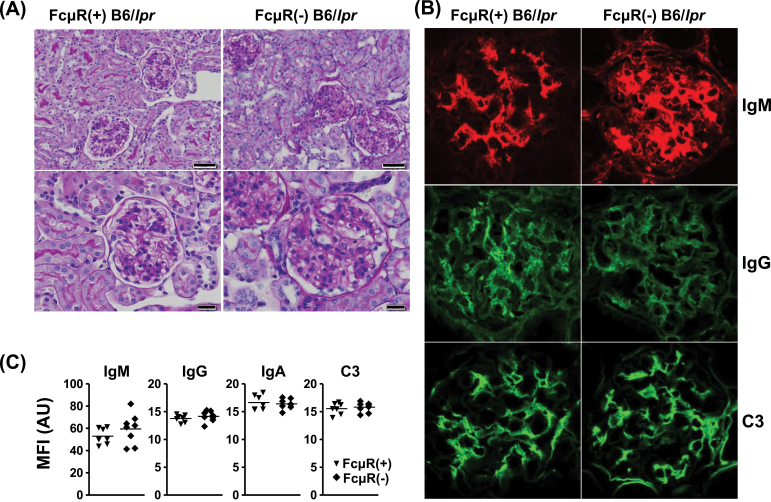
To determine whether the early increase in serum auto-antibody titers and alterations of certain lymphocyte subsets in FcμR(−) B6/lpr mice affect the autoimmune pathology, we performed immunohistopathological examination of major organs in FcμR(−) or FcμR(+) B6/lpr mice at two different ages. At ~20 weeks of age, in both groups of mice, the pathological changes in glomeruli were generally minimal and no significant changes were observed in the urinary tubules. Immunohistological analysis of glomeruli revealed weakly stained IgM deposits in both groups of mice. Perivascular lymphoid infiltration in the interstitium was detectable in only one out of five mice in each group. Perivascular lymphoid infiltration in liver was observed in three FcμR(+) mice and in one FcμR(−) B6/lpr mouse. By contrast, at ~50 weeks of age, many glomeruli in all mice of both groups were equally affected to variable degrees, including mesangial cell proliferation, increased mesangial matrix with homogeneous PAS+ substances and focal necrosis (Fig. 5A), but very few changes in urinary tubules were observed [1/7 for FcμR(+) and 0/8 for FcμR(−) B6/lpr mice]. Perivascular interstitial lymphoid infiltration was also observed in some mice of both groups [4/7 for FcμR(+) and 3/8 for FcμR(−) B6/lpr mice]. Immunohistological analysis of glomeruli revealed variable deposition of IgM, IgG, IgA and C3 primarily in the mesangium from both groups of mice (Figs 5B and 5C). When the fluorescence was assessed quantitatively, no significant differences were observed between FcμR(+) and FcμR(−) B6/lpr mice (Fig. 5C). In liver, variable degrees of perivascular lymphoid infiltration [6/7 for FcμR(+) and 7/8 for FcμR(−) B6/lpr] and of piecemeal necrosis of hepatocytes [5/7 for FcμR(+) and 8/8 for FcμR (−) B6/lpr] were observed in both groups of mice. Tiny perivascular lymphoid infiltrates were also observed in lungs [3/7 for FcμR(+) and 6/8 for FcμR(−) B6/lpr]. These findings suggest that the presence or absence of FcμR had no significant effect on the autoimmune pathology associated with B6/lpr mice.
Fig. 5.
Pathological changes of kidney. (A) Representative PAS-stained kidney sections from FcμR(+) (left panel) and FcμR(−) (right panel) B6/lpr mice of 50 weeks of age at two different magnifications. Scale bars indicate 50 μm (upper panel) and 20 μm (lower panel). Note mesangial cell proliferation and increase mesangial matrix with homogeneous PAS+ substances in both groups of mice. (B) Representative immunofluorescence images of glomerular deposition of IgM (top), IgG (middle) and C3 (bottom) from FcμR(+) (left panel) and FcμR(−) (right panel) B6/lpr mice. Note the presence of immune deposits primarily in the mesangium of both groups of mice. (C) Mean fluorescence intensity (MFI) of deposition of IgM, IgG, IgA and C3 in glomeruli from FcμR(+) (closed inverted triangles) and FcμR(−) (closed diamonds) B6/lpr mice. Results are shown as arbitrary units.
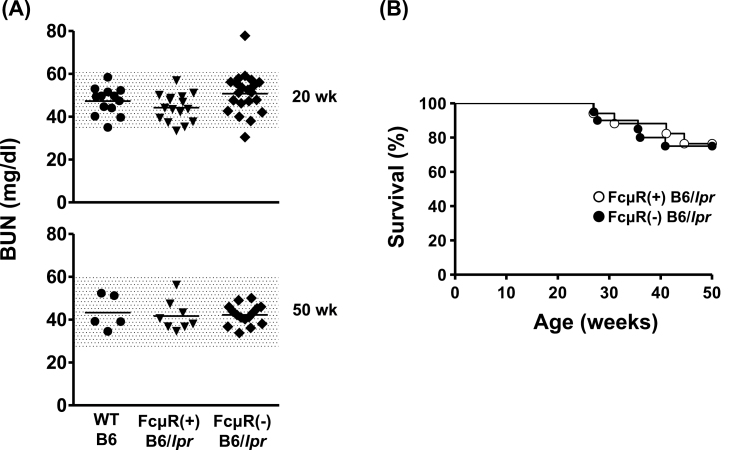
To evaluate renal function, levels of BUN were assessed. As shown in Fig. 6A, these were comparable in both groups of B6/lpr mice at either 20 or 50 weeks of age and not significantly increased as compared with those of WT B6 mice. This is consistent with the histopathological findings that nearly three quarters of the B6/lpr mice, irrespective of FcμR expression, had many prominent glomerular lesions but very few urinary tubular changes. Notably, there were also no significant differences in lifespan between FcμR(−) and FcμR(+) B6/lpr mice, as 20% of both groups of mice died during the first year of life (Fig. 6B). [Unfortunately, the cause of death, e.g. asphyxia due to massive enlargement of cervical LNs, hemodynamic changes or cardiac failure due to generalized lymphadenopathy and splenomegaly, renal insufficiency due to severe glomerulonephritis, or other reasons, was unclear.]
Fig. 6.
Kidney function and survival. (A) Results show the BUN levels (mg/dl) in WT B6 (n = 5–13; closed circles), FcμR(+) B6/lpr (n = 8–16; closed inverted triangles) and FcμR(−) B6/lpr (n = 15–21; closed diamonds) mice at 20 (top) and 50 (bottom) weeks of ages. Each symbol represents data from an individual mouse. The shaded area represents the range within 2 SDs of the mean of WT B6 mice. (B) Survival of FcμR(+) B6/lpr (n = 17; unfilled circles) and FcμR(−) B6/lpr (n = 20; filled circles) mice up to 50 weeks of age.
Discussion
The results from previous studies by us and others have demonstrated that FcμR has an important role in IgM homeostasis as evidenced by significant increases in serum levels of IgM and natural auto-antibodies of both IgM and IgG isotypes in naive Fcmr KO mice (11, 12, 19). The goal of the present study was to determine the impact of Fcmr ablation in Fas-deficient autoimmune-prone B6/lpr mice, which have a milder form of lupus-like disease than MRL Fas lpr/lpr mice (25, 35). Our hypothesis was that the introduction of the Fcmr null mutation onto B6/lpr background would affect the autoimmune process depending on the balance of protective IgM versus pathologic IgG auto-antibodies. Fcmr deficiency in B6/lpr mice led to (i) a rapid increase in serum auto-antibody levels of IgM and, to a lesser extent, IgG isotypes at a relatively early age, (ii) normalization of the splenic B2 subset and the BM B-lineage cell compartment, mostly mature B cells, (iii) a marked reduction of the MZ B-cell subset and (iv) increased formation of Mott cells, an aberrant form of plasma cells. The reduction of MZ B cells in the absence of FcμR seemed to be due to their rapid differentiation into plasma cells, as evidenced by an increase in serum IgM anti-Sm antibodies in both groups (B6/lpr and B6) of FcμR(−) mice. The FcμR deficiency, however, did not affect the severity of glomerulonephritis, renal function or survival.
Serum auto-antibody titers of both IgM and IgG isotypes, though the latter was less evident, were consistently higher in FcμR(−) B6/lpr mice than FcμR(+) B6/lpr mice during the entire period examined. Serum levels of total IgM and IgA, but not of total IgG, were also higher in FcμR(−) B6/lpr than FcμR(+) B6/lpr mice. On the other hand, the total CD138+ plasma cell numbers in all three tissues examined (spleen, LNs and BM) were consistently lower in FcμR(−) B6/lpr mice than FcμR(+) B6/lpr mice, although the difference was not statistically significant. This apparent discrepancy cannot easily be explained at moment. In our previous studies, the half-life of injected IgM was identical regardless of the FcμR expression, suggesting no obvious involvement of the B-cell FcμR in IgM catabolism (11). The half-life of secreted IgM antibody in mice is estimated as ~2 days, similar to that of short-lived plasmablasts (<3 days), which express FcμR on their cell surface (36). Thus, the involvement of FcμR in antibody secretion by plasma cells as the consequence of B-cell responses to foreign or self-antigens may be very complex and occurring at multiple levels and remains to be further explored.
Another interesting feature of plasma cells in B6/lpr mice was their massive expansion (~40- to ~3,800-fold) in peripheral sites (spleen and LNs) but only 2-fold in the BM, confirming the concept of the limited capacity of the BM microenvironment for plasma cells, especially the long-lived variety. Furthermore, the peripheral expansion occurred in both FcμR(+) and FcμR(−) B6/lpr mice, whereas the BM expansion was only observed in FcμR(+) B6/lpr mice. In this regard, an inhibitory FcγR, FcγRIIb, is also expressed on BM plasma cells, and its engagement with IgG immune complexes induces apoptosis through an antigen-independent mechanism, thereby reducing the number of pre-existing plasma cells in the limited BM niche and creating space for newly formed plasma cells to take up residency and to become part of the long-lived plasma cell repertoire (37). Notably, however, autoimmune-prone mice including MRL Fas lpr/lpr express almost negligible levels of surface FcγRIIb on GC B and BM plasma cells (37–39), hence suggesting the existence of additional regulator(s) that must control the homeostasis of BM plasma cells more strongly than FcγRIIb. Further studies are required to determine whether FcμR and its signals are involved in the differentiation and/or survival of plasmablasts.
The findings that several B-cell subsets in B6/lpr mice were affected by FcμR deficiency could be important with regard to the potential function of this receptor. Fcmr ablation in B6/lpr mice led to a reduction of the number of splenic B2 (or FO) B cells to the normal level, but did not alter the B1 B-cell populations. By contrast, in our previous studies, we observed that Fcmr ablation in B6 mice resulted in a 2-fold increase in splenic B1 B cells (11). Interestingly, similar changes, ~25% reduction of the FO B-cell number and a 2-fold increase in B1 B cells, have been demonstrated in the spleen of mice unable to secrete IgM (μs−/−) (40, 41). These findings thus suggest that despite the equal surface FcμR levels on both B2- and B1-cell subsets, FcμR or its signals may differentially regulate the homeostasis of B2 versus B1 B cells. In support of this idea, it has been well documented that upon engagement of the BCR, B2 B cells exhibit robust intracellular Ca2+ mobilization and cell proliferation, whereas B1 B cells display modest Ca2+ mobilization, impaired proliferation and increased apoptosis (42). The present analysis also revealed a marked (~10- to ~100-fold) increase of B1b B cells, defined as CD5−/CD11b+, in spleen and LNs in B6/lpr mice regardless of FcμR expression. B1b B cells, a minor subset of mature B cells, express very low levels of FcμR on their cell surface and are known to mediate long-lasting antibody responses to T-cell-independent antigens expressed on many bacterial pathogens (43). It should be elucidated whether the increase of B1b B cells is restricted to Fas-deficient MRL Fas lpr/lpr mice or is common to other autoimmune-prone mice. In this regard, an unusual type of mature B cell (CD11c+/CD11b+/CD5−/lo/CD21−/CD23−) that accumulates in aged female mice as a result of stimulation through TLR7 or TLR9 was recently identified and shown to be important for the development of autoimmunity (44, 45). It will be of interest to determine if this population, termed age-associated B cells, includes the B1b B cells that we describe here.
Another remarkable finding is the marked reduction of MZ B cells in FcμR(−) B6/lpr mice as compared with FcμR(+) B6/lpr mice, similar to our previous findings with FcμR(−) B6 mice (11). By contrast, the number of MZ B cells in μs−/− mice is increased by ~3-fold and this increase can be normalized by passive administration of natural or polyclonal, but not monoclonal, IgM preparations (41). It remains unclear, however, whether this normalization results from differentiation of MZ B cells into plasma cells or apoptosis of MZ B cells upon possible engagement of multiple receptors including TLRs with natural IgM. To explore the basis for reduction of the MZ B-cell compartment in the absence of FcμR, we first examined their surface expression of adhesion molecules LFA1 and VLA4, which are known to be crucial for their precursor’s homing to the marginal sinus (34). Both adhesion molecules were equally expressed irrespective of FcμR expression, ruling out the possibility for homing impairment of MZ B-cell precursors in such mutant mice. Next, we assessed serum antibodies against an Sm/RNP antigen because the results from analysis of immunoglobulin-transgenic mice suggested that while both B1 and MZ B cells have the potential to produce anti-Sm antibodies, the MZ B cell was found to be the major source as a consequence of its interaction with apoptotic cell-ingested dendritic cells in the marginal sinuses (32, 33). In the present studies, serum titers of IgM, but not IgG, anti-Sm antibodies at 10 weeks of age were increased by >10-fold in FcμR(−) B6/lpr mice as compared with FcμR(+) B6/lpr mice. This finding, along with the result of equivalent number of B1 cells in both genotypes of B6/lpr mice, suggested that the reduced number of MZ B cells in both groups (B6/lpr and B6) of FcμR(−) mice resulted from their rapid differentiation into plasma cells secreting anti-Sm antibodies. This in turn implies that FcμR and its signals may play an important regulatory role in the differentiation of MZ B cells into plasma cells secreting natural IgM antibodies against apoptotic cells.
The finding of enhanced Mott cell formation in the absence of FcμR is also intriguing. Mott cells containing immunoglobulin aggregates termed Russell bodies are rarely observed in normal lymphoid tissues, but are found in various pathological conditions, including neoplasms, inflammatory diseases and autoimmune disorders (46–50). Several mechanisms for Mott cell formation have been suggested: (i) structural alterations of immunoglobulin heavy chains (HCs) preventing their appropriate processing; (ii) impairment of immunoglobulin light chains, which normally prevent immunoglobulin HC chain aggregation; and (iii) inability to degrade or to export immunoglobulin, leading to its aggregation. As to the molecular basis for enhanced Mott cell formation in FcμR-deficient mice, the possibility that FcμR may be involved in the assembly process of nascent immunoglobulin μ HCs in the ER is unlikely because IgG+ Mott cells were also observed, albeit less frequently, in the mutant mice. There is a precedent that certain B-cell hybridomas accumulate IgM in the Golgi due to formation of IgM/glycosaminoglycan complexes and release of large spherical IgM complexes, termed spherons, of up to 2 μm in diameter (51, 52). Thus, it is possible that some of the IgM produced by plasma cells in both groups (B6/lpr and B6) of FcμR(−) mice may recognize determinant(s) on the ER membrane, thereby generating Mott cells. In support of this idea, it has been suggested that Mott cells predominantly occur in the B1-cell compartment (47, 49).
There is compelling evidence for a protective role of IgM in autoimmune processes. For example, in μs−/− mice, there was an increase in spontaneous autoimmunity as judged by the age-dependent development of serum IgG auto-antibodies and their glomerular deposition (53). When the μs−/− mice were crossed with MRL Fas lpr/lpr mice, the resultant μs−/− MRL/lpr mice developed elevated serum levels of IgG auto-antibodies with glomerular deposition, suffered more severe glomerulonephritis and succumbed to the disease at an earlier age as compared with control MRL/lpr mice (54). Thus, secreted IgM, including naturally produced IgM auto-antibody, appears to lessen the severity of autoimmune pathology associated with IgG auto-antibodies. Conversely, MRL/lpr mice deficient in activation-induced deaminase, an enzyme required for immunoglobulin somatic hypermutation and class switch recombination, lacked IgG auto-antibodies but had elevated autoreactive IgM and experienced a significant reduction in glomerulonephritis and a dramatic increase in survival as compared with control MRL/lpr mice (55). These findings suggest that IgM, including autoreactive IgM, may not promote nephritis but rather provides a protective role in MRL/lpr mice.
Given these precedents and our previous findings that Fcmr ablation led to an increase in the concentrations of pre-immune serum IgM and natural auto-antibodies of both IgM and IgG isotypes (11), we expected that FcμR deficiency would have a major impact on the phenotype of B6/lpr mice. Indeed, compared with FcμR(+) B6/lpr mice, the increase in serum levels of IgM and IgG antibodies to dsDNA or chromatin in FcμR(−) B6/lpr mice was clearly evident as early as 10 weeks. However, this difference became less dramatic, especially for IgG auto-antibodies, in older mice. The severity of glomerulonephritis, the renal function and the lifespan were all comparable between FcμR(−) and FcμR(+) B6/lpr mice. Thus, despite the early elevation of autoreactive IgM and IgG, the FcμR null mutation did not affect the Fas lpr/lpr-associated pathology. This is quite distinct from the strong association between the null mutation of the inhibitory FcγRIIb and risk for developing severe autoimmune manifestations; Fcgr2b-deficient B6/lpr mice developed severe systemic autoimmune diseases and died within 30–40 weeks of age (56–58). However, it was also suggested that the chromosome 1 region around the Fcgr2b, called Sle16, contributed directly to the development of lethal lupus as determined by comparison of the severity of autoimmunity-associated pathology between 129 ES-derived versus B6 ES-derived Fcgr2b −/− mice, and that Fcgr2b was a modifier, rather than a causative gene, of autoimmune susceptibility (30, 31, 59, 60). Indeed, the absence of 129-derived Sle16 locus in our FcμR(−) B6/lpr mice would explain why they failed to develop severe lupus-like autoimmune disease. Furthermore, it has also been suggested that autoreactive B cells rather than their products, the auto-antibodies, play a more essential role in developing autoimmune pathology, possibly by presenting auto-antigens to T cells (61, 62). In this regard, the cellular infiltrates in the renal interstitium and around the blood vessels, which are considered as T-cell mediated immune responses (61), were comparable between FcμR(−) and FcμR(+) B6/lpr mice. This finding in turn implies that unlike FcγRs, FcμR may not have a function in antigen presentation as suggested by Ouchida et al. (12), although the receptor is rapidly internalized into the lysosomal compartment upon IgM binding (8).
Supplementary data
Supplementary data are available at International Immunology Online.
Funding
National Institute of Health /National Institute of Allergy and Infectious Diseases (R56 AI82249 and R21 AI094625 to H.K.).
Acknowledgements
We wish to thank Dr Peter D. Burrows, Dr Hui-Chen Hsu and Dr John F. Kearney for critical reading and valuable suggestions; Ms Mary Ann Hamilton for preparation of tissue sections; Dr Robert W. Hardy for BUN reagents and Mr Stewart New for immunofluorescence microscopical analysis. Both S.I. and H.K. would like to dedicate this paper as a token of our immense gratitude to our respected mentor Dr Yoshihiro Hamashima, Professor Emeritus of Kyoto University, who passed away at the age of 90 on December 14, 2013.
Conflict of interest statement: The authors declared no conflicts of interest.
References
- 1. Ehrenstein M. R., Notley C. A. 2010. The importance of natural IgM: scavenger, protector and regulator. Nat. Rev. Immunol. 10:778. [DOI] [PubMed] [Google Scholar]
- 2. Czajkowsky D. M., Shao Z. 2009. The human IgM pentamer is a mushroom-shaped molecule with a flexural bias. Proc. Natl Acad. Sci. USA 106:14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ravetch J. V., Nimmerjahn F. 2008. Fc receptors and their role in immune regulation and inflammation. In Paul W. E. ed., Fundamental Immunology, p. 684 Lippincott Williams & Wilkins, Philadelphia, PA, USA. [Google Scholar]
- 4. Sanders S. K., Kubagawa H., Suzuki T., Butler J. L., Cooper M. D. 1987. IgM binding protein expressed by activated B cells. J. Immunol. 139:188. [PubMed] [Google Scholar]
- 5. Ohno T., Kubagawa H., Sanders S. K., Cooper M. D. 1990. Biochemical nature of an Fcμ receptor on human B-lineage cells. J. Exp. Med. 172:1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakamura T., Kubagawa H., Ohno T., Cooper M. D. 1993. Characterization of an IgM Fc-binding receptor on human T cells. J. Immunol. 151:6933. [PubMed] [Google Scholar]
- 7. Kubagawa H., Oka S., Kubagawa Y., et al. 2009. Identity of the elusive IgM Fc receptor (FcμR) in humans. J. Exp. Med. 206:2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vire B., David A., Wiestner A. 2011. TOSO, the Fcμ receptor, is highly expressed on chronic lymphocytic leukemia B cells, internalizes upon IgM binding, shuttles to the lysosome, and is downregulated in response to TLR activation. J. Immunol. 187:4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Murakami Y., Narayanan S., Su S., et al. 2012. Toso, a functional IgM receptor, is regulated by IL-2 in T and NK cells. J. Immunol. 189:587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shima H., Takatsu H., Fukuda S., et al. 2010. Identification of TOSO/FAIM3 as an Fc receptor for IgM. Int. Immunol. 22:149. [DOI] [PubMed] [Google Scholar]
- 11. Honjo K., Kubagawa Y., Jones D. M., et al. 2012. Altered Ig levels and antibody responses in mice deficient for the Fc receptor for IgM (FcμR). Proc. Natl Acad. Sci. USA 109:15882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ouchida R., Mori H., Hase K., et al. 2012. Critical role of the IgM Fc receptor in IgM homeostasis, B-cell survival, and humoral immune responses. Proc. Natl Acad. Sci USA 109:E2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lang K. S., Lang P. A., Meryk A., et al. 2013. Involvement of Toso in activation of monocytes, macrophages, and granulocytes. Proc. Natl Acad. Sci. USA 110:2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Honjo K., Kubagawa Y., Kubagawa H. 2013. Is Toso/IgM Fc receptor (FcμR) expressed by innate immune cells? Proc. Natl Acad. Sci. USA 110:E2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lang K. S., Lang P. A., Meryk A., et al. 2013. Reply to Honjo et al.: functional relevant expression of Toso on granulocytes. Proc. Natl Acad. Sci. USA 110:E2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hitoshi Y., Lorens J., Kitada S. I., et al. 1998. Toso, a cell surface, specific regulator of Fas-induced apoptosis in T cells. Immunity 8:461. [DOI] [PubMed] [Google Scholar]
- 17. Honjo K., Kubagawa Y., Kubagawa H. 2012. Is Toso an antiapoptotic protein or an Fc receptor for IgM? Blood 119:1789. [DOI] [PubMed] [Google Scholar]
- 18. Nguyen X. H., Lang P. A., Lang K. S., et al. 2011. Toso regulates the balance between apoptotic and nonapoptotic death receptor signaling by facilitating RIP1 ubiquitination. Blood 118:598. [DOI] [PubMed] [Google Scholar]
- 19. Choi S. C., Wang H., Tian L., et al. 2013. Mouse IgM Fc receptor, FCMR, promotes B cell development and modulates antigen-driven immune responses. J. Immunol. 190:987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shimada S., Kawaguchi-Miyashita M., Kushiro A., et al. 1999. Generation of polymeric immunoglobulin receptor-deficient mouse with marked reduction of secretory IgA. J. Immunol. 163:5367. [PubMed] [Google Scholar]
- 21. Honda S., Kurita N., Miyamoto A., et al. 2009. Enhanced humoral immune responses against T-independent antigens in Fcα/μR-deficient mice. Proc. Natl Acad. Sci. USA 106:11230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nimmerjahn F., Ravetch J. V. 2008. Fcγ receptors as regulators of immune responses. Nat. Rev. Immunol. 8:34. [DOI] [PubMed] [Google Scholar]
- 23. Mackay M., Stanevsky A., Wang T., et al. 2006. Selective dysregulation of the FcγRIIB receptor on memory B cells in SLE. J. Exp. Med. 203:2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Su K., Yang H., Li X., et al. 2007. Expression profile of FcγRIIb on leukocytes and its dysregulation in systemic lupus erythematosus. J. Immunol. 178:3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cohen P. L., Eisenberg R. A. 1991. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu. Rev. Immunol. 9:243. [DOI] [PubMed] [Google Scholar]
- 26. Suda T., Nagata S. 1997. Why do defects in the Fas-Fas ligand system cause autoimmunity? J. Allergy Clin. Immunol. 100:S97. [DOI] [PubMed] [Google Scholar]
- 27. Oliver A. M., Grimaldi J. C., Howard M. C., Kearney J. F. 1999. Independently ligating CD38 and FcγRIIB relays a dominant negative signal to B cells. Hybridoma 18:113. [DOI] [PubMed] [Google Scholar]
- 28. Nimmerjahn F., Bruhns P., Horiuchi K., Ravetch J. V. 2005. FcγRIV: a novel FcR with distinct IgG subclass specificity. Immunity 23:41. [DOI] [PubMed] [Google Scholar]
- 29. Hashimoto A., Suzuki Y., Suzuki H., et al. 2012. Determination of severity of murine IgA nephropathy by glomerular complement activation by aberrantly glycosylated IgA and immune complexes. Am. J. Pathol. 181:1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boross P., Arandhara V. L., Martin-Ramirez J., et al. 2011. The inhibiting Fc receptor for IgG, FcγRIIB, is a modifier of autoimmune susceptibility. J. Immunol. 187:1304. [DOI] [PubMed] [Google Scholar]
- 31. Sato-Hayashizaki A., Ohtsuji M., Lin Q., et al. 2011. Presumptive role of 129 strain-derived Sle16 locus in rheumatoid arthritis in a new mouse model with Fcγ receptor type IIb-deficient C57BL/6 genetic background. Arthritis Rheum. 63:2930. [DOI] [PubMed] [Google Scholar]
- 32. Clarke S. H. 2008. Anti-Sm B cell tolerance and tolerance loss in systemic lupus erythematosus. Immunol. Res. 41:203. [DOI] [PubMed] [Google Scholar]
- 33. Kishi Y., Higuchi T., Phoon S., et al. 2012. Apoptotic marginal zone deletion of anti-Sm/ribonucleoprotein B cells. Proc. Natl Acad. Sci. USA 109:7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lu T. T., Cyster J. G. 2002. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science 297:409. [DOI] [PubMed] [Google Scholar]
- 35. Izui S., Kelley V. E., Masuda K., Yoshida H., Roths J. B., Murphy E. D. 1984. Induction of various autoantibodies by mutant gene lpr in several strains of mice. J. Immunol. 133:227. [PubMed] [Google Scholar]
- 36. Vieira P., Rajewsky K. 1988. The half-lives of serum immunoglobulins in adult mice. Eur. J. Immunol. 18:313. [DOI] [PubMed] [Google Scholar]
- 37. Xiang Z., Cutler A. J., Brownlie R. J., et al. 2007. FcγRIIb controls bone marrow plasma cell persistence and apoptosis. Nat. Immunol. 8:419. [DOI] [PubMed] [Google Scholar]
- 38. Pritchard N. R., Cutler A. J., Uribe S., Chadban S. J., Morley B. J., Smith K. G. 2000. Autoimmune-prone mice share a promoter haplotype associated with reduced expression and function of the Fc receptor FcγRII. Curr. Biol. 10:227. [DOI] [PubMed] [Google Scholar]
- 39. Jiang Y., Hirose S., Abe M., et al. 2000. Polymorphisms in IgG Fc receptor IIB regulatory regions associated with autoimmune susceptibility. Immunogenetics 51:429. [DOI] [PubMed] [Google Scholar]
- 40. Boes M., Esau C., Fischer M. B., Schmidt T., Carroll M., Chen J. 1998. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J. Immunol. 160:4776. [PubMed] [Google Scholar]
- 41. Baker N., Ehrenstein M. R. 2002. Cutting edge: selection of B lymphocyte subsets is regulated by natural IgM. J. Immunol. 169:6686. [DOI] [PubMed] [Google Scholar]
- 42. Dal Porto J. M., Burke K., Cambier J. C. 2004. Regulation of BCR signal transduction in B-1 cells requires the expression of the Src family kinase Lck. Immunity 21:443. [DOI] [PubMed] [Google Scholar]
- 43. Alugupalli K. R. 2008. A distinct role for B1b lymphocytes in T cell-independent immunity. Curr. Top. Microbiol. Immunol. 319:105. [DOI] [PubMed] [Google Scholar]
- 44. Hao Y., O’Neill P., Naradikian M. S., Scholz J. L., Cancro M. P. 2011. A B-cell subset uniquely responsive to innate stimuli accumulates in aged mice. Blood 118:1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rubtsov A. V., Rubtsova K., Fischer A., et al. 2011. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c⁺ B-cell population is important for the development of autoimmunity. Blood 118:1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shultz L. D., Coman D. R., Lyons B. L., Sidman C. L., Taylor S. 1987. Development of plasmacytoid cells with Russell bodies in autoimmune “viable motheaten” mice. Am. J. Pathol. 127:38. [PMC free article] [PubMed] [Google Scholar]
- 47. Tarlinton D., Förster I., Rajewsky K. 1992. An explanation for the defect in secretion of IgM Mott cells and their predominant occurrence in the Ly-1 B cell compartment. Eur. J. Immunol. 22:531. [DOI] [PubMed] [Google Scholar]
- 48. Jäck H. M., Beck-Engeser G., Sloan B., Wong M. L., Wabl M. 1992. A different sort of Mott cell. Proc. Natl Acad. Sci. USA 89:11688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jiang Y., Hirose S., Hamano Y., et al. 1997. Mapping of a gene for the increased susceptibility of B1 cells to Mott cell formation in murine autoimmune disease. J. Immunol. 158:992. [PubMed] [Google Scholar]
- 50. Corcos D., Osborn M. J., Matheson L. S., et al. 2010. Immunoglobulin aggregation leading to Russell body formation is prevented by the antibody light chain. Blood 115:282. [DOI] [PubMed] [Google Scholar]
- 51. Khan S. N., Cox J. V., Nishimoto S. K., et al. 2011. Intra-Golgi formation of IgM-glycosaminoglycan complexes promotes Ig deposition. J. Immunol. 187:3198. [DOI] [PubMed] [Google Scholar]
- 52. Radic M., Weigert M. G., Khan S. N., Han J., Kalinina O., Luning Prak E. T. 2013. Antibodies that bind complex glycosaminoglycans accumulate in the Golgi. Proc. Natl Acad. Sci. USA 110:11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ehrenstein M. R., Cook H. T., Neuberger M. S. 2000. Deficiency in serum immunoglobulin (Ig)M predisposes to development of IgG autoantibodies. J. Exp. Med. 191:1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Boes M., Schmidt T., Linkemann K., Beaudette B. C., Marshak-Rothstein A., Chen J. 2000. Accelerated development of IgG autoantibodies and autoimmune disease in the absence of secreted IgM. Proc. Natl Acad. Sci. USA 97:1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jiang C., Foley J., Clayton N., et al. 2007. Abrogation of lupus nephritis in activation-induced deaminase-deficient MRL/lpr mice. J. Immunol. 178:7422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bolland S., Ravetch J. V. 2000. Spontaneous autoimmune disease in FcγRIIB-deficient mice results from strain-specific epistasis. Immunity 13:277. [DOI] [PubMed] [Google Scholar]
- 57. Yajima K., Nakamura A., Sugahara A., Takai T. 2003. FcγRIIB deficiency with Fas mutation is sufficient for the development of systemic autoimmune disease. Eur. J. Immunol. 33:1020. [DOI] [PubMed] [Google Scholar]
- 58. McGaha T. L., Karlsson M. C., Ravetch J. V. 2008. FcγRIIB deficiency leads to autoimmunity and a defective response to apoptosis in Mrl-MpJ mice. J. Immunol. 180:5670. [DOI] [PubMed] [Google Scholar]
- 59. Fossati-Jimack L., Cortes-Hernandez J., Norsworthy P. J., Cook H. T., Walport M. J., Botto M. 2008. Regulation of B cell tolerance by 129-derived chromosome 1 loci in C57BL/6 mice. Arthritis Rheum. 58:2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Carlucci F., Cortes-Hernandez J., Fossati-Jimack L., et al. 2007. Genetic dissection of spontaneous autoimmunity driven by 129-derived chromosome 1 Loci when expressed on C57BL/6 mice. J. Immunol. 178:2352. [DOI] [PubMed] [Google Scholar]
- 61. Chan O. T., Hannum L. G., Haberman A. M., Madaio M. P., Shlomchik M. J. 1999. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J. Exp. Med. 189:1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shlomchik M. J. 2009. Activating systemic autoimmunity: B’s, T’s, and tolls. Curr. Opin. Immunol. 21:626. [DOI] [PMC free article] [PubMed] [Google Scholar]