Abstract
The effect of nitrogen source (N2 or nitrate) on carbon assimilation by photosynthesis and on carbon partitioning between shoots and roots was investigated in pea (Pisum sativum L. ‘Baccara’) plants at different growth stages using 13C labelling. Plants were grown in the greenhouse on different occasions in 1999 and 2000. Atmospheric [CO2] and growth conditions were varied to alter the rate of photosynthesis. Carbon allocation to nodulated roots was unaffected by N source. At the beginning of the vegetative period, nodulated roots had priority for assimilates over shoots; this priority decreased during later stages and became identical to that of the shoot during seed filling. Carbon allocation to nodulated roots was always limited by competition with shoots, and could be predicted for each phenological stage: during vegetative and flowering stages a single, negative exponential relationship was established between sink intensity (percentage of C allocated to the nodulated root per unit biomass) and net photosynthesis. At seed filling, the amount of carbon allocated to the nodulated root was directly related to net photosynthesis. Respiration of nodulated roots accounted for more than 60 % of carbon allocated to them during growth. Only at flowering was respiration affected by N supply: it was significantly higher for strictly N2‐fixing plants (83 %) than for plants fed with nitrate (71 %). At the vegetative stage, the increase in carbon in nodulated root biomass was probably limited by respiration losses.
Key words: Roots, nodules, legumes, C partitioning, symbiotic nitrogen fixation, Pisum sativum L., 13C labelling
INTRODUCTION
It has long been suggested that symbiotic N2 fixation requires more carbon than absorption of nitrate by roots and so would impair carbon accumulation by whole plants (Minchin and Pate, 1973; Silsbury, 1977; Ryle et al., 1979b). However, relationships between C and N nutrition remain unclear. Some authors have reported symptoms of N deficiency (stress) in the early stages of growth for strictly N2‐fixing pea plants (Oghoghorie and Pate, 1971); others have observed optimal growth and N nutrition for field‐grown pea plants regardless of the N source (Sagan et al., 1993; Crozat et al., 1994; Voisin et al., 2002a). Nitrate supply to pea in the field was rarely found to increase yields, except when provided late in the growth cycle (Jensen, 1986). The same was observed for soybean (Isfan, 1991; Yinbo, 1997) even though it is more sensitive to N supply and N source than other legumes (Crozat et al., 1994). Biomass accumulation and partitioning within the plant also differ with N source, particularly in the glasshouse where light may be more limiting than it is in the field, with strictly N2‐fixing plants having less root biomass but more nodule biomass than plants supplied with nitrate (Oghoghorie and Pate, 1971; Pate et al., 1979; Ryle et al., 1979b; Butler and Ladd, 1985; Jensen, 1986; Schulze et al., 1999). Understanding the relationship between biomass accumulation and the mode of N nutrition of legumes (i.e. symbiotic N2 fixation vs. absorption of mineral N from the soil) requires a better understanding of carbon partitioning and use within the plant. However, photosynthetic carbon input is difficult to compare among plants relying on different N sources, since nitrate often increases both leaf area and photosynthesis and thus stimulates shoot and root biomass (Mahon and Child, 1979; Lodeiro et al., 2000).
Differences in shoot and nodulated root biomass could be explained by differences in carbon use efficiency (i.e. the ratio of C increment in biomass over C increment in biomass plus respiration). Carbon use efficiency may be unaffected by N source, as in cowpea (Atkins et al., 1980), or it may be greater for nitrate‐assimilating than for N2‐fixing plants, as in lupin (Pate et al., 1979), pea and faba bean (Schulze et al., 1999). Carbon allocated to below‐ground organs has various possible fates which can be modified by nitrate supply: carbon may be incorporated into root and nodule biomass; respired for synthesis and maintenance processes; or used to provide carbon skeletons for the amino compounds that are translocated to the shoots. It should be noted that in temperate legumes, such as pea, nitrate is largely reduced in roots, whereas it is mainly reduced in shoots of most tropical legumes (Wallace, 1986). The percentage of photosynthetic C allocated to nodulated roots and the amount of C respired may be higher for nodulated than non‐nodulated roots (Pate et al., 1979; Ryle et al., 1979a, b; Atkins et al., 1980; Schulze et al., 1999). These results are likely to vary during the growth cycle, with the degree of variation depending on the species (Herridge and Pate, 1977; Atkins et al., 1978). These observations have led to the hypothesis, mentioned above, that higher C costs are incurred by symbiotic N2 fixation than nitrate assimilation (Pate et al., 1979; Ryle et al., 1979a, b; Atkins et al., 1980; Schulze et al., 1999). However, it remains difficult to quantify and compare carbon costs associated with both symbiotic N2‐fixation activity and nodule biosynthesis and maintenance (Phillips, 1980). The strategy of adaptation to the higher C costs of symbiotic N2 fixation varies among species, with some having greater photosynthetic capacities when relying on symbiotic N2 fixation (e.g. faba bean), whilst others (e.g. pea) suffer the additional C costs at the expense of root tissue (Schulze et al., 1994, 1999).
The objectives of this study were to determine how nitrogen sources modify carbon fluxes during growth of pea plants, and to understand the rules that drive C distribution and use. Carbon partitioning between shoots and nodulated roots, and C loss by respiration of nodulated roots were quantified in relation to the mode of N nutrition using 13C. Atmospheric [CO2] and sowing date were altered to provide treatments to alter net photosynthesis and biomass partitioning within the plant.
MATERIALS AND METHODS
Plant material and growing conditions
Pea plants (Pisum sativum L. ‘Baccara’) were sown in 5 l plastic pots (eight seeds per pot) on three occasions (15 Sep. 1999, 2 Mar. 2000, 26 May 2000) and were inoculated with Rhizobium leguminosarum bv vicieae. The growing medium was a 1 : 1 (v/v) mixture of sterilized attapulgite and clay balls (diameter 2–6 mm). This medium has no buffering effect, allows gases to diffuse easily and is inert to gas, thus avoiding interference with CO2 and O2 measurements. At emergence, plants were thinned to four per pot. Since plants were grown in a naturally lit glasshouse (Dijon, France), delaying the date of sowing was a means of varying growing conditions, owing to natural variations in temperature, radiation and photoperiod. The temperature in the glasshouse was maintained at over 5 °C during winter, and the roof opened automatically when the temperature exceeded 20 °C. The genotype used (Baccara) requires long days for flower initiation and has a facultative vernalization requirement. Supplementary artificial light was provided in autumn 1999 (total day length 16 h d–1) to induce flowering. Plants were watered regularly and maintained at water‐retention capacity using nutrient solution (P, K, S, Mg, Ca, Na, Fe, oligoelements: Co, B, Mn, Zn, Mo, Cu) according to plant transpiration needs. Timing of irrigation was controlled by two balances carrying one pot each, which automatically triggered watering of all pots of a treatment when the cumulated transpiration since the last watering exceeded one‐tenth of the soil water reserve.
Experimental treatments
For each sowing date, two different N treatments were applied to obtain various rates of symbiotic N2 fixation. As it is difficult to preclude nodulation on pea roots throughout the growth cycle, a level of nitrate supply that reduces nodule biomass (and symbiotic N2 fixation) by 50 % compared with that of control plants (only fixing N2) was chosen during a preliminary experiment: half of the plants (90 pots) were provided with N‐free nutrient solution (N0 treatment, plants fixing N2 only) and the other half with 5 mol m–3 N as KNO3 in the nutrient solution (N5 treatment, plants assimilating both NO3 and N2).
For each group of plants corresponding to different sowing dates, three pots of each treatment were selected for 13C labelling at three characteristic stages: ‘vegetative’ (ten nodes on the main stem); ‘flowering’ (flowers on the sixth node of the main stem, before the beginning of seed filling); and ‘seed filling’ (between the final stage of seed abortion and the start of physiological maturity). The early flowering stage (two reproductive nodes) was also studied in 1999.
In 2000, in order to obtain a range of photosynthetic rates for a given phenological stage, three different concentrations of CO2 in air (150, 360 and 750 µl l–1) were applied to the different plant sets (three sowing dates) during 13C labelling.
13C labelling
The equipment used for 13C labelling was similar to that used by Warembourg et al. (1982) and described by Voisin et al. (2000). Roots and shoots were maintained in separate compartments: pots of plants were inserted into individual air‐tight plastic containers to separate root and shoot atmospheres. Air leaks between aerial and root atmospheres were prevented by using physiological moulding material (Qubitac; Qubit Systems Inc., Kingston, Canada) and silicone rubber (RTV; Zundel Kohler, Illzach, France) around the stems of plants. The pots with separate root atmospheres were placed in a transparent, air‐tight labelling chamber (0·95 × 0·95 × 1·5 m) made of Plexiglas.
At each chosen CO2 concentration (150, 360 and 750 µl l–1), shoots were exposed to a 13C‐enriched atmosphere for 10 h: the CO2 concentration was measured continuously using an infrared gas analyser (IRGA; PP system; Ciras, Montigny le Bretonneux, France) and maintained by automatic CO2 injection. To obtain a constant and uniformly labelled atmosphere, all CO2 in the atmosphere within the chamber was trapped for 20 min at the beginning of the experiment; the required CO2 concentration (150, 360 or 750 µl l–1) was then achieved and maintained by injecting a mixture of CO2 with a constant 13C : 12C ratio (10 atom %13C). 13C enrichment of the atmosphere around the shoot during the labelling period was assessed by mass spectrometry of samples taken regularly.
To study the fate of photosynthates, labelling was followed by a non‐labelling period (chase) lasting 4 d in 1999 and 2 d in 2000 (Montange et al., 1981; Kouchi et al., 1986), with plants being held at 360 µl l–1 CO2. Within the chamber, temperature and humidity of the air around the shoots were maintained using an air‐conditioning unit. Light was supplied by four 400 W sodium lamps placed on each side of the enclosure and two 1000 W mercury lamps situated above it. The resultant photosynthetic active radiation varied from 600–900 µmol m–2 from the bottom to the top of the canopy. Soil water was adjusted gravimetrically each day. Throughout labelling and during the chase, the soil atmosphere entering the pots was CO2‐free air. Respiration of nodulated roots was measured by trapping CO2 from the pots by bubbling the gas stream through NaOH solution (0·5 mol.l–1). The whole system was monitored by computer using Dasylab software (Newport Omega, Trappes, France).
Harvesting and measurements
Plants were harvested at the beginning of the labelling experiment (control plants) and at the end of the chase period (labelled plants). All plants in each pot were harvested together and separated into shoots, roots and nodules. Dry matter was determined after oven drying at 80 °C for 48 h. Concentrations of C and N were determined by the Dumas procedure on ground samples. Their 13C enrichment (atom%13C) was measured using a dual‐inlet mass spectrometer (Fisons Isochron; Micromass, Villeurbanne, France).
Respiration of nodulated roots was measured by titration of the NaOH solution used to trap the CO2 evolved. 13C enrichment of the respired CO2 was measured using a mass spectrometer on a gaseous sample produced by acidification of the trap solutions. The total amount of respiration was obtained by summation of the daily respiration values from the start of labelling until harvest.
Calculations
Isotopic determinations were carried out according to the isotope dilution principle. The percentage of carbon that was derived from the labelled source (%13C; I) can be calculated by:
I = 100 (S – C)/(A – C)
where C is atom%13C untreated plants (control) and A is atom%13C labelled atmosphere, where S is atom%13C sample in [plant material (shoot, roots, nodules) or respired C] (Deléens et al., 1994). For each component (plant sample, CO2), the quantity of carbon derived from photosynthesis during the labelling period (QC) was calculated using I, dry matter (M) and carbon (C) determinations:
QC = M(C/100)(I/100)
Statistics
For each treatment, values were the average of three measurements (three pots). Analysis of variance was performed using the GLM procedure of SAS, and means were compared using the least significant difference test (LSD) at the 0·05 probability level (SAS Institute, 1987).
RESULTS
Plant biomass partitioning related to N source and growing conditions
An example of the isotopic content of pea plants is given in Table 1. The different N treatments and growing conditions altered both plant biomass accumulation and partitioning (Table 2). Total biomass was significantly larger for plants grown with nitrate (N5 treatment) than for those fixing N2 only (N0 treatment; Table 2). For each sowing date, plants from the N5 treatment had greater (30–60 %) shoot biomass than those from the N0 treatment. Roots of plants in both N treatments were nodulated, but plants supplied with nitrate produced 35–110 % more root biomass and 60–70 % less nodule biomass compared with those only fixing N2 (Table 2). Considering the different growing periods, biomass of all parts was significantly smaller for plants grown in autumn 1999 compared with that of plants grown in spring and summer 2000 (Table 2). Overall, total growth decreased as the sowing date was delayed from March to September.
Table 1.
δ13C isotopic content (δ‰13C) of carbon in strictly N2‐fixing pea plants (N0) and in plants supplied with nitrate
| Vegetative stage | Flowering | Seed filling | ||||
| Isotopic content of C (δ‰ 13C) | Control | Labelled | Control | Labelled | Control | Labelled |
| N0 treatment | ||||||
| Leaves and stems | –28·7 | 597 | –29·5 | 528 | –22·0 | 157 |
| Pod walls | – | – | –28·3 | 1022 | –24·0 | 71 |
| Seeds | – | – | –27·3 | 1289 | –27·9 | 452 |
| Roots | –27·5 | 127 | –28·4 | 122 | –28·6 | 94 |
| Nodules | –28·3 | 396 | –28·5 | 96 | –29·0 | 206 |
| N5 treatment | ||||||
| Leaves and stems | –28·9 | 614 | –29·3 | 558 | –27·0 | 250 |
| Pod walls | – | – | –28·4 | 1040 | –26·9 | 156 |
| Seeds | – | – | –26·2 | 1232 | –27·0 | 682 |
| Roots | –27·9 | 214 | –28·2 | 267 | –28·6 | 126 |
| Nodules | –27·9 | 197 | –28·0 | 229 | –28·5 | 109 |
Pea plants were sown on 26 May 2002. Values are means for three pots before (control plants) and after (labelled) exposure to 13CO2 labelling.
Table 2.
Biomass and total N concentration at the seed filling stage for strictly N2‐fixing plants (N0 treatment) and for plants supplied with nitrate (N5 treatment) for each experiment
| Plant set (sowing date) | Total biomass (g per plant) | Shoot biomass (g per plant) | Root biomass (g per plant) | Nodule biomass (g per plant) |
| 15 September 1999 | ||||
| N5 | 8·9 ± 2·1 | 8·4 ± 2·1 | 0·39 ± 0·12 | 0·04 ± 0·02 |
| N0 | 6·6 ± 0·3 | 6·2 ± 0·2 | 0·29 ± 0·06 | 0·13 ± 0·09 |
| 3 March 2000 | ||||
| N5 | 22·9 ± 1·8 | 21·6 ± 1·8 | 1·21 ± 0·40 | 0·07 ± 0·05 |
| N0 | 17·3 ± 0·7 | 16·4 ± 0·7 | 0·73 ± 0·03 | 0·17 ± 0·01 |
| 16 March 2000 | ||||
| N5 | 20·5 ± 1·7 | 19·4 ± 1·7 | 1·02 ± 0·11 | 0·05 ± 0·02 |
| N0 | 14·4 ± 1·9 | 13·5 ± 1·8 | 0·71 ± 0·09 | 0·16 ± 0·01 |
| 26 May 2000 | ||||
| N5 | 13·0 ± 1·6 | 12·1 ± 1·5 | 0·83 ± 0·07 | 0·04 ± 0·01 |
| N0 | 8·2 ± 1·4 | 7·7 ± 1·3 | 0·40 ± 0·07 | 0·10 ± 0·03 |
Plant were grown in a glasshouse on different occasions in 1999 and 2000.
Net photosynthesis related to 13CO2 concentration
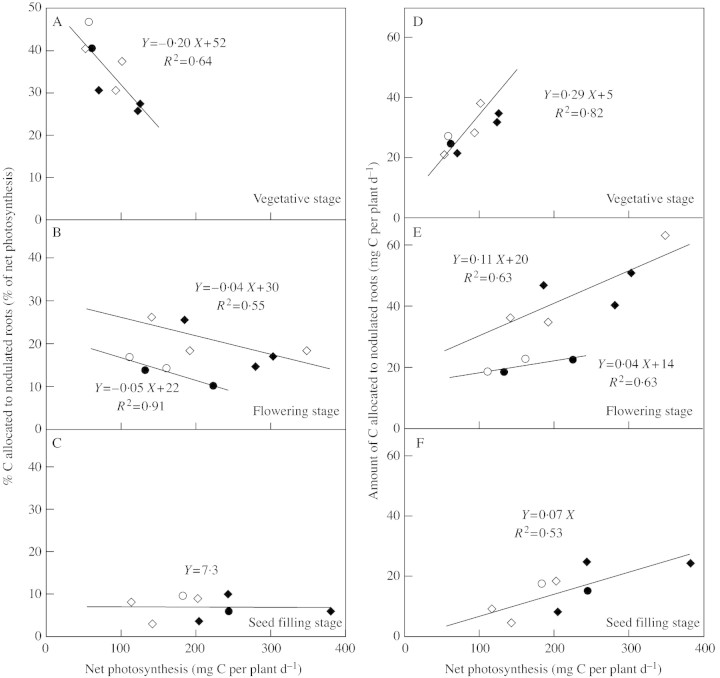
The amount of labelled C in plant parts at harvest, and in CO2 from root respiration after each 13C experiment, was used to estimate net C assimilation by photosynthesis during each period of exposure. Combining the different sowing dates and CO2 concentrations, net C assimilation by photosynthesis ranged from 51 to 124 mg per plant d–1 at the vegetative stage, from 110 to 346 mg per plant d–1 at flowering and from 112 to 378 mg per plant d–1 at seed filling (Fig. 1).
Fig. 1. Carbon allocation to nodulated roots (calculated as C increase in biomass plus nodulated root respiration) expressed as a percentage of net photosynthesis (A–C), or as mg C (D–F) during growth (A and D, vegetative; B and E, flowering; C and F, seed filling stages) of Pisum sativum, plotted as a function of net C‐assimilation for two N treatments: N2‐fixing plants (N0 treatment) (open symbols) or plants supplied with nitrate (N5 treatment) (closed symbols), for experiments conducted in 1999 (circles) and 2000 (diamonds).
C allocation to nodulated roots
Carbon partitioning between shoots and nodulated roots was related to daily total net C assimilated at each phenological stage (Fig. 1). Carbon allocated to nodulated roots was calculated as the sum of C increment in nodulated root biomass plus nodulated root respiration that originated from the labelling period.
The percentage of photosynthetic C allocated to nodulated roots decreased during growth for both N treatments, from around 45 % at the vegetative stage (Fig. 1A) to less than 10 % at seed filling (Fig. 1C). During the vegetative (Fig. 1A) and flowering (Fig. 1B) stages, C allocation to nodulated roots decreased linearly when net C‐photosynthesis increased, regardless of N treatment. Slopes of the regressions between C allocation to nodulated roots and net C‐photosynthesis decreased with phenology. At the seed filling stage (Fig. 1C), allocation remained stable (around 7 % of net C‐photosynthesis) regardless of the rate of net C‐photosynthesis. At flowering (Fig. 1B), values were systematically lower in 1999 than in 2000, as was nodulated root biomass (Table 2), suggesting that relationships between C allocation to nodulated roots and net C‐photosynthesis depend upon initial biomass of nodulated roots.
At each phenological stage, the amount of C allocated to nodulated roots increased with net photosynthesis (Fig. 1D–F), despite the fact that the percentage of C allocated to the nodulated roots decreased (or remained stable) with increasing net C‐photosynthesis (Fig. 1A–C). In all cases, allocation followed the same relationships irrespective of the different nitrogen sources (Fig. 1).
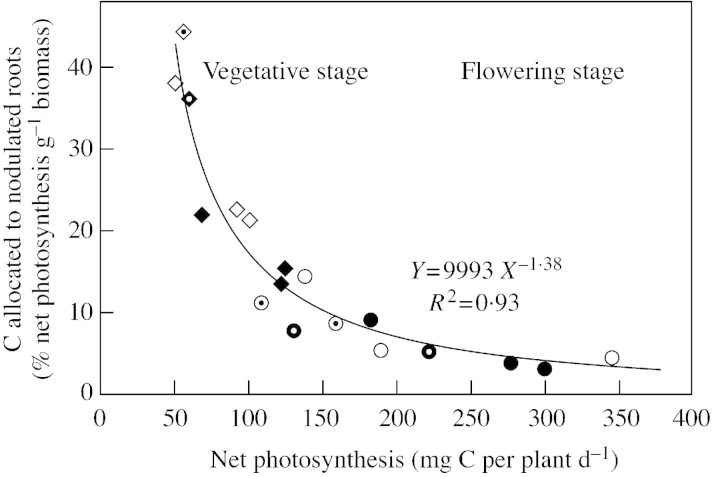
As mentioned above, carbon allocation to nodulated roots depended upon nodulated root biomass (Fig. 1B and E): for vegetative and flowering points, there was a single relationship between allocation per unit biomass (% C allocated to the nodulated roots per unit of nodulated root biomass) and net photosynthesis, regardless of N treatment or growth stage (Fig. 2).
Fig. 2. Percentage of net photosynthesis allocated to nodulated roots of Pisum sativum expressed per unit of nodulated root biomass and plotted as a function of net C‐assimilation for two N treatments: N2‐fixing plants (N0 treatment) (open symbols), or plants supplied with nitrate (N5 treatment) (closed symbols), at the vegetative (diamonds) and flowering (circles) stages. Symbols with a dot in the centre represent data from experiments conducted in 1999; all other data are from 2000.
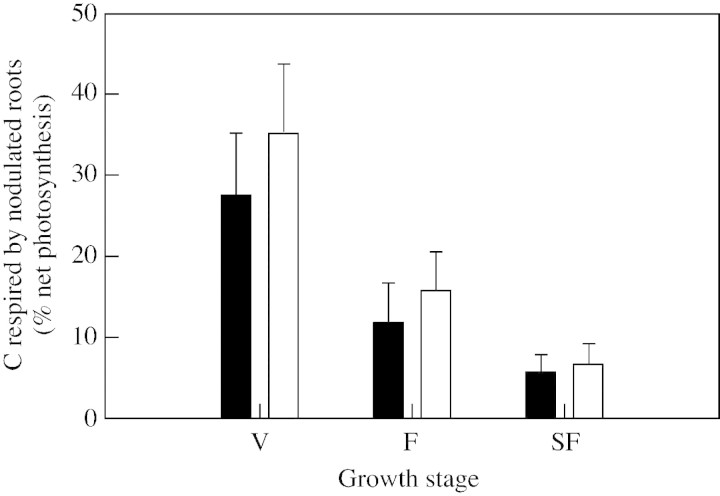
C‐respiration by nodulated roots
Nodulated root respiration was normalized by expressing it as a percentage of net C‐photosynthesis, thus enabling comparisons to be made among plants of different sizes at different stages (Fig. 3). Differences among N treatments were not significant, regardless of the growth stage (Fig. 3). Averaged over both N treatments, the percentage of net photosynthesis respired by nodulated roots decreased during growth from 31 % at the vegetative stage to 6 % at seed filling (Fig. 3).
Fig. 3. Percentage of daily net photosynthesis respired by nodulated roots of Pisum sativum during growth in two N treatments: N2‐fixing plants (N0 treatment) (open bars) or plants supplied with nitrate (N5 treatment) (filled bars). Values are means for the different growing conditions. V, Vegetative stages; F, flowering; SF, seed filling stages. Bars represent s.d.
To investigate C utilization within the root systems, nodulated root respiration was then expressed as a percentage of C allocated to nodulated roots. Nodulated root respiration always accounted for more than 60 % of the total C allocated to nodulated roots (Fig. 4). At the vegetative stage this percentage decreased with increasing net photosynthesis (Fig. 4A), whereas it remained constant during flowering (Fig. 4B) and seed filling (Fig. 4C). At flowering, and only at that stage, the percentage of C allocated to nodulated roots that was respired differed significantly between the two N treatments, being approx. 83 % for the strictly N2‐fixing plants and 71 % for those supplied with nitrate (Fig. 4B).
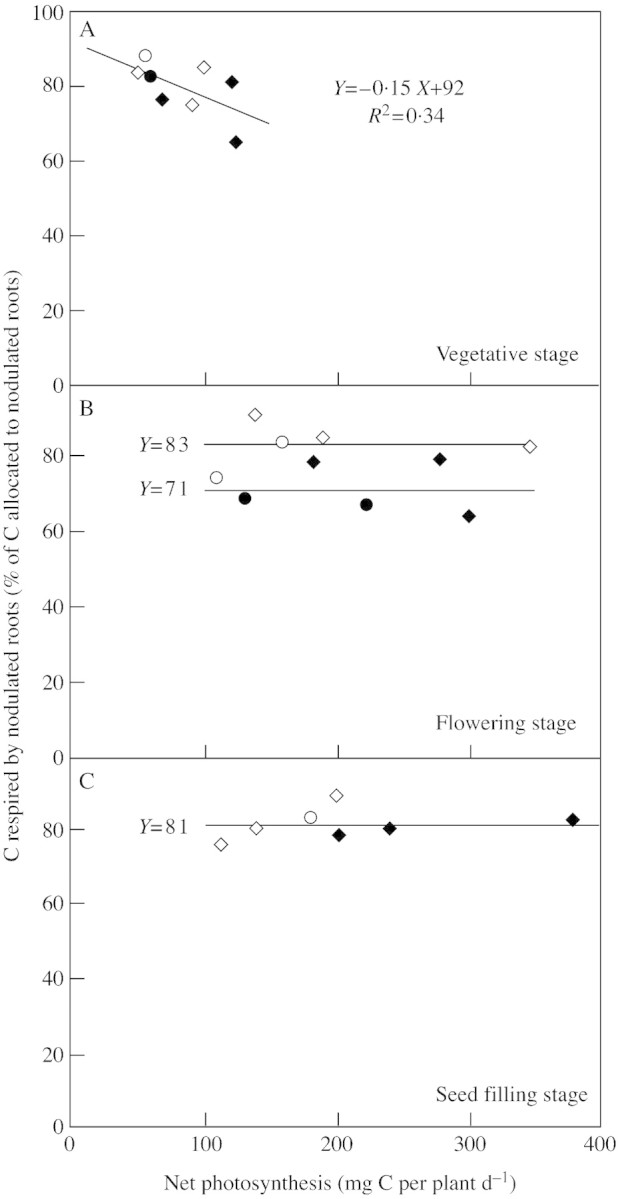
Fig. 4. Carbon respiration by nodulated roots, expressed as a percentage of carbon allocated to nodulated roots during growth (A, vegetative; B, flowering; C, seed filling stages) of Pisum sativum, plotted as a function of net C‐assimilation for two N treatments: N2‐fixing plants (N0 treatment) (open symbols), or plants supplied with nitrate (N5 treatment) (closed symbols), for experiments conducted in 1999 (circles) and 2000 (diamonds).
DISCUSSION
Our calculations of net photosynthesis included carbon incorporated into both shoot and nodulated root biomass, plus nodulated root respiration, but did not account for shoot respiration. However, in pea plants, shoot respiration is usually a constant proportion of net photosynthesis (15 %) and is not influenced by N nutrition (Pate et al., 1979; Ryle et al., 1979b; Schulze et al., 1999). Thus, if there is a difference in carbon use efficiency, it is more likely to appear in below‐ground organs. The amount of carbon allocated to nodulated roots included both nodulated root respiration and C accumulated in biomass. It is a net value since some of the carbon that reaches nodulated roots via the phloem is transported back to the shoot through the xylem as amino‐compound skeletons (Pate and Herridge, 1978).
Carbon partitioning between shoots and nodulated roots
Percentages (5–50 %) and patterns of net C allocated to nodulated roots of pea (Fig. 1A–C) are similar to those described for cowpea (Herridge and Pate, 1977). However, they contrast with the high (around 50 %) and stable values reported for lupin (Atkins et al., 1978; Pate and Herridge, 1978). Although several authors have suggested that C supply to nodulated roots was higher than to non‐nodulated roots (Pate et al., 1979; Ryle et al., 1979b; Atkins et al., 1980), and was therefore dependent upon the N source, this was not the case in the present study (Fig. 1A–C). However, until flowering, the percentage of C allocated to nodulated roots varied with photosynthetic rate (Fig. 1A and B). Since plants grown with nitrate usually had more shoot and leaf biomass, differences previously observed (Pate et al., 1979; Ryle et al., 1979b; Atkins et al., 1980) among plants relying on different N sources can be explained by differences in photosynthetic rate.
The percentage of net photosynthetic C allocated to nodulated roots was considered to be an indicator of the sink strength of nodulated roots compared with that of shoots. Variations of this indicator with net photosynthetic rate allowed a general relationship to be established for carbon partitioning between shoots and nodulated roots. The increased sink strength of nodulated roots when net photosynthesis decreased indicated the priority of C allocation to nodulated roots over that to shoots (Fig. 1A–C). This priority decreased as the plant aged; the slope of the linear regression between % C allocated to nodulated roots and net photosynthesis decreased to zero between the vegetative (Fig. 1A) and seed filling (Fig. 1C) stages. At seed filling, despite low sink strength (7 % of net photosynthesis), the priority of nodulated roots for C equalled that of shoots. Regression slopes at flowering (Fig. 1B), established separately for data collected in 1999 and 2000 (which differed in nodulated root biomass; Table 2), were not significantly different, suggesting that C partitioning between shoots and nodulated roots did not depend on the biomass of nodulated roots or on the form of N supplied (Table 2).
Is the sink strength of nodulated roots limited by photosynthesis?
At each phenological stage, the amount of photosynthetic C allocated to nodulated roots increased when net photosynthesis was enhanced (Fig. 1D–F). This suggests that nodulated roots were never saturated with assimilate, despite having a higher priority than the shoots, as discussed above. The slope of the linear regression between the amount of C allocated to nodulated roots and net photosynthesis decreased during growth (Fig. 1–F), indicating that the nodulated root was probably less well supplied with C as growth progressed, presumably in relation to variation in demand for C for synthesis and/or functioning of nodulated roots.
From the present data, it was impossible to determine the sink size of nodulated roots, as stabilization of C uptake by nodulated roots (C allocation to nodulated roots) with C supply by photosynthesis was not observed. At seed filling (Fig. 1F), carbon allocation to nodulated roots may have reached a plateau at approx. 20 mg per plant d–1 when net photosynthesis was over 250 mg C per plant d–1.
CO2 respiration by nodulated roots
The percentage of net photosynthesis that was respired by nodulated roots was not affected by N nutrition (Fig. 3), as previously shown for cowpea (Atkins et al., 1978). However, the proportion, and its variation during growth, may differ among species. The percentage of net photosynthesis respired by nodulated roots increased from 30 to 50 % during growth for lupin (Pate and Herridge, 1978), and from 20 to 40 % for cowpea (Herridge and Pate, 1977), whereas it decreased from 31 to 6 % for pea in the present experiment (Fig. 3).
Respiration was the main component of C utilization within nodulated roots (Fig. 4), probably limiting C accumulation in nodulated root biomass. Within nodulated roots, the percentage of C lost by respiration decreased with net photosynthesis at the vegetative stage in both N treatments (Fig. 4A) and followed the same pattern as % C allocated to nodulated roots (Fig. 1A). From flowering, the percentage of C allocated to nodulated roots that was respired did not vary, despite changes in net photosynthesis, showing identical priority for respiration and increase in biomass. At flowering, however, the percentage of C allocated to nodulated roots that was respired was significantly higher for the strictly N2‐fixing plants than for those supplied with nitrate (Fig 4B), suggesting a difference in carbon use and/or carbon use efficiency according to the N source, i.e. a higher C cost for the process of symbiotic fixation. At the seed filling stage, the percentage of C allocated to the nodulated roots that was respired remained constant and was independent of N nutrition regime.
C partitioning at each growth stage
At the vegetative stage, establishment of nodulated roots seemed to take priority over shoot growth (Fig. 1A), even if shoot growth obviously limited the amount of C allocated to the nodulated root (Fig. 1D). At this early stage, when organs are forming, demand for assimilates may never totally be satisfied for shoots or nodulated roots. Nodulated root growth may be limited by the high C costs incurred by synthetic processes in the whole plant (Fig. 4A).
Sink strength of nodulated roots was lower at flowering than during vegetative growth (Fig. 1B) owing to the appearance of reproductive organs that constitute a competitive sink for assimilates (Jeuffroy and Warembourg, 1991). Growth of nodulated roots may also have been almost complete at this stage (Tricot, 1993). Carbon would then be used mainly for maintenance and symbiotic N2‐fixation activity within nodulated roots. Since these processes both depend upon biomass (Tricot‐Pellerin et al., 1994), C allocation to nodulated roots at flowering may depend on the biomass of nodulated roots, as shown by the single relationship established between the percentage of C allocated to the nodulated root per unit biomass (sink intensity) and net photosynthesis (Fig. 2) for both years, with plants differing in biomass of nodulated roots (Table 2). Data from the vegetative stage followed the same single relationship (Fig. 2). Thus, carbon allocation to nodulated roots at the vegetative stage can be calculated as a function of net photosynthesis using two different relationships (Figs 1D and Fig. 2, derived from Fig. 1). Of these, only the relationship shown in Fig. 1D depends upon nodulated root biomass. This suggests that there are two components that determine carbon allocation to nodulated roots, and that the component linked to biomass is dominant at the flowering stage only. At flowering, the greater proportion of the percentage C supplied to the nodulated root respired in strictly N2‐fixing plants compared with those supplied with nitrate (Fig. 4B) suggests higher C costs for the strictly N2‐fixing plants, induced by demands related to nodule maintenance and/or nodule activity.
At seed filling (Fig. 1F), the amount of C allocated to the nodulated root was directly related to photosynthesis. In contrast to previous stages when C allocation to the nodulated root depended upon nodulated root biomass (Fig. 2), this showed that nodulated roots behaved as a passive sink at the end of the growth cycle (Fig. 1F). Carbon may be mainly used for maintenance at this late stage, as nodulated roots become less active and senesce (Voisin et al., 2002b) at a rate that depends on C supply to them.
The predictive relationships established in this study will be useful for a better understanding of legume C accumulation patterns since, as pointed out by Pate and Armstrong (1996), C integration and use in nodulated roots have often been neglected, despite their obvious requirement for assimilates and their importance in water and nutrient uptake. Moreover, the percentage of net photosynthesis allocated to nodulated roots can be substantial (up to 45 % at the vegetative stage) and respiratory losses can be more than 60 % of this allocation. It was shown that C use within nodulated roots may depend upon N nutrition at flowering. However, further investigation is needed to determine whether synthesis, maintenance or N accumulation, associated either with N2 fixation or with nitrate assimilation, were affected.
ACKNOWLEDGEMENTS
Our grateful thanks are due to V. Durey, J. Gonthier and P. Mathey for excellent technical assistance. We also thank B. Mary and O. Delfosse (ENSAIA, Nancy) for vegetal 13C isotopic analysis and J. N. Thibault and P. Garnier (INRA, Rennes) for gaseous 13C isotopic analysis. This work was partly funded by INRA, UNIP and Conseil Régional de Bourgogne.
Supplementary Material
Received: 16 October 2002; Returned for revision: 8 November 2002; Accepted: 17 December 2002 Published electronically: 29 January 2003
References
- AtkinsCA, Herridge DF, Pate JS.1978. The economy of carbon and nitrogen in nitrogen‐fixing annual legumes Vienna: International Atomic Agency, 211–242. [Google Scholar]
- AtkinsCA, Pate JS, Griffiths GJ, White ST.1980. Economy of carbon and nitrogen in nodulated and nonnodulated (NO3– grown) cowpea (Vigna unguiculata L. Walp.). Plant Physiology 66: 978–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ButlerJH, Ladd JN.1985. Symbiotically‐fixed and soil‐derived nitrogen in legumes grown in pots in soils with different amounts of available nitrate. Soil Biology and Biochemistry 17: 47–55. [Google Scholar]
- CrozatY, Aveline A, Coste F, Gillet JP, Domenach AM.1994. Yield performance and seed production pattern of field‐grown pea and soybean in relation to N nutrition. European Journal of Agronomy 3: 135–144. [Google Scholar]
- DeléensE, Cliquet JB, Prioul J‐L.1994. Use of 13C and 15N plant label near natural abundance for monitoring carbon and nitrogen partitioning. Australian Journal of Plant Physiology 21: 133–146. [Google Scholar]
- HerridgeDF, Pate JS.1977. Utilisation of net photosynthate for nitrogen fixation and protein production in an annual legume. Plant Physiology 60: 759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IsfanD.1991. Fertilizer nitrogen uptake by soybean as related to cultivars and time of application using 15N dilution technique. Journal of Plant Nutrition 14: 1369–1380. [Google Scholar]
- JensenES.1986. The influence of rate and time of supply on nitrogen fixation and yield in pea (Pisum sativum L.). Fertilizer Research 10: 193–202. [Google Scholar]
- JeuffroyMH, Warembourg FR.1991. Carbon transfer and partitioning between vegetative and reproductive organs in Pisum sativum L. Plant Physiology 97: 440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KouchiH, Akao S, Yoneyama T.1986. Respiratory utilisation of 13C‐labelled photosynthate in nodulated root systems of soybean plants. Journal of Experimental Botany 37: 985–993. [Google Scholar]
- LodeiroAR, Gonzalez P, Hernandez A, Balague LJ, Faveukes G.2000. Comparison of drought tolerance in nitrogen‐fixing and inorganic nitrogen‐grown common beans. Plant Science 154: 31–41. [DOI] [PubMed] [Google Scholar]
- MahonJD, Child JF.1979. Growth response of inoculated peas (Pisum sativum) to combined nitrogen. Canadian Journal of Botany 57: 1687–1693. [Google Scholar]
- MinchinRR, Pate JS.1973. The carbon balance of a legume and the functional economy of its root nodules. Journal of Experimental Botany 24: 259–271. [Google Scholar]
- MontangeD, Warembourg FR, Bardin R.1981. Utilisation du 15N2 pour estimer la fixation d’azote et sa répartition chez les légumineuses. Plant and Soil 63: 131–139. [Google Scholar]
- OghoghorieCGO, Pate JS.1971. The nitrate stress syndrome of the nodulated field pea (Pisum arvense L.). Techniques for measurement evaluation in physiological terms. In: Lie TA, Muller EG, eds. Biological nitrogen fixation in natural and agricultural habitats Plant and Soil, Special Issue. The Hague: Martinus Nijhoff, 185–202. [Google Scholar]
- PateJS, Armstrong EL.1996. Pea. In: Zamski E, Schaffer AA, eds. Photoassimilate distribution in plants and crops source‐sink relationships, New York: Dekker Inc. 625–642. [Google Scholar]
- PateJS, Herridge DF.1978. Partitioning and utilization of net photosynthate in a nodulated legume. Journal of Experimental Botany 29: 401–412. [Google Scholar]
- PateJS, Layzell DB, Atkins CA.1979. Economy of carbon and nitrogen in a nodulated and non nodulated (NO3– grown) legume. Plant Physiology 64: 1083–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PhillipsDA.1980. Efficiency of symbiotic nitrogen fixation in legumes.Annual Review of Plant Physiology 31: 29–49. [Google Scholar]
- RyleGJA, Powell CE, Gordon AJ.1979a The respiratory costs of nitrogen fixation in soybean, cowpea and white clover. I. Nitrogen fixation and the respiration of nodulated roots. Journal of Experimental Botany 30: 113–118. [Google Scholar]
- RyleGJA, Powell CE, Gordon AJ.1979b The respiratory costs of nitrogen fixation in soybean, cowpea and white clover. II. Comparison of nitrogen fixation and the utilisation of combined nitrogen. Journal of Experimental Botany 30: 145–153. [Google Scholar]
- SaganM, Ney B, Duc G.1993. Plant symbiotic mutants as a tool to analyse nitrogen and yield relationship in field‐grown peas (Pisum sativum L.). Plant and Soil 153: 33–45. [Google Scholar]
- SAS Institute.1987. SAS/STAT Guide for Personal Computer. 6th edition. Cary, NC: SAS Institute. [Google Scholar]
- SchulzeJ, Adgo E, Merbach W.1999. Carbon costs associated with N2 fixation in Vicia faba L. and Pisum sativum L. over a 14‐day period. Plant Biology 1: 625–631. [Google Scholar]
- SchulzeJ, Adgo E, Shilling G.1994. The influence of N2 fixation on the carbon balance of leguminous plants. Experientia 50: 906–916. [Google Scholar]
- SilsburyJH.1977. Energy requirement for symbiotic nitrogen fixation. Nature 267: 149–150. [DOI] [PubMed] [Google Scholar]
- TricotF.1993. Mise en place des nodosités du pois protéagineux de printemps (Pisum sativum L.). Influence de la nutrition carbonée. PhD Thesis, Université Paris‐Sud Orsay, France. [Google Scholar]
- Tricot‐PellerinF, Angevin F, Crozat Y.1994. Elaboration de la biomasse des nodosités: Influence de la nutrition carbonée. In: UNIP, ITCF, INRA, eds. Agrophysiologie du pois protéagineux Paris: INRA Editions, 75–91. [Google Scholar]
- VoisinAS, Salon C, Munier‐Jolain NG, Ney B.2002a Effect of mineral nitrogen on nitrogen nutrition and biomass partitioning between the shoot and roots of pea (Pisum sativum L.). Plant and Soil 242: 251–262. [Google Scholar]
- VoisinAS, Salon C, Munier‐Jolain NG, Ney B.2002b Quantitative effect of soil nitrate, growth potential and phenology on symbiotic nitrogen fixation of pea (Pisum sativum L.). Plant and Soil 243: 31–42. [Google Scholar]
- VoisinAS, Warembourg FR, Jeudy C, Salon C.2000. Construction d’une chambre phytotronique de marquage isotopique double C/N, pour l’étude du fonctionnement des légumineuses: description du dispositif. Cahiers Techniques de l’ I.N.R.A. 45: 3–13. [Google Scholar]
- WallaceW.1986. Distribution and nitrate assimilation of between the root and shoot of legumes and comparison with wheat. Physiologia Plantarum 66: 630–636. [Google Scholar]
- WarembourgFR, Montange D, Bardin R.1982. The simultaneous use of 14CO2 and 15N2 labelling techniques to study the carbon and nitrogen economy of legumes grown under natural conditions. Physiologia Plantarum 56: 46–55. [Google Scholar]
- YinboG, Peoples MB, Rerkasem B.1997. The effect of N fertilizer on N2 fixation, growth and yield of vegetable soybean. Field Crop Research 51: 221–229. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.