Abstract
Background:
Elevators are ubiquitous and active inside hospitals, potentially facilitating bacterial transmission. The objective of this study was to estimate the prevalence of bacterial colonization on elevator buttons in large urban teaching hospitals.
Methods:
A total of 120 elevator buttons and 96 toilet surfaces were swabbed over separate intervals at 3 tertiary care hospitals on weekdays and weekends in Toronto, Ontario. For the elevators, swabs were taken from 2 interior buttons (buttons for the ground floor and one randomly selected upper-level floor) and 2 exterior buttons (the "up" button from the ground floor and the "down" button from the upper-level floor). For the toilet surfaces, swabs were taken from the exterior and interior handles of the entry door, the privacy latch, and the toilet flusher. Samples were obtained using standard bacterial collection techniques, followed by plating, culture, and species identification by a technician blind to sample source.
Results:
The prevalence of colonization of elevator buttons was 61% (95% confidence interval 52%–70%). No significant differences in colonization prevalence were apparent in relation to location of the buttons, day of the week, or panel position within the elevator. Coagulase-negative staphylococci were the most common organisms cultured, whereas Enterococcus and Pseudomonas species were infrequent. Elevator buttons had a higher prevalence of colonization than toilet surfaces (61% v. 43%, p = 0.008).
Conclusions:
Hospital elevator buttons were commonly colonized by bacteria, although most pathogens were not clinically relevant. The risk of pathogen transmission might be reduced by simple countermeasures.
Hospital-acquired infections are a substantial cause of morbidity and mortality.1,2 The point prevalence of nosocomial infection among hospital inpatients is estimated to be as high as 10%.3 Furthermore, even brief exposure to a hospital emergency department can increase the risk of such an infection.4 A variety of inanimate objects, including white coats, computer keyboards, cellular telephones, stethoscopes, adhesive tape, ultrasound transducers, and radiographic equipment, harbour bacteria.5–12 Previous studies have most commonly identified colonization by skin bacteria, such as coagulase-negative staphylococci.6,7,9–11 Surface contamination has also been implicated in the propagation of drug-resistant bacteria.13,14 Moreover, bacteria can persist on inanimate objects for days.15
We hypothesized that buttons in hospital elevators may be an additional under-recognized site of microbial contamination. At a single university in a community setting, for example, about one-third of elevator buttons were colonized by bacteria.16 The corresponding frequency of colonization in hospitals has not been described. If present, such colonization creates the potential for pathogen transmission, given the ubiquity of elevators in large hospitals, the necessity of using the buttons to operate the elevator, and repeated contacts by diverse individuals. Pathogen transmission may occur if use of the buttons is associated with ineffective hand hygiene by individuals who interact with hospital inpatients. The objective of this study was to estimate the prevalence of bacterial colonization of elevator buttons in large teaching hospitals.
Methods
Study setting.
We performed this study at 3 large urban teaching hospitals located in Toronto, Ontario. These hospitals represented a combined total of 1490 acute inpatient hospital beds (range 353 to 677 per hospital). Eligible elevators selected for inclusion had the following characteristics: connected to the majority of patient floors; opened to the main floor of the hospital (defined as street level); considered the most used (as judged by a service attendant at the hospital information desk); and available to patients, visitors, and health care professionals. The research ethics board of Sunnybrook Health Sciences Centre approved the study.
Collection of specimens.
At each hospital, 4 elevator buttons were swabbed for the presence of bacteria on 10 separate days between 5 and 21 November 2012. Specimens were collected midmorning from Monday to Wednesday, so that each sample could be incubated for 48 hours. To ensure a robust sampling strategy, one weekend day (a Sunday) was included. All samples were collected by a single individual with training in microbial collection techniques (C.E.K.).17,18
Each individual elevator button was swabbed in a standardized fashion with a sterile, single-use Transystem Culture Swab and Transport System (Copan Diagnostics, Inc., Murrieta, Calif.).19 The dry swab was removed from its sterile packaging by the individual collecting the samples, who immediately used it to swab the entire surface of one button for 3 seconds in a continuous motion while rotating the tip. For each elevator on each sampling day, a total of 4 buttons were swabbed: 2 exterior buttons and 2 interior buttons. Specifically, the exterior "up" button outside the elevator on the main floor, the exterior "down" button on a randomly selected upper-level floor, the interior "number" button to travel to the selected upper-level floor, and the interior "ground" button to return to the main floor were swabbed.
A random number generator ("Undecided," Apple Computer Company iTunes App Store) that used the Lehmer algorithm was employed to determine the random upper-level floor destination at each hospital. To do so, the individual collecting the specimens activated the random number generator when approaching a hospital's elevator bank to determine which destination floor to select at that hospital. Because each elevator had 2 interior button panels and each elevator bank had 2 exterior button panels, an additional randomization was undertaken to determine the panel to be swabbed by activating the same random number generator application. This randomized selection process was repeated every day during the study period, with no exceptions or anomalies.
Collection of control samples.
We returned to the same hospitals a few months later to assess bacterial colonization of the public washrooms closest to the elevators, using the same sample collection techniques. Surfaces in the men's washroom were swabbed (which may have introduced bias, although the difference in surface colonization between men's and women's washrooms is minimal20). Four toilet surfaces were swabbed over 8 separate days from 17 to 27 March 2013. Collections occurred daily from Sunday to Wednesday with the same time constraints for sample processing. From each public washroom, swabs were taken from the exterior and interior entry-door handles, the privacy latch, and the toilet flusher. When more than one toilet was available, randomization was performed to select the stall to be swabbed. If the designated washroom had automatic toilet-flushing mechanisms, we substituted the nearest manually operated toilet.
Processing of specimens.
The specimens were maintained at room temperature for a maximum of 2 hours until the daily collection was completed, after which they were stored at 4°C until inoculated for culture. Each specimen was inoculated separately on blood agar and MacConkey agar, followed by aerobic incubation at 37°C for up to 48 hours. Plates were examined on 2 sequential days, and the organisms grown were identified to the genus level at a minimum. We specifically assessed for the following organisms: Staphylococcus aureus, coagulase-negative staphylococci, Streptococcus spp., Pseudomonas spp., enterococci, diphtheroids, coliform bacteria, and other (miscellaneous) organisms. Neither Gram staining nor growth quantification was undertaken. Susceptibility testing was reserved for detection of methicillin-resistant Staphylococcus aureus ( MRSA) a nd v ancomycinresistant enterococcus (VRE) using the polymerase chain reaction for the nuc and mec genes (for MRSA) and the vanA and vanB genes (for VRE).21,22 We did not perform advanced cultures because of a lack of personnel. All samples were cultured and characterized by the same trained laboratory technician, who was unaware of the study hypothesis or swab origin.
Statistical analysis.
The primary outcome was the prevalence of bacteria on elevator buttons. We used a 2-tailed χ2 test to assess for significant differences in colonization prevalence between the interior buttons and the exterior buttons as the primary comparison. Interior buttons are challenging to sterilize, are more likely to be touched by every passenger, and may be more likely to lead to pathogen transmission. In secondary analyses, we compared left and right elevator panels, days of the week, and individual hospitals. Analogous statistics were replicated for the control toilet surfaces, with a prespecified secondary analysis comparing the 4 surfaces (exterior and interior entry-door handles, individual privacy latch, toilet flusher). All p values were 2-tailed and were calculated using Stat- View version 5.0 (SAS Institute Inc., Cary, N.C.), with 0.05 defined as the threshold of statistical significance.
Results
A total of 120 elevator buttons were swabbed over the study period, a completion rate of 100%, with 108 (90%) of the samples collected on weekdays. Specimen collection was evenly distributed by hospital and date. The most common randomized elevator destinations were the ninth floor at hospital 1, the ninth floor at hospital 2, and the third and fourth floors at hospital 3. About half of the swabbed elevator buttons were located on the left-hand panel (exterior and interior). No adverse events or service disruptions occurred during the study.
A total of 73 samples from the 120 cultures showed microbiological growth, equivalent to a colonization prevalence of 61% (95% confidence interval 52%–70%). The most common organisms cultured were coagulasenegative staphylococci, followed by Streptococcus spp. (Table 1). The distribution of coagulase-negative staphylococci was relatively even across the buttons, whereas Streptococcus spp. and coliform bacteria were predominately isolated from the interior elevator buttons. One sample grew Pseudomonas sp., 2 samples grew Enterococcus spp., and another grew a fungal species. No specimens were positive for Staphylococcus aureus, MRSA, or VRE.
Table 1.
Bacteria cultured from elevator buttons and toilet surfaces*
| Organism | Sampling site; no. (%) of samples* | |
|---|---|---|
| Elevators n = 120 |
Toilet surfaces n = 96 |
|
| Staphylococcus† | 67 (56) | 35 (36) |
| Streptococcus | 11 (9) | 7 (7) |
| Coliform bacteria | 10 (8) | 2 (2) |
| Enterococcus | 2 (2) | 0 (0) |
| Pseudomonas | 1 (1) | 1 (1) |
| Miscellaneous‡ | 2 (2) | 4 (4) |
Samples were collected on different dates. The sum of percentages in each column is greater than overall prevalence because multiple organisms were cultured from some sites (polymicrobial colonization).
Coagulase-negative staphylococci in all cases.
Includes other Gram-negative bacilli and fungi.
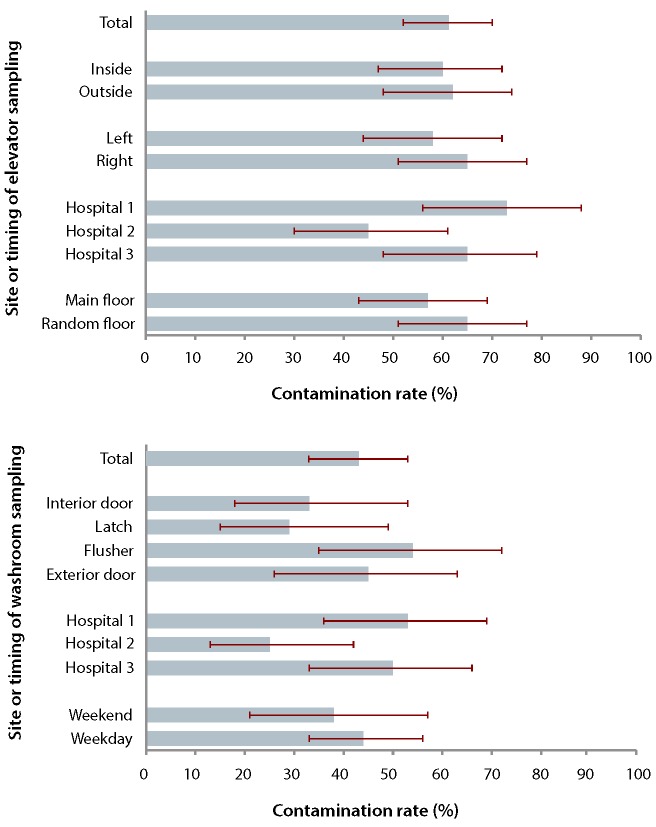
We found no statistically significant difference in the prevalence of colonization between the interior and exterior elevator buttons (60% v. 62%, p = 0.85). Findings were also consistent by day of the week (weekend 60% v. 58% weekday, p = 0.85), button panel side (left 58% v. right 65%, p = 0.46), a nd selected floor (street level 57% v. upper level 65%, p = 0.35). Among the interior and exterior buttons, we found similar colonization prevalence between the main floor and the random upper floor (Figure 1). Colonization prevalence varied somewhat among the 3 hospitals (range 45% to 73%, p = 0.034).
Figure 1.
Prevalence of bacterial colonization of elevator buttons (top) and toilet surfaces (bottom). Bars show bacterial growth for samples from different surface locations, in relation to different variables (e.g., hospital, day of sample collection). For each bar, the corresponding 95% confidence interval is denoted by a solid line. For toilet surfaces, "interior door" refers to the inside handle of the entry door, and "exterior door" refers to the outer handle of the entry door.
Of the 96 toilet surface specimens, 72 (75%) were collected on weekdays. A total of 41 specimens showed microbiological growth, equivalent to a colonization prevalence of 43% (95% confidence interval 33%– 53%). Toilet surfaces had significantly lower colonization prevalence than elevator buttons (61% v. 43%, p = 0.008). The colonization prevalence varied marginally among the 4 surfaces (range 29% to 54%, p = 0.47). We observed no significant variation by day of the week (weekend 38% v. weekday 44%, p = 0 .55). There was modest variability among the 3 hospitals (range 25% to 53%, p = 0.063). Coagulase-negative staphylococci were the most frequently cultured organisms, and the distribution of bacterial species was approximately even across the toilet surfaces. Four surfaces grew a fungus and one grew Pseudomonas sp.
Interpretation
Our elevator buttons in 3 large urban hospitals were colonized with bacteria typical of skin commensal organisms. Relative to prior research (based on different collection and culture techniques), which documented a prevalence rate of 30% at a large university,16 we found that hospital elevator buttons were more likely to be colonized and less likely to grow Staphylococcus aureus. We also found that samples were unlikely to demonstrate significant asymmetry between interior and exterior buttons. Individual hospitals varied somewhat, yet the prevalence of colonization in all hospital elevators exceeded that observed in the community.16 Elevator buttons had higher colonization rates than toilet surfaces in the same buildings. In turn, the washroom surfaces sampled in our study had higher contamination rates than those reported previously.23
The majority of colonizing bacteria had low pathogenicity. This pattern is reassuring and in keeping with the extremely low rates of hospital-acquired MRSA and VRE at the participating hospitals.24 Absence of pathogenic organisms on elevator buttons is a testament to the prevailing cleaning services combined with widespread hand hygiene. Although the prevalence of colonization of elevator buttons in our study was lower than that for computer keyboards6 and ultrasound transducers11 in previous studies, patients remain at potential risk of cross-contamination because of the frequent use of these buttons by diverse individuals. In addition, a visitor is more likely to come into contact with an elevator button or a toilet than with inanimate hospital equipment and may transmit organisms if interacting with inpatients.
Our study had several limitations that merit emphasis. Sample collection occurred during the influenza season, which may have prompted increased use of hand sanitizer. In addition, cold autumn weather may have increased the use of gloves, which block the transmission of hand organisms. Alternatively, the influenza season may have resulted in increased hospital traffic and generalized exposures. Sampling hospitals from a single geographic area may limit generalizability, and replication of the study elsewhere would be valuable. Swabbing the elevator buttons mostly in the morning might have introduced additional bias if, for example, environmental cleaning varied (individual hospital cleaning schedules were unavailable, but could account for variation in colonization prevalence). The optimum standards for cleaning elevator buttons are unknown.25
Additional bias might have resulted from collecting elevator button and toilet surface specimens during 2 different seasons, as nosocomial infections fluctuate on a monthly basis.26 Only some bacterial species were assessed, which precluded identification of other potential pathogens such as Clostridium difficile, viral respiratory pathogens, and enteric viruses (all of which require specialized culture techniques that were unavailable in this study). Smaller inocula of microorganisms may not have been detected because broth enhancement techniques were not used.17 Together, these limitations may have led to underestimation of colonization prevalence, yielding an unduly reassuring assessment.
Although many inanimate objects harbour bacteria, hospital elevator buttons represent a frequently encountered fomite because of their ubiquity and frequency of use. Alcohol-based hand sanitizers are effective for removing surface bacteria,27 and their strategic placement inside and outside elevators might attenuate some of the potential risk of pathogen transmission. Additional countermeasures could include enlarging the buttons to allow for elbow activation or installing touchless proximity sensors. A fourth approach to mitigate risk could be increased public education about hand hygiene targeted to individuals exiting elevators and visitors who tend to exhibit poor hand hygiene.28 Ultimately, an awareness of risk might spur greater attention throughout a hospital.
Acknowledgments
We are indebted to Christine Watt, the microbiology technician without whom we would not have been able to conduct this study. We thank Gabor Kandel, Rita Kandel, Mark Cheung, Kevin Imrie, Adina Weinerman, and Wayne Gold for helpful comments on earlier drafts.
Footnotes
None declared.
This project was supported by a Canada Research Chair in Medical Decision Sciences, the Canadian Institutes of Health Research, and the University of Toronto, Faculty of Medicine. The funding organizations had no role in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation, review, or approval of the manuscript.
Contributor Information
Christopher E Kandel, Christopher E. Kandel, MD, is a resident in internal medicine at the University of Toronto, Toronto, Ontario..
Andrew E Simor, Andrew E. Simor, MD, FRCPC, is a Professor of Medicine and of Laboratory Medicine and Pathobiology at the University of Toronto, Head of the Department of Microbiology and the Division of Infectious Diseases at Sunnybrook Health Sciences Centre, and a Senior Scientist at the Sunnybrook Research Institute, Toronto, Ontario..
Donald A Redelmeier, Donald A. Redelmeier, MD, FRCPC, MSHSR, FACP, is a Professor of Medicine at the University of Toronto, the Director of Evaluative Clinical Sciences at the Sunnybrook Research Institute, a Staff Physician in the Division of General Internal Medicine at Sunnybrook Health Sciences Centre, and a Senior Scientist at the Institute for Clinical Evaluative Sciences, Toronto, Ontario..
References
- 1.Gravel D, Taylor G, Ofner M, Johnston L, Loeb M, Roth VR, et al. Point prevalence survey for healthcare-associated infections within Canadian adult acute-care hospitals. J Hosp Infect. 2007;66(3):243–248. doi: 10.1016/j.jhin.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Klevens RM, Edwards JR, Richards CL, Jr, Horan TC, Gaynes RP, Pollock DA, et al. Estimating health care-associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122(2):160–166. doi: 10.1177/003335490712200205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler-Jones D. The chief public health officer's report on the state of public health in Canada 2013. Final report. Ottawa (ON): Public Health Agency of Canada; 2013. Sep, Report No.: 130329. Cat. No.: HP2- 10/2013E. [Google Scholar]
- 4.Quach C, McArthur M, McGeer A, Li L, Simor A, Dionne M, et al. Risk of infection following a visit to the emergency department: a cohort study. CMAJ. 2012;184(4):E232–E239. doi: 10.1503/cmaj.110372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Treakle AM, Thom KA, Furuno JP, Strauss SM, Harris AD, Perencevich EN. Bacterial contamination of health care workers' white coats. Am J Infect Control. 2009;37(2):101–105. doi: 10.1016/j.ajic.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schultz M, Gill J, Zubairi S, Huber R, Gordin F. Bacterial contamination of computer keyboards in a teaching hospital. Infect Control Hosp Epidemiol. 2003;24(4):302–303. doi: 10.1086/502200. [DOI] [PubMed] [Google Scholar]
- 7.Brady RR, Verran J, Damani NN, Gibb AP. Review of mobile communication devices as potential reservoirs of nosocomial pathogens. J Hosp Infect. 2009;71(4):295–300. doi: 10.1016/j.jhin.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 8.Kei J, Richards JR. The prevalence of methicillin-resistant Staphylococcus aureus on inanimate objects in an urban emergency department. J Emerg Med. 2011;41(2):124–127. doi: 10.1016/j.jemermed.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Tang PH, Worster A, Srigley JA, Main CL. Examination of staphylococcal stethoscope contamination in the emergency department (pilot) study (EXSSCITED pilot study) CJEM. 2011;13(4):239–244. doi: 10.2310/8000.2011.110242. [DOI] [PubMed] [Google Scholar]
- 10.Redelmeier DA, Livesley NJ. Adhesive tape and intravascular-catheter- associated infections. J Gen Intern Med. 1999;14(6):373–375. doi: 10.1046/j.1525-1497.1999.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mullaney PJ, Munthali P, Vlachou P, Jenkins D, Rathod A, Entwisle J. How clean is your probe? Microbiological assessment of ultrasound transducers in routine clinical use, and cost-effective ways to reduce contamination. Clin Radiol. 2007;62(7):694–698. doi: 10.1016/j.crad.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Levin PD, Shatz O, Sviri S, Moriah D, Or-Barbash A, Sprung CL, et al. Contamination of portable radiograph equipment with resistant bacteria in the ICU. Chest. 2009;136(2):426–432. doi: 10.1378/chest.09-0049. [DOI] [PubMed] [Google Scholar]
- 13.Lowe C, Willey B, O'Shaughnessy A, Lee W, Lum M, Pike K, et al. Outbreak of extended-spectrum ß-lactamase-producing Klebsiella oxytoca infections associated with contaminated handwashing sinks. Emerg Infect Dis. 2012;18(8):1242–1247. doi: 10.3201/eid1808.111268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hota B. Contamination, disinfection, and cross-colonization: Are hospital surfaces reservoirs for nosocomial infection? Clin Infect Dis. 2004;39(8):1182–1189. doi: 10.1086/424667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate objects? A systematic review. BMC Infect Dis. 2006;6:130–130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooke JS, Annand JW, Hammer A, Dembkowski K, Shulman ST. Investigation of bacterial pathogens on 70 frequently used environmental surfaces in a large urban U.S. university. J Environ Health. 2009;71(6):17–22. [PubMed] [Google Scholar]
- 17.Dolan A, Bartlett M, McEntee B, Creamer E, Humphreys H. Evaluation of different methods to recover methicillin-resistant Staphylococcus aureus from hospital environmental surfaces. J Hosp Infect. 2011;79(3):227–230. doi: 10.1016/j.jhin.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Galvin S, Dolan A, Cahill O, Daniels S, Humphreys H. Microbial monitoring of the hospital environment: Why and how? J Hosp Infect. 2012;82(3):143–151. doi: 10.1016/j.jhin.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Perry JL. Assessment of swab transport systems for aerobic and anaerobic organism recovery. J Clin Microbiol. 1997;35(5):1269–1271. doi: 10.1128/jcm.35.5.1269-1271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flores GE, Bates ST, Knights D, Lauber CL, Stombaugh J, Knight R, et al. Microbial biogeography of public restroom surfaces. PLoS One. 2011;6(11):e28132–e28132. doi: 10.1371/journal.pone.0028132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McClure JA, Conly JM, Lau V, Elsayed S, Louie T, Hutchins W, et al. Novel multiplex PCR assay for detection of staphylococcal virulence marker Panton-Valentine leukocidin genes and simultaneous discrimination of methicillin-susceptible from -resistant staphylococci. J Clin Microbiol. 2006;44(3):1141–1144. doi: 10.1128/JCM.44.3.1141-1144.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sloan LM, Uhl JR, Vetter EA, Schleck CD, Harmsen WS, Manahan J, et al. Comparison of the Roche LightCycler vanA/vanB detection assay and culture for detection of vancomycin-resistant enterococci from perianal swabs. J Clin Microbiol. 2004;42(6):2636–2643. doi: 10.1128/JCM.42.6.2636-2643.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendes MF, Lynch DJ. A bacteriological survey of washrooms and toilets. J Hyg (Lond) 1976;76(2):183–190. doi: 10.1017/s002217240005508x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patient safety [webpage] Toronto (ON): Heath Quality Ontario; 2014. Available from: www.hqontario.ca/public-reporting/patient-safety (accessed 2014 Feb 16). [Google Scholar]
- 25. Ontario Agency for Health Protection and Promotion, Provincial Infectious Diseases Advisory Committee , author. Best practices for environmental cleaning for prevention and control of infections in all health care settings. 2nd revision. Final report. Toronto (ON): Queen's Printer for Ontario; 2012. May, [Google Scholar]
- 26.Richet H. Seasonality in Gram-negative and healthcare-associated infections. Clin Microbiol Infect. 2012;18(10):934–940. doi: 10.1111/j.1469-0691.2012.03954.x. [DOI] [PubMed] [Google Scholar]
- 27.WHO guidelines on hand hygiene in health care. Geneva (Switzerland): World Health Organization; 2009. Available from: http://whqlibdoc.who.int/publications/2009/9789241597906_eng.pdf (accessed 2013 Mar 7). [Google Scholar]
- 28.Randle J, Arthur A, Vaughan N. Twenty-four-hour observational study of hospital hand hygiene compliance. J Hosp Infect. 2010;76(3):252–255. doi: 10.1016/j.jhin.2010.06.027. [DOI] [PubMed] [Google Scholar]