Abstract
C4 photosynthesis is characterized by a division of labour between two different photosynthetic cell types, mesophyll and bundle‐sheath cells. Relying on phosphoenolpyruvate carboxylase (PEPC) as the primary carboxylase in the mesophyll cells a CO2 pump is established in C4 plants that concentrates CO2 at the site of ribulose 1,5‐bisphosphate carboxylase/oxygenase in the bundle‐sheath cells. The C4 photosynthetic pathway evolved polyphyletically implying that the genes encoding the C4 PEPC originated from non‐photosynthetic PEPC progenitor genes that were already present in the C3 ancestral species. The dicot genus Flaveria (Asteraceae) is a unique system in which to investigate the molcular changes that had to occur in order to adapt a C3 ancestral PEPC gene to the special conditions of C4 photosynthesis. Flaveria contains not only C3 and C4 species but also a large number of C3–C4 intermediates which vary to the degree in which C4 photosynthetic traits are expressed. The C4 PEPC gene of Flaveria trinervia, which is encoded by the ppcA gene class, is highly expressed but only in mesophyll cells. The encoded PEPC protein possesses the typical kinetic and regulatory features of a C4‐type PEPC. The orthologous ppcA gene of the C3 species Flaveria pringlei encodes a typical non‐photosynthetic, C3‐type PEPC and is weakly expressed with no apparent cell or organ specificity. PEPCs of the ppcA type have been detected also in C3–C4 intermediate Flaveria species. These orthologous PEPCs have been used to determine the molecular basis for C4 enzyme characteristics and to understand their evolution. Comparative and functional analyses of the ppcA promoters from F. trinervia and F. pringlei make it possible to identity the cis‐regulatory sequences for mesophyll‐specific gene expression and to search for the corresponding trans‐regulatory factors.
Key words: Phosphoenolpyruvate carboxylase, C4 photosynthesis, evolution, Flaveria
INTRODUCTION
The C4 photosynthetic carbon cycle is a sophisticated addition to the C3 photosynthetic pathway and enables C4 plants to cope well with high light intensities, high temperatures and dryness. For this reason, C4 plants dominate grassland floras and biomass production in the warmer climates of the tropical and subtropical regions. It is no surprise that C4 plants, such as sugar cane, maize and sorghum are among the most productive crops in agriculture (Brown, 1999; Sage, 1999).
The high photosynthetic capacity of C4 plants is due to their unique mode of carbon assimilation which involves two different photosynthetic cell types, mesophyll and bundle‐sheath cells. CO2 is initially fixed by phosphoenolpyruvate carboxylase (PEPC) in the mesophyll cells into the C4 acids malate and/or aspartate, which are then transported to the bundle‐sheath. In the bundle‐sheath cells the C4 acids are decarboxylated, and the CO2 is refixed by ribulose 1,5‐bisphosphate carboxylase/oxygenase (Hatch, 1987).
As a consequence of this CO2 concentration at the site of ribulose 1,5‐bisphosphate carboxylase/oxygenase, the competitive inhibition of this enzyme by oxygen, which becomes prominent at higher temperatures, is largely excluded and C4 plants show drastically reduced rates of photorespiration. In C3 plants this process may be responsible for a reduction in photosynthesis of up to 40 % (Ehleringer and Monson, 1993). The CO2 pump ensures high rates of photosynthesis even when CO2 concentrations are low in the intercellular air spaces of the leaf. With this, C4 plants are able to limit the opening of their stomata and thereby minimize water loss due to transpiration. As the CO2 pump delivers saturating concentrations of CO2 to the site of ribulose 1,5‐bisphosphate carboxylase/oxygenase high photosynthetic rates are maintained with less enzyme than is required in C3 species. This is reflected in a higher nitrogen use efficiency (Long, 1999).
The functioning of C4 photosynthesis depends upon strict compartmentation of the CO2 assimilatory enzymes into the photosynthetic cell types, mesophyll and bundle sheath cells. The primary carboxylating enzyme, phosphoenolpyruvate carboxylase, occurs exclusively in the mesophyll cells while the secondary carboxylase, ribulose 1,5‐bisphosphate carboxylase/oxygenase, and the decarboxylating enzymes NADP‐dependent malic enzyme, NAD‐dependent malic enzyme or phosphoenolpyruvate carboxykinase are restricted to the bundle‐sheath cells (Hatch, 1987; Kanai and Edwards, 1999). Enzymes of the photorespiratory C2 carbon cycle (Baldy and Cavalié, 1984), the nitrogen (Rathnam and Edwards, 1976) and sulphur (Schmutz and Brunold, 1984) assimilation pathways and of carbohydrate metabolism (Lunn and Hatch, 1995) also accumulate differentially in mesophyll and bundle‐sheath cells. Even the photosynthetic electron transport chains of mesophyll and bundle sheath chloroplasts of the NADP‐malic enzyme type of C4 plants differ. While thylakoid membranes of mesophyll chloroplasts possess a fully developed linear electron transport chain, those of the bundle‐sheath chloroplasts are devoid of grana and severely depleted in photosystem II (Woo et al., 1970; Meierhoff and Westhoff, 1993). These facts demonstrate that the C4 mode of photosynthesis involves a restructuring of the entire metabolic pathway of the mesophyll and bundle‐sheath cells and leads to a single, highly integrative metabolic system.
The vast majority of C4 plants uses this two‐cell mode, i.e. Kranz anatomy, to concentrate CO2 at the site of ribulose 1,5‐bisphosphate carboxylase/oxygenase. How ever, there are plants where inducible or permanent CO2 pumps have been established within a single cell (Holaday and Bowes, 1980; Freitag and Stichler, 2000, 2002). These one‐cell types of C4 photosynthesis are rare exceptions which apparently evolved only under very special environmental conditions.
The division of labour between mesophyll and bundle‐sheath cells is governed by differential gene expression. It is known that this differential expression of the genes encoding the C4 cycle enzyme is largely due to transcriptional control (Dengler and Nelson, 1999; Sheen, 1999). Differential expression has also been reported for nuclear as well as plastid‐encoded genes for photosystem II proteins (Sheen et al., 1987; Kubicki et al., 1994). Other genes are expressed differentially in C4 photosynthesis too (Wyrich et al., 1998; Furumoto et al., 2000; Renné et al., 2003) but the extent has still to be elucidated.
All gene expression data indicate that the two cell types follow distinct but complementary differentiation pathways. To date it is not known how this interdependence of mesophyll and bundle sheath cells is achieved during leaf development. Cell‐lineage analyses indicate that the two cells do not necessarily depend on a single clonal relationship. The available data suggest that positional information and light play important roles in determining the differentiation of mesophyll and bundle sheath cells (Dengler and Nelson, 1999).
C4 plants occur in at least 18 families of mono‐ and dicotyledonous plants (Sage et al., 1999). These families are phylogenetically quite separate from each other. This indicates that C4 plants must have evolved several times independently from C3 ancestors during the evolution of angiosperms. There is strong evidence that even within a single taxon, for instance the Gramineae, this transition from C3 to C4 may have occurred more than once (Kellogg, 1999; Monson, 1999). The multiple independent origin of C4 photosynthesis suggests that the evolution of a C3 into a C4 species must have been relatively easy in genetic terms and that just a few master genes, if any, would have been involved. The available molecular data on the C4 cycle enzymes support this point of view. None of the C4 enzymes are unique to C4 plants. Non‐photosynthetic isoforms of these enzymes are also present in C3 species and in the non‐photosynthetic tissues of C4 species. The ubiquity of these non‐photosynthetic isoforms of the C4 cycle enzymes in C3 plants strongly indicates that these ‘C3 isoforms’ served as the starting point for the evolution of the C4 genes (Monson, 1999).
At least three major changes must have occurred during the evolution to adapt the C4 progenitor gene for its function in C4 photosynthesis. First, C4 isoform genes are highly expressed (Harpster and Taylor, 1986; Hermans and Westhoff, 1990; Crétin et al., 1991) but C3 isoform genes are only moderately transcribed (Crétin et al., 1991; Kawamura et al., 1992; Ernst and Westhoff, 1996). The effectiveness of gene expression had therefore to be increased. Secondly, the C4 isoform genes had to evolve an organ‐ and cell‐specific expression pattern, because strict compartmentation of the C4 enzymes is imperative for proper functioning of the C4 cycle (Hatch, 1987). Thirdly, it is known, at least for PEPC, that the C4 cycle enzymes differ from their C3 counterparts in kinetic and regulatory characteristics (Ting and Osmond, 1973a; Svensson et al., 2003). Therefore, the coding regions had to be changed to achieve the required adaptations of the enzymatic properties.
To gain insight into the evolution of C4 genes the entry enzyme of the C4 cycle, PEPC is being used as our model C4 enzyme/gene and the genus Flaveria (Asteraceae; Powell, 1978) as our experimental system. Flaveria contains C3 and C4 species and, in addition, a large number of C3–C4 photosynthetic intermediates (Edwards and Ku, 1987). These intermediates differ quantitatively in the expression of C4 photosynthetic traits, and there is convincing evidence that at least some of these are true evolutionary intermediates (Monson and Moore, 1989). Their presence suggests that evolution towards C4 photosynthesis is still continuing in this genus.
Phosphoenolpyruvate carboxylase was selected for this evolutionary analysis because the biochemistry and molecular biology of this enzyme have been studied intensively both in C4 and C3 plants (Rajagopalan et al., 1994; Chollet et al., 1996). PEPC catalyses the irreversible carboxylation of phosphoenolpyruvate (PEP) to form oxaloacetate. The enzyme needs Mg2+ as an essential cofactor and requires that the inorganic carbon be supplied as bicarbonate. Consequently, mesophyll cells of C4 plants contain high amounts of carbonic anhydrase to fulfil the demands of PEPC when the carbon flux through the C4 cycle is high (Badger and Price, 1994). Native PEPC is a tetramer comprising four identical subunits each with a molecular mass of about 100 000 Da (Kai et al., 2003). PEPC activity is regulated by metabolites (Kai et al., 2003), but also post‐translationally by phosphorylation (Nimmo, 2003).
This brief review is summary of our work on the evolution of the enzymatic characteristics of C4 PEPC and of the transcriptional regulation of its gene in Flaveria.
THE GENUS FLAVERIA AND C4 PHOTOSYNTHESIS
The PEPC gene family
The photosynthetic and non‐photosynthetic PEPCs of the C4 plant F. trinervia are encoded by a small gene family which consists of three distinct classes, named ppcA, ppcB and ppcC (Fig. 1). The ppcA gene class contains two members and encodes the C4 isoform of PEPC (Poetsch et al., 1991; Hermans and Westhoff, 1992). The ppcB and ppcC gene classes probably consist of only one gene each and code for non‐photosynthetic PEPC isoforms. The exact physiological roles of the ppcB and ppcC PEPCs have not been determined (Ernst and Westhoff, 1996). Phylogenetic analysis of cDNA sequences revealed that ppcA and ppcB genes are sister gene classes. This indicates that they were derived from an ancestral ppcB‐like gene by gene duplication (Fig. 1; Bläsing et al., 2002). There may be a fourth gene class, named ppcD. However, the existence of this remains doubtful since neither genomic nor cDNA sequences have been analysed and no expression has been detected (Hermans and Westhoff, 1990).
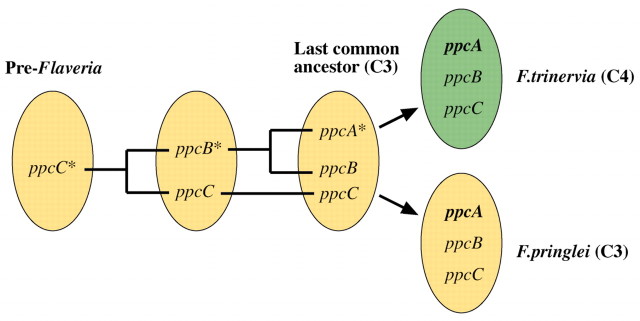
Fig. 1. Evolution of PEPC genes (ppc) in the genus Flaveria. Sequence analyses suggest that the ppcA gene class was formed by gene duplication of a ppcB‐type PEPC gene (ppcB*) already present in the last common ancestral C3 plant that gave rise to F. pringlei (C3) and F. trinervia (C4) (Bläsing et al., 2002). The ppcA genes of F. trinervia (C4) encode the C4 PEPC of this species. The orthologous ppcA gene class of the closely related C3 species F. pringlei is used as a reference C3 gene for studying the evolution of C4 PEPC.
The same three classes of ppc genes are also present in the C3 plant F. pringlei (Hermans and Westhoff, 1990, 1992). This indicates that the last common ancestor of F. pringlei and F. trinervia contained the same set of genes as those in present C3 and C4 flaverias (Fig. 1). The nearest neighbour to the ppcA genes of F. trinervia is the ppcA gene class of F. pringlei, which appears to be represented by a single copy gene (Hermans and Westhoff, 1990; Bläsing et al., 2002). The ppcA gene classes of F. trinervia and F. pringlei therefore represent evolutionary orthologues. If it is assumed that the ppcA gene of F. pringlei (C3) was similar, both in function and expression behaviour, to the ppcA gene of the last common C3 ancestor of present C3 and C4 Flaveria species, the ppcA gene class of F. pringlei provides the reference C3 gene that defines the starting point of C4 evolution.
The systematics of the genus Flaveria and the evolution of C4 photosynthesis
Based on the number of phyllaries, Powell (1978) divided the genus Flaveria into two major branches (Fig. 2A). The section with species that possess three to four phyllaries contains C3, C3–C4 and C4 species. Within this group are F. trinervia and F. pringlei, which serve as model C4 and C3 species of this genus, respectively. The three to four phyllaries branch also contains F. bidentis which is the only C4 flaveria that is amenable to genetic engineering by tissue‐culture‐based Agrobacterium tumefaciens‐mediated transformation techniques (Chitty et al., 1994). The group with five to six phyllaries is composed of only C3–C4 intermediate species. Within this group is F. brownii, a C4‐like species but with the expression of C4 photosynthesis dependent on environmental conditions, i.e. light intensity and growth temperature (Cheng et al., 1989). The group with five to six phyllaries also contains the C3–C4 intermediate species F. pubescens which is the only transformable C3–C4 intermediate flaveria (Chu et al., 1997).
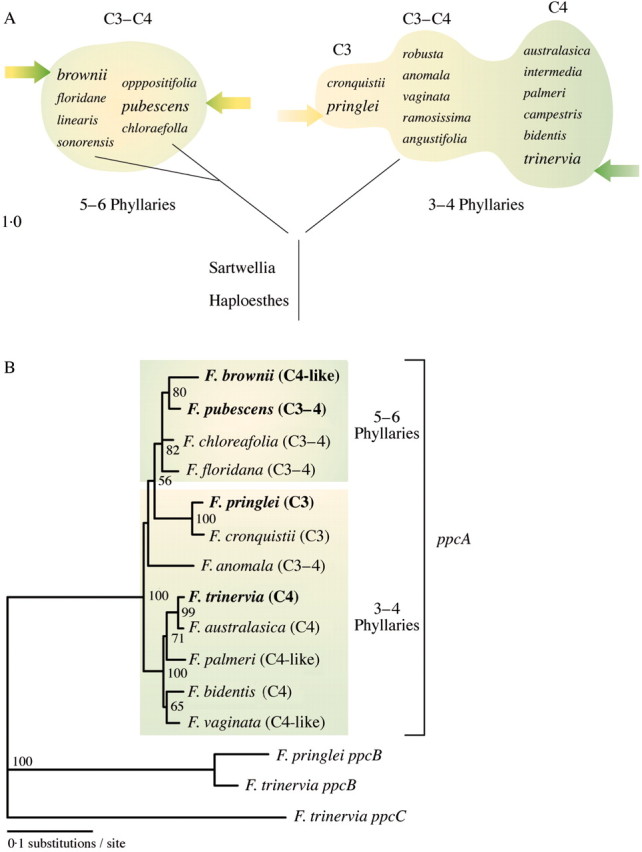
Fig. 2. Systematics and evolution of the genus Flaveria. A, Occurrence of C3, C3–C4 and C4 species. The figure is based on the systematics of the genus Flaveria as presented by Powell (1978). B, Phylogenetic tree of Flaveria species based on ppcA1 promoter sequences. The 500 base pairs of proximal ppcA1 promoter sequences were aligned using Clustal W (Thompson et al., 1994). The phylogenetic tree was generated by the neighbour‐joining method as implemented in PAUP 4·0 (Swofford, 2002).
Kopriva et al. (1996) proposed a molecular phylogeny of the genus Flaveria by using the H‐protein of the glycine cleavage system as a gene marker. Their findings indicate that the group with five to six phyllaries forms a separate clade, thus confirming Powell’s classification (Powell, 1978). Two to three thousand base pairs of ppcA1 promoter sequences were isolated from the C4 plants F. trinervia and F. bidentis, the C4‐like species F. palmeri, F. vaginata and F. brownii, the C4–C3‐like species F. pubescens, F. floridana, F. anomala and F. chloraefolia, and the C3 plants F. pringlei and F. cronquistii. The overall structure of the promoters clearly separates the species with five to six phyllaries, i.e. F. brownii, F. pubescens, F. floridana and F. chloraefolia from the other Flaveria species analysed (U. Gowik and P. Westhoff, unpublished data). The 500 base pairs of proximal promoter sequences which can be aligned to each other without major gaps were used to construct a phylogenetic tree. The phylogram obtained supports the above classification and confirms that the species with five to six phyllaries form a distinct group (Fig. 2B). Based on these ppcA PEPC data one may propose that the evolution from C3 to C4 photosynthesis was initiated at least twice in this genus. However, to confirm this conclusion additional C4‐related genes should be investigated at the phylogenetic level.
EVOLUTION OF THE PEPC PROTEIN
Differences between C3 and C4 PEPCs
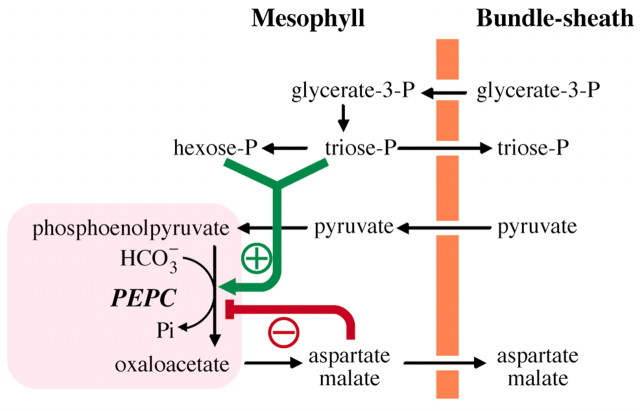
PEPC is positively and negatively controlled by its metabolic context. The enzyme is activated by hexose‐ and triose‐phosphates and feedback inhibited by malate and aspartate (Fig. 3; Kai et al., 2003). In addition, PEPC is regulated by phosphorylation at a specific serine residue near the amino terminus thereby increasing its general activity and decreasing its inhibition by malate (Nimmo, 2003).
Fig. 3. The physiological context of C4 PEPC and its metabolic regulation. Metabolite products of photosynthesis, i.e. sugar phosphates, stimulate the enzyme and decrease Km for the substrate PEP. Malate and other four‐carbon organic acids (oxaloacetate, aspartate) are feedback inhibitors.
The PEPCs involved in C4 photosynthesis differ from their non‐photosynthetic isoforms in C3 plants (collectively named C3 PEPCs) in kinetic and regulatory properties. C4 PEPCs exhibit substrate saturation constants (Km) for PEP that are usually about ten times larger than those of their C3 counterparts (Ting and Osmond, 1973b). On the other hand, the saturation constants for bicarbonate, the second substrate, are lower in C4 than C3 PEPCs (Bauwe, 1986). Finally, C4 PEPCs are more tolerant to malate when compared with the C3 PEPC isozymes (Dong et al., 1998; Bläsing et al., 2002).
Molecular determinants of C4 properties
The differences in substrate affinity and inhibition by malate suggest that C4 PEPCs harbour specific C4 determinants that were acquired during the evolution of C4 photosynthesis. The use of the genus Flaveria as a model system permitted the first analyses of the molecular nature of these determinants. Recombinant C4, C3–C4 and C3 ppcA PEPCs and their chimeras were produced by functional expression of the respective cDNAs in a PEPC‐negative E. coli strain, and the enzymatic properties of the purified proteins were investigated (Svensson et al., 2003).
The ppcA PEPC of F. trinervia (C4) revealed a Km(PEP) which is about nine‐fold higher than the Km(PEP) of the ppcA PEPC of F. pringlei (C3). The C4 enzyme is also ten times more tolerant to malate than its C3 counterpart (Fig. 4). The two orthologous PEPC enzymes, therefore, show the expected differences in kinetic and regulatory properties which are typical for C4 and C3 PEPC isoforms (Svensson et al., 1997).
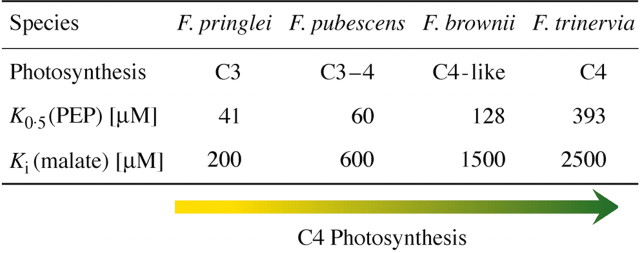
Fig. 4. Evolutionary changes of the affinity of ppcA PEPCs for the substrate PEP and in the inhibition by malate. Data from Engelmann et al. (2003).
To unravel how PEPC enzyme characteristics changed during evolution towards C4 photosynthesis, ppcA PEPCs from the C3–C4 intermediate plant F. pubescens and the C4‐like C3–C4 intermediate F. brownii were investigated. Both the Km(PEP) values and the malate inhibition constants, Ki, are intermediate between the C3 and C4 ppcA PEPCs (Fig. 4). This indicates that the C3 PEPC evolved step by step into a C4 enzyme (Engelmann et al., 2003).
Since the C3 and C4 ppcA isoforms share 96 % identical amino acid positions it should be feasible to pinpoint changes in the amino acid sequence responsible for the C4 characteristics (Svensson et al., 1997). Therefore, reciprocal domain swapping experiments were conducted with the two ppcA PEPCs to locate regions in the enzyme that influence Km(PEP) (Bläsing et al., 2000). With this approach two regions, from amino acids 296 to 437 (region 2) and from amino acids 645 to 966 (region 5), were identified that contain the major C4 determinants for the saturation kinetics of the substrate PEP (Fig. 5). This was confirmed by inserting region 2 of the C4 enzyme and the C4 determinant of region 5 (see below) into an otherwise C3 background. The resulting chimeric enzyme possessed about two thirds of C4 PEPC characteristics with respect to Km(PEP) (Engelmann et al., 2002).
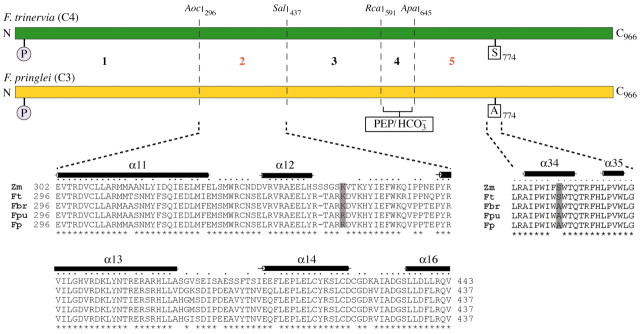
Fig. 5. An evolutionary model of C4 PEPC in Flaveria. From five investigated enzyme domains, region 2 (positions 296–437) and region 5 (amino acids 645–966) contain the major C4 determinants for the saturation kinetics of PEP. P indicates the target phosphorylation site at position 11. The secondary structures indicated on top of the sequence alignments (black bars) were obtained from the recently published 3D structure of the C4 PEPC of Zea mays (Matsumura et al., 2002). The dots above the maize sequence show those amino acid residues that are conserved in all displayed sequences. Sequence positions which are identical in all four Flaveria ppcA PEPCs are marked by stars below the strings of sequences. Note that position 347 (grey column) of the PEPC isoenzymes harbours a lysine in the C4 species F. trinervia and Z. mays, and in the C4‐like plant F. brownii, while the C3 species F. pringlei and the C3–C4 intermediate plant F. pubescens both instead hold an arginine at this site. At position 774 (grey column) serine occurs only in C4 PEPCs, while PEPCs from C3 and C3–C4 intermediate plants contain an alanine at this position. The amino acid numbering follows that of the F. trinervia protein.
In region 5, the C4‐specific properties were confined to a single amino acid, serine 774 (Fig. 5). All C4 enzymes studied to date contain a serine at this position while in all non‐photosynthetic and CAM PEPCs this site is occupied by an alanine (Svensson et al., 2003). It has to be concluded that serine 774 is of central importance for the evolution of C4 characteristics, at least with regard to the Km(PEP). All investigated C3–C4 intermediate PEPCs, even from the C4‐like species F. brownii, still show an alanine at this position (Engelmann et al., 2003). This suggests that the change from alanine to serine occurred only recently during evolution from C3 to C4 photosynthesis.
In region 2, 16 differences were detected between the C3 and C4 ppcA PEPCs (Fig. 5). There is only one amino acid residue, a lysine at position 347, which both F. trinervia and F. brownii have in common and which differs from the arginine in this position in F. pubescens and F. pringlei (Fig. 5; Engelmann et al., 2003). This lysine is also conserved in the C4 PEPC of maize where it is located between helices 12 and 13 (Matsumura et al., 2002). This suggests that a lysine at this position is essential for C4 enzyme properties and hence this C4‐associated lysine residue is a prime candidate for a C4 determinant of region 2.
Determinants for malate sensitivity and the affinity constant of bicarbonate have not been identified. Since the chimeric C3–C4 enzyme strategy in combination with phylogenetic comparisons of C3–C4 intermediate PEPCs, was very successful in identifying molecular determinants for Km(PEP) at the amino acid level, this approach should also give new insights into the evolution of C4 PEPC characteristics for malate sensitivity and Km(bicarbonate).
EVOLUTION OF C4 PEPC GENE REGULATION
Expression patterns of ppcA genes
The ppcA genes of F. trinervia are highly expressed but only in leaves (Ernst and Westhoff, 1996), where expression is restricted to the mesophyll cells (Höfer et al., 1992; Koprivova et al., 2001). This contrasts with the ppcA genes of F. pringlei which are weakly expressed similarly in leaves, stems and roots (Ernst and Westhoff, 1996). The increased gene expression in only the leaves, and the restriction of expression to the mesophyll cells were therefore two major steps taken during the evolution of the C3 ancestral ppcA gene into the present C4 ppcA genes. Elevated amounts of ppcA RNAs can already be detected in less advanced C3–C4 intermediates, i.e. F. chloraefolia (Engelmann et al., 2003), while the corresponding ppcA PEPC is still almost C3‐like (Bauwe and Chollet, 1986). This suggests that an increase in ppcA gene expression occurred first during C4 evolution and preceded the conversion of the C3 PEPC into the C4 enzyme. As far as the specificity of ppcA gene expression is concerned, no detailed in situ studies have been conducted on proteins and RNAs and it remains to be shown when the specific elements controlling mesophyll expression evolved.
Cis‐regulatory elements for mesophyll specific ppcA gene expression
The mesophyll‐specific expression of the ppcA1 gene of F. trinervia is controlled at the transcriptional level. About 2200 base pairs of 5′ flanking sequences (with reference to the AUG translational start codon) are sufficient to cause high β‐glucuronidase (GUS) expression exclusively in the mesophyll cells of the closely related C4 plant F. bidentis. In contrast, the 2538 base pairs (with reference to the AUG start codon) of the 5′ flanking sequences of the ppcA1 gene of F. pringlei were found to be a weak promoter and did not direct any organ‐ or cell‐specific expression (Stockhaus et al., 1997; Fig. 6). Both promoters thus exhibited all the attributes expected from the accumulation patterns of their corresponding RNAs and proteins. The increase in gene expression, but exclusively in the leaves, and the confinement of expression to the mesophyll cells were, therefore, the two prominent steps taken to convert the C3 ancestral ppcA gene into the present C4 ppcA genes.
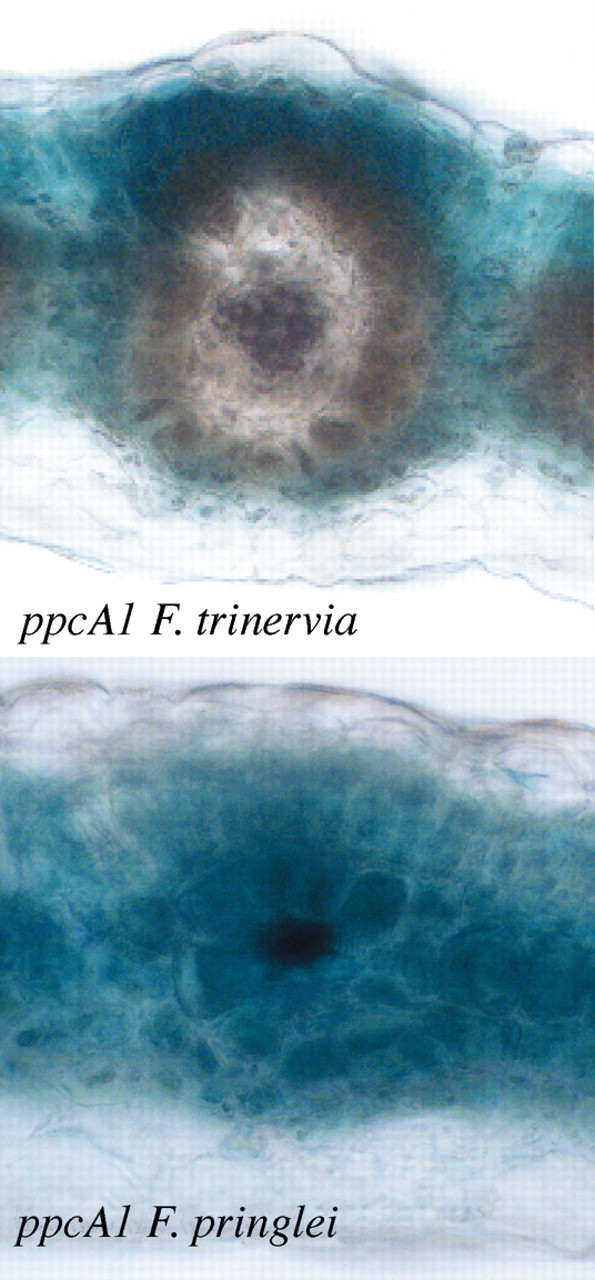
Fig. 6. Histochemical analysis of the activities of the ppcA1 promoters of F. trinervia (C4) and F. pringlei (C3) in transgenic F. bidentis (C4) (cf. Stockhaus et al., 1997). The β‐glucuronidase gene (GUS) was used as a reporter gene (Jefferson et al., 1987).
Promoter deletion and recombination studies (U. Gowik, J. Burscheidt, M. Akyildiz and P. Westhoff, unpublished data) revealed that the distal sequences between base pairs –1565 to –2188 (DR segment) in combination with the proximal 570 base pairs (PR segment) of 5′ flanking sequences of the ppcA1 gene (Fig. 7A) are sufficient to achieve elevated mesophyll expression of the GUS reporter gene, i.e. the nucleotide sequences between –570 and –1566 are essentially dispensable for ppcA1 promoter activity. The C4‐DR functions both in the correct and the inverse orientation; this cis‐regulatory region, therefore, shows the typical features of a transcriptional enhancer (Blackwood and Kadonaga, 1998). When the C4‐DR was inserted into the ppcA1 promoter of F. pringlei a mesophyll expression component was added to that promoter, but its expression strength was only slightly increased (U. Gowik, J. Burscheidt, M. Akyildiz and P. Westhoff, unpublished data). Thus the C4‐DR contains mesophyll expression determinants which are lacking in the C3 ppcA1 promoter. However, a high expression in the mesophyll cells is only observed, when the C4‐DR is combined with its corresponding PR segment. It also follows that the PR segments of the two promoters differ in mesophyll expression determinants.
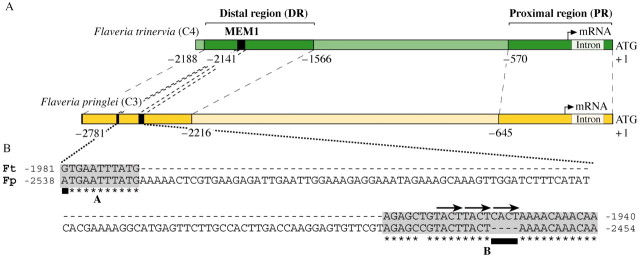
Fig. 7. The structures of the ppcA1 promoters from F. trinervia (C4) and F. pringlei (C3) and the nucleotide composition of the mesophyll expression module MEM1. A, Schematic comparisons of the 5′ flanking sequences of the ppcA1 genes of F. trinervia and F. pringlei. The numbers of nucleotides refer to the translation initiation codon. The proximal (PR) and distal regions (DR) of the two promoters are strongly coloured. The positions of MEM1 and its homologue in F. pringlei are marked by black boxes. B, Sequence comparison of MEM1 of F. trinervia (Ft) and its homologue in F. pringlei (Fp). Asterisks label identical nucleotides in the A or B segments of MEM1. Black bars indicate the single nucleotide difference in A and the CACT tetra nucleotide in B. The two tandem TACT repeats and the third imperfect CACT repeat are marked by arrows. The C/T difference in the B segment is not correlated with C3/C4 photosynthesis, because all C4 flaverias except F. trinervia contain a C at that position. Data from U. Gowik, J. Burscheidt, M. Akyildiz and P. Westhoff, unpublished data
The following scenario for the evolution of the C3 into the C4 ppcA1 promoter may be derived from these findings. The evolutionary changes occurred both in the DR and PR segments of the promoter (Fig. 7A), and resulted in a high and mesophyll‐specific expression of the C4 ppcA gene (Fig. 7A). First, cis‐regulatory elements had to evolve that led to mesophyll specificity. Since the C3 ppcA1 promoter is active in all leaf cells (Stockhaus et al., 1997; Fig. 6) mesophyll specificity required that cis‐regulatory elements for expression in non‐mesophyll cells became inactivated. In addition, novel cis‐regulatory elements for mesophyll expression may have been created. Secondly and concomitantly, the quantity of ppcA gene expression had to be increased. This could have been achieved by adding novel mesophyll specificity elements to the promoter whose interaction with the corresponding trans‐regulatory factors was optimized step by step when progressing from C3 to C4 photosynthesis. Alternatively, and/or in addition, novel quantitative elements were created that interacted specifically with the mesophyll quality elements.
Detailed analyses allowed more precise definition of determinants in the C4‐DR responsible for mesophyll expression. A 41‐base‐pair segment, named MEM1 (mesophyll expression module 1), was identified in the DR segment of the F. trinervia promoter that, in combination with the PR segment of that promoter, was sufficient to confer specificity for expression of the GUS reporter gene in the mesophyll (U. Gowik, J. Burscheidt, M. Akyildiz and P. Westhoff, unpublished data).
MEM1 homologous sequences were also detected in the ppcA1 promoter of F. pringlei (Fig. 7B) and in other C3, C4 and C4‐like Flaveria species (Gowik et al., 2003). Their comparison revealed that MEM1 sequences consist of two parts, A and B, which are contiguous in F. trinervia, but are separated by 97–108 base pairs in the C3 species F. pringlei and F. cronquistii, the C4 plant F. bidentis (C4) and the C4‐like species F. palmeri and F. vaginata (U. Gowik, J. Burscheidt, M. Akyildiz and P. Westhoff, unpublished data). The A parts of all C4 and C4‐like species show a guanine at their first nucleotide position, while an adenine is present in the A‐homologues of the two C3 species. A similar C4‐to‐C3 associated difference is also found for the tetra nucleotide CACT. This assemblage is present in the B parts of all C4 and C4‐like species but absent in both C3 promoters. These C4‐to‐C3 correlated differences in MEM1 composition are likely candidates for cis‐regulatory elements governing mesophyll specific gene expression.
Trans‐regulatory factors for mesophyll specific ppcA gene expression
Analysis of trans‐regulatory factors that are necessary for mesophyll‐specific expression of the ppcA gene is in its infancy. By using the yeast one‐hybrid system (Vidal and Legrain, 1999), homeodomain proteins were identified that belong to a hitherto uncharacterized class containing a zinc finger domain. Such proteins specifically interact with the PR segment of the ppcA1 promoter of F. trinervia but not with the corresponding region of the ppcA1 promoter of F. pringlei (Windhövel et al., 2001; Fig. 8). They are, therefore, prime candidates for transcription factors that are required for establishing the C4 specific expression pattern of the C4 ppcA genes. However, in planta experiments to probe their participation in C4 ppcA1 gene expression are still lacking.
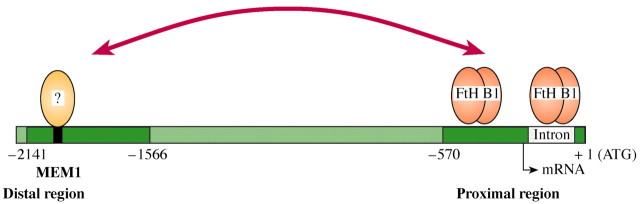
Fig. 8. A working model of the C4 ppcA1 promoter of F. trinervia. FtHB1 denotes the zinc finger homeodomain transcription factors that have been shown to bind to the proximal region of the C4 ppcA1 promoter (Windhövel et al., 2001). The trans‐regulatory factors which bind to the enhancer‐like distal region are unknown.
Similarly, one‐hybrid approaches have been performed with MEM1 of F. trinervia as the bait (M. Akyildiz and P. Westhoff, unpublished data). The isolated MEM1‐binding proteins still need to be completely characterized and their specific binding properties verified (Fig. 8). Taken together, the data suggest that the one‐hybrid screening assay is a good heuristic tool to use in the search for transcription factor candidates. Reverse genetic approaches based on RNAi‐triggered gene inactivation (Waterhouse and Helliwell, 2003) or ‘gain‐of‐function’ experiments which ectopically express the putative transcription factor (Schwechheimer et al., 1998) are required to verify the in planta relevance of these possible trans‐regulatory factors.
CONCLUSIONS AND OUTLOOK
C4 photosynthesis is a fascinating system for studying the evolution of a new metabolic capability. The C4 photosynthetic pathway evolved polyphyletically and provides insight into the flexibility of the genetic system of angiosperms. This system independently created C4 photosynthesis several times, the main feature—indeed the central pillar—of which is the efficient CO2 pump, based on PEPC, that concentrates CO2 where ribulose 1,5‐bisphosphate carboxylase/oxygenase is located. The CO2 pump normally requires two different cell‐types, mesophyll and bundle sheath cells, which unite to a metabolic system that is characterized by a division of labour.
The differentiation of these two photosynthetic cell‐types required an alteration of the developmental programmes. This makes C4 photosynthesis an attractive system which reveals how regulatory circuits of gene expression are modified to give new, specific expression patterns. Also, evolution of the C4 assimilatory pathway requires adaptation of enzymes to a new metabolic role which involves changes in enzyme kinetics and their regulation. The case study with C4 PEPC, which is reviewed here, demonstrates that the pursuit of this evolution‐oriented approach leads to new insights into structure–function relationships of this enzyme (Svensson et al., 2003) and the molecular nature of cis‐ and trans‐regulatory factors that are required for the mesophyll specific expression of the corresponding gene.
The polyphyletic origin of C4 photosynthesis implies that, in genetic terms, it has been comparatively simple to evolve C4 plants from C3 ancestors. The data obtained with Flaveria support this point of view. Small alterations sufficed to convert a C3 into a C4 PEPC enzyme and change a C3 into a C4 PEPC promoter. Comparison of the enzymatic properties of C3, C3–C4 and C4 PEPC enzymes shows that the changes proceed gradually. To date comparable data on the changes within the gene regulation system are not available. Investigation of ppcA RNA amounts in C3, C3–C4, C4‐like and C4 Flaveria species suggests that at least the changes in the promoter strength also happened step by step (Engelmann et al., 2003).
This gradual transition from C3 to C4 photosynthesis, which occurred in Flaveria, can easily be explained if the C4 syndrome is viewed as a quantitative trait. Quantitative traits (Mauricio, 2001) are of a polygenic nature and their gene components are called quantitative trait loci (QTL). In contrast to Mendelian traits, which are discrete, quantitative traits are affected by multiple genes and by environmental factors. Individual QTL may have only small effects on the expression of the trait, i.e. are minor genes. However, recent studies with crop and model plants show that individual loci may behave as major genes and determine a large part of the trait variation. These studies also reveal that regulatory loci play a prominent role within the genetic architecture of quantitative traits (Remington and Purugganan, 2003).
Unfortunately, the quantitative trait concept of C4 photosynthesis cannot be easily tested experimentally, because there is no genetic system consisting of easily crossable C3 and C4 species. A segregating population between Atriplex hastata (C3) and A. rosea (C4) was constructed and individual C4 traits segregated in that population (Björkman et al., 1971). However, the F2 plants were aneuploid and a genetic mapping of the C4 QTL failed for this reason (Nobs, 1976). Within the genus Flaveria crosses between C3 and C3–C4 species have been achieved, but crosses between C3 and C4 Flaveria species have not succeeded (Brown and Bouton, 1993). Clearly, a genetic system which allows the construction of segregating populations from C3 and C4 species is highly desirable. The number of genes which make up the genetic architecture of C4 photosynthesis, and their molecular nature, could be identified with this strategy. Whether mutational studies, for instance with maize, will be a good substitute for the QTL approach remains to be seen.
In his influential review on biochemical evolution, A. C. Wilson pointed out that ‘quantitative mutations affecting enzyme levels may have had a major role in the adaptative metabolic evolution of multicellular organisms’, and that ‘these quantitative effects can result from point mutations in control genes’ (Wilson et al., 1977). He believed that these regulatory mutations happened before mutations in protein coding regions. The studies with the molecular evolution of C4 PEPC in Flaveria are in line with this concept. These investigations have shown that a C4‐type pattern of gene expression evolved before the C4 enzyme characteristics became established. This is best illustrated by the C4‐like species F. brownii. The ppcA PEPC of this species is rather C3‐like, most notably its amino acid position 774 is still occupied by an alanine and not by the C4‐typical serine. On the other hand, the expression levels of the ppcA mRNA (Engelmann et al., 2003) and the distribution of the PEPC protein (Cheng et al., 1988) are essentially of C4 type.
Evolutionary biologists have collected convincing evidence which support the view that changes in the spatio‐temporal expression patterns of genes are the principal mechanisms for novel morphological and biochemical traits (Doebley and Lukens, 1998; Carroll, 2000). Flaveria is a unique system which allows detailed tests of this concept by using C4 photosynthesis as the model trait.
ACKNOWLEDGEMENTS
Work summarized here was supported by the Deutsche Forschungsgenmeinschaft (DFG) within the Sonder forschungsbereich SFB590 and the Graduiertenkolleg Molekulare Physiologie both at the Heinrich‐Heine‐Universität of Düsseldorf. Additional support from the Fonds der Chemischen Industrie is greatfully acknowledged.
Supplementary Material
Received: 6 August 2003; Returned for revision: 8 September 2003; Accepted: 16 September 2003 Published electronically: 26 November 2003
References
- BadgerMR, Price GD.1994. The role of carbonic anhydrase in photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 45: 369–392. [Google Scholar]
- BaldyP, Cavalié G.1984. Compartmentation of photorespiratory enzymes in a C4 photosynthesis plant, Zea mays Zeitschrift für Pflanzenphysiologie 114: 255–259. [Google Scholar]
- BauweH.1986. An efficient method for the determination of Km values for HCO3– of phosphoenolpyruvate carboxylase. Planta 169: 356–360. [DOI] [PubMed] [Google Scholar]
- BauweH, Chollet R.1986. Kinetic properties of phosphoenolpyruvate carboxylase from C3, C4, and C3–C4 intermediate species of Flaveria (Asteraceae). Plant Physiology 82: 695–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BjörkmanO, Nobs MA, Berry JA.1971. Further studies on hybrids between C3 and C4 species of Atriplex Carnegie Institution of Washington, Year Book 70: Carnegie Institution, 507–511. [Google Scholar]
- BlackwoodEM, Kadonaga JT.1998. Going the distance: a current view of enhancer action. Science 281: 60–63. [DOI] [PubMed] [Google Scholar]
- BläsingOE, Ernst K, Streubel M, Westhoff P, Svensson P.2002. The non‐photosynthetic phosphoenolpyruvate carboxylases of the C4 dicot Flaveria trinervia – implications for the evolution of C4 photosynthesis. Planta 215: 448–456. [DOI] [PubMed] [Google Scholar]
- BläsingOE, Westhoff P, Svensson P.2000. Evolution of C4 phosphoenolpyruvate carboxylase in Flaveria, a conserved serine residue in the carboxyl‐terminal part of the enzyme is a major determinant for C4‐specific characteristics. Journal of Biological Chemistry 275: 27917–27923. [DOI] [PubMed] [Google Scholar]
- BrownRH.1999. Agronomic implications of C4 photosynthesis. In: Sage RF, Monson RK, eds. C4 Plant Biology San Diego: Academic Press, 473–507. [Google Scholar]
- BrownRH, Bouton JH.1993. Physiology and genetics of interspecific hybrids between photosynthetic types. Annual Review of Plant Physiology and Plant Molecular Biology 44: 435–456. [Google Scholar]
- CarrollSB.2000. Endless forms: the evolution of gene regulation and morphological diversity. Cell 101: 577–580. [DOI] [PubMed] [Google Scholar]
- ChengSH, Moore Bd, Edwards GE, Ku MSB.1988. Photosynthesis in Flaveria brownii, a C4‐like species. Leaf anatomy, characteristics of CO2 exchange, compartmentation of photosynthetic enzymes, and metabolism of 14CO2 Plant Physiology 87: 867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ChengSH, Moore Bd, Wu J, Edwards GE, Ku MSB.1989. Photosynthetic plasticity in Flaveria brownii Growth irradiance and the expression of C4 photosynthesis. Plant Physiology 89: 1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ChittyJA, Furbank RT, Marshall JS, Chen Z, Taylor WC.1994. Genetic transformation of the C4 plant, Flaveria bidentis Plant Journal 6: 949–956. [Google Scholar]
- CholletR, Vidall J, O’Leary MH.1996. Phosphoenol pyruvate carboxylase: a ubiquitous, highly regulated enzyme in plants. Annual Review of Plant Physiology and Plant Molecular Biology 47: 273–298. [DOI] [PubMed] [Google Scholar]
- ChuCC, Qu N, Bassüner B, Bauwe H.1997. Genetic transformation of the C3–C4 intermediate plant, Flaveria pubescens (Asteraceae). Plant Cell Reports 16: 715–718. [DOI] [PubMed] [Google Scholar]
- CrétinC, Santi S, Keryer E, Lepiniec L, Tagu D, Vidal J, Gadal P.1991. The phosphoenolpyruvate carboxylase gene family of Sorghum: promoter structures, amino acid sequences and expression of genes. Gene 99: 87–94. [DOI] [PubMed] [Google Scholar]
- DenglerNG, Nelson T.1999. Leaf structure and development in C4 plants. In: Sage RF, Monson RK, eds. C4 Plant Biology San Diego: Academic Press, 133–172. [Google Scholar]
- DoebleyJ, Lukens L.1998. Transcriptional regulators and the evolution of plant form. Plant Cell 10: 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DongLY, Masuda T, Kawamura T, Hata S, Izui K.1998. Cloning, expression, and characterization of a root‐form phosphoenolpyruvate carboxylase from Zea mays: comparison with the C4‐form enzyme. Plant and Cell Physiology 39: 865–873. [DOI] [PubMed] [Google Scholar]
- EdwardsGE, Ku MSB.1987. Biochemistry of C3–C4 intermediates. In: Hatch MD, Boardman NK, eds. The Biochemistry of Plants, Vol. 10 New York: Academic Press, 275–325. [Google Scholar]
- EhleringerJR, Monson RK.1993. Evolutionary and ecological aspects of photosynthetic pathway variation. Annual Review of Ecology and Systematics 24: 411–439. [Google Scholar]
- EngelmannS, Bläsing OE, Gowik U, Svensson P, Westhoff P.2003. Molecular evolution of C4 phosphoenolpyruvate carboxylase in the genus Flaveria – a gradual increase from C3 to C4 characteristics. Planta 217: 717–725. [DOI] [PubMed] [Google Scholar]
- EngelmannS, Bläsing OE, Westhoff P, Svensson P.2002. Serine 774 and amino acids 296 to 437 comprise the major C4 determinants of the C4 phosphoenolpyruvate carboxylase of Flaveria trinervia. FEBS Letters 524: 11–14. [DOI] [PubMed] [Google Scholar]
- ErnstK, Westhoff P.1996. The phosphoenolpyruvate carboxylase (ppc) gene family of Flaveria trinervia (C4) and F. pringlei (C3): molecular characterization and expression analysis of the ppcB and ppcC genes. Plant Molecular Biology 34: 427–443. [DOI] [PubMed] [Google Scholar]
- FreitagH, Stichler W.2000. A remarkable new leaf type with unusual photosynthetic tissue in a Central Asiatic genus of Chenopodiaceae. Plant Biology 2: 154–160. [Google Scholar]
- FreitagH, Stichler W.2002.Bienertia cycloptera Bunge ex Boiss. Chenopodiaceae, another C4 plant without Kranz tissues. Plant Biology 4: 121–134. [Google Scholar]
- FurumotoT, Hata S, Izui K.2000. Isolation and characterization of cDNAs for differentially accumulated transcripts between mesophyll cells and bundle sheath strands of maize leaves. Plant and Cell Physiology 41: 1200–1209. [DOI] [PubMed] [Google Scholar]
- HarpsterMH, Taylor WC.1986. Maize phosphoenolpyruvate carboxylase. Cloning and characterization of mRNAs encoding isozymic forms. Journal of Biological Chemistry 261: 6132–6136. [PubMed] [Google Scholar]
- HatchMD.1987. C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochimica et Biophysica Acta 895: 81–106. [Google Scholar]
- HermansJ, Westhoff P.1990. Analysis of expression and evolutionary relationships of phosphoenolpyruvate carboxylase genes in Flaveria trinervia (C4) and F. pringlei (C3). Molecular & General Genetics 224: 459–468. [DOI] [PubMed] [Google Scholar]
- HermansJ, Westhoff P.1992. Homologous genes for the C4 isoform of phosphoenolpyruvate carboxylase in a C3‐ and a C4‐Flaveria species. Molecular & General Genetics 234: 275–284. [DOI] [PubMed] [Google Scholar]
- HöferMU, Santore UJ, Westhoff P.1992. Differential accumulation of the 10‐, 16‐ and 23‐kDa peripheral components of the water‐splitting complex of photosystem II in mesophyll and bundle‐sheath chloroplasts of the dicotyledonous C4 plant Flaveria trinervia (Spreng.) C. Mohr. Planta 186: 304–312. [DOI] [PubMed] [Google Scholar]
- HoladayAS, Bowes G.1980. C4 acid metabolism and dark CO2 fixation in a submersed aquatic macrophyte (Hydrilla verticillata). Plant Physiology 65: 331–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JeffersonRA, Kavanagh TA, Bevan MW.1987. GUS fusions: β‐glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO Journal 6: 3901–3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KaiY, Matsumura H, Izui K.2003. Phosphoenolpyruvate carboxylase: three‐dimensional structure and molecular mechanisms. Archives of Biochemistry and Biophysics 414: 170–179. [DOI] [PubMed] [Google Scholar]
- KanaiR, Edwards GE.1999. The biochemistry of C4 photosynthesis. In: Sage RF, Monson RK, eds. C4 Plant Biology San Diego: Academic Press, 49–87. [Google Scholar]
- KawamuraT, Shigesada K, Toh H, Okumura S, Yanagisawa S, Izui K.1992. Molecular evolution of phosphoenolpyruvate carboxylase for C4 photosynthesis in maize: comparison of its cDNA sequence with a newly isolated cDNA encoding an isozyme involved in anaplerotic function. Journal of Biochemistry 112: 147–154. [DOI] [PubMed] [Google Scholar]
- KelloggEA.1999. Phylogenetic aspects of the evolution of C4 photosynthesis. In: Sage RF, Monson RK, eds. C4 Plant Biology San Diego: Academic Press, 411–444. [Google Scholar]
- KoprivaS, Chu CC, Bauwe H.1996. Molecular phylogeny of Flaveria as deduced from the analysis of nucleotide sequences encoding the H‐protein of the glycine cleavage system. Plant Cell and Environment 19: 1028–1036. [Google Scholar]
- KoprivovaA, Melzer M, vonBallmoos P, Mandel T, Brunold C, Kopriva S.2001. Assimilatory sulfate reduction in C‐3, C‐3‐C‐4, and C‐4 species of Flaveria. Plant Physiology 127: 543–550. [PMC free article] [PubMed] [Google Scholar]
- KubickiA, Steinmüller K, Westhoff P.1994. Differential transcription of plastome‐encoded genes in the mesophyll and bundle‐sheath chloroplasts of the monocotyledonous NADP‐malic enzyme type C4 plants maize and Sorghum. Plant Molecular Biology 25: 669–679. [DOI] [PubMed] [Google Scholar]
- LongSP.1999. Environmental responses. In: Sage RF, Monson RK, eds. C4 Plant Biology San Diego: Academic Press, 215–249. [Google Scholar]
- LunnJE, Hatch MD.1995. Primary partitioning and storage of photosynthate in sucrose and starch in leaves of C4 plants. Planta 197: 385–391. [Google Scholar]
- MatsumuraH, Xie Y, Shirakata S, Inoue T, Yoshinaga T, Ueno Y, Izui K, Kai Y.2002. Crystal structures of C4 form maize and quaternary complex of E. coli phosphoenolpyruvate carboxylases. Structure 10: 1721–1730. [DOI] [PubMed] [Google Scholar]
- MauricioR.2001. Mapping quantitative trait loci in plants: uses and caveats for evolutionary biology. Nature Reviews Genetics 2: 370–381. [DOI] [PubMed] [Google Scholar]
- MeierhoffK, Westhoff P.1993. Differential biogenesis of photosystem II in mesophyll and bundle‐sheath cells of monocotyledonous NADP‐malic enzyme‐type C4 plants: the non‐stoichiometric abundance of the subunits of photosystem II in the bundle‐sheath chloroplasts and the translational activity of the plastome‐encoded genes. Planta 191: 23–33. [Google Scholar]
- MonsonRK.1999. The origins of C4 genes and evolutionary pattern in the C4 metabolic phenotype. In: Sage RF, Monson RK, eds. C4 Plant Biology San Diego: Academic Press, 377–410. [Google Scholar]
- MonsonRK, Moore Bd.1989. On the significance of C3–C4 intermediate photosynthesis to the evolution of C4 photosynthesis. Plant Cell and Environment 12: 689–699. [Google Scholar]
- NimmoHG.2003. Control of the phosphorylation of phosphoenolpyruvate carboxylase in higher plants. Archives of Biochemistry and Biophysics 414: 189–196. [DOI] [PubMed] [Google Scholar]
- NobsMA.1976. Hybridizations in Atriplex Carnegie Institution of Washington, Year Book 75: Carnegie Institution, 421–423. [Google Scholar]
- PoetschW, Hermans J, Westhoff P.1991. Multiple cDNAs of phosphoenolpyruvate carboxylase in the C4 dicot Flaveria trinervia FEBS Letters 292: 133–136. [DOI] [PubMed] [Google Scholar]
- PowellAM.1978. Systematics of Flaveria (Flaveriinae‐Asteraceae). Annals of the Missouri Botanical Garden 65: 590–636. [Google Scholar]
- RajagopalanAV, Devi MT, Raghavendra AS.1994. Molecular biology of C4 phosphoenolpyruvate carboxylase: structure, regulation and genetic engineering. Photosynthesis Research 39: 115–135. [DOI] [PubMed] [Google Scholar]
- RathnamCKM, Edwards GE.1976. Distribution of nitrate‐assimilating enzymes between mesophyll protoplasts and bundle sheath cells in leaves of three groups of C4 plants. Plant Physiology 57: 881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RemingtonDL, Purugganan MD.2003. Candidate genes, quantitative trait loci, and functional trait evolution in plants. International Journal of Plant Sciences 164: Suppl. S7–S20. [Google Scholar]
- RennéP, Dreßen U, Hebbeker U, Hille D, Flügge U‐I, Westhoff P, Weber A.2003. The Arabidopsis mutant dct is deficient in the plastidic glutamate/malate translocator DiT2. Plant Journal 35: 316–341. [DOI] [PubMed] [Google Scholar]
- SageRF.1999. Why C4 photosynthesis? In: Sage RF, Monson RK, eds. C4 Plant Biology San Diego: Academic Press, 3–16. [Google Scholar]
- SageRF, Li M, Monson RK.1999. The taxonomic distribution of C4 photosynthesis. In: Sage RF, Monson RK, eds. C4 Plant Biology San Diego: Academic Press, 551–584. [Google Scholar]
- SchmutzD, Brunold C.1984. Intercellular localization of assimilatory sulfate reduction in leaves of Zea mays and Triticum aestivum Plant Physiology 74: 866–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SchwechheimerC, Zourelidou M, Bevan MW.1998. Plant transcription factor studies. Annual Review of Plant Physiology and Plant Molecular Biology 49: 127–150. [DOI] [PubMed] [Google Scholar]
- SheenJ.1999. C4 gene expression. Annual Review of Plant Physiology and Plant Molecular Biology 50: 187–217. [DOI] [PubMed] [Google Scholar]
- SheenJY, Sayre RT, Bogorad L.1987. Differential expression of oxygen‐evolving polypeptide genes in maize leaf cell types. Plant Molecular Biology 9: 217–226. [DOI] [PubMed] [Google Scholar]
- StockhausJ, Schlue U, Koczor M, Chitty JA, Taylor WC, Westhoff P.1997. The promoter of the gene encoding the C4 form of phosphoenolpyruvate carboxylase directs mesophyll specific expression in transgenic C4 Flaveria spp. Plant Cell 9: 479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SvenssonP, Bläsing O, Westhoff P.1997. Evolution of the enzymatic characteristics of C4 phosphoenolpyruvate carboxylase: a comparison of the orthologous ppcA phosphoenolpyruvate carboxylases of Flaveria trinervia (C4) and F. pringlei (C3). European Journal of Biochemistry 246: 452–460. [DOI] [PubMed] [Google Scholar]
- SvenssonP, Bläsing OE, Westhoff P.2003. Evolution of C4 phosphoenolpyruvate carboxylase. Archives of Biochemistry and Biophysics 414: 180–188. [DOI] [PubMed] [Google Scholar]
- SwoffordDL.2002.PAUP*: Phylogenetic Analysis Using Parsimony (and other methods) 4.0. Sinauer Associates, Sunderland, MA, USA. [Google Scholar]
- ThompsonJD, Higgins DG, Gibson TJ.1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position‐specific gap penalties and weight matrix choice. Nucleic Acids Research 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TingIP, Osmond CB.1973a. Multiple forms of plant phosphoenolpyruvate carboxylase associated with different metabolic pathways. Plant Physiology 51: 448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TingIP, Osmond CB.1973b. Photosynthetic phosphoenolpyruvate carboxylase. Characteristics of allozymes from leaves of C3 and C4 plants. Plant Physiology 51: 439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VidalM, Legrain P.1999. Yeast forward and reverse ‘n’‐hybrid systems. Nucleic Acids Research 27: 919–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WaterhousePM, Helliwell CA.2003. Exploring plant genomes by RNA‐induced gene silencing. Nature Reviews Genetics 4: 29–38. [DOI] [PubMed] [Google Scholar]
- WilsonAC, Carlson SS, White TJ.1977. Biochemical evolution. Annual Review of Biochemistry 46: 573–639. [DOI] [PubMed] [Google Scholar]
- WindhövelA, Hein I, Dabrowa R, Stockhaus J.2001. Characterization of a novel class of plant homeodomain proteins that bind to the C4 phosphoenolpyruvate carboxylase gene of Flaveria trinervia Plant Molecular Biology 45: 201–214. [DOI] [PubMed] [Google Scholar]
- WooKC, Anderson JM, Boardman NK, Downton WJS, Osmond CB, Thorne SW.1970. Deficient photosystem II in agranal bundle sheath chloroplasts of C4 plants. Proceedings of the National Academy of Sciences of the USA 67: 18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WyrichR, Dreßen U, Brockmann S, Streubel M, Chang C, Qiang D, Paterson AH, Westhoff P.1998. The molecular basis of C4 photosynthesis in sorghum: isolation, characterization and RFLP mapping of mesophyll‐ and bundle‐sheath‐specific cDNAs obtained by differential screening. Plant Molecular Biology 37: 319–335. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.