Abstract
• Background and aims The scarcity and unpredictability of active pollinators during late winter in temperate areas tends to favour extended flowering seasons and increased floral longevity in early blooming species, which are usually pollinated by diverse sets of insects. Daphne laureola is a gynodioecious woody perennial that flowers from January to April in southern Spain, a period characterized by cold temperatures, frequent rains and irregular snowfalls.
• Methods Pollinators were excluded at four different times during the flowering season in order to determine the effect of decreased exposure to pollinators on fruit set in female and hermaphrodite individuals. The role of nocturnal and diurnal pollination on reproductive success in each gender was simultaneously evaluated by selective exclusion.
• Key results A 50 % reduction in the flowering period decreased fruit set of females by 50 %, whereas the corresponding decrease in self‐compatible hermaphrodites was only approx. 25 %. Day‐active hymenopterans and lepidopterans were infrequent visitors, and nocturnal pollinators were inefficient, suggesting that pollen beetles, Meligethes elongatus, were the main pollinators of D. laureola in the study region.
• Conclusions Beetles were less abundant in pollenless females, although discrimination did not apparently result in pollination limitation of female reproduction. A preference of beetles for sunny locations emphasized the relevance of abiotic conditions for pollination of this early blooming shrub.
Key words: Daphne laureola, gynodioecy, Mediterranean, Nitidulidae, nocturnal pollination, plant reproductive system, Thymelaeaceae
INTRODUCTION
Generalization in plant–pollinator systems is expected to be favoured whenever the availability of different pollinators is unpredictable from year to year (Johnson and Steiner, 2000) and if, in addition, they are recurrently scarce. This is the scenario in Mediterranean mountains for most late winter/early spring flowering plants that bloom during harsh and variable weather conditions characterized by cold temperatures, frequent rain and irregular snowfall (Romero et al., 1998). Two known exceptions to these expectations are Narcissus longispathus Pugsley (Amaryllidaceae) and Helleborus foetidus L. (Ranunculaceae), which are mainly pollinated by a taxonomically restricted set of floral visitors (Herrera, 1995; Herrera et al., 2001). Both species illustrate that extensive self‐compatibility and long‐lasting flowers can also provide mechanisms to cope with the scarcity of pollinators in the forest understorey under adverse abiotic conditions (for discussion, see Herrera, 2002).
In southern Spain, Daphne laureola produces a large number of small, tubular green‐yellowish flowers that are open from late January to early April. Flowers are visited by a diverse pollinator assemblage comprising Hymenoptera, Lepidoptera and Coleoptera. In such a generalized pollination system, differences in pollinators’ foraging behaviour will probably determine their efficiency in pollen transfer and can have a measurable effect on plant fitness (e.g. Herrera, 2000, and references therein; Utelli and Roy, 2000). The best example of differences in pollinators’ behaviour is between diurnal and nocturnal insects (e.g. Young, 2002). Nocturnal pollination is usually thought to be both specific and limited to plant species bearing flowers with particular morphological and functional traits, such as a whitish or pale colour, a strong, sweet scent, long and narrow corollas or spurs, and abundant evening nectar production (Faegri and Van der Pijl, 1971). These flowers are often able to attract moths, bats or both. However, some of these typical night‐pollinated species are also visited by diurnal insects of variable quality as pollinators (e.g. Baker, 1961; Jennersten and Morse, 1991; Guitián et al., 1993; Groman and Pellmyr, 1999; Young, 2002). The role of nocturnal pollinators in plant species whose flowers are pollinated by diurnal insects, or appear to be so, has rarely been studied in detail (but see Anderson, 1976; Navarro, 1999; Pelletier et al., 2001). Although both the timing of the flowering season and the characters of the flowers would have never suggested a priori any role of nocturnal pollination, occasional observations of noctuid moths feeding on D. laureola flowers at night suggested the potential for such an unexpected pollination relationship, thus providing a study case to test for the effects of nocturnal pollination in a presumed diurnal system.
Furthermore, the effects of pollinator behaviour are expected to be stronger in sexually dimorphic species (Ashman, 2000), where an efficient vector is a strict necessity for some morphs to reproduce successfully. Daphne laureola is a gynodioecious species whose hermaphrodite individuals are self‐compatible, although flowers require a pollination vector to become fertilized (Alonso and Herrera, 2001). Thus, pollinator behaviour may affect the magnitude of selfing in hermaphrodites, which might in turn determine their success as both females (due to inbreeding depression), and males (because of pollen discounting). In addition, a negative discrimination against female plants by pollinators searching for pollen can increase pollen limitation of females and consequently modify both genders’ relative fecundity (Charlesworth, 1993; Ashman, 2000; but see Young, 2002).
Thus Daphne laureola combines several notable characteristics allowing a simultaneously evaluation of the effect of pollination quantity and quality on the reproductive success of different sex morphs under adverse conditions for pollination. These features include the ability to bloom very early in the season, to be gynodioecious, and to exhibit nocturnal and diurnal pollination. In this study, diurnal pollinator discrimination between female and hermaphrodite D. laureola flowers was investigated. An experiment was designed (a) to quantify the effect of shortening the pollination period on the fruit set of female and hermaphrodite D. laureola individuals; and (b) to evaluate the relative role of diurnal and nocturnal pollinators on the fruit set of both sex morphs by selectively excluding nocturnal visitors. The experiment tested the following two hypotheses: (1) shortening the flowering season should have negative consequences for the fruit set of females and hermaphrodites; and (2) if moths actually were the primary pollinators, their selective exclusion should decrease the fruit set of hermaphrodite and female plants.
MATERIALS AND METHODS
Study species
Daphne laureola L. (Thymelaeaceae) is a 0·5–1·5 m tall, evergreen shrub that occupies the understorey of coniferous and mixed‐montane forests of the Mediterranean region (Nieto Feliner, 1997). In the Natural Park of Sierras de Cazorla‐Segura‐Las Villas (Jaén province, south‐east Spain) where the study was conducted, D. laureola is in flower from late January to April. Each plant produces a large number of small, tubular green‐yellowish flowers aggregated into a number of compact inflorescences per stem. Female flowers have vestigial stamens that do not produce pollen and have also shorter corolla tubes (7·7 ± 1·0 mm, n = 632 flowers from 65 plants) than perfect hermaphrodite flowers (12·9 ± 1·8 mm, n = 629 flowers from 68 plants). Hermaphrodites are self‐compatible but require a pollination vector. Self‐pollen may clog up the stigma of hermaphrodites, reducing their fruit set (Alonso and Herrera, 2001). Individual flowers have a single ovule, do not produce any strong scent, and remain open continuously for approx. 1 month. Direct observations on D. laureola flowers indicated that bees in the families Andrenidae, Apidae and Megachilidae were infrequent visitors. Adults of the pollen beetle Meligethes elongatus Rosenhauer (Nitidulidae) were frequently observed in the flowers, feeding on pollen and occasionally mating. Noctuid moths [including Autographa gamma L. (Plusiinae); Agrotis sp., Cerastis faceta Treitschke (Noctuinae); and Xylocampa areola Esper (Cuculliinae)] were also observed feeding on D. laureola flowers at night.
Preference of diurnal pollinators
Preference of diurnal pollinators for either female or hermaphrodite flowers of D. laureola was evaluated experimentally during spring 2001 by cutting a flowering branch of each sex‐morph and placing them together with their stems in a glass bottle filled with water. Two of these experimental bottles were placed at 30 cm distance from each other within flowering D. laureola populations and watched for pollinator visits during 30‐min periods. The procedure was repeated 23 times, between 0900 and 1300 h, on different sunny days and within two different populations (Cuevas Bermejas and Roblehondo; located within a well‐preserved pine–oak forest at 1210 and 1235 m elevation, respectively), using as background either female or hermaphrodite D. laureola flowering individuals and other nearby flowering species, such as Helleborus foetidus (Ranunculaceae), Narcissus longispathus (Amaryllida ceae) and Rosmarinus officinalis L. (Lamiaceae).
Preference of nitidulid beetles for the two plant genders was studied in March 2003, when plants were at their flowering peak. Discrimination was determined by counting the number of beetles found in a plant during a 3‐min searching interval on a sunny day, recording whether the plant was in sun or shade during this period. Observations were conducted on 12 female and 12 hermaphrodite unpaired individuals at the Roblehondo site. A more detailed test was carried out the following day at the Cañada del Espino population (1575 m) where eight females and eight hermaphrodites were selected in pairs of close and similar‐sized individuals to prevent differences in size or shading from modifying the results. Both populations showed similar trends and, thus, they were combined in a single analysis.
All statistical analyses were performed using the SAS statistical package (SAS Institute, 1996). The fixed effects that population, sex and sun had on beetle frequency were tested by a General Linear Model (Proc GLM) on squared‐root transformed data. Interactions between factors were not statistically significant and hence were excluded from the final model.
Pollinator exclusion experiment
The experiment was conducted between 12 February and 9 April 2001 at the Roblehondo site. Temperature and precipitation during this period were estimated from data collected at Cazorla Torre del Vinagre Meteorological Station (740 m elevation). Despite its lower altitude, this was the closest station to the study site, located approx. 6 km away. The average maximum temperature recorded was 20·2 ± 6·1 °C (mean ± 1 s.d., this notation will be used henceforth unless otherwise stated) and the average minimum was 5·4 ± 3·9 °C, with 38·5 % of the days recording minimum nocturnal temperatures lower than 4 °C (because of its higher elevation, this value was presumably greater at the study site). Rain was recorded on 41 % of the days and the average daily precipitation of rainy days was 14·6 ± 15·0 mm.
At the beginning of the study, 15 female and 15 hermaphrodite D. laureola individuals were selected. Three branches per plant were selected and tagged, each one with two to five inflorescences (mostly three) and most of their flowers closed. Every inflorescence of a marked branch was individually identified with a numbered tag tied to its pedicel and its number of flowers recorded. Each marked branch per plant was assigned to one of the following treatments: control (C), unselective pollination exclusion (UPE), and nocturnal pollination exclusion (NPE). The C branches were exposed to natural pollination during the whole flowering period. The UPE branches were exposed to day and night pollinators for only half of the flowering period to evaluate the effect of pollination reduction. Because it was impractical to conduct nocturnal pollinators’ exclusion over the whole flowering season, which lasted for more than 2 months, the NPE treatment was combined with the shortening of the flowering period experiment (UPE). Thus, the NPE branches were exposed to only diurnal pollinators over exactly the same dates that the UPE branches were exposed to day and night pollinators in order to evaluate the effect of specifically excluding nocturnal pollinators. UPE branches were used as a control for this effect (see below).
Pollinators were excluded by using mesh bags tied with a wire to the leafless portion of the stems. Because the specific weather conditions of a single period within the season could affect the results of the treatments, pollinator exclusion (including both UPE and NPE treatments) was conducted over four different non‐consecutive periods. Altogether this comprised 28 days out of the approx. 57 days that the study plants bore open flowers (see Fig. 1). This resulted in different‐aged flowers being exposed to pollinators. For the other 29 days, the UPE branches where uncovered and thus available to both diurnal and nocturnal pollinators. Meanwhile, bags on NPE branches were closed between dawn and dusk and remained open only during daytime. The comparison between branches where nocturnal pollinators were selectively excluded (NPE) and those that were available to all pollinators during the same study dates (UPE) was adequate to evaluate nocturnal pollination effect because those dates represented random ‘temporal samples’ over the entire flowering period; they were evenly distributed over the course of the flowering season (Fig. 1); the average temperatures did not differ from those recorded during the whole period (data not shown); and they comprised roughly half a priori favourable (i.e. sunny) and unfavorable (i.e. cloudy, rainy, snowy) days for pollination. The numbers of flowers closed, opened and withered in every marked inflorescence were periodically recorded during the flowering period. All C, UPE and NPE branches remained bagged during the fruit development period to avoid predation by mice and caterpillars. On 18 June, when approx. 98 % of fruits were mature, counts of unripe and mature fruit were used to estimate the fruit set.
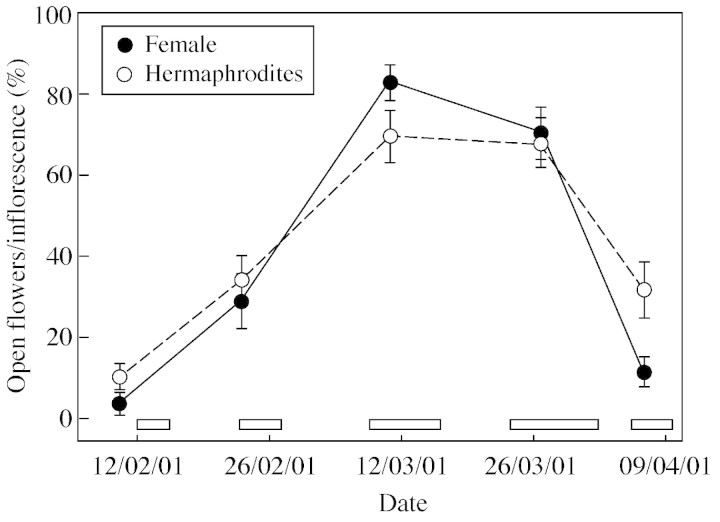
Fig. 1. Flowering phenology of female and hermaphrodite Daphne laureola plants at the Roblehondo study site. Data are mean percentage of open flowers per inflorescence (n = 15 individuals per sex); error bars denote ± 1 s.e. Boxes at the bottom of the graph indicate the periods in which treated branches were exposed to pollinators.
Differences in final fruit set between C and UPE branches, and between UPE and NPE branches, were analysed separately by fitting Generalized Linear Mixed Models (GLIMMIX macro; Littell et al., 1996) with binomial error distribution and probit link function. The covariance structure was defined as compound symmetric for each individual plant, i.e. the plant random effect was defined as the subject term (Littell et al., 1996). Sex, treatment and their interaction were the fixed factors of the model, whereas the plant × treatment interaction was included as a random effect. One female and one hermaphrodite plant that did not produce any fruit were excluded from the analyses. The comparison between C and NPE branches did not have any specific biological meaning in the context of this study and was not analysed.
RESULTS
The flowering phenology of D. laureola females and hermaphrodites, based on flower counts conducted on the open‐pollinated control branches (C), is shown in Fig. 1. Individual plants bore open flowers for more than 2 months. On average, females tended to have a shorter flowering period, because they started to open flowers later and ended their flowering period earlier than the hermaphrodites. The difference between females and hermaphrodites in the percentage of open flowers per inflorescence varied between positive and negative on different dates (Fig. 1).
Preference of diurnal pollinators
Visits to D. laureola flowers were very infrequent, although insects were actually observed flying at the study sites. Only seven short visits to flowers were recorded during the total 23‐h observation period and, in most of these cases, only one flower was visited. This low number of visits precluded any analysis of pollinator preference for female or hermaphrodite flowers.
Nitidulid pollen beetles were present in 87·5 % of the D. laureola individuals examined. Abundance of beetles was higher on hermaphrodite plants (15·2 ± 14·1 vs. 7·3 ± 10·5) with statistically significant differences between sexes (F1,36 = 5·63, P = 0·02). The sun/shade location of individual plants at the time of monitoring had only a marginally significant effect on the number of beetles recorded (F1,36 = 3·88, P = 0·06), with abundance higher at sunny locations. The two study populations were similar in the frequency of nitidulid beetles recorded (F1,36 = 1·18, P = 0·28).
Unselective reduction of pollinators
The effects of a 50 % reduction in the opportunities for pollination in female and hermaphrodite D. laureola flowers, as estimated by comparing fruit set of C and UPE branches, were examined.
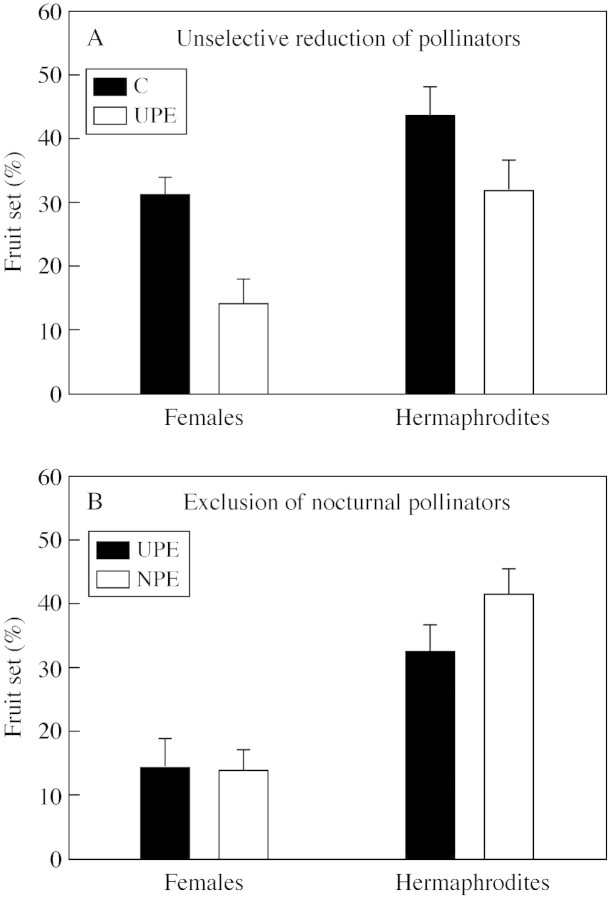
When exposed to natural pollination, females set a lower proportion of fruits than hermaphrodites (Fig. 2A; F1 117 = 4·53, P = 0·03). Inflorescences that were available to pollinators during only half of the flowering period also set a lower proportion of fruits in both female and hermaphrodite individuals (F1,51 = 6·42, P = 0·01). Reduced pollinator availability decreased fruit set of females by half, and by approx. one‐quarter in hermaphrodites (Fig. 2A). Despite this, the sex × treatment interaction was not statistically significant (F1,117 = 0·54, P = 0·46).
Fig. 2. Mean percentage fruit set (+ 1 s.e.) of inflorescences of female (n = 14 plants) and hermaphrodite (n = 14 plants) Daphne laureola individuals. A, Open‐pollinated control branches available to pollinators during the whole flowering period (C), and branches bagged for half of the flowering period and open to both diurnal and nocturnal pollinators for the rest of the time (UPE). B, Branches bagged for half of the flowering period and available to both diurnal and nocturnal pollinators for the rest of the time (UPE), and branches bagged for half of the flowering period and only available to diurnal pollinators for the rest of the time (NPE).
Exclusion of nocturnal pollinators
The effects of the selective exclusion of nocturnal pollinators by comparing fruit set of UPE and NPE branches were examined. Exposure to nocturnal pollinators had a negative effect on the fruit set of hermaphrodite flowers but not on females. Exclusion of nocturnal insects increased fruit set of hermaphrodites approx. 10 % but did not alter the fruit set of female inflorescences (Fig. 2B). However, neither the effect of treatment (F1,50 = 0·69, P = 0·41) or the sex × treatment interaction (F1,124 = 0·73, P = 0·40) was statistically significant.
Overall, comparison of the fruit sets of C, UPE and NPE treatments shows that, in hermaphrodites, the negative effect of reduced pollinator availability (UPE) is compensated for when nocturnal pollinators are also excluded (NPE). In contrast, females are more strongly affected by a reduction in pollinators than hermaphrodites, but nocturnal exclusion does not add any quantitative effect.
DISCUSSION
Plant interactions with pollinators are often shaped by the abiotic conditions in which they occur (Heinrich and Raven, 1972; Herrera, 1996; Herrera et al., 2001, and references therein; Totland, 2001). In seasonal environments, early flowering species tend to have long flowering seasons and long‐lived flowers in order to achieve cost‐efficient seed production (Schemske et al., 1978; Herrera, 2002). Shortening exposure of D. laureola flowers to pollinators significantly decreased fruit set in both female and hermaphrodite individuals, thus demonstrating the significance of the long flowering season to this species for reproductive success. In particular, female function was decreased two‐fold in female plants as compared with hermaphrodites. Thus a long flowering season promotes higher reproductive success by increasing the opportunity for seed fertilization and furthermore, by particularly favouring females’ seed production, it contributes to the maintenance of gynodioecy. Extensive flowering might also have consequences in terms of male fitness if the opportunity to export pollen successfully to other plants also increases with length of flowering period, hence promoting outcrossing in hermaphrodites.
Visitation of D. laureola by diurnal flying insects in 2001 was too low to allow for any inference regarding their possible selectivity between sex morphs. Despite the limited observations, it was clear that not all visits were made by the same insect species. It is worth mentioning that regular observations were only conducted during sunny days and at times when insects were most active. On some occasions bumble bees were observed feeding on nearby Helleborus foetidus for a while, moving to D. laureola to visit just a couple of flowers, and then returning to H. foetidus. Moreover, several Andrena bees were observed actively visiting Narcissus longispathus flowers, but never shifted to feeding on D. laureola flowers nearby. These few observations serve at least to illustrate that D. laureola flowers appear to be a low‐valued resource in the area for most day‐flying floral visitors.
Nocturnal visitors could also be disregarded as effective pollinators of D. laureola because their selective exclusion did not modify the fruit set of female plants and slightly increased it in hermaphrodites. It is possible that moths visiting hermaphrodite flowers during cold nights may cause the deposition of large amounts of self‐pollen, which clogs up the stigma and prevents an effective pollination (Kikuzawa, 1989; Thompson, 1989), in a way similar to that observed when adding pollen to non‐emasculated hermaphrodites by using a paintbrush (Alonso and Herrera, 2001). Such a negative outcome for nocturnal pollination has only been reported for a few species which are mostly pollinated during the daytime (Morse and Fritz, 1983; Navarro, 1999). In other plants pollinated by nocturnal and diurnal insects, nocturnal pollination has always been beneficial (e.g. Guitián et al., 1993; Groman and Pellmyr, 1999; Young, 2002). As previously stated, the comparison between day and night pollination effectiveness has been mostly conducted on species apparently ‘specialized’ for night pollination (see Groman and Pellmyr, 1999) for which a negative effect of nocturnal pollinators would not be expected.
Although suggestive, the results presented here have to be taken with caution. Logistical limitations precluded the inclusion of a treatment consisting of flowers available only to nocturnal pollinators. This treatment would have allowed discrimination between the two hypotheses, enabling a full explanation of the results reported, namely whether nocturnal pollinators discriminated against females, thus explaining the difference in the outcome of the treatment for both sexes, or whether moths are infrequent visitors to both genders and the unexpected increase of hermaphrodites’ fruit set was an artefact. The last option cannot be discounted but seems unlikely. For instance, the nocturnal exclusion of pollinators might have increased the resources available for diurnal pollinators (e.g. Morse and Fritz, 1983), which in turn would increase fruit set. However, with the current treatment design similar effects would be expected in the unselectively excluded branches, because both were closed to pollinators for longer periods than a single night and, thus, were not likely to modify the comparison between the unselective and nocturnal pollination‐excluded branches.
Despite the low flower visitation rates by Hymenopterans and Lepidopterans at the study area in 2001, hermaphrodites naturally set an average of 44 % of their flowers, while females set 31 %. Thus, although the figures must be qualified because counts of pollen beetles were conducted in a different year, the pollen predator, Meligethes elongathus, that was present at 80 % of the examined females and 95 % of the hermaphrodites, appeared to be the major pollinator of D. laureola in the study region. A single beetle can carry more than 400 D. laureola pollen grains. Their preference for hermaphrodite flowers is likely because, in addition to being a food source, they might also be more attractive if pollen emanates an odour (Cook et al., 2002).
The relative high fruit set of the species, the low impact that hand‐pollination had on it, and the absence of gender differences, at least in some years (Alonso and Herrera, 2001; C. Alonso, unpub. res.) indicates that reproduction of D. laureola females is not particularly limited by pollination deficit, despite the discrimination of the main pollinator. It is likely that D. laureola flowers may not require more than one or two pollinator visits in order to develop their single‐seeded fruits (C. Alonso, unpub. res.). With regard to the self‐compatible hermaphrodite, the behaviour of the beetles in walking around over individual plants of D. laureola, combined with the characteristic high number of simultaneously open flowers per plant, will probably promote geitonogamy (M. Medrano, unpub. res.), with apparently low short‐term consequences (Alonso and Herrera, 2001) but whose long‐term consequences are still under study. Similar predictions have been also made for the congeneric D. mezereum, also mainly pollinated by minute sedentary insects (Borg‐Karlsson et al., 1996, and references therein).
Finally, the preference of beetles for sunny places leads us back to the initial consideration that D. laureola pollination is shaped by abiotic conditions. In addition to the year and population sources of variability already documented for other species (e.g. Eckhart, 1992; Utelli and Roy, 2000), pollen beetles introduce a further step in the case of D. laureola, namely intra‐population individual variability. Through this pollinator behaviour, site‐related abiotic conditions are able to modify individual plant fitness, thus decreasing the opportunity of D. laureola genotypes to respond to natural selection acting on flowering‐related traits.
ACKNOWLEDGEMENTS
I would like to thank Rocío Ruíz for her help during field work, Marina García for technical assistance, J. Carlos Otero for Nitidulidae identification, Carlos M. Herrera and Estación Biológica de Doñana for the support and facilities provided to conduct the study, Consejería de Medio Ambiente for authorizing the work in Cazorla, and Carlos M. Herrera, Mónica Medrano, Alfonso M. Sánchez‐Lafuente, Don Levin and two anonymous referees for their constructive comments on the manuscript. The study was funded by a Marie Curie Fellowship (HMPF‐CT‐2000‐01095) and a Ramón y Cajal grant from the Spanish Ministerio de Ciencia y Tecnología.
Supplementary Material
Received: 21 July 2003; Returned for revision: 29 August 2003; Accepted: 10 September 2003 Published electronically: 5 November 2003
References
- AlonsoC, Herrera CM.2001. Neither vegetative nor reproductive advantages account for high frequency of male‐steriles in southern Spanish gynodioecious Daphne laureola (Thymelaeaceae). American Journal of Botany 88: 1016–1024. [PubMed] [Google Scholar]
- AndersonGJ.1976. The pollination biology of Tilia American Journal of Botany 63: 1203–1212. [Google Scholar]
- AshmanT‐L.2000. Pollinator selectivity and its implications for the evolution of dioecy and sexual dimorphism. Ecology 81: 2577–2591. [Google Scholar]
- BakerHG.1961. The adaptation of flowering plants to nocturnal and crepuscular pollinators. Quarterly Review of Biology 36: 64–73. [Google Scholar]
- Borg‐KarlsonA, Unelius C, Valterova I, Nilsson L.1996. Floral fragance chemistry in the early flowering shrub Daphne mezereum Phytochemistry 41: 1477–1483. [Google Scholar]
- CharlesworthD.1993. Why are unisexual flowers associated with wind pollination and unspecialized pollinators? American Naturalist 141: 481–490. [Google Scholar]
- CookSM, Bartlet E, Murray DA, Williams IH.2002. The role of pollen odour in the attraction of pollen beetles to oilseed rape flowers. Entomologia Experimentalis et Applicata 104: 43–50. [Google Scholar]
- EckhartVM.1992. Spatio‐temporal variation in abundance and variation in foraging behavior of the pollinators of gynodioecious Phacelia linearis (Hydrophyllaceae). Oikos 64: 573–586. [Google Scholar]
- FaegriK, Van der Pijl L.1971.The principles of pollination ecology. Oxford, UK: Pergamon Press. [Google Scholar]
- GromanJD, Pellmyr O.1999. The pollination biology of Manfreda virginica (Agavaceae): relative contribution of diurnal and nocturnal visitors. Oikos 87: 373–381. [Google Scholar]
- GuitiánP, Guitián J, Navarro L.1993. Pollen transfer and diurnal versus nocturnal pollination in Lonicera etrusca Acta Oecologica 14: 219–227. [Google Scholar]
- HeinrichB, Raven PH.1972. Energetics and pollination ecology. Science 176: 597–602. [DOI] [PubMed] [Google Scholar]
- HerreraCM.1995. Floral biology, microclimate, and pollination by ectothermic bees in an early‐blooming herb. Ecology 76: 218–228. [Google Scholar]
- HerreraCM.1996. Floral traits and plant adaptation to insect pollinators: a devil’s advocate approach. In: Lloyd DG, Barrett SCH, eds. Floral biology New York: Chapman and Hall, 65–87. [Google Scholar]
- HerreraCM.2000. Flower‐to‐seedling consequences of different pollination regimes in an insect‐pollinated shrub. Ecology 81: 15–29. [Google Scholar]
- HerreraCM.2002. Censusing natural microgametophyte populations: variable spatial mosaics and extreme fine‐graininess in winter‐flowering Helleborus foetidus (Ranunculaceae). American Journal of Botany 89: 1570–1578. [DOI] [PubMed] [Google Scholar]
- HerreraCM, Sánchez‐Lafuente AM, Medrano M, Guitián J, Cerdá X, Rey P.2001. Geographical variation in autonomous self‐pollination levels unrelated to pollinator service in Helleborus foetidus (Ranunculaceae). American Journal of Botany 88: 1025–1032. [PubMed] [Google Scholar]
- JennerstenO, Morse DH.1991. The quality of pollination by diurnal and nocturnal insects visiting common milkweed, Asclepias syriaca American Midland Naturalist 125: 18–28. [Google Scholar]
- JohnsonSD, Steiner KE.2000. Generalization versus specialization in plant pollination systems. Trends in Ecology and Evolution 15: 140–143. [DOI] [PubMed] [Google Scholar]
- KikuzawaK.1989. Floral biology and evolution of gynodioecism in Daphne kamtchatica var. jezoensis Oikos 56: 196–202. [Google Scholar]
- LittellRC, Milliken GA, Stroup WW, RD Wolfinger.1996.SAS System for mixed models. Cary, NC, USA: SAS Institute. [Google Scholar]
- MorseDH, Fritz RS.1983. Contributions of diurnal and nocturnal insects to the pollination of common milkweed (Asclepias syriaca L.) in a pollen limited system. Oecologia 60: 190–197. [DOI] [PubMed] [Google Scholar]
- NavarroL.1999. Pollination ecology and effect of nectar removal in Macleania bullata (Ericaceae). Biotropica 31: 618–625. [Google Scholar]
- Nieto FelinerG 1997. Thymelaeaceae. In: Castroviejo S, Aedo C, Benedí C, Laínz M, Muñoz Garmendia F, Nieto Feliner G, Paiva J, eds. Flora Iberica, Vol. VIII, Haloragaceae‐Euphorbiaceae Madrid, Spain: Real Jardín Botánico, CSIC, 32–69. [Google Scholar]
- PelletierL, Brown A, Otrysko B, McNeil JN.2001. Entomophily of the cloudberry (Rubus chamaemorus). Entomologia Experimentalis et Applicata 101: 219–224. [Google Scholar]
- RomeroR, Guijarro JA, Ramis C, Alonso S.1998. A 30 year (1964–1993) daily rainfall data base for the Spanish Mediterranean regions: First exploratory study. International Journal of Climatology 18: 541–560. [Google Scholar]
- SAS Institute.1996. SAS/STAT software: changes and enhancements through Release 6·11. Cary, NC, USA: SAS Institute. [Google Scholar]
- SchemskeDW, Willson MF, Melampy MN, Miller LJ, Verner L, Schemske KM, Best LB.1978. Flowering ecology of some spring woodland herbs. Ecology 59: 351–366. [Google Scholar]
- ThompsonJD.1989. Germination schedules of pollen grains: impli cations for pollen selection. Evolution 43: 220–223. [DOI] [PubMed] [Google Scholar]
- TotlandØ.2001. Environment‐dependent pollen limitation and selection on floral traits in an alpine species. Ecology 82: 2233–2244. [Google Scholar]
- UtelliA‐B, Roy BA.2000. Pollinator abundance and behavior on Aconitum lycoctonum (Ranunculaceae): an analysis of the quantity and quality components of pollination. Oikos 89: 461–470. [Google Scholar]
- YoungHJ.2002. Diurnal and nocturnal pollination of Silene alba (Caryophyllaceae). American Journal of Botany 89: 433–440. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.