Abstract
• Background and aims To understand better the basic growth characteristics of pines and the fundamental properties of the shoot apical meristem (SAM), variations within the shoot apex of buds were studied.
• Methods A detailed structural comparison of meristem dimensions, organogenetic activity, and the presence of lipids, starch grains and tannins was performed on shoot apices of juvenile, and male and female adult Pinus pinaster at five different times in the annual growth cycle.
• Key results There were significant correlations among traits and differences in the pattern for juvenile and adult shoots. In juvenile shoots, peaks of organogenesis were present in spring and autumn, but not in summer. In adult shoots, one peak, characterized by an increase in meristem dimensions, was present in summer. The accumulation of starch grains beneath the SAM and of tannin in sub‐apical pith parenchyma were at their maximum when organogenetic activity was high in spring and autumn in juvenile plants, and in summer in adult plants. In juvenile and adult plants, lipids were stored within the SAM in autumn, filling a large part of the bud in winter, and were depleted in the cortical parenchyma and then in the pith during shoot elongation.
• Conclusions Depending on the sites of accumulation within the SAM and on the stage of the annual growth cycle, lipids, starch and tannins may be involved in different processes. In spring, energy and structural materials released by lipid hydrolysis may contribute to stem elongation and/or cell‐to‐cell communication. During organogenesis, energy and structural materials released by starch hydrolysis may influence developmental programmes in the SAM and adjacent cells. Tannins may be involved in cellular detoxification. At the end of the growing season, accumulation of lipid and starch is positively correlated with the onset of dormancy.
Key words: Pine, Pinus pinaster, organogenesis, reserve allocation, shoot apical meristem, starch, tannins, triacylglycerol
INTRODUCTION
Pines are an ecologically and economically important group of trees. As their exploitation occurs some years after their establishment, an understanding of their growth is important for plant improvement programmes. Important variations in both the growth pattern and the morphological characteristics of shoots have been observed in maturing Pinus pinaster, P. radiata and P. sylvestris trees (David, 1966; Debazac, 1966; Kremer, 1990). Different stages of development can be defined. In P. pinaster, mostly cultivated for wood production in the Mediterranean area, the juvenile stage is observed from 1–3 years old, the adult male stage from 3 years old, and the adult female stage from 5 years old (Debazac, 1966; Kremer et al., 1990).
In the juvenile stage, leaf primordia differentiate into photosynthetically active primary needles. Axillary buds develop either into long shoots (auxiblasts) in basal positions or into randomly distributed short shoots (brachyblasts) in more distal positions on the stem. Later, when trees reach the adult stage, leaf primordia differentiate into scale leaves (cataphylls), and photosynthetic activity is shifted to long needles (pseudophylles) differentiated on short shoots. The position of axillary buds is also changed. As a result, the shoot growth units (GU) exhibit an acropetal sequence of scale leaves with empty axils in the basal position, short shoots in the middle section, and a pseudo‐whorl of long shoot buds in the terminal position. The first male strobili appear in place of some of the short shoots when the trees reach 3 years old, but this is dependent upon environmental conditions. Female cones appear in a position similar to the long shoots when the trees reach 4–5 years old (Debazac, 1966). Depending on the age of the trees and the environment in which they are growing, a second GU may also be added (David, 1966; Kremer et al., 1990).
The first aim of the present study was to gather more data to understand better the basic growth characteristics of pines. Buds were studied as morphological modifications resulting from the organogenetic activity occurring in the shoot apical meristem (SAM). This activity varies according to several parameters such as the position of the bud on the plant, the age of the plant, and the time of year. Initiation and elongation of shoots may occur in juvenile plants immediately (free growth), after a short period of latency (discontinuous growth), or after the winter rest period (predetermined growth) (Kremer et al., 1990). In adult plants, shoot elongation occurs only following the predetermined growth pattern (Kremer and Roussel, 1986; Kremer et al., 1990). A detailed structural comparison of meristem dimensions and organogenetic activity was performed on shoot apices of juvenile, and male and female adult P. pinaster trees at five different times during their annual growth cycle.
Physiological modifications of the SAM have been described concomitantly with organogenetic activity. In germinating Pinus seeds, the first signs of the resumption of this activity in the SAM is at the radicle emergence stage (Riding, 1972; Riding and Gifford, 1973; Cecich, 1977) and consists of a decrease in SAM size and the production of a rosette of photosynthetic needles (primary needles) (Riding, 1972; Cecich and Horner, 1977). Simultaneously, the triacylglycerol reserves, which have been stored in oleosomes distributed in the whole embryo and mega gametophyte, are progressively depleted following a precise spatio‐temporal pattern (Cecich, 1980; Stone and Gifford, 1999; Jordy et al., 2000). In the SAM, lipid hydrolysis begins in the cells of the peripheral zone (PZ) which is involved in the initiation of needles. Subsequently, the hydrolysis continues in the rib meristem (RM) and the central mother cells (CMC) until the oleosomes are completely depleted. Concurrently, newly synthesized starch and tannins accumulate, particularly in the sub‐apical pith parenchyma underlying the SAM (Mia and Durzan, 1974; Murphy and Hammer, 1994; Jordy, 2000). This suggests that the allocation of reserves and SAM organogenetic activity are interdependent phenomena, a hypothesis that is consistent with the sink/source regulation of the SAM activity (Honkanen and Haukioja, 1998; Obeso, 2002).
Starch, lipid reserves and tannins are known to accumulate in the shoot tip as pines become older. During the first growing season, triacylglycerols accumulate in the SAM (Riding and Gifford, 1973; Cecich, 1977; Stone and Gifford, 1999; Jordy et al., 2000) and starch grains remain stored in the pith parenchyma (Jordy, 2000). In 15‐year‐old P. sylvestris (Scots pine) trees, starch and polyphenols accumulate within the shoot tip during the growing season (Hejnowicz, 1979). Unfortunately, these studies did not evaluate SAM organogenetic activity.
The second aim of the present study was to estimate the presence of starch, lipid reserves and tannins within the shoot apex of buds collected for structural comparison. A combination of these observations with the shoot apex activities is useful in determining the possible involvement of these compounds in shoot apical development and in providing data for understanding the fundamental properties of SAMs in vascular plants.
MATERIALS AND METHODS
Plant material
Plant material was collected from 1‐year‐old (juvenile), 3‐year‐old (adult male reproductive) and 20‐year‐old (adult female reproductive) P. pinaster trees artificially sown in experimental stands of AFOCEL at Castelnau‐le‐Médoc (Aquitaine, south‐west France).
Thirty terminal shoot segments (20 cm long) were harvested randomly from the last whorl of branches at the top of the trees. In line with David (1966), Kremer and Roussel (1986) and Kremer et al. (1990), who all worked on adult plants, there were five sampling dates (Fig. 1): (A) rest period (10 December 1997), (B) bud burst (11 March 1998), (C) shoot elongation (29 April 1998), (D) end of shoot elongation (5 July 1998) and (E) end of the growing season (28 October 1998). In south‐west France, the mean temperature on each date was: (A) 8 °C, (B) 10 °C, (C) 12 °C, (D) 20 °C and (E) 12 °C. Temperatures below 0 °C may be recorded from September.
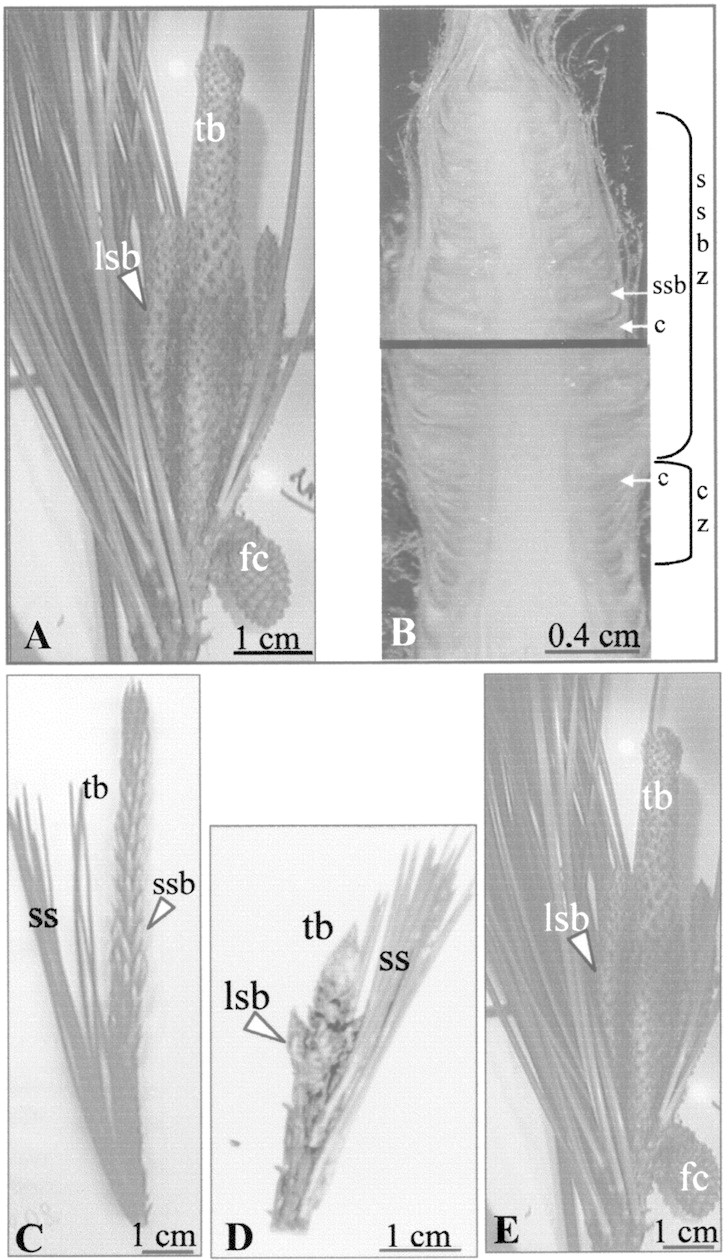
Fig. 1. Time series of the external morphology of the terminal bud of an adult pine during one year. A, Bud burst (March); long, terminal bud subtended by three long shoot buds and one female cone developed during the previous growing season. B, Bud burst; longitudinal section of the terminal bud showing preformed tissues. Scale leaves alone or in association with a short shoot bud formed the greater part of the future growth unit. C, Shoot elongation; the lower part of the terminal bud was elongated and the short shoot buds were enlarging. D, End of shoot elongation (July); the terminal bud was small, and a lateral long shoot bud was well differentiated. Each short shoot bud had burst and bore two long needles. E, End of the growing season (October and December); the terminal bud and the axillary long shoot buds were enlarged. tb, Terminal bud; lsb, long shoot bud; ssb, short shoot bud; fc, female cone; ss, short shoot; c, scale leaf; ssbz, short shoot bud zone; cz, scale leaves with empty axils.
Histology
At each growth stage, ten samples were fixed in FAA [10 % formaldehyde (v/v), 5 % acetic acid (v/v) and 50 % ethanol 95 % (v/v)] and used for further RNA staining. Twenty other specimens were fixed in CRAF solution (Randolph, 1935): ten for starch and tannin analysis, and ten for the lipid reserves study.
For the identification of lipid reserves, median‐longitudinal sections, 30 µm thick, were cut from ten samples using a freezing microtome. Sections were stained for 10 min in a filtered solution containing 60 % saturated Oil red O in isopropanol and 40 % distilled water (Clark, 1981).
Ten other samples were used for RNA staining. The Brachet test was performed on Paraplast Plus (Sherwood Medical Co., St Louis, USA) embedded sections (8 µm thick) using 0·5 % methyl green (w/v) and 0·05 % pyronine (w/v). The test stains RNA purple, the intensity of the colour correlating positively with the RNA concentration (Fig. 2). Controls were assessed after sections were treated with 50 µg ml–1 ribonuclease (Sigma) for 1 h at 37 °C (Clark, 1981).
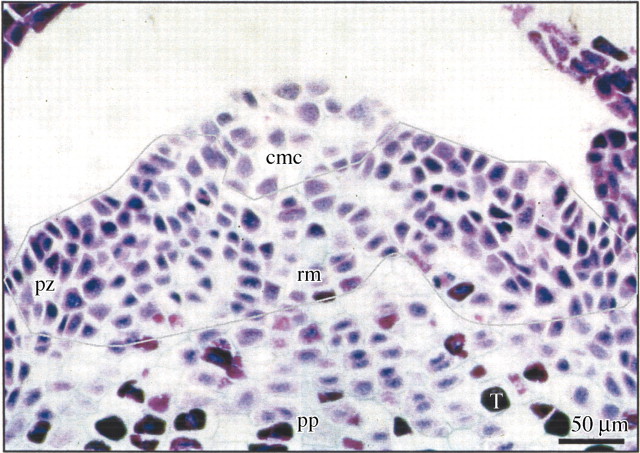
Fig. 2. SAM zonation with RNA staining (Brachet test). Deep purple stained cells contained high concentrations of RNA. Lines surround areas with the highest staining, which indicates regions of the most intense cell activity, located in the rib meristem and in the peripheral zone. cmc, Central mother cells; pz, peripheral zone; rm, rib meristem; pp, pith parenchyma; T, tannins.
An estimation of starch content was done on embedded sections (8 µm thick) from the last ten samples, using the periodic acid–Schiff reaction (Lillie and Fullmer, 1976 in Clark, 1981). On the same sections, polyphenols were indicated by brown staining after incubation in CRAF solution (Locquin and Langeron, 1978) and tannin precipitates were stained pink by the Brachet test (Jordy, 2000).
Stained areas from median‐longitudinal sections were photographed using a digital camera (Nikon D1) attached to a microscope (Nikon Optiphot 2, Champigny sur Marne, France) and estimates of the stained areas were made using Visilog 5·1 software developed by Noesis (Courtaboeuf, France).
The distribution of starch, tannins and lipids was recorded and the surface area of starch, tannins and RNA was measured.
Morphology and morphometry
Shoot activities were characterized by observing morphological traits to estimate the period of organ initiation and elongation, by measuring the SAM size (David, 1966), and by estimating the metabolic activity of SAM cells (Cecich et al., 1972; Monteuuis and Gendraud, 1987). Morphological characteristics of the growth unit (leaves, axillary buds and lateral shoots) were noted immediately after sampling. SAM height, width and area were measured just above the needle primordia with Visilog 5.1 image processing software. Variations in the ratio between the RNA‐stained surface and the dome surface were used to evaluate the surface of metabolically active cells in the SAM (Riding and Gifford, 1973). Analysis of variance (ANOVA) was used to test for significant differences between means obtained during 1 year for each category of plant material. If an ANOVA F‐test resulted in the rejection of the overall null hypothesis, the protected t‐test was used for pairwise comparison with 95 % confidence (Welkowitz et al., 1988).
RESULTS
Evaluation of activities within the shoot apex
During the rest period (December), juvenile material had a short terminal bud surrounded by primary needles, in contrast with the terminal scaly buds of male and female reproductive material which were 10 cm long and consisted of the preformed sequence of one growth unit (Fig. 1A, B and E). The SAM was characterized by a low metabolic activity with 10–15 % of cells with strong, positive staining for RNA (Fig. 3) and a minimum size (Fig. 4) in both the juvenile and adult plants. On the periphery of the SAM, axillary buds containing several well‐differentiated leaf primordia were observed (Fig. 5E, AB).
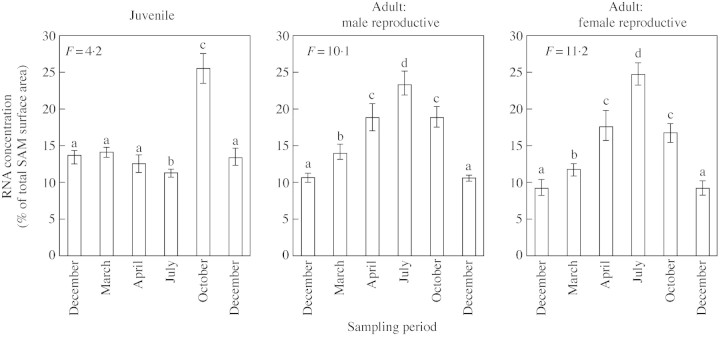
Fig. 3. Seasonal variation in the proportion of cells with strong positive staining of RNA in the SAM. Vertical bars correspond to the standard errors, letters indicating the result of the protected Student’s t‐test (P = 0·005).
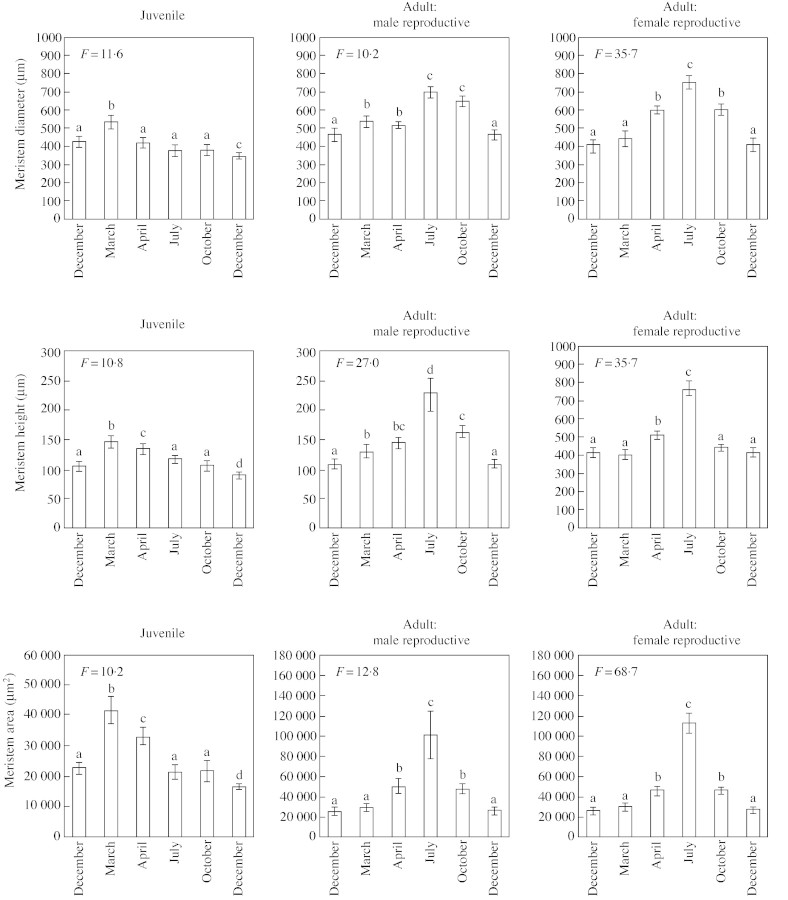
Fig. 4. Seasonal variations in the SAM size in juvenile, adult male and adult female reproductive plants. Vertical bars correspond to the standard errors, letters indicating the result of the protected Student’s t‐test (P = 0·005).
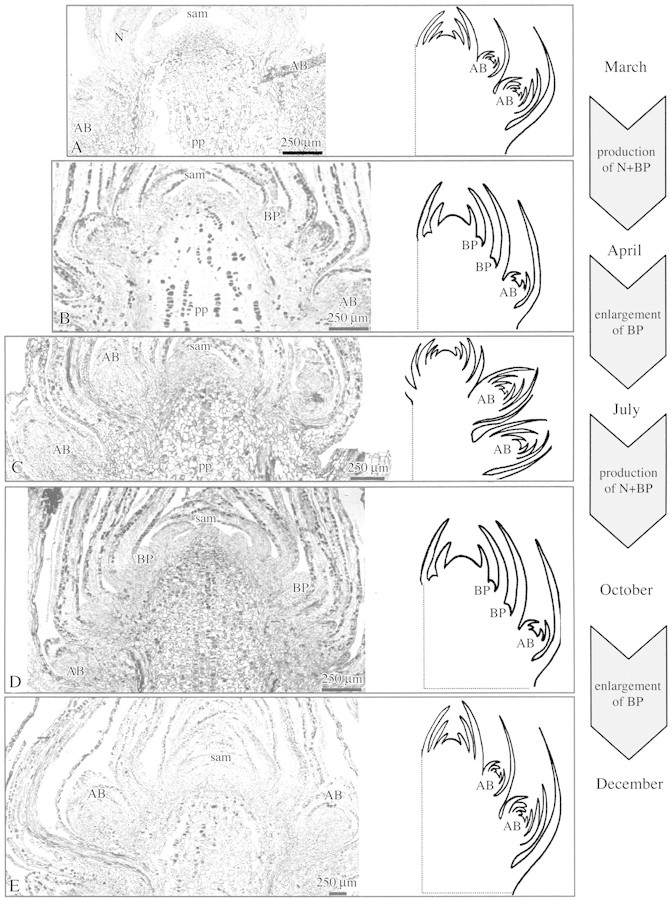
Fig. 5. Photomicrographs and schematics of bud apex development in juvenile plants. The SAM was surrounded by primary needles and lateral buds in March (A); by primary needles and bud primordia in April (B); by well‐developed lateral buds in July (C); by primary needles and bud primordia in October (D); and by well‐developed lateral buds in December (E). On the right of the figure, the main events occurring between each sampling period are represented. AB, Axillary buds; BP, bud primordia; N, primary needles; pp, pith parenchyma.
During the growing season, SAM characteristics changed progressively. In juvenile material, shoot elongation occurred in spring (from March to late June), stopped in summer (July) and resumed until the autumn (late September). Concomitant with this activity, histological variations in the SAM were detected. The variations observed in morphometric parameters (SAM diameter, height and area) reflected a slight increase in meristem size during bud burst (March) and, to a lesser extent, during shoot elongation (April) (Fig. 4). In contrast, the percentage of cells with strong, positive staining for RNA within the SAM did not show significant variation until the end of the growing season (October) (Fig. 3). Emerging bud primordia without visible needle primordia (BP) were observed in the axils of most needle primordia in April and October (Fig. 5B and D), indicating two periods of lateral organogenetic activity. In contrast, axillary bud primordia with well‐differentiated leaf primordia appeared proximal to the SAM in July.
In both male and female adult reproductive material, terminal shoot elongation began in April (Fig. 6B). During the same period, newly formed scale leaf primordia were visible within the shoot tip (Fig. 6B) and axillary bud primordia exhibited well‐differentiated scale leaves. Terminal shoot elongation progressed acropetally and ceased in July (Fig. 1C). Meanwhile, a set of scale leaves with empty axils was formed within the shoot tip, followed by scale leaves with emerging bud primordia in their axils (Fig. 6C). SAM size increased during this period, beginning earlier in male than in female reproductive material and culminating in July (Fig. 4). The metabolic activity, estimated by the relative RNA surface area, increased from April in both male and female reproductive material, and also reached a maximum in July (Fig. 3). Summer was, therefore, a peak period of organogenetic activity.
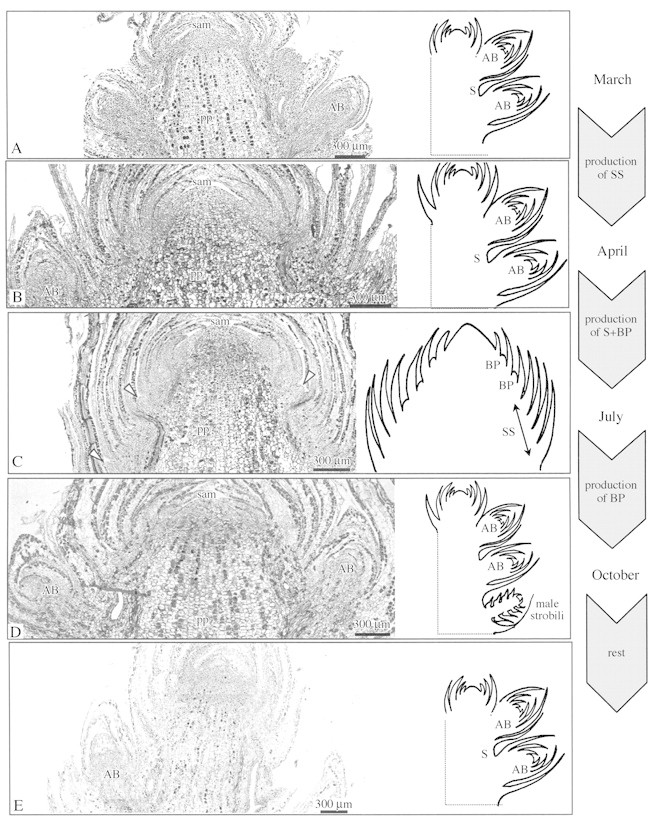
Fig. 6. Photomicrographs and schematics of terminal bud apex development during one growing season in adult female reproductive plants. By March, axillary buds were well developed close to the SAM (A). By April, the SAM was surrounded by several newly formed scale leaves (B); by July, by several scale leaves with axillary bud primordia (arrows) (C); and by October, by scale leaves located above well‐developed lateral buds (D). Amongst these buds and further down, future male strobili can be seen (see schematic). By December, the SAM was surrounded by several scale leaves above well‐differentiated axillary buds (E). On the right of the figure, the main events occurring between each sampling period are represented. AB, Axillary buds; BP, bud primordia; S, scale leaf; ss, scale leaves with empty axils; pp, pith parenchyma.
In October, the terminal shoot reached a length of 10 cm, confirming that organogenetic activity remained high after July (Fig. 1D). The SAM was surrounded by new scale leaves and axillary buds with well‐differentiated scale leaves. Basal to this zone, some differentiating male strobili were observed occasionally among the axillary buds (Fig. 6D). After October, SAM size and relative RNA areas were reduced (Figs 3 and 4), correlating with the decreased organogenetic activity of the SAM before entering the winter rest period.
To summarize, the bud apex of juvenile plants was organogenetically active in April and October and slowed down in July and December. This activity was associated with an increase in the percentage of cells with strong, positive staining for RNA within the SAM in October. In adult plants, the SAM was organogenetically active at all stages of the growing season. It produced scale leaves with empty axils during the elongation phase in spring, scale leaves with short shoots and male strobili in summer, and scale leaves with long shoot buds and female cones on female reproductive material in autumn.
Lipid reserves, starch and tannins in the shoot apex
Lipid reserves, starch and tannin distribution varied during the annual growth cycle, but followed different seasonal patterns. Lipid reserve distribution was similar in shoot tips of juvenile and adult male and female reproductive plants (not shown). Oleosomes were abundant in December and March, mainly in the pith and cortical parenchyma, in procambial strands, and in the SAM except in the peripheral zone (Fig. 7A–C). After bud break, lipid reserves were progressively depleted. This depletion was observed first in the cortex in April (Fig. 7D), and then in both the pith parenchyma and the rib meristem in July. Thus, lipid storage was at its minimum during summer (Fig. 7E). The central mother cells (CMC) of the SAM remained a permanent site of lipid accumulation. Oleosomes started accumulating in both the pith parenchyma and the rib meristem in October (Fig. 7F and G) and in the cortical tissue in December.
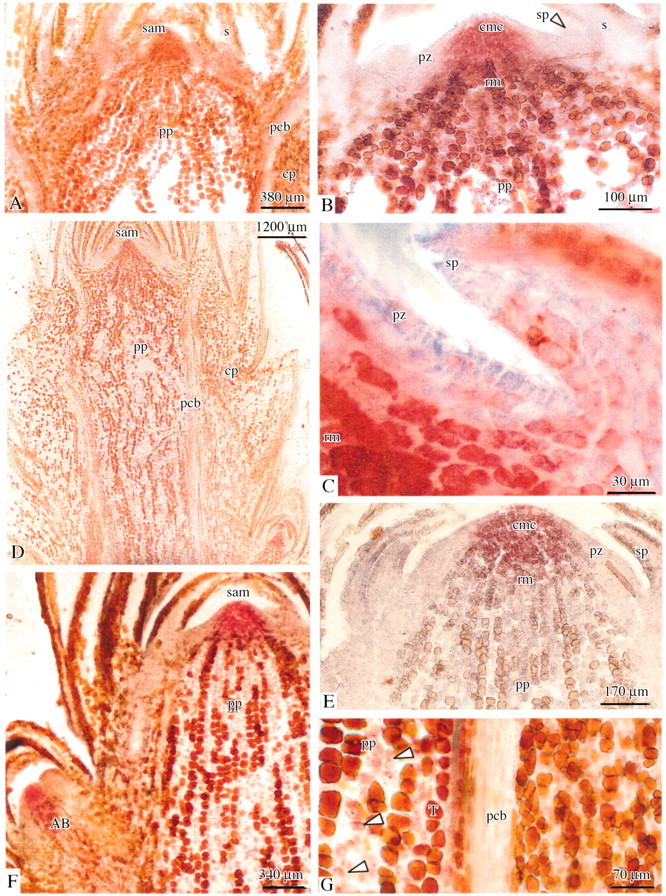
Fig. 7. Photomicrographs of longitudinal sections of the bud apex sampled from adult male plants. Lipid reserves are stained with Oil red O. A, Rest period (December); bud apices were fully enriched with lipid reserves except in scale leaves and in some regions of the SAM. B, Higher magnification of the SAM; scale primordia and the peripheral zone of the SAM were free from lipid reserves. C, Higher magnification of the peripheral zone. D, Shoot elongation period (April); lipids were abundant in the SAM and in the pith parenchyma, but were not detected in the cortex. E, End of shoot elongation period (July); oleosomes were abundant but only in the central mother cells. F, End of growth season (October); lipid reserves were located in the SAM, in the pith parenchyma and in a young lateral bud. G, October; higher magnification of the pith and the cortical parenchyma, lipid reserves were mainly detected in the pith parenchyma (arrows). ab, Axillary bud; s, scale leaf; sam, shoot apical meristem; rm, rib meristem; pz, peripheral zone; cmc, central mother cells; pp, pith parenchyma; pcb, procambium; cp, cortical parenchyma.
During winter, amyloplasts, confined to the sub‐apical pith parenchyma, were smallest and least abundant in all plant material studied (Table 1 and Fig. 8I and J). During the growing season, large and abundant starch grains accumulated in the sub‐apical pith parenchyma and occasionally in the rib meristem in all material studied but with different temporal patterns depending on the material (Table 1). In the shoot tips of juvenile plants two maxima were observed, the first appearing in spring (March–April) (Fig. 8A and B), and the second in autumn (October) (Fig. 8G). Between these two peak periods (July), the abundance and size of amyloplasts decreased dramatically, falling back to their winter minimum (Table 1). In some cases, starch grains totally disappeared during this period (Fig. 8D). In shoot tips of adult male and female reproductive plants starch levels increased progressively during spring, reached a maximum in summer (July) (Fig. 8E and F), then decreased gradually until attaining their minimum levels during winter (Fig. 8H and I). Pairwise comparisons of adult material showed that the spring/summer increase in numbers and size of amyloplasts occurred more rapidly in the male (April) than in the female reproductive material (July) (Fig. 8C and Table 1). Similarly, in autumn (October), the decrease was more pronounced in male than in female reproductive material (Table 1).
Table 1.
Size and abundance of starch grains located in sub‐apical pith parenchyma
| March | April | July | October | December | ||||||
| Abundance(%) | Size(µm2) | Abundance(%) | Size(µm2) | Abundance(%) | Size(µm2) | Abundance(%) | Size(µm2) | Abundance(%) | Size(µm2) | |
| Juvenile | 100 (II) | 8·1 | 100 | 10·0 | 10 | 5·1 | 100 | 14·5 | 10 | 4·5 |
| (± 2·2) | (II) | (± 2·5) | (I) | (± 0·8) | (II) | (± 2·7) | (I) | (± 0·6) | ||
| Adult male | 10 | 3·3 | 50 | 6·1 | 100 | 14·2 | 10 | 3·3 | 10 | 3·5 |
| (I) | (± 0·8) | (II) | (± 1·1) | (II) | (± 2·8) | (II) | (± 1·0) | (I) | (± 0·7) | |
| Adult female | 10 | 3·3 | 10 | 5·9 | 100 | 15·2 | 50 | 5·5 | 10 | 4·5 |
| (I) | (± 0·7) | (I) | (± 1·3) | (II) | (± 3·0) | (II) | (± 1·5) | (I) | (± 0·8) | |
Grain size was evaluated by measuring the grain surface on longitudinal section.
Abundance was evaluated by calculating the percentage of cells containing amyloplasts and the number of amyloplasts per cell: (I), less than five amyloplasts per cell; (II), more than five amyloplasts per cell.
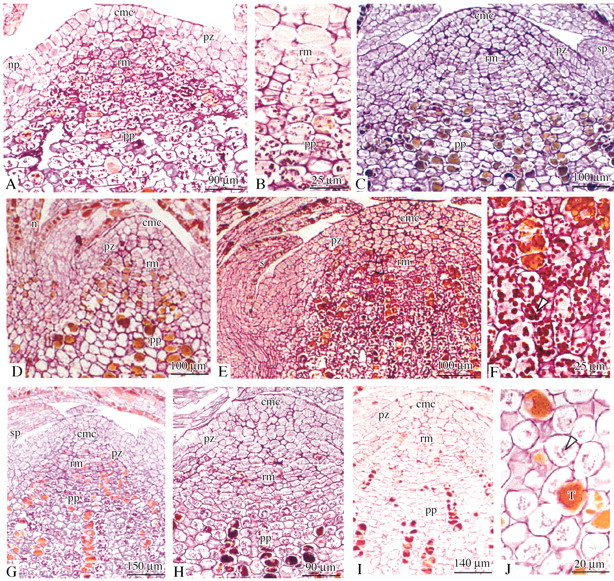
Fig. 8. Photomicrographs of longitudinal sections of the bud apex. Polysaccharides are stained pink by the PAS reaction. A, Shoot elongation period (April); juvenile plant in which starch grains were abundant in the rib meristem and the pith parenchyma. B, April; higher magnification of large starch grains in the rib meristem and the pith parenchyma. C, April; adult male plant in which only a few starch grains were present in some pith parenchyma cells. D, End of shoot elongation period (July); juvenile plant in which no starch grains were detected. E, July; adult female plant in which abundant large starch grains were distributed in the pith parenchyma and the adjacent rib meristem. F, July; higher magnification of large starch grains in the sub‐apical pith parenchyma. G, End of the growing season (October); a juvenile plant in which starch grains were abundant in the rib meristem and the pith parenchyma. H, October; adult female plant in which some starch grains appeared in the sub‐apical pith parenchyma. I, Rest period (December); a juvenile plant in which a few small starch grains appeared in some pith parenchyma cells. J, December; higher magnifications of small starch grains from I (arrow). ab, Axillary bud; s, scale leaf; sp, scale leaf primordium; np, needle primordium; n, needles; rm, rib meristem; pz, peripheral zone; cmc, central mother cells; pp, pith parenchyma; pcb, procambium; cp, cortical parenchyma; t, tannins.
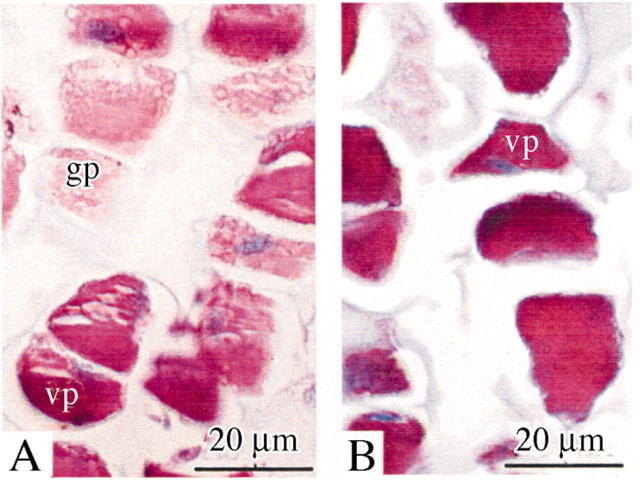
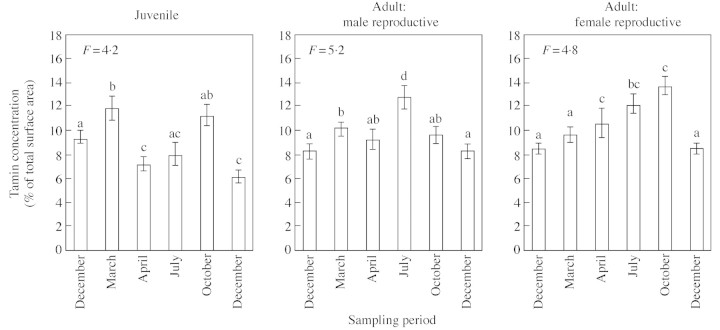
Tannins were identified in the vacuoles in two forms. Polyphenolic globules were located in a restricted number of cells within the rib meristem and in the parenchyma cells of the adjacent pith. Tannins filling whole vacuoles were observed only in pith cells (Fig. 9A and B). The amount of tannins in the pith parenchyma underlying the SAM also varied during the growing season and according to the age of the plant (Fig. 10). During winter, tannin levels were low in both juvenile and adult material. In juvenile plants, the relative amount of tannin increased in March, decreased in April and July, and increased again in October. In contrast, in adult plants, the relative amount increased progressively until maximum levels were reached in July. The relative amount decreased in October but remained higher in female material.
Fig. 9. Tannins in the sub‐apical pith parenchyma. Precipitates of tannins stained pink. A, Globular and vacuolar polyphenols in vacuolate pith cells adjacent to the rib meristem. B, Vacuolar polyphenols in vacuoles of pith parenchyma cells. gp, Globular polyphenols; vp, vacuolar polyphenols.
Fig. 10. Seasonal variations in the proportion of cells with strong staining of tannins in the sub‐apical pith parenchyma. Vertical bars correspond to the standard errors, letters indicating the result of the protected Student’s t‐test (P = 0·005).
DISCUSSION
All parameters used to estimate the organogenetic activity within the shoot apex showed that winter is a rest period for both juvenile and adult plants, thus confirming the results of David (1966) on mature P. pinaster trees. During this period, initiation of new leaf primordia was interrupted and axillary buds were recognizable only at some distance basal from the SAM. This must be due to the onset of the endodormancy state corresponding to the inability to grow probably imposed by the SAM, which in another gymnosperm, Picea abies, begins in November (De Fay et al., 2000).
Organogenetic activity began in spring and then continued, exhibiting temporal variations that could be related to the shoot growth patterns described by Kremer et al. (1990). In juvenile material, two maxima were observed. The first occurred in spring (March–April) and led to the formation of shoots that elongated immediately. It was characterized by a slight increase in SAM size and a steady RNA level. The second occurred in autumn (October) after the shoot elongation break. The newly formed organs did not elongate immediately but remained unextended until the following spring. No significant variation in SAM size was observed, but the metabolic activity, estimated by RNA staining, increased in this zone.
Between these two periods of organogenetic activity (July), shoot elongation was interrupted, confirming the previous observations of Illy and Castaing (1966). Furthermore, the shoot apex exhibited similar characteristics to those observed during the winter rest period. Since the SAM is not dormant, its inability to grow, given that temperatures are favourable for growth, was probably imposed by other parts of the plant (paradormancy) (Champagnat, 1992) such as the roots. In juvenile pines, the active period of root growth has been shown to correlate with decreased leaf production (Drew and Ledig, 1980).
In both male and female reproductive adult trees the organogenetic activity of the shoot tip began in spring, but a little later than in juvenile material (April). This activity continued without break until autumn with a single maximum period in summer (July). Plastochron measurements performed in spring and summer confirmed that SAM activity was highest during the summer period (Kremer and Roussel, 1986). SAM size and RNA content followed the same pattern of variation as organ initiation. Therefore, the seasonal variation in organogenetic activity in the SAM is different between juvenile and adult plants.
When these variations are compared with the morphological sequence observed on the resulting growth unit of adult material, it can be shown that the increase of organogenetic activity during the elongation phase of the terminal shoot (April) corresponds to the production of scale leaves with empty axils. The summer maximum of organogenetic and metabolic activity of the SAM was noted when shoot elongation was completed (July) and corresponded to the initiation of short shoots and male strobili. The autumnal decrease of SAM activities (October) corresponds to the initiation of the distal part of the GU, i.e. long shoot buds and female cones on adult female reproductive plants. In polycyclic plants, initiation of the second GU has been shown to occur during this autumnal period (David, 1966). In adult material, organ initiation and elongation are, thus, temporally separated as observed in the second period of organogenetic activity of juvenile plants.
When these variations of organogenetic activity within shoot tips are cross‐compared with the variations recorded in the accumulation of lipid reserves, starch and tannins, differences are observed. As in seedling shoot tips, in which lipids represent the major reserve for post‐germinative growth (Cecich, 1977; Jordy et al., 2000), oleosomes were abundant during the winter rest period in the shoot apex of both juvenile and adult plants. In P. banksiana, the entire meristem becomes filled with triacylglycerols in autumn and winter and, thus, can be considered a storage organ (Cecich, 1980). Such lipid storage has also been observed in quiescent buds of Douglas fir (Krasowski and Owens, 1990), and in some deciduous tree species (Catesson, 1964; Cragg and Willison, 1980). Lipids contained in oleosomes constitute a minimal mass for a maximal releasing of energy (Mohr and Shopfer, 1995) and confer frost tolerance to buds (Krasowski and Owens, 1990). The large numbers of oleosomes observed in overwintering nodules and root apical meristems of beach pea (Lathyrus maritimus) have been interpreted as sources of energy for various metabolic functions of cells which could protect these organs from freezing (Gurusamy et al., 2000). Furthermore, it has been suggested that lipid bodies are involved in dormancy processes in root nodules (Gurusamy et al., 2000) and in SAMs (Rinne et al., 2001). In SAMs of birch (Betula pubescens), the lipid bodies contained 1,3‐β‐d‐glucanase which is digested, resulting in closure of the plasmodesmata and cessation of cell‐to‐cell communication (Rinne et al., 2001).
During spring, with the resumption of shoot elongation and organ initiation, both processes requiring high energy and substrates for cell enlargement, the accumulated lipid reserves depleted progressively following an acropetal sequence from the cortex to the SAM. Minimum lipid storage was observed during summer. During autumn, depleted lipid reserves were restored again, reaching their maximum winter level. These seasonal variations of lipid body concentration within shoot tips were observed in both juvenile and adult material in which the organogenetic activity followed contrasting patterns of variation. This discrepancy between lipid reserve levels and organogenetic activity was clearly illustrated in July when reserves were low and organ initiation was interrupted in juvenile plants but were maximum in adult material. Variation in lipid reserve levels was mainly seasonally dependent without clear connection with paradormancy or variations of the within‐shoot‐tip organogenetic activity. However, it should be noted that these seasonal variations did not occur in all parts of the SAM. Two zones remained stable throughout the year. These were the mitotically active cells of the peripheral zone (PZ) and leaf primordial, which remain free from lipid reserves, and the quiescent central mother cells (CMC) which are permanent storage sites of lipid reserves. Catesson (1964) made similar observations in the shoot tips of plane tree (Platanus acerifolia) and suggested that mitotically active cells could be a determining factor of oleosome distribution within the SAM: the higher the cell activity, the lower the stored amounts of lipid reserve and vice versa. However, the situation seems to be more complex as in seedlings the histochemical localization of peroxidase and catalase indicates that CMC cells may be metabolically active (Riding and Gifford, 1973; Jordy et al., 2000). The proximity of these contrasting zones suggests that the CMC was a lipid source for the adjacent dividing cells which required energy and fatty acids for membrane synthesis. These lipid bodies may also be involved in lipid trafficking as reported by Murphy (2001). Consequently, oleosomes in pine buds would have multiple roles during the annual growth cycle.
For starch and tannins, the spatio‐temporal variations were quite different. Their accumulation occurred preferentially in pith parenchyma cells located just below the SAM, and their within‐shoot‐tip accumulation followed closely the variations of organogenetic activity. For example, starch and tannin accumulation in adult material increased in spring during scale leaf emergence, reached its maximum in summer when numerous short shoots were initiated, and decreased in October before the winter rest.
Whether or not tannins are related to organogenetic processes at the shoot tip level is not clear. Tannins are probably involved in detoxification processes during the initiation of leaves (Takahama and Oniki, 1997; Jordy et al., 2000). The correspondence between female cone formation and the autumnal tannin accumulation within shoot tips might support this hypothesis. In addition, it has been suggested that tannins are involved in frost tolerance and dormancy (Bilkovà et al., 1999).
The high accumulation of starch in pith parenchyma underlying the mitotically and metabolically active SAM may indicate an important flux of carbohydrates to the shoot apex, as observed during the rapid translocation of carbohydrates from the megagametophyte to the pine embryo (Stone and Gifford, 1999; Jordy, 2000). Thus, starch allocation within pine shoot tips is probably related to the seasonal variation in the strength of sources and sinks (Drew and Ledig, 1980; Jach and Ceuleman, 2000). During spring, triacylglycerol hydrolysis may supply substrates for starch synthesis, as suggested for germinating pine seeds (Stone and Gifford, 1999; Jordy et al., 2000). The most favourable period for carbohydrate accumulation in the shoot tip is probably during summer and autumn, as the newly elongated long needles are photosynthetically active during these seasons. This is in agreement with our observations within the shoot tips of adult plants (present study). Furthermore, growing roots constitute a sink during the growing season but this growth slows down during summer in adult plants (Jach and Ceuleman, 2000). In contrast, in juvenile plants, the lack of starch in summer may be attributed to the low SAM activity observed and to intensive root growth (Drew and Ledig, 1980).
Several important developmental processes can be related to starch accumulation within the shoot tip. This pool of carbohydrates located a short distance from the SAM can either be used as a direct source of energy or to provide carbon needed for organogenesis. In addition, it may influence meristem activity since glucose levels can impact on gene expression programmes (Smeekens, 2000). The seasonal pattern of starch storage within shoot tips can also relate to the acquisition of cold hardiness. Indeed, it has been shown that starch storage in P. radiata stems correlates with the acquisition of frost tolerance in autumn (Greer et al., 2000). The transitory accumulation of starch observed in shoot tips during the same period may also act as a precursor of lipid synthesis and thereby contribute to winter acclimatization.
In summary, strong correlations between the growth pattern and the accumulation of lipids, starch and tannins within the shoot apex were shown. Depending on the sites of accumulation within the SAM and the stage of the annual growth cycle, lipids, starch and tannins may be involved in different processes. During spring, energy and structural material may be supplied for shoot elongation by lipid hydrolysis, which may regulate cell‐to‐cell communication. During organogenesis, energy and structural material may be supplied for the SAM and the adjacent cells by starch hydrolysis, which may impact on gene expression programmes. Tannins may be involved in the detoxification of these cells. At the end of the growing season, lipids and starch may also be involved in dormancy and freezing‐tolerance processes.
ACKNOWLEDGEMENTS
I thank J. M. Favre for fruitful discussions and AFOCEL for providing the experimental material. This work was carried out with financial support from the Commission of the European Communities, Agriculture and Fisheries (FAIR) RTD programme 3‐CT‐96‐1445.
Supplementary Material
Received: 24 May 2003; Returned for revision: 26 August 2003; Accepted: 30 September 2003
References
- BilkovàJ, Albrechtovà J, Opartnà J.1999. Histochemical detection and image analysis of non‐specific esterase activity and the amount of polyphenols during annual bud development in Norway spruce. Journal of Experimental Botany 336: 1129–1138. [Google Scholar]
- CatessonAM.1964. Origine, fonctionnement, et variation cytologiques saisonnières du cambium. Annales Sciences Naturelles 5: 368–375. [Google Scholar]
- CecichRA.1977. An electron microscopic evaluation of cytohistological zonation in the shoot apical meristem of Pinus banksiana American Journal of Botany 64: 1263–1271. [Google Scholar]
- CecichRA.1980. The apical meristem. In: Proceeding of the joint workshop of IUFRO working party on xylem and shoot growth physiology Vancouver: Little C.H.A., 1–12. [Google Scholar]
- CecichRA, Horner HT.1977. An ultrastructural and microspectrophotometric study of the shoot apex during the initiation of the first leaf in germinating Pinus banksiana American Journal of Botany 64: 207–222. [Google Scholar]
- CecichRA, Lersten NR, Miksche JP.1972. A cytophotometric study of nucleic acids and proteins in the shoot apex of white spruce. American Journal of Botany 59: 442–449. [Google Scholar]
- ChampagnatP.1992. Dormance des bourgeons chez les végétaux ligneux. In: Côme D, ed. Les végétaux et le froid Paris: Hermann, 203–262. [Google Scholar]
- ClarkM.1981.Staining procedures 2nd edn. London: Williams and Wilkins, 199–200. [Google Scholar]
- CraggFJ, Willison JHM.1980. The ultrastructure of quiescent buds of Tilia europaea Canadian Journal of Botany 58: 1804–1813. [Google Scholar]
- DavidR.1966. Physiologie du pin maritime: 3 aspects de la physiologie du pin maritime: la genèse de la racine et de la tige, la formation de l’oléorésine. Bulletin de la Société Botanique de France 114: 137–164. [Google Scholar]
- DebazacEF.1966. Les modalités de la croissance en longueur chez les Pins. Bulletin de la Société Botanique de France 114: 3–14. [Google Scholar]
- DeFayE, Vacher V, Humbert F.2000. Water‐related phenomena in winter buds and twigs of Picea abies L. (Karst.) until bud burst: a biological, histological and NMR study. Annals of Botany 86: 1097–1107. [Google Scholar]
- DrewAP, Ledig FT.1980. Episodic growth and relative shoot: root balance in loblolly pine seedlings. Annals of Botany 45: 143–148. [Google Scholar]
- GreerDH, Robinson LA, Hall AJ, Klages K, Donnison H.2000. Frost hardening of Pinus radiata seedlings: effects of temperature on relative growth rate, carbohydrate concentration. Tree Physiology 20: 107–114. [DOI] [PubMed] [Google Scholar]
- GurusamyC, Davis PJ, Bal AK.2000. Seasonal changes in perennial nodules of beach pea (Lathyrus maritimus [L.] Bigel.) with special reference to oleosomes. International Journal of Plant Science 161: 631–638. [Google Scholar]
- HejnowiczA.1979. Tannin vacuoles and starch in the development of Scots pine (Pinus sylvestris) vegetative buds. Acta Societatis Botanicorum Poloniae 2: 195–203. [Google Scholar]
- HonkanenT, Haukioja E.1998. Intra‐plant regulation of growth and plant herbivore interactions. Ecoscience 5: 470–479. [Google Scholar]
- IllyG, Castaing JP.1966. Rythme sainonnier de croissance en diamètre et en hauteur du Pin maritime. Bulletin de la Société Botanique de France 114: 173–179. [Google Scholar]
- JachME, Ceulemans R.2000. Effects of season, needle age and elevated CO2 in photosynthesis in Scots pine (Pinus sylvestris). Tree Physiology 20: 145–157. [DOI] [PubMed] [Google Scholar]
- JordyM‐N.2000.Les variations saisonnières au sein des apex caulinaires du Pin maritime (Pinus pinaster Ait.) depuis la graine jusqu’à l’état adulte. PhD Thesis, Université H. Poincaré, Nancy, France. [Google Scholar]
- JordyM‐N, Danti S, Favre JM, Racchi ML.2000. Histological and biochemical changes in Pinus spp. seeds during germination and post‐germinative growth: triacylglycerol distribution and catalase activity. Australian Journal of Plant Physiology 27: 1109–1117. [Google Scholar]
- KrasowskiMJ, Owens JN.1990. Seasonal changes in the apical zonation and ultrastructure of coastal Douglas fir seedlings (Pseudotsuga menziesii). American Journal of Botany 77: 245–260. [DOI] [PubMed] [Google Scholar]
- KremerA, Roussel G.1986. Décomposition de la croissance en hauteur du pin maritime (Pinus pinaster Ait.). Variabilité géographique des composantes morphogénétiques et phénologiques. Annales des Sciences Forestières 43: 15–34. [Google Scholar]
- KremerA, Nguyen A, Lascoux M, Roussel G.1990. Morphogenèse de la tige principale et croissance primaire du pin maritime (Pinus pinaster Ait.) In: Actes du 3ième colloque ARBORA Sciences et Industries du bois, 14–15 mai 1990, Bordeaux. De la forêt cultivée à l’industrie de demain, 333–349. [Google Scholar]
- LocquinM, Langeron M.1978.Manuel de microscopie Paris: Masson, 247–248. [Google Scholar]
- MiaAJ, Durzan DJ.1974. Cytochemical and subcellular organization of the shoot apical meristem of dry and germinating Jack pine embryos. Canadian Journal of Forest Research 4: 39–54. [Google Scholar]
- MohrH, Schopfer A.1995. Respiratory metabolism. In: Plant physiology Berlin: Springer Verlag, 206–207. [Google Scholar]
- MonteuuisO, Gendraud M.1987. Nucleotide et nucleic status in shoot tips from juvenile and mature clones of Sequoiadendron giganteum during rest and growth phases. Tree Physiology 3: 257–263. [DOI] [PubMed] [Google Scholar]
- MurphyJB, Hammer MF.1994. Starch synthesis and localization in post‐germination Pinus edulis seedlings. Canadian Journal of Forest Research 24: 1457–1463. [Google Scholar]
- MurphyDJ.2001. The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Progress in Lipid Research 40: 325–438. [DOI] [PubMed] [Google Scholar]
- ObesoJR.2002. The cost of reproduction in plants. New Phytologist 155: 321–348. [DOI] [PubMed] [Google Scholar]
- RandolphLF.1935. A new fixing fluid and a revised schedule for the paraffin method in plant cytology. Stain Technology 10: 95–96. [Google Scholar]
- RidingRT.1972. Early ontogeny of seedlings of Pinus radiata Canadian Journal of Botany 50: 2381–2387. [Google Scholar]
- RidingRT, Gifford EM.1973. Histochemical changes occuring at the seedling shoot apex of Pinus radiata Canadian Journal of Botany 51: 501–512. [Google Scholar]
- RinnePLH, Kaikuranta PM, van der Schoot C.2001. The shoot apical meristem restores its symplasmic organization during chilling – induced release from dormancy. Plant Journal 26: 249–264. [DOI] [PubMed] [Google Scholar]
- SmeekensS.2000. Sugar‐induced signal transduction in plants. Annual Review Plant Molecular Biology 51: 49–81. [DOI] [PubMed] [Google Scholar]
- StoneSL, Gifford DJ.1999. Structural and biochemical changes in loblolly pine (Pinus taeda L.) seeds during germination and early seedling growth. II. Storage triacylglycerols and carbohydrates. International Journal of Plant Science 160: 663–671. [Google Scholar]
- TakahamaU, Oniki T.1997. A peroxidase/phenolics/ascorbate system can scavenge hydrogen peroxide in plant cells. Physiologia Plantarum 101: 845–852. [Google Scholar]
- WelkowitzJ, Ewen RB, Cohen J.1988.Introductory statistics for the behavioral sciences Orlando, FL: Harcourt Brace Jovanovich, 242–259. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.