Abstract
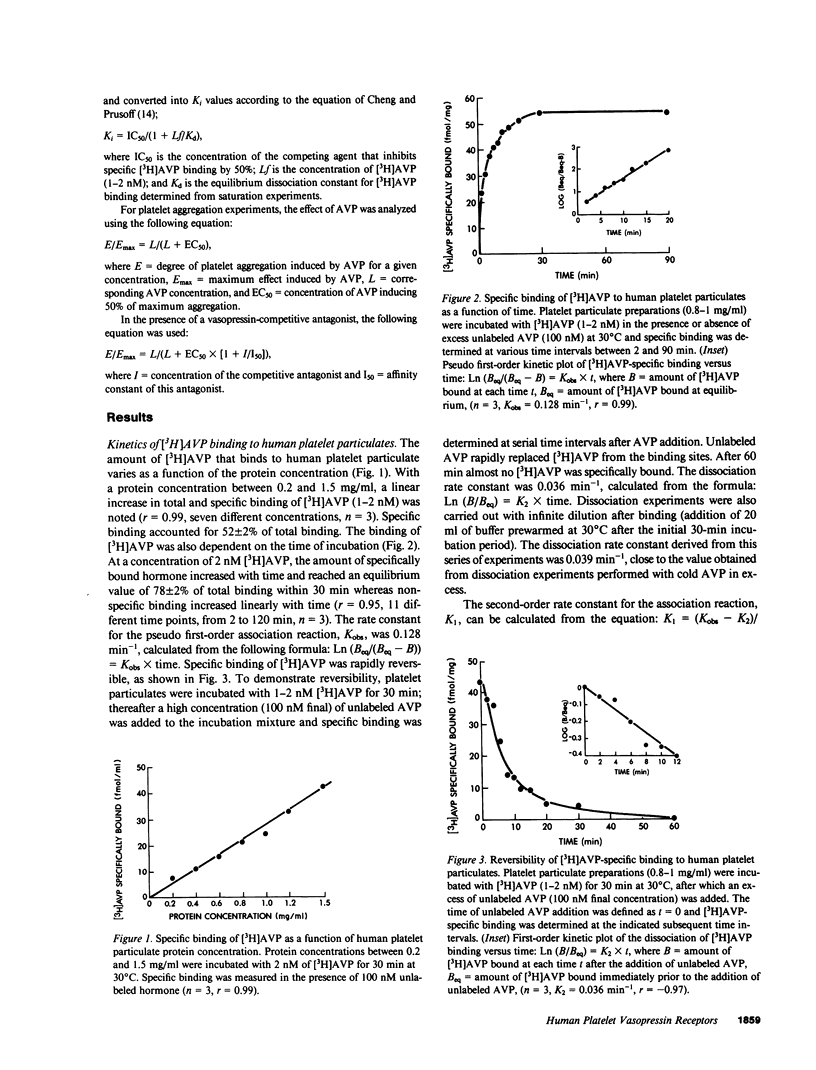
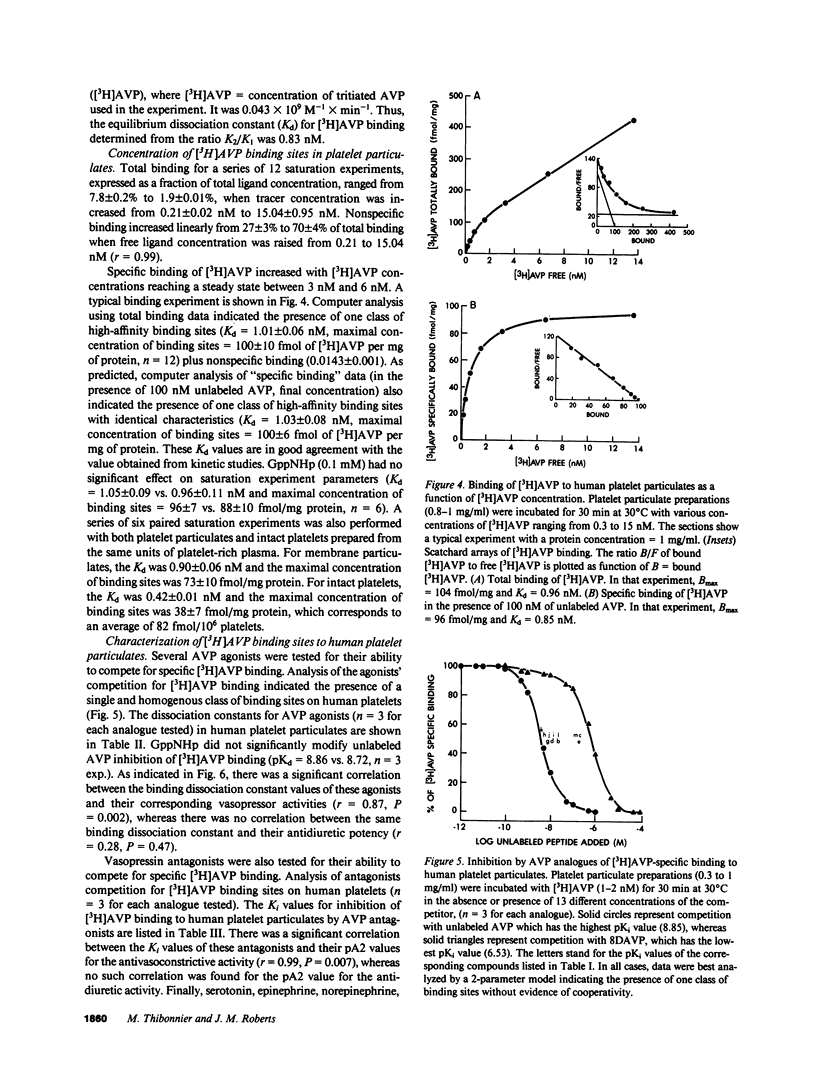
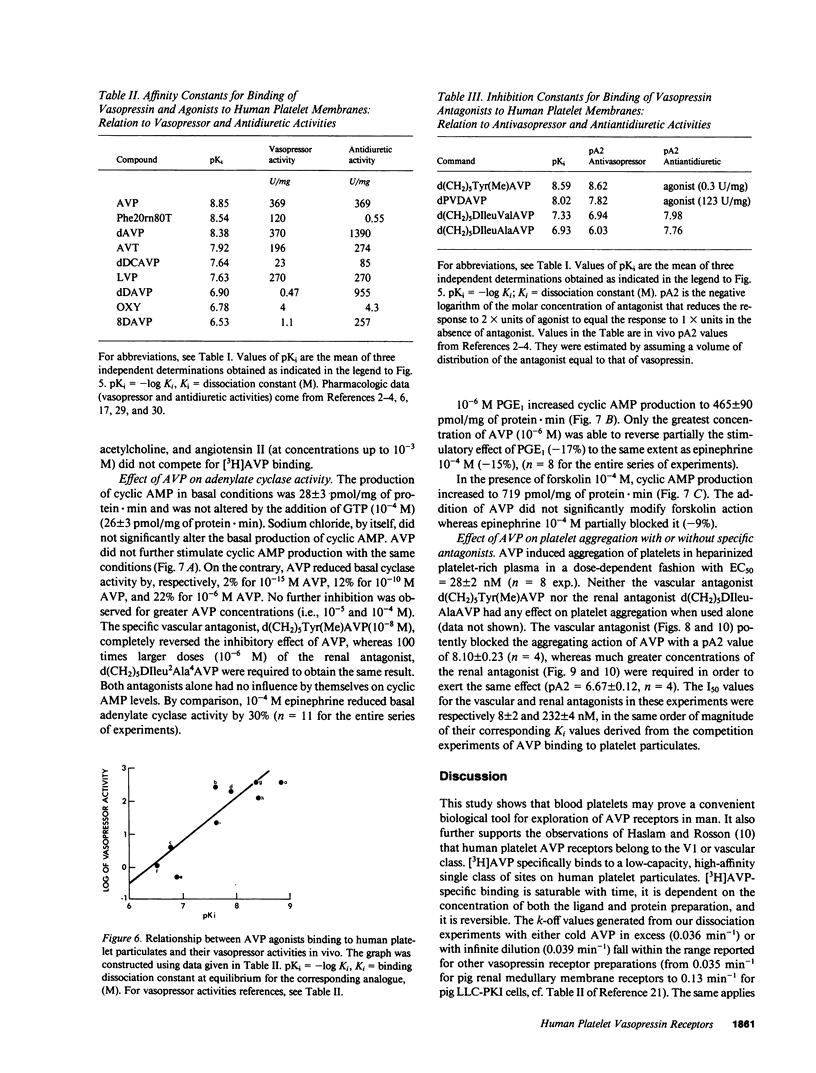
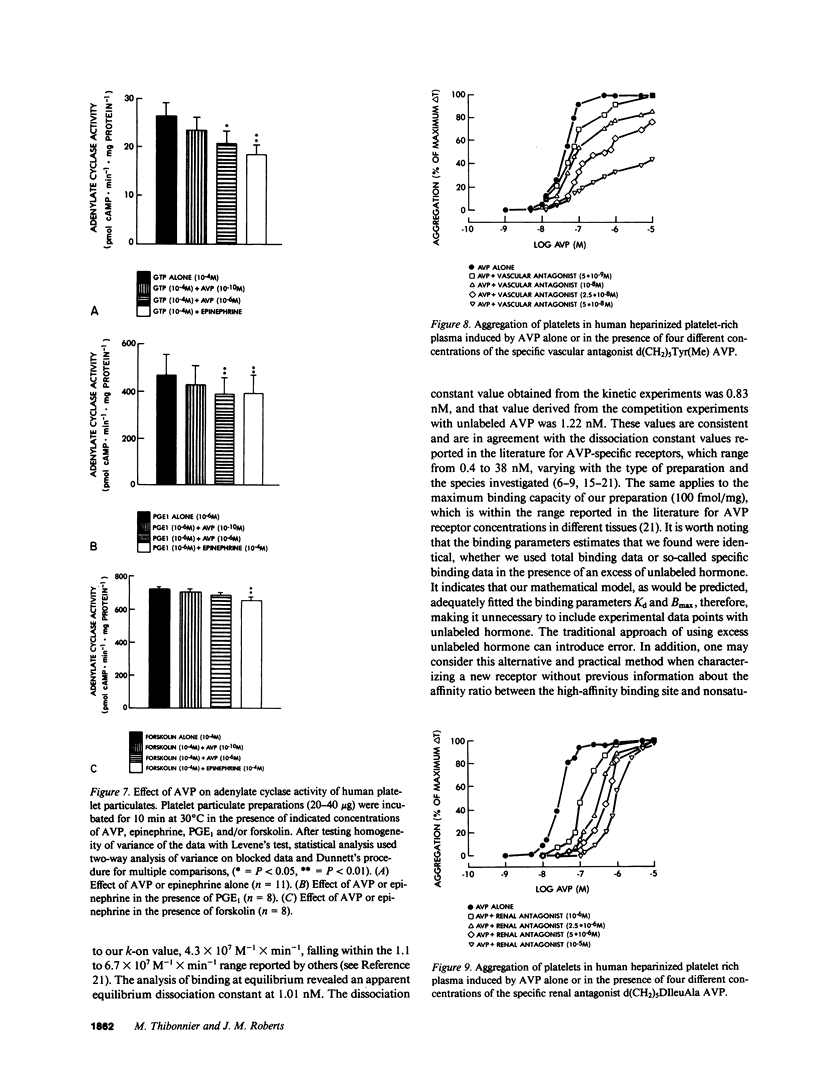
Using tritiated arginine-8-vasopressin [3H]AVP, vasopressin-specific binding sites were detected on human platelet membranes. One class of high-affinity binding sites was characterized with an equilibrium dissociation constant of 1.01 +/- 0.06 nM and a maximal binding capacity of 100 +/- 10 fmol/mg of protein (n = 12). Highly significant correlations were found between the relative agonistic (r = 0.87, P = 0.002) or antagonistic (r = 0.99, P = 0.007) vasopressor activities of a series of 13 AVP structural analogues and their relative abilities to inhibit [3H]AVP binding to platelet receptors whereas no such relationship existed when antidiuretic activities were considered (r = 0.28, P = 0.47). AVP did not stimulate cyclic AMP production of human platelets; on the contrary, high AVP concentrations (10(-6) M) inhibited cyclic AMP production measured in basal and prostaglandin E1-stimulated conditions. AVP caused intact platelet aggregation with a half-maximal aggregation (EC50) of 28 +/- 2 nM. This effect was more potently reversed by the specific vascular antagonist d(CH2)5Tyr(Me)AVP (pA2 = 8.10 +/- 0.23) than by the specific renal antagonist d(CH2)5IleuAlaAVP (pA2 = 6.67 +/- 0.12). The pA2 values of these two antagonists in platelets are in close agreement with the pKi values obtained in competition experiments (respectively 8.59 and 6.93) and with pA2 values reported in the literature for their in vivo antivasopressor activity (respectively 8.62 and 6.03). The observation that human platelets bear AVP receptors belonging to the vascular class suggests that platelet receptors can be used to further explore the role of vasopressin in cardiovascular homeostasis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berrettini W. H., Post R. M., Worthington E. K., Casper J. B. Human platelet vasopressin receptors. Life Sci. 1982 Feb 1;30(5):425–432. doi: 10.1016/0024-3205(82)90458-1. [DOI] [PubMed] [Google Scholar]
- Block L. H., Locher R., Tenschert W., Siegenthaler W., Hofmann T., Mettler E., Vetter W. 125I-8-L-arginine vasopressin binding to human mononuclear phagocytes. J Clin Invest. 1981 Aug;68(2):374–381. doi: 10.1172/JCI110265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Butlen D., Guillon G., Rajerison R. M., Jard S., Sawyer W. H., Manning M. Structural requirements for activation of vasopressin-sensitive adenylate cyclase, hormone binding, and antidiuretic actions: effects of highly potent analogues and competitive inhibitors. Mol Pharmacol. 1978 Nov;14(6):1006–1017. [PubMed] [Google Scholar]
- Cantau B., Keppens S., De Wulf H., Jard S. (3H)-vasopressin binding to isolated rat hepatocytes and liver membranes: regulation by GTP and relation to glycogen phosphorylase activation. J Recept Res. 1980;1(2):137–168. doi: 10.3109/10799898009044096. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Dorsa D. M., Majumdar L. A., Petracca F. M., Baskin D. G., Cornett L. E. Characterization and localization of 3H-arginine8-vasopressin binding to rat kidney and brain tissue. Peptides. 1983 Sep-Oct;4(5):699–706. doi: 10.1016/0196-9781(83)90021-9. [DOI] [PubMed] [Google Scholar]
- Guillon G., Couraud P. O., Butlen D., Cantau B., Jard S. Size of vasopressin receptors from rat liver and kidney. Eur J Biochem. 1980 Oct;111(1):287–294. doi: 10.1111/j.1432-1033.1980.tb06104.x. [DOI] [PubMed] [Google Scholar]
- Hase S., Morikawa T., Sakakibara S. Synthesis of a biologically active analog of deamino-8-arginine-vasopressin which does not contain a disulphide bond. Experientia. 1969 Dec 15;25(12):1239–1240. doi: 10.1007/BF01897469. [DOI] [PubMed] [Google Scholar]
- Haslam R. J., Davidson M. M., Davies T., Lynham J. A., McClenaghan M. D. Regulation of blood platelet function by cyclic nucleotides. Adv Cyclic Nucleotide Res. 1978;9:533–552. [PubMed] [Google Scholar]
- Haslam R. J., Rosson G. M. Aggregation of human blood platelets by vasopressin. Am J Physiol. 1972 Oct;223(4):958–967. doi: 10.1152/ajplegacy.1972.223.4.958. [DOI] [PubMed] [Google Scholar]
- Jard S. Vasopressin: mechanisms of receptor activation. Prog Brain Res. 1983;60:383–394. doi: 10.1016/S0079-6123(08)64405-2. [DOI] [PubMed] [Google Scholar]
- Keppens S., De Wulf H. Vasopressin and angiotensin control the activity of liver phosphodiesterase. Biochem J. 1984 Aug 15;222(1):277–280. doi: 10.1042/bj2220277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppens S., de Wulf H. The nature of the hepatic receptors involved in vasopressin-induced glycogenolysis. Biochim Biophys Acta. 1979 Nov 15;588(1):63–69. doi: 10.1016/0304-4165(79)90371-4. [DOI] [PubMed] [Google Scholar]
- Manning M., Lammek B., Kruszynski M., Seto J., Sawyer W. H. Design of potent and selective antagonists of the vasopressor responses to arginine-vasopressin. J Med Chem. 1982 Apr;25(4):408–414. doi: 10.1021/jm00346a015. [DOI] [PubMed] [Google Scholar]
- Manning M., Olma A., Klis W. A., Kolodziejczyk A. M., Seto J., Sawyer W. H. Design of more potent antagonists of the antidiuretic responses to arginine-vasopressin. J Med Chem. 1982 Jan;25(1):45–50. doi: 10.1021/jm00343a009. [DOI] [PubMed] [Google Scholar]
- Manning M., Sawyer W. H. Antagonists of vasopressor and antidiuretic responses to arginine vasopressin. Ann Intern Med. 1982 Apr;96(4):520–522. doi: 10.7326/0003-4819-96-4-520. [DOI] [PubMed] [Google Scholar]
- Murayama N., Werness J. L., Kusano E., Christensen S., Dousa T. P. Interaction of forskolin with vasopressin-sensitive cyclic AMP system in renal medullary tubules. J Cyclic Nucleotide Protein Phosphor Res. 1983;9(6):427–433. [PubMed] [Google Scholar]
- Murlas C., Nadel J. A., Roberts J. M. The muscarinic receptors of airway smooth muscle: their characterization in vitro. J Appl Physiol Respir Environ Exerc Physiol. 1982 Apr;52(4):1084–1091. doi: 10.1152/jappl.1982.52.4.1084. [DOI] [PubMed] [Google Scholar]
- Penit J., Faure M., Jard S. Vasopressin and angiotensin II receptors in rat aortic smooth muscle cells in culture. Am J Physiol. 1983 Jan;244(1):E72–E82. doi: 10.1152/ajpendo.1983.244.1.E72. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Schiffrin E. L., Genest J. 3H-vasopressin binding to the rat mesenteric artery. Endocrinology. 1983 Jul;113(1):409–411. doi: 10.1210/endo-113-1-409. [DOI] [PubMed] [Google Scholar]
- Stassen F. L., Erickson R. W., Huffman W. F., Stefankiewicz J., Sulat L., Wiebelhaus V. D. Molecular mechanisms of novel antidiuretic antagonists: analysis of the effects on vasopressin binding and adenylate cyclase activation in animal and human kidney. J Pharmacol Exp Ther. 1982 Oct;223(1):50–54. [PubMed] [Google Scholar]
- Thibonnier M., Aldigier J. C., Soto M. E., Sassano P., Menard J., Corvol P. Abnormalities and drug-induced alterations of vasopressin in human hypertension. Clin Sci (Lond) 1981 Dec;61 (Suppl 7):149s–152s. doi: 10.1042/cs061149s. [DOI] [PubMed] [Google Scholar]
- Thomas M. E., Osmani A. H., Scrutton M. C. Some properties of the human platelet vasopressin receptor. Thromb Res. 1983 Dec 15;32(6):557–566. doi: 10.1016/0049-3848(83)90057-9. [DOI] [PubMed] [Google Scholar]
- Vanderwel M., Lum D. S., Haslam R. J. Vasopressin inhibits the adenylate cyclase activity of human platelet particulate fraction through V1-receptors. FEBS Lett. 1983 Dec 12;164(2):340–344. doi: 10.1016/0014-5793(83)80313-5. [DOI] [PubMed] [Google Scholar]



