Abstract
• Background and Aims The labellar papillae and trichomes of Maxillaria Ruiz & Pav. show great diversity. Although papillae also occur upon other parts of the flower (e.g. column and anther cap), these have not yet been studied. Labellar trichomes of Maxillaria are useful in taxonomy, but hitherto the taxonomic value of floral papillae has not been assessed. The aim of this paper is to describe the range of floral papillae found in Maxillaria and to determine whether papillae are useful as taxonomic characters.
• Methods Light microscopy, histochemistry, low‐vacuum scanning and transmission electron microscopy.
• Key Results A total of 75 taxa were studied. Conical papillae with rounded or pointed tips were the most common. The column and anther cap usually bear conical, obpyriform or villiform papillae, whereas those around the stigmatic surface and at the base of the anther are often larger and swollen. Labellar papillae show greater diversity, and may be conical, obpyriform, villiform, fusiform or clavate. Papillae may also occur on multiseriate trichomes that perhaps function as pseudostamens. Labellar papillae contain protein but most lack lipid. The occurrence of starch, however, is more variable. Many papillae contain pigment or act as osmophores, thereby attracting insects. Rewards such as nectar or a protein‐rich, wax‐like, lipoidal substance may be secreted by papillae onto the labellar surface. Some papillae may have a protective role in preventing desiccation. Species of diverse vegetative morphology may have identical floral papillae, whereas others of similar vegetative morphology may not.
• Conclusions Generally, floral papillae in Maxillaria have little taxonomic value. Nevertheless, the absence of papillae from members of the M. cucullata alliance, the occurrence of clavate papillae with distended apices in the M. rufescens alliance and the presence of papillose trichomes in some species may yet prove to be useful.
Key words: Anther, column, histochemistry, labellum, low‐vacuum scanning electron microscopy, papillae, pseudopollen, pseudostamen
INTRODUCTION
Orchids largely attract specific pollinators by means of a combination of visual and olfactory cues (e.g. van der Pijl and Dodson, 1969). However, on alighting upon the labellum, tactile stimuli and rewards often take on an equally important role. Such rewards include pollen, nectar, oils and pseudopollen (van der Pijl and Dodson, 1969; Dressler, 1993). However, it has been estimated that approx. one‐third of orchid species provide no reward, merely ‘empty promises’ (Ackerman, 1984). Nevertheless, those species that reward pollinators often double their chances of fruiting (Neiland and Wilcock, 1998).
Pollen of epidendroid orchids is inaccessible to pollinators as it is bound into pollinia. Consequently, nectar is the most common reward amongst these orchids (Dressler, 1993). Even so, Porsch (1908) and van der Pijl and Dodson (1969) have estimated that one‐third of orchid species produce little or no nectar. Indeed, until recently, it was generally believed that all members of the Neotropical genus Maxillaria lacked nectar. However, it is now known that this is incorrect since, lately, nectar has been demonstrated for a number of species including M. coccinea (Jacq.) L. O. Williams ex Hodge (Stpiczyñska et al., 2004), M. jenischiana (Rchb.f.) C. Schweinf., M. imbricata Barb. Rodr., M. sophronitis (Rchb.f.) Garay (Davies et al., 2003a, b), M. parviflora (Poepp. & Endl.) Garay (Singer, 2003; Singer and Koehler, 2003; Stpiczyñska et al., 2004), M. pendens Pabst and M. rigida Barb. Rodr. (Singer and Koehler, 2003). Similarly, pseudopollen has been reported from Maxillaria (Janse, 1886; Porsch, 1905; van der Pijl and Dodson, 1969; Davies and Winters, 1998; Davies et al., 2000; Davies et al., 2003b) and occurs in the M. grandiflora and M. discolor alliances as well as in M. longissima Lindl. In each case, pseudopollen is formed by the fragmentation of uniseriate, moniliform hairs into individual component cells or short chains of cells rich in protein (Davies et al., 2000, 2003b). Members of the M. splendens alliance are also thought, solely on morphological grounds, to possess pseudopollen‐forming labellar hairs (Davies and Winters, 1998; Davies et al., 2000, 2003a, b) which differ from the above in that they are few‐celled and the component cells are elongate rather than elliptical or lemon‐shaped as in the M. grandiflora alliance or fusiform as in M. longissima. So far, however, labellar hairs from the M. splendens alliance have not been tested for food substances. Other species of Maxillaria, such as members of the M. acuminata and M. discolor alliances, produce a wax‐like material upon their labella and this is thought to be collected by bees for nest‐building (van der Pijl and Dodson, 1969), although, in that it contains lipids and aromatic amino acids, it clearly also has nutritional value (Davies et al., 2003a, b). Pheromone‐like compounds have been demonstrated for some Maxillaria spp. (Roubik, 2000), but hitherto, pseudocopulation is not known to occur in Maxillaria sensu stricto. However, this phenomenon has been demonstrated for Trigonidium obtusum Lindl., which molecular evidence suggests is ‘nested’ within Maxillaria (Singer, 2002).
Although many Maxillaria spp. offer rewards, the vast majority seemingly do not, and these are thought to attract insects by ‘deceit’ using a combination of features such as colour, fragrance and pilosity. The labella of such flowers generally lack trichomes but are clothed with abundant epidermal papillae. These papillae show great morphological diversity (Davies and Winters, 1998; Davies et al., 2003a) but the reasons for such differences are not clear. It is possible that some may function as osmophores. For example, the flower of M. picta Hook. has a strong honey‐like fragrance but lacks nectar or any other reward (Singer and Cocucci, 1999). However, its labellum, whilst lacking trichomes, is heavily clothed with villiform papillae (Davies and Winters, 1998). Singer and Cocucci (1999) report that M. picta is pollinated by the stingless bee Trigona spinipes, which, on backing out of the flower, appears somewhat drowsy. It may be that the papillae produce an intoxicating fragrance which, by partially anaesthetizing the insect, facilitates pollination. Indeed, van der Pijl and Dodson (1969) report that vanillin production in M. rufescens Lindl. is confined to those parts of the labellum around the ‘hair’. Another possibility is that even in apparently rewardless species, food substances may indeed be present but these are located within papillae and are only accessible to gnawing insects. Direct evidence for this is lacking. Nevertheless, this may be the case in M. rufescens, where, according to Porsch (1905) the unicellular ‘hairs’ have delicate walls and contain aleurone and oil droplets. In most cases, however, regardless of whether rewards are present or not, it is probable that labellar papillae play an important role in both attracting and guiding visiting insects deeper into the flower, thus facilitating pollination. This is accomplished by means of olfactory and tactile stimuli and, since many papillae are highly pigmented, visual cues. The main pollinators of Maxillaria are stingless bees (Meliponini) (Singer and Cocucci, 1999; Roubik, 2000), although euglossine bees (van der Pijl and Dodson, 1969), Ponerinae ants (Singer, 2003) and hummingbirds (Dodson, 1965—cited in van der Pijl and Dodson, 1969) have also been observed visiting these flowers. To date, however, there is no concrete evidence that hummingbirds pollinate Maxillaria spp.
Davies and Winters (1998) proposed that labellar features could provide useful taxonomic characters for determining infrageneric relations within Maxillaria. Subsequent work has proven this to be the case in that pseudopollen appears to be restricted to a handful of infrageneric alliances, and that these can be distinguished, to a degree, on the basis of this feature (Davies et al., 2000, 2003b). By contrast, the taxonomic value of papillae has, hitherto, not been assessed.
The present paper examines the morphological diversity of floral papillae in Maxillaria; those occurring on the lip as well as those on the column and anther cap. Moreover, it discusses their possible functions and their value as taxonomic characters.
MATERIALS AND METHODS
A total of 75 taxa were examined for floral papillae and/or trichomes using light microscopy and/or low‐vacuum scanning electron microscopy. Wherever possible, the column, anther cap and labellum were examined. The labellum of one specimen of M. vernicosa Barb. Rodr. was also examined using transmission electron microscopy (TEM) and a number of species, having different types of labellar papillae, were selected for histochemistry. Authorities for plant names follow Brummitt and Powell (1992). Plants with accession numbers prefixed ‘KLD’ were obtained from the first author’s collection, whereas those prefixed ‘S’ were grown at Swansea Botanical Complex, Swansea, UK. Those prefixed ‘MM’ were obtained from Dr M. McIllmurray at the National Collection of Maxillarias, Shirley, Croydon, UK and those prefixed ‘BSNS’, ‘XX’, ‘G’ or ‘GXX’ from the National Botanic Garden, Glasnevin, Eire. Further plants were obtained from The Royal Botanic Garden Edinburgh, UK and from Dr E. D. L. Schmidt, Wageningen, The Netherlands and these are prefixed ‘E’ and ‘ED’, respectively. Herbarium specimens were deposited at the National Museum of Wales, Cardiff, UK.
Low‐vacuum SEM
Following preliminary examination by means of light microscopy, specimens were prepared for low‐vacuum SEM. Examples showing different types of papillae were dissected and immediately examined by means of back‐scattered electron imaging using a JSM 5200 LV‐SEM in low‐vacuum mode at an accelerating voltage of 20–25 kV.
TEM
Pieces of labella of M. vernicosa (S20010100) were removed, prepared for TEM as described in previous papers (Davies et al., 2000, 2003a) and examined using a JEOL 1201 TEM at an accelerating voltage of 80 kV.
Histochemistry
Labellar papillae were tested for starch, lipids and aromatic amino acids using IKI, saturated ethanolic Sudan III and the xanthoproteic test, respectively (Davies et al., 2000, 2003a, b).
RESULTS
Morphology
The labellum, column and anther cap may be glabrous or papillose but, whereas the unicellular papillae found near the anther and on the column and anther cap are almost invariably conical, obpyriform or villiform (Fig. 1A, B and D), those occurring on the labellum show greater diversity and may be conical with rounded (Figs 2A–G and 4B–E) or pointed tips (Fig. 3A, C and D), obpyriform to clavate with greatly distended apices (Fig. 6A and B), villiform (Fig. 4A), fusiform or borne upon multiseriate, labellar trichomes (Fig. 6C). In M. lepidota Lindl., labellar papillae, as previously described (Davies and Winters, 1998), become modified into unicellular, spherical glands. These are more abundant on the ventral surface of the labellum than the dorsal.
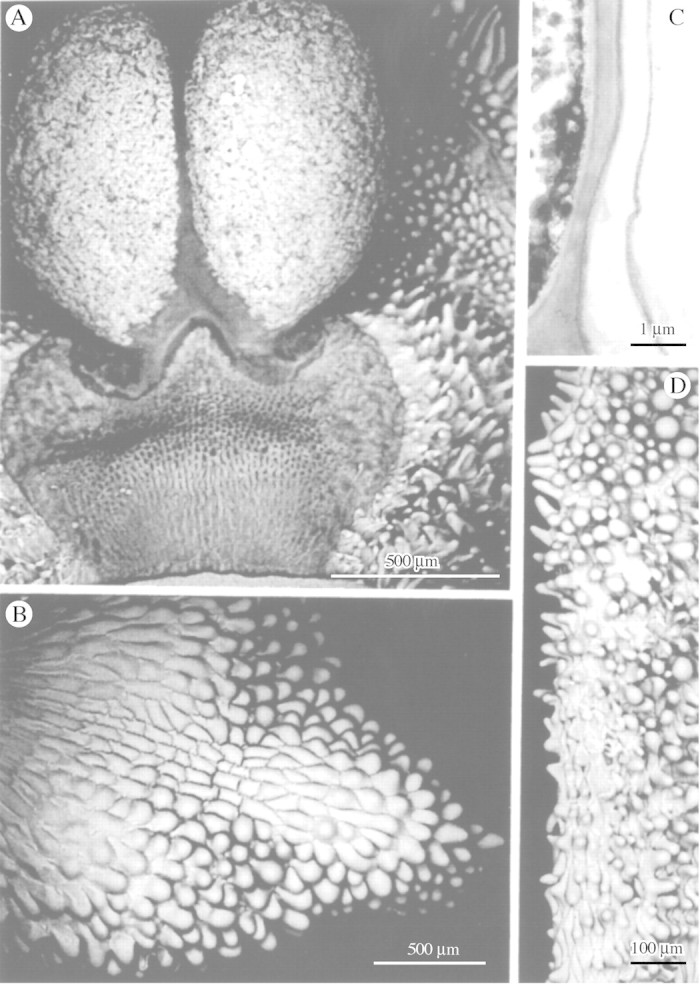
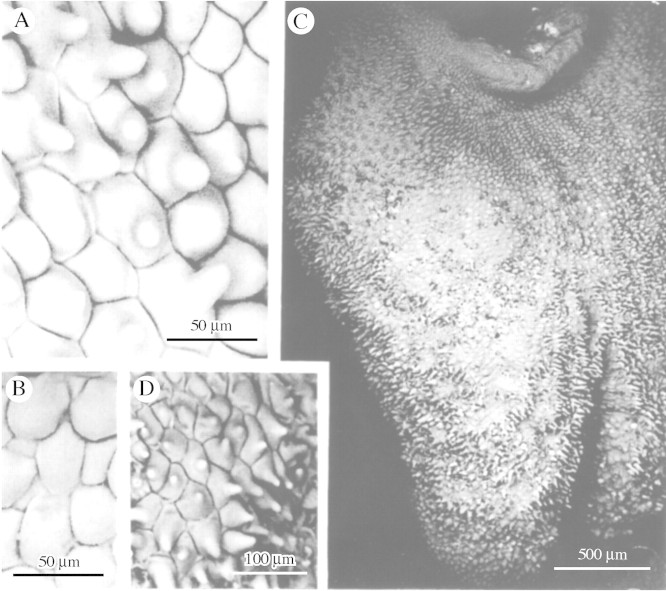
Fig. 1. A, Pollinia of Maxillaria cf. notylioglossa with associated conical and villiform papillae. B, Anther cap of M. seidelii with conical and obpyriform papillae. C, TEM of cell wall of labellar papilla of M. vernicosa showing relatively thick cuticle. D, Column of M. seidelii with conical and obpyriform papillae. Scale bars = 500 µm (A and B), 100 µm (D) and 1 µm (C).
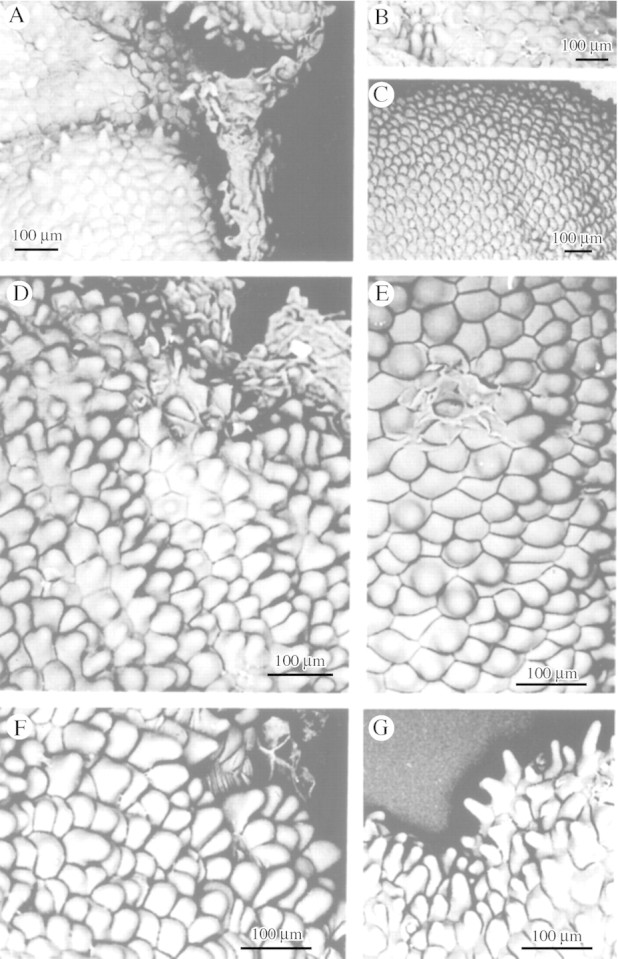
Fig. 2. Conical labellar papillae with broad points of insertion and rounded apices of M. mosenii (A), M. cogniauxiana (B), M. vernicosa (C), M. minuta (D) and M. seidelii (E). F and G, M. cf. minuta showing typical and marginal labellar papillae, respectively. Scale bar = 100 µm.
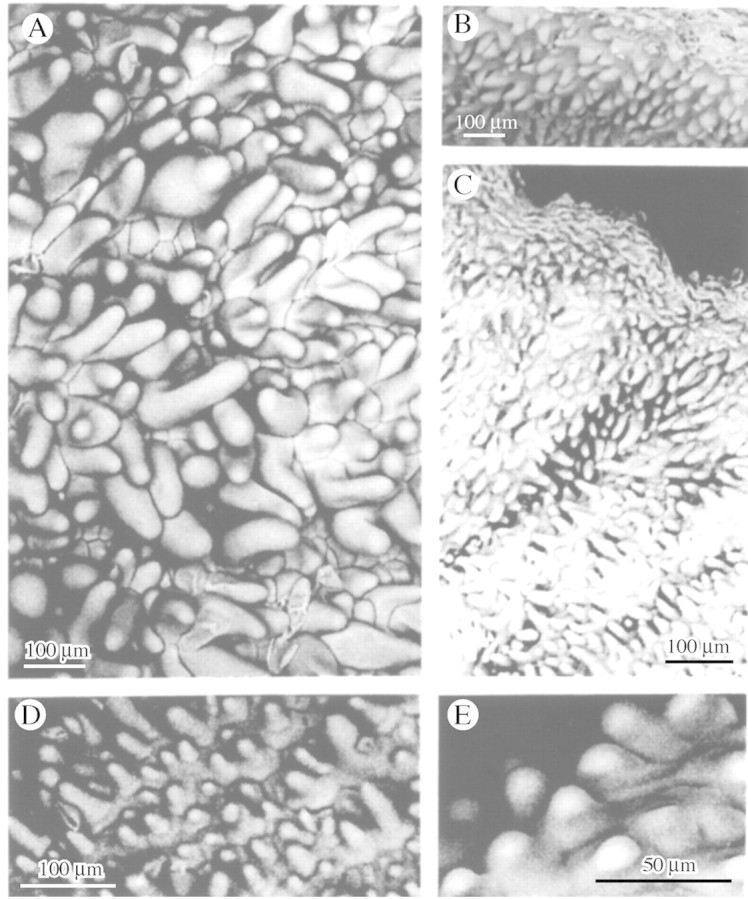
Fig. 4. A, Villiform labellar papillae of M. pulchra. B–E, Short conical labellar papillae of M. elatior (B), M. desvauxiana (C), M. procurrens (D) and M. coccinea (E). Scale bars = 100 µm (A–D) and 50 µm (E).
Fig. 3. A, Conical labellar papillae of M. meleagris. B, Some parts of the labellum of M. meleagris may lack papillae. C, Papillose labellum of M. densa. D, Conical labellar papillae of M. densa with pointed apices. Scale bars = 500 µm (C), 100 µm (D) and 50 µm (A and B).
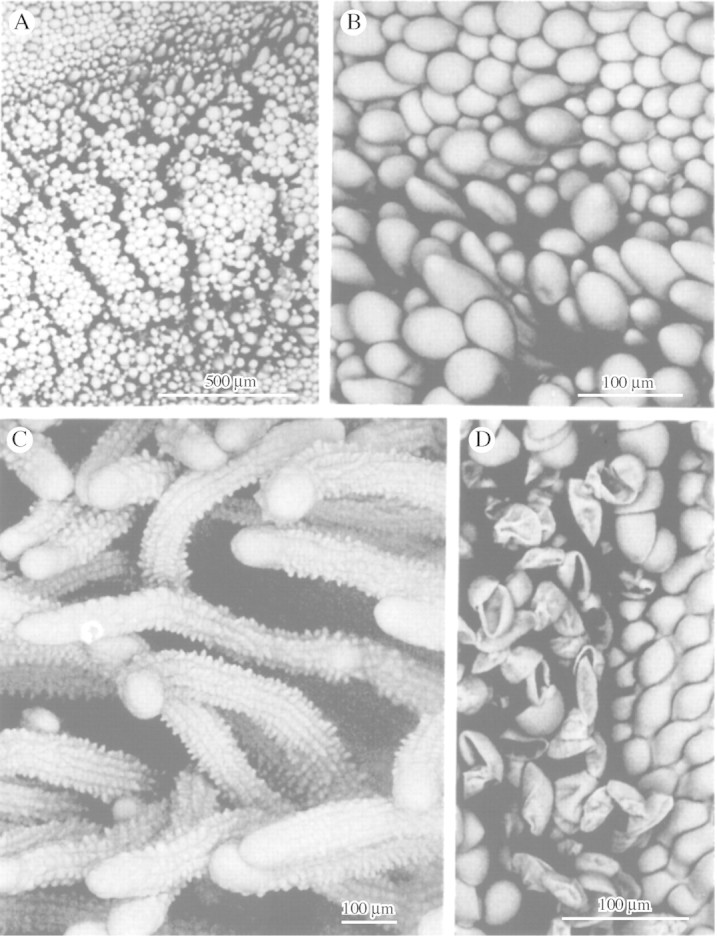
Fig. 6. A and B, Labellar surface of M. rufescens showing small, peripheral, obpyriform papillae and larger, central obpyriform to clavate papillae with distended apices. C, Papillose, multiseriate trichomes of M. camaridii that may function as pseudostamens. D, Labellar papillae of M. johniana with associated pseudopollen. Scale bars = 500 µm (A) and 100 µm (B–D).
Around the anther and stigmatic surface, papillae may become enlarged or swollen and appear to contain a thin layer of peripheral cytoplasm and a large vacuole with watery cell sap that occupies most of the cell. Moreover, in many species (e.g. M. lindleyana Schltr. and M. villosa (Barb. Rodr.) Cogn.), many of these conical papillae are replaced by villiform papillae, some of which are curved like a scythe.
Conical papillae (Figs 1A, B and D, 2A–G, 3A, C and D and 4B–E) are the most common in Maxillaria and are ubiquitous, occurring on the column, the anther cap and the labellum of a great many species, regardless of their vegetative morphology. Thus, xeromorphic species such as M. minuta Cogn. (Fig. 2D, F and G), M. pumila Hook., M. ferdinandiana Barb. Rodr., M. plebeja Rchb.f. (M. pumila alliance), M. vitelliniflora Barb. Rodr., M. vernicosa Barb. Rodr. (Fig. 2C), M. seidelii Pabst (Fig. 2E) (M. subulata alliance), M. mosenii Kraenzl. (Fig. 2A) and M. cogniauxiana Hoehne (Fig. 2B) (M. madida alliance), together with mesomorphic ascending types such as M. densa Lindl. (Fig. 3C and D), M. jenischiana (Rchb.f.) C. Schweinf., M. elatior Rchb.f. (Fig. 4B) and M. coccinea (Fig. 4E), caespitose types such as M. meleagris Lindl. (Fig. 3A) and M. desvauxiana Rchb.f. (Fig. 4C), and cane types such as M. procurrens Lindl. (Fig. 4D) all have conical labellar papillae, although even papillose labella may have some glabrous regions (Fig. 3B).
Wherever pseudopollen‐forming trichomes occur, labellar papillae tend to be obpyriform (Fig. 6D). However, this type of papilla may occur in the absence of pseudopollen hairs as in M. rufescens Lindl. (Fig. 6A and B), M. acutifolia Lindl, M. tenuibulba E.A. Christenson and M. moralesii Carnevali & Atwood; all members of the M. rufescens alliance. Here, however, the apices of the papillae, in particular the larger, central papillae, are greatly distended and the papilla assumes a clavate profile. The exception is M. hedwigae Hamer & Dodson, where the papillae tend to be somewhat fusiform or villiform.
Glabrous labella completely devoid of papillae were found only in M. cucullata Lindl. (Fig. 5A and B), M. hematoglossa A. Rich & Galeottii (Fig. 5C–E) and M. lexarzana Soto Arenas & F. Chiang (Fig. 5F); all members of the M. cucullata alliance. Members of this group are often misidentified and since M. hematoglossa is such a variable species, forms with dark, light, striped and spotted flowers were examined with identical results. However, in M. meleagris (Fig. 3A and B), a species thought to be closely related to members of the M. cucullata alliance, the labellum is papillose and the conical papillae which have pointed tips resemble those of M. densa (Fig. 3C and D).
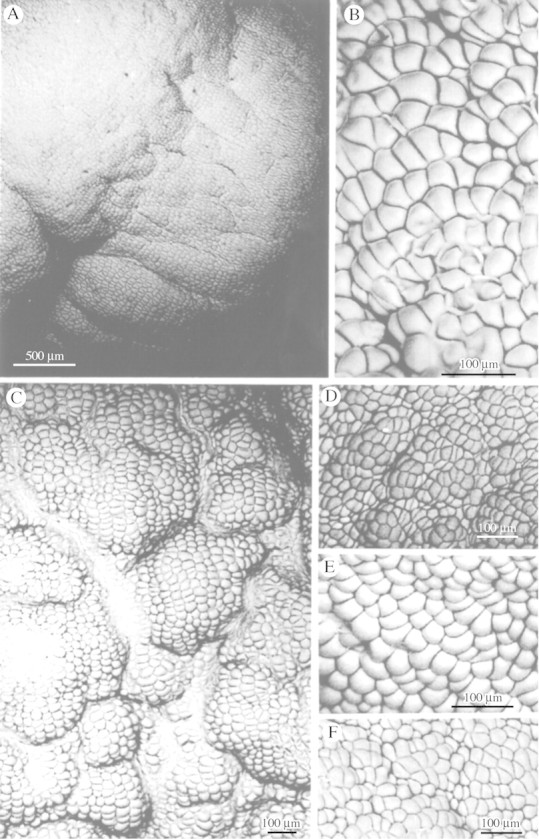
Fig. 5. Glabrous labellar surfaces of M. cucullata (A and B), M. hematoglossa with striped, dark flowers (C), striped flowers (D) and yellow, spotted flowers (E) and M. lexarzana (F). Scale bars = 500 µm (A) and 100 µm (B–F).
Thus, papillae not only vary greatly within a particular species but also according to their position upon a given organ. For example, conical papillae on the column often become villiform around the anther (Fig. 1A) and stigmatic surface. Similarly, conical papillae on the lip surface form a fringe of longer, often villiform papillae, around the labellar margin (Fig. 2G). Detailed data for individual species are shown in Table 1.
Table 1.
Distribution and classification of papillae and trichomes
| Type of papilla | |||||
| Taxon | Accession no. | Column | Anther cap | Labellum | Type of labellar trichome |
| Maxillaria acuminata Lindl. | MMB14 | c, o | – | ||
| M. acutifolia Lindl. | MMB64 | o | o | o, cl | – |
| M. buchtienii Schltr. | KLD199813 | c | c | o | s (3–4 cells) |
| M. camaridii Rchb.f. | KLD199920 | c | c | c | mu |
| M. cerifera Barb. Rodr. | ED95–70 | c | – | ||
| M. chartacifolia Ames & C. Schweinf. | MMA18 | o | o | – | – |
| M. chlorantha Lindl. | S0000079 | o | s (3–4 cells) | ||
| M. chrysantha Barb. Rodr. | S19940012 | c | c | – | – |
| M. coccinea (Jacq.) L.O. Williams ex Hodge | KLD199803, S19950015 | – | c | c | – |
| M. cogniauxiana Hoehne | KLD199701 | c | – | ||
| M. cucullata Lindl. | GXXF012515 | – | – | ||
| M. dalessandroi Dodson | KLD199908 | o | m | ||
| M. densa Lindl. | S19970029 | – | c | c | – |
| M. desvauxiana Rchb.f. | KLD199804 | c | – | ||
| M. discolor (Lodd. ex Lindl.) Rchb.f. | S19990261, MMB62 | c | c | c, o | m |
| M. elatior Rchb.f. | KLDX19971, KLD200005 | c | – | ||
| M. elegantula Rolfe | S19980054 | c, v | o | m | |
| M. ferdinandiana Barb. Rodr. | MMC17 | o, v | c,o | c, v | – |
| M. fractiflexa Rchb.f. | S19980052 | c | c | c, v | s (3–4 cells) |
| M. fucata Rchb.f. | KLD199916, KLD199933 | o | m | ||
| M. cf. gracilis Lodd. | S19980072 | c | c | c, o | s (3–4 cells) |
| M. hedwigae Hamer & Dodson | BSNS089 | f, v | – | ||
| M. hematoglossa A. Rich & Galeottii | KLD198601, KLD199811,KLD199704, S19920439,S19910275, KLD1998100,E19191019, KLD199602,S199930276, KLD1998101 | c | c, o | – | – |
| M. hillsii Dodson | S19990292 | o | m | ||
| M. huancabambe (Kraenzl.) C. Schweinf. | S20010484 | c, o | |||
| M. imbricata Barb. Rodr. | MMA21 | c | c, o | ||
| M. infausta Rchb.f. | MMA13 | c, o | c, o, v | c | – |
| M. irrorata Rchb.f. | KLD199824 | c | c, v | o | m |
| M. jenischiana (Rchb.f.) Garay & C. Schweinf. | S19980077 | c, o | c, o | c | – |
| M. jocunda Lehm. & Kraenzl. | S19990297 | – | c | o | m |
| M. johniana Kraenzl. | MMA41 | o | m | ||
| M. cf. lehmanii Rchb.f. | KLD199911 | o | m | ||
| M. lepidota Lindl. | S19950277 | o with glands | s (2–3 cells) | ||
| M. lexarzana Soto Arenas & F. Chiang | KLD199102, KLD199706 | – | c | – | – |
| M. cf. lilliputana D.E. Benn. & E.A. Christenson | S19980062 | c, v | – | – | m |
| M. lindleyana Schltr. | KLD199806 | c | c | o | – |
| M. longissima Lindl. | S20010485 | o | m | ||
| M. meleagris Lindl. | KLD199603, KLD199604,KLD199919, S20000208 | c | c | c | – |
| M. minuta Cogn. | S19960283 | – | c | c | – |
| M. cf. minuta Cogn. | S19980011 | – | c | c | – |
| M. molitor Rchb.f. | KLD199828, KLD199929 | o | m | ||
| M. moralesii Carnevali & Atwood | BSNS number unknown | – | – | c, o, cl | – |
| M. mosenii Kraenzl. | S19970030 | c | – | ||
| M. mosenii Kraenzl. var. hatschbachii (Schltr.) Hoehne | S19990245, S19980051 | c | c | c | – |
| M. notylioglossa Rchb.f. | ED97‐16, S20030488 | o, v | o | c, o | – |
| M. cf. notylioglossa Rchb.f. | S19990147 | c, v | c | c, o | – |
| M. nutans Lindl. | KLD199909 | o | m | ||
| M. ochroleuca Lodd. ex Lindl. | KLD199714 | o | s (3–4 cells) | ||
| M. oreocharis Schltr. | S19980012 | – | c | c | – |
| M. parviflora (Poepp. & Endl.) Garay | MMC8 | c | – | c | – |
| M. picta Hook. | KLD199917 | v | – | ||
| M. plebeja Rchb.f. | KLD2000001 | – | – | c | – |
| M. ponerantha Rchb.f. | MMC9 | c | c, v | – | |
| M. procurrens Lindl. | KLD199812 | – | – | c | – |
| M. pseudoreichenheimiana Dodson | MMC16 | c, v | o, v | o | s (2 cells) |
| M. pulchra (Schltr.) L.O. Williams | S19910274 | c | c, v | mu | |
| M. pumila Hook. | MMB60 | c, o | o | c, o | – |
| M. reichenheimiana Endres & Rchb.f. | MMA50 | c, v | v | o | s (2 cells) |
| M. rufescens Lindl. | BSNS532, KLDX20001,S19980055, BSNS230 | c | c | o ,cl | – |
| M. sanderiana Rchb.f. | KLD199815, KLD199816 | o | m | ||
| M. schunkeana Campacci & Kautsky | S19980056 | – | c | c | – |
| M. seidelii Pabst | S19970500 | c, o | c, o, v | c | – |
| M. cf. setigera Lindl. | S19980057 | c with simple 2–3celled trichomes | c | o | m (2–3 cells) |
| M. sophronitis (Rchb.f.) Garay | S19950281 | c | – | ||
| M. striata Rolfe | KLD199910 | v | |||
| M. tenuibulba E.A. Christenson | MMB16 | o | o | c, o, cl | – |
| M. tenuifolia Lindl. | S00000282, KLDX20002,MMA48 | c, v | c, v | v | – |
| M. tenuis C. Schweinf. | KLD199923 | s | |||
| M. cf. triloris E. Morren | KLD199707 | o | s (2–3 cells) | ||
| M. uncata Lindl. | S20000209 | – | c | c | – |
| M. variabilis Bateman ex Lindl. | XX012504, XX012505,MMA28, KLD199105,KLD199714 | c, v | c | c, v | – |
| M. vernicosa Barb. Rodr. | G1960002066, S20010100 | c | c | c | – |
| M. villosa (Barb. Rodr.) Cogn. | S19990262 | c | c,v | o | s (3–5 cells) |
| M. violaceopunctata Rchb.f. | S19990263 | – | c | o | s (4–6 cells) |
| M. vitelliniflora Barb. Rodr. | MMB12 | c, o | c, o | c | – |
Types of papillae: c = conical; cl = clavate; f = fusiform; – = absent (i.e. glabrous); o = obpyriform; v = villiform. Types of trichome: m = moniliform; mu = multiseriate; s = simple; – = absent.
TEM
TEM sections through the labellar epidermis of M. vernicosa revealed the presence of a relatively thick cuticle upon its surface (Fig. 1C).
Histochemistry
The labella of a number of species selected to show a wide range of papillar morphology were tested for food substances. Without exception, all types of papillae contained protein and most contained very little or no lipid. Starch, however was more variable, occurring in several species such as M. jenischiana, M. jocunda Lehm. & Kraenzl. and M. buchtienii Schltr. Some species (e.g. M. rufescens, M. acutifolia and M. tenuibulba) have obpyriform papillae containing all three food substances, protein, starch and lipid; those papillae at the centre of the lip often containing more starch than the others. Moreover, the labella of M. acutifolia and M. tenuibulba produce a lipoidal secretion much like that found in the M. acuminata alliance and some members of the M. discolor alliance (Davies et al., 2003a, b). Simple, bicellular trichomes, whose walls stain selectively with Sudan III, occur on the labella of M. reichenheimiana Endres & Rchb.f. and M. pseudoreichenheimiana Dodson. Morphologically, these resemble the food hairs described by Davies et al. (2002) for certain species of Polystachya. The histochemical results obtained for papillae and trichomes are presented in Tables 2 and 3, respectively.
Table 2.
Histochemical analysis of labellar papillae
| Foods present in papillae | |||||
| Taxon | Accession no. | Type of papilla | Protein | Starch | Lipid |
| M. acutifolia Lindl. | MMB64 | o, cl | + | + ce | + s |
| M. buchtienii Schltr. | KLD199813 | o | + | + | – |
| M. camaridii Rchb.f. | KLD199920 | c | + | – | – |
| M. chrysantha Barb. Rodr. | S19940012 | a | + | – | – |
| M. jenischiana (Rchb.f.) Garay & C. Schweinf. | S19980077 | c | + | + | – |
| M. jocunda Lehm. & Kraenzl. | S19990297 | o | + | + | – |
| M. lexarzana Soto Arenas & F. Chiang | KLD199706 | a | + | – | – |
| M. mosenii Kraenzl. var. hatschbachii (Schltr.) Hoehne | S19980051 | c | + | – | – |
| M. pseudoreichenheimiana Dodson | MMC16 | o | + | – | – |
| M. reichenheimiana Endres & Rchb.f. | MMA50 | o | + | – | – |
| M. rufescens Lindl. | S19980055 | o, cl | + | + | + |
| M. cf. setigera Lindl. | S19980057 | o | + | + | – |
| M. tenuibulba E.A. Christenson | MMB16 | c, o, cl | + | + ce | + s |
| M. tenuifolia Lindl. | S00000282 | v | + | – | – |
Types of papillae: c = conical; cl = clavate; o = obpyriform; v = villiform; a = absent.
+ and – indicate presence and absence, respectively, of food substance, whereas ce indicates that the food substance is largely concentrated in the central papillae of the labellum and s that the substance is secreted onto the labellar surface.
Table 3.
Histochemical analysis of labellar trichomes
| Type of labellar trichome | |||||
| Taxon | Accession no. | Type of trichome | Protein | Starch | Lipid |
| M. buchtienii Schltr. | KLD199813 | s (3–4 cells) | – | – | – |
| M. jocunda Lehm. & Kraenzl. | S19990297 | m | + | + | – |
| M. johniana Kraenzl. | MMA41 | m | + | + | – |
| M. lilliputana D.E. Benn. & E.A. Christenson | S19980062 | m | + | + | – |
| M. longissima Lindl. | S20010485 | m | + | – | + |
| M. pseudoreichenheimiana Dodson | MMC16 | s (2 cells) | + | – | * |
| M. reichenheimiana Endres & Rchb.f. | MMA50 | s (2 cells) | + | – | * |
| M. cf. setigera Lindl. | S19980057 | m (2–3 cells) | + | + | – |
| M. tenuis C. Schweinf. | KLD199923 | s | + | + | – |
Types of trichome: m = moniliform; s = simple.
+ and – indicate presence and absence, respectively, of food substance, whereas * indicates that the cell wall only selectively stained for this substance.
DISCUSSION
As in other angiosperms (Kay et al., 1981), the conical papilla is the most common type of papilla found in Maxillaria. Conical papillae occur on the labellum, the column and the anther cap and their persistence on the last two structures, even when the labellar papillae show modification, would indicate that this is a conservative character and that conical papillae are probably less derived than other papillae or indeed the glabrous condition. It is presumed that morphological modifications of papillae reflect underlying changes in their physiology, although, in many cases, identical papillae clearly perform different roles. For example, both M. jenischiana and M. coccinea have conical papillae but they cannot serve exactly the same purpose since the first species is probably insect‐pollinated, whereas the second is thought to be ornithophilous (Roubik, 2000; Stpiczyñska et al., 2004). Conversely, different types of papillae may have the same function and therefore determining the specific role of a particular type of papilla can be problematical. Nevertheless, much can be achieved by means of histochemistry used in conjunction with comparative morphology. Using this dual approach, we have been able to relate structure to function, although it must be remembered that the function of a particular type of papilla may vary from species to species, organ to organ and even according to its position upon that organ. Papillae may be involved in:
Attraction
In most Maxillaria spp., labellar papillae may become modified towards the centre and margin of the lip. Thus, in a species whose labellar papillae are largely conical, there would generally be a tendency for marginal papillae to become villiform, whereas the central ones would tend to be more or less obpyriform. In this way, from the moment it alights upon the lip, the visiting insect is guided by means of tactile cues towards and along the median axis of the labellum. Many papillae are pigmented and act as nectar guides, drawing the insect further into the flower. Moreover, preliminary data obtained by the in vivo staining of flowers with a dilute aqueous solution of neutral red (Stern et al., 1986) would indicate that some papillae, especially those at the margins of the tepals and labellum, may perhaps function as osmophores and provide olfactory cues (K.L. Davies, unpublished data). Similar osmophores have been observed in Cymbidium tracyanum L. Castle and Gymnadenia conopsea (L.) R. Br. (Stpiczyñska 1993, 2001). Perhaps the most remarkable papillae are to be found in M. camaridii Rchb.f and are seemingly involved in mimicry. These conical papillae are not unusual in themselves but are noteworthy in that they occur on the surface of multiseriate, labellar trichomes, which, in terms of size, position and colour, resemble a tuft of stamens. These hairs may thus perhaps function as pseudostamens but direct evidence for this is lacking. Such structures also occur in a similar position in M. pulchra (Schltr.) L.O. Williams, but here hairs tend to be glabrous. Nevertheless, the floral morphology of both species would perhaps suggest that they attract insects by deceit.
Rewards
The intensity of staining following the xanthoproteic test would indicate that the papillae of all species tested contain relatively high concentrations of aromatic amino acids. In contrast, most contain no lipid but the occurrence of starch is more variable. Protein, then, appears to be the most common food substance found within the floral papillae of Maxillaria and the results presented here closely resemble those obtained for pseudopollen (Davies et al., 2000, 2003b). Usually, pseudopollen‐forming hairs and papillae are inextricably associated and it is noteworthy that M. johniana Kraenzl., M. jocunda and M. lilliputana D.E. Benn. & E.A. Christenson produce pseudopollen identical to those other members of the M. grandiflora alliance that have already been studied (Davies et al., 2000). Furthermore, these species, together with M. cf. setigera Lindl., resemble the latter in that their pseudopollen and labellar papillae stain for protein and starch but not for lipid.
Members of the M. splendens alliance such as M. buchtienii, M. chlorantha Lindl. and M. ochroleuca Lodd. ex Lindl. have labella with uniseriate, relatively few‐celled hairs as well as obpyriform papillae, and it has been speculated, solely on morphological grounds, that these hairs may become detached or fragment to form pseudopollen (Davies et al., 2000, 2003a, b). However, histochemistry failed to demonstrate the presence of protein, starch or lipid within such hairs in M. buchtienii. Nevertheless, identical hairs occur in M. ochroleuca (Davies et al., 2000) and Singer has observed Trigona bees (workers only) collecting these from the tip of the labellum (R. B. Singer, pers. comm.). Moreover, Singer reports that these long, yellowish hairs were chewed and stored in a paste‐like form on the corbiculae. It may be that the bees actually collected papillae rather than hairs, especially since the labellar papillae of the closely related M. buchtienii are known to contain numerous starch grains. Similarly, abundant starch occurs in the papillae of M. jenischiana and, since this species produces copious nectar, the starch is probably hydrolysed to form sugars during nectar production (Durkee, 1983). Again, starch is found in the labellar papillae of M. rufescens as well as in other members of that alliance.
In yet other species, such as members of the M. acuminata and M. discolor alliances, histochemistry indicated that the labellar papillae are involved in the secretion of a viscid, wax‐like material rich in lipids and protein and this is gathered by visiting bees (Davies et al., 2003a, b). A similar secretion also occurs in M. acutifolia and M. tenuibulba.
Protection
The exact function of the spherical glands on the ventral surface of the labellum of M. lepidota is still not known. Although they seemed to contain aromatic amino acids, histochemical analysis was frustrated by the intense pigmentation of the labellum. However, their position on the ventral surface of the lip would suggest that these glands are not involved in the attraction of pollinators. Instead, it may be that by secreting sugars (much like the sugary droplets found at the tips of tepals), they attract ants and these defend the plant from herbivory (Dressler, 1993; Davies et al., 2003b). However, evidence for this is lacking.
The labellar epidermis of M. chrysantha Barb. Rodr. produces wax upon its surface and this protects the plant from desiccation. Similarly, in some xeromorphic species with somewhat dull‐coloured flowers, such as those assigned to the M. pumila, M. subulata and M. madida alliances (Pabst and Dungs, 1977), the labellar papillae have a relatively thick cuticle. This serves not only to protect the flower from desiccation but also gives it a glossy appearance. Although it has been suggested that the glossy surface of the labellum may mimic water (Warren, 1999), or a shallow nectary, thereby attracting insects, it is perhaps more likely that insects are attracted simply because of its reflective nature.
The modified papillae associated with the stigmatic surface, anther and anther cap contain a little peripheral cytoplasm and a large vacuole with watery cell sap. They thus, in many ways, resemble water‐storing or ‘aqueous’ tissue. These papillae tend to be larger and more swollen than those found elsewhere and may function like the paraphyses of mosses, trapping a layer of moist air. In this way, they prevent desiccation of the delicate reproductive structures.
Taxonomy
The occurrence of conical papillae on the column, anther cap and labellum of a wide range of species that differ in their vegetative morphology would indicate that this feature has little value as a taxonomic character. Moreover, obpyriform and villiform papillae are also found in species that are clearly unrelated on morphological grounds and these may have evolved in response to similar pollinator pressures. Nevertheless, labellar papillae, or the lack thereof, could prove useful as a taxonomic character in exceptional cases and these cases deserve mention.
Glabrous labella are not common in Maxillaria and tend to occur largely in species assigned to the M. cucullata alliance. However, in M. chartacifolia Ames & C. Schweinf., the upper surface of the labellum is glabrous, whereas the lower is papillose with obpyriform papillae. A distinctive type of labellar papilla is also found in members of the M. rufescens alliance. Here, the labellar surface is clothed with obpyriform papillae, whereas those towards the centre of the lip are clavate and much larger with distended apices. Remarkably, some papillae occur upon multiseriate trichomes. Since, to date, multiseriate trichomes have been found in only two species of Maxillaria, namely, M. camaridii and M. pulchra, these hairs are probably far more important as taxonomic characters than the papillae they may bear.
Finally, the papillae of Maxillaria are highly adaptable and fulfil a variety of roles. They attract and guide visiting insects along the flower using a combination of visual, olfactory and tactile cues. They are rich in aromatic amino acids and provide rewards in the form of nectar or a viscid wax‐like material containing protein and lipids, and may even offer protection from desiccation and herbivorous insects. Of course the exact function of papillae in a particular species can only be established for certain by observing how pollinators respond to them in the field. Until such data are forthcoming, morphological studies such as this can only provide half the story. Even so, of all the cell types to be found in Maxillaria, floral papillae must surely rank amongst the most intriguing.
ACKNOWLEDGEMENTS
The authors are grateful to A. Gregg, Dr M. McIllmurray, Dr E. D. L. Schmidt and B. Sayers for providing many of the plants needed for this study.
Supplementary Material
Received: 18 July 2003; Returned for revision: 1 September 2003; Accepted: 22 September 2003 Published electronically: 20 November 2003
References
- AckermanJD.1984. Pollination of tropical and temperate orchids. In: Tan KW, ed. Proceedings of the Eleventh World Orchid Conference. Miami, Florida: American Orchid Society, 98–101. [Google Scholar]
- BrummittRK, Powell CE.1992.Authors of plant names. Royal Botanic Gardens, Kew. [Google Scholar]
- DaviesKL, Winters C.1998. Ultrastructure of the labellar epidermis in selected Maxillaria species (Orchidaceae). Botanical Journal of the Linnean Society 126: 349–361. [Google Scholar]
- DaviesKL, Roberts DL, Turner MP.2002. Pseudopollen and food‐hair diversity in Polystachya Hook. (Orchidaceae) Annals of Botany 90: 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaviesKL, Turner MP, Gregg A.2003a. Lipoidal labellar secretions in Maxillaria Ruiz & Pav. (Orchidaceae). Annals of Botany 91: 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaviesKL, Turner MP, Gregg A.2003b. Atypical pseudopollen‐forming hairs in Maxillaria Ruiz & Pav. (Orchidaceae). Botanical Journal of the Linnean Society 143: 151–158. [Google Scholar]
- DaviesKL, Winters C, Turner MP.2000. Pseudopollen: its structure and development in Maxillaria (Orchidaceae). Annals of Botany 85: 887–895. [Google Scholar]
- DresslerRL.1993.Phylogeny and classification of the orchid family. Cambridge Massachusetts: Dioscorides Press. [Google Scholar]
- DurkeeLT.1983. Ultrastructure of floral and extrafloral nectaries. In: Bentley B, Elias T, eds. The biology of nectaries. New York: Columbia University Press. [Google Scholar]
- JanseJM.1886. Imitirte pollenkörner bei Maxillaria sp. Deutsche Botanische Gesellschaft Berichte 4: 277–283. [Google Scholar]
- KayQON, Daoud HS, Stirton CH.1981. Pigment distribution, light reflection and cell structure in petals. Botanical Journal of the Linnean Society 83: 57–84. [Google Scholar]
- NeilandMR, Wilcock CC.1998. Fruit set, nectar reward and rarity in the Orchidaceae. American Journal of Botany 85: 1657–1671. [PubMed] [Google Scholar]
- PabstGFJ, Dungs F.1977.Orchidaceae Brasilienses Band II. Hildesheim: Brücke‐Verlag Kurt Schmersow. [Google Scholar]
- PorschO.1905. Beiträge zur ‘histologischen’ Blütenbiologie I. Österreichische Botanische Zeitschrift 55: 253–260. [Google Scholar]
- PorschO.1908. Neuere Untersuchungen über die Insektenan lockungsmittel der Orchideenblüte. Mittelungen Naturwissen schaftlichen Vereines für Steiermark 45: 346–370. [Google Scholar]
- RoubikDW.2000. Deceptive orchids with Meliponini as pollinators. Plant Systematics and Evolution 222: 271–279. [Google Scholar]
- SingerRB.2002. The pollination mechanism in Trigonidium obtusum Lindl. (Orchidaceae: Maxillariinae): sexual mimicry and trap‐flowers. Annals of Botany 89: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SingerRB.2003. Orchid pollination: recent developments from Brazil. Lankesteriana 7: 111–114. [Google Scholar]
- SingerRB, Cocucci AA.1999. Pollination mechanisms in four sympatric southern Brazilian Epidendroideae orchids. Lindleyana 14: 47–56. [Google Scholar]
- SingerRB, Koehler S.2003. Toward a phylogeny of Maxillariinae orchids: multidisciplinary studies with emphasis on Brazilian species. Lankesteriana 7: 57–60. [Google Scholar]
- SternWL, Curry KJ, Whitten WM.1986. Staining fragrance glands in orchid flowers. Bulletin of the Torrey Botanical Club 113: 288–297. [Google Scholar]
- StpiczyñskaM.1993. Anatomy and ultrastructure of osmophores of Cymbidium tracyanum Rolfe (Orchidaceae). Acta Societatis Botanicorum Poloniae 62: 5–9. [Google Scholar]
- StpiczyñskaM.2001. Osmophores of the fragrant orchid Gymnadenia conopsea L. (Orchidaceae). Acta Societatis Botanicorum Poloniae 70: 91–96. [Google Scholar]
- StpiczyñskaM, Davies KL, Gregg A.2004. Nectary structure and nectar secretion in Maxillaria coccinea (Jacq.) L.O. Williams ex Hodge (Orchidaceae). Annals of Botany 93: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der PijlL, Dodson CH.1969.Orchid flowers: their pollination and evolution. Coral Gables, Florida: University of Miami Press. [Google Scholar]
- WarrenR.1999.Equatorial Plants Newsletter, Vol. 16, No. 1. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.