Abstract
• Background and Aims It had previously been assumed that Maxillaria spp. produce no nectar. However, nectar has recently been observed in Maxillaria coccinea (Jacq.) L.O. Williams ex Hodge amongst other species. Furthermore, it is speculated that M. coccinea may be pollinated by hummingbirds. The aim of this paper is to investigate these claims further.
• Methods Light microscopy, histochemistry, scanning and transmission electron microscopy.
• Key Results This is the first detailed account of nectar secretion in Maxillaria Ruiz & Pav. A ‘faucet and sink’ arrangement occurs in M. coccinea. Here, the nectary is represented by a small protuberance upon the ventral surface of the column and nectar collects in a semi‐saccate reservoir formed by the fusion of the labellum and the base of the column‐foot. The nectary comprises a single‐layered epidermis and three or four layers of small subepidermal cells. Beneath these occur several layers of larger parenchyma cells. Epidermal cells lack ectodesmata and have a thin, permeable, reticulate cuticle with associated swellings that coincide with the middle lamella between adjoining epidermal cells. Nectar is thought to pass both along the apoplast and symplast and eventually through the stretched and distended cuticle. The secretory cells are collenchymatous, nucleated and have numerous pits with plasmodesmata, mitochondria, rough ER and plastids with many plastoglobuli but few lamellae. Subsecretory cells have fewer plastids than secretory cells. Nectary cells also contain large intravacuolar protein bodies. The floral morphology of M. coccinea is considered in relation to ornithophily and its nectary compared with a similar protuberance found in the entomophilous species M. parviflora (Poepp. & Endl.) Garay.
• Conclusions Flowers of M. coccinea produce copious amounts of nectar and, despite the absence of field data, their morphology and the exact configuration of their parts argue strongly in favour of ornithophily.
Key words: Hummingbird pollination, Maxillaria coccinea, nectary ultrastructure, Orchidaceae, scanning electron microscopy, transmission electron microscopy
INTRODUCTION
Rewards often play an important role in the pollination of orchids. Indeed, it has been demonstrated that orchid species that reward potential pollinators may increase their chances of producing fruit by almost two‐fold (Neiland and Wilcock, 1998). Such rewards include pollen, nectar, oils and pseudopollen (van der Pijl and Dodson, 1969). Pollen, however, cannot serve as a reward in epidendroid orchids since it occurs in discrete masses within pollinia and is generally inaccessible to foraging insects. Consequently, in these species potential pollinators are usually rewarded with nectar (van der Pijl and Dodson, 1969; Arditti, 1992; Dressler, 1993). However, although nectar is the most common reward amongst these orchids, it has been estimated that as many as one‐third of orchid species produce little or no nectar (Porsch, 1908) and a similar number produce no reward whatsoever (van der Pijl and Dodson, 1969; Ackerman, 1984).
The neotropical genus Maxillaria Ruiz & Pav. is represented throughout the American tropics and subtropics (Bechtel et al., 1981) and some of its members have evolved a number of strategies for rewarding potential pollinators. These rewards include the production of pseudopollen (Davies and Winters, 1998; Davies et al., 2000, 2002, 2003b) and a wax‐like material secreted by the labellum (van der Pijl and Dodson, 1969; Davies et al., 2003a). Pseudopollen is formed in the M. grandiflora and M. discolor alliances as well as in M. longissima Lindl. by the fragmentation of multicellular hairs into individual cells rich in protein and starch (Davies and Winters, 1998; Davies et al, 2000, 2002, 2003b) and is collected by Meliponini (stingless bees: R. B. Singer, pers. comm., 2003) or euglossine bees (Dodson and Frymire, 1961; Dodson, 1962). Similarly, the wax‐like secretion produced by the labella of members of the M. acuminata alliance is rich in lipids and aromatic amino acids (Davies et al., 2003a) and is said to be collected by bees for nest building (van der Pijl and Dodson, 1969), although it clearly has a nutritive function too. Remarkably, those species of Maxillaria that produce pseudopollen and lipoidal labellar secretions tend not to produce nectar (van der Pijl and Dodson, 1969; Davies et al., 2000, 2003a). Indeed, it was once generally thought that Maxillaria spp. did not produce nectar. Recently, however, nectar has been reported in a number of species, including M. coccinea (Jacq.) L.O. Williams ex Hodge, M. jenischiana (Rchb.f.) C. Schweinf., M. imbricata Barb. Rodr., M. sophronitis (Rchb.f.) Garay (Davies et al, 2003a, b), M. parviflora (Poepp. & Endl.) Garay, M. rigida Barb. Rodr. and M. pendens Pabst (Singer, 2003; Singer and Koehler, 2003).
Although most Maxillaria spp. are pollinated by stingless bees (Roubik, 2000), the floral morphology of some red‐flowered species such as M. coccinea and M. sophronitis and the abundant nectar they produce would suggest that these plants may be pollinated by hummingbirds. However, published evidence for this is seemingly based solely on an observation reported by van der Pijl and Dodson (1969), where the hummingbird Pantrope insignis (sic Panterpe insignis) was seen visiting an unidentified species of Maxillaria with tubular pink flowers. Nevertheless, Ackerman and del Castillo Mayda (1992) too have stated that hummingbird pollination is likely to occur in M. coccinea, although the present authors are not aware of any direct observations that would confirm this.
By now, it is generally thought that nectar secretion in Orchidaceae may be a derived condition and that primitive orchids rewarded pollinators with pollen (Kocyan and Endress, 2001). Thus, the morphology of orchid nectaries has been widely studied (van der Pijl and Dodson, 1969), and Dressler (1993) believes that the ‘lily‐like ancestors of the orchids probably had shallow nectar glands between the perianth and the ovary’. In extant orchids, however, nectar is not produced in septal glands but in a relatively shallow nectary on the lip or tepals or between the column and the lip (e.g. Bulbophyllum Thouars, Cirrhopetalum Lindl., Epipactis Sw., Listera R. Br., Pleurothallis R. Br., Stelis Sw.), in glandular ring‐like nectaries at the top of the receptacle or in spurs (e.g. Angraecum Bory) or in tubular nectaries embedded in the ovaries (cuniculus) (e.g. Brassavola R. Br., Rhyncholaelia Schltr.). Other orchids (e.g. Cymbidium Sw., Grammatophyllum Blume, Vanda Jones) produce nectar at the base of the outer surface of the tepals, and it has been proposed (van der Pijl and Dodson, 1969; Dressler, 1993) that the mentum may also function as a nectar spur (e.g. Scaphyglottis Poepp. & Endl., Dendrobium Sw.). However, only rarely has the column ever been observed to secrete nectar (e.g. Stelis Sw.; Porsch 1908, cited in van der Pijl and Dodson, 1969). Conversely, in contrast to gross morphological studies, detailed, ultrastructural studies of the orchid nectary are rare and have been largely confined to a handful of insect‐pollinated terrestrial and mainly European species (e.g. Figueiredo and Pais, 1992; Pais and Figueiredo, 1994; Stpiczyñska, 1997; Stpiczyñska and Matusiewicz, 2001).
Thus, the present paper differs from previous studies in that it considers, for the first time, the ultrastructure of the nectary of a presumed hummingbird‐pollinated, epiphytic orchid and presents, again for the first time, a detailed account of nectary structure and nectar secretion in the genus Maxillaria.
MATERIALS AND METHODS
Plants of M. coccinea (Jacq.) L.O. Williams ex Hodge (accession no. S19950015) were grown at Swansea Botanical Complex, UK. Herbarium specimens were prepared and deposited at the National Museum and Gallery of Wales, Cardiff, UK.
Authorities for plant names follow Brummitt and Powell (1992). Flowers were sampled well into the secretory stage (approx, day 6 of anthesis) and preliminary determination of the position of the nectary was achieved using a hand lens.
Light microscopy and histochemistry
Further detailed study of the position of the nectary in complete, fresh flowers was achieved using an Olympus SZX12 stereo‐microscope. Hand‐cut sections through the living nectary were tested for starch and lipids using IKI and a saturated alcoholic solution of Sudan III, respectively. Small pieces of nectary were fixed in 2·5 % glutaraldehyde/5 % sucrose in phosphate buffer (pH 6·8; 0·075 m) at 20 °C for 4 h, washed in phosphate buffer and post‐fixed in 1 % osmium tetroxide at 0 °C for 2 h. The fixed material was then dehydrated using a graded ethanol series, infiltrated and embedded in Spurr resin. Semi‐thin sections (1 µm) were stained for general histology (O’Brien et al., 1965) using 1 % (w/v) toluidine blue in 1 % (w/v) aqueous sodium tetraborate solution (Vaughn, 1987). Staining with Coomassie brilliant blue R‐250 (Fisher, 1968) and ruthenium red (Jensen, 1962) revealed the presence of protein and mucilage/acidic polysaccharides, respectively. Micrometry and photomicrography of nectaries were accomplished using a Nikon Eclipse 600 microscope.
TEM and SEM
Sections (approx. 60 nm) were stained with uranyl acetate and lead citrate and examined using a TESLA BS‐340 transmission electron microscope at an accelerating voltage of 60 kV. Fixed pieces of column and labellar tissue were dehydrated through a graded ethanol series and, following critical‐point drying using liquid CO2, were sputter‐coated with gold and examined using a TESLA BS‐300 scanning electron microscope at an accelerating voltage of 20 kV.
RESULTS
Floral morphology
The flowers of M. coccinea are produced in axillary fascicles. They are globose with scarlet tepals and a scarlet and yellow labellum. The sepals are ovate‐lanceolate, acuminate and +/– forwardly pointing. The dorsal sepal is 10 mm long × 5 mm broad and the lateral sepals are oblique and measure 11 mm long × 5 mm broad. Mentum absent. Petals are ovate‐lanceolate, acuminate and oblique, 7 mm long × 4 mm broad and form a hood over the column. Labellum arcuate, 3‐lobed and immovable, 7 mm long × 4 mm broad, the mid‐lobe is 4 mm long × 3 mm broad, linguiform and strongly reflexed with a simple, hemispherical callus. Lateral lobes erect, 1·5 mm long × 1 mm broad. The base of the labellum is sub‐saccate due to the partial fusion of the lip to the column foot. A transverse labellar ridge separates the reflexed mid‐lobe of the labellum from the steeply sloping, proximal region of the lip, thereby partially closing the floral tube directly beneath the reproductive structures. Column short, white, 4 mm long × 2 mm broad at tip and with prominent, basal protuberance. Pollinia 4, of equal size.
The nectary is represented by a pronounced protuberance at the base of the column (Fig. 1A and B). Exuded nectar accumulates beneath the column in the semi‐saccate reservoir formed by the fusion of the labellum and the base of the column‐foot.
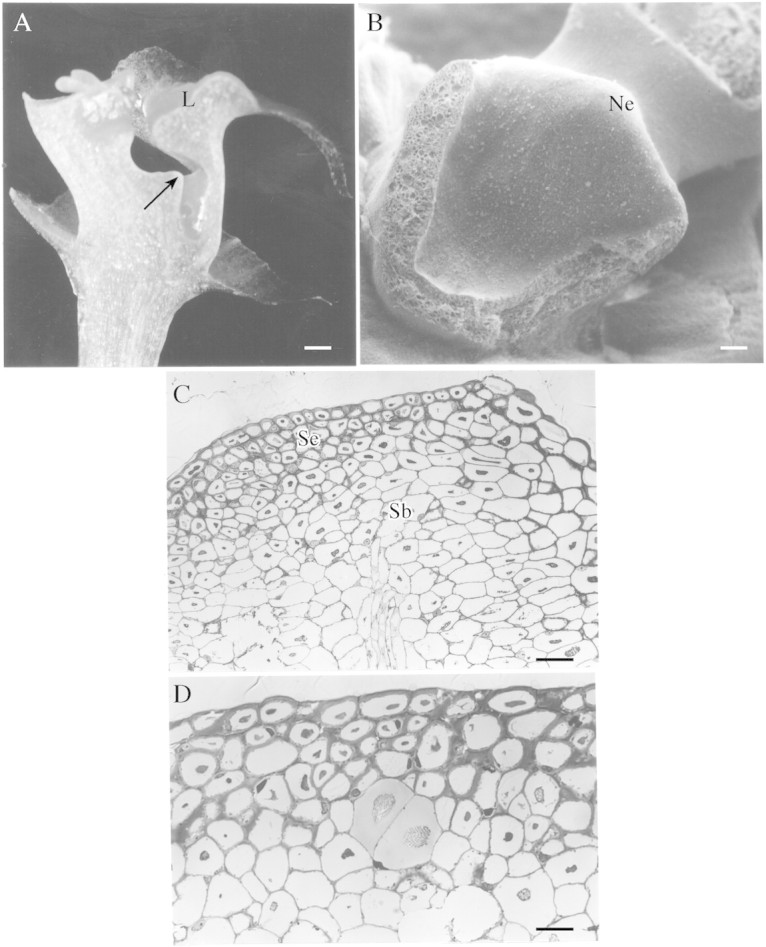
Fig. 1. A, Median longitudinal section of flower of M. coccinea. Nectar is secreted by the protuberance (arrow) and accumulates in the reservoir beneath the column. Scale bar = 1 mm. B, Part of column showing the nectary protuberance. Scale bar = 100 µm. C, Section through nectary protuberance showing secretory and subsecretory tissues and vascular bundle. Scale bar = 50 µm. D, Section through nectary showing secretory tissue; the subsecretory parenchyma containing two cells with raphides. Scale bar = 20 µm. Key (all figures): Ne = nectary; L = labellum; Se = secretory tissue; Sb = subsecretory tissue; N = nucleus; P = plastid; m = mitochondrion; c = cuticle; V = vacuole; Pb = protein body; Cs = cuticular swelling; CW = cell wall.
Light microscopy and histochemistry
The nectary consists of a single‐layered epidermis and three or four layers of subepidermal secretory cells (Fig. 1C). Beneath these are several layers of parenchyma. Secretory cells are small (approx. 16·38 µm diameter, range 14·17–18·42 µm), whereas subsecretory parenchyma cells are larger (30 µm diameter, range 26·5–38·2 µm). Some of the subsecretory parenchyma cells contain raphides. The nectary is supplied by a single vascular bundle comprising xylem and phloem (Fig. 1C). Staining with toluidine blue indicated that the walls of secretory cells consist of cellulose whereas staining with ruthenium red showed that the middle lamella and the outer tangential walls of epidermal cells contain acidic polysaccharides. A characteristic feature of these nectary cells is the presence of a large, intravacuolar, protein body that is finely granular and irregular in shape (Fig. 1C and D) and stains with Coomassie brilliant blue R‐250. Such cells, whilst lacking accumulated starch, nevertheless contain numerous lipid droplets.
TEM and SEM
The secretory cells are collenchymatous in that they possess unusually thick walls (2·5 µm, range 2·3–3·26 µm). Numerous pits with associated plasmodesmata connect the protoplasts of contiguous epidermal and secretory cells (Fig. 2A and B). The cytoplasm contains numerous mitochondria and rough ER profiles, which are associated with the plasmodesmata. The plastids contain many small plastoglobuli but few lamellae (Fig. 2C). Secretory vesicles associated with the cell wall tend to be absent.
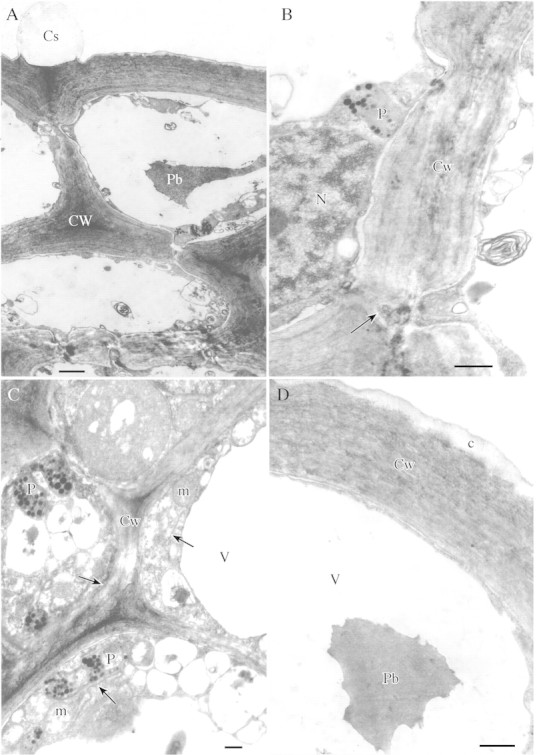
Fig. 2. A, Cell walls of secretory cells with numerous pits; the epidermal cell showing swelling of cuticle. Scale bar = 2 µm. B, Section through thick cell wall of secretory epidermis showing plasmodesma within pit (arrow). Scale bar = 1 µm. C, Cytoplasm of secretory cell with abundant mitochondria, ER (arrows) and plastids containing numerous plastoglobuli. Scale bar = 1 µm. D, Outer tangential wall of epidermis with reticulate cuticle. Note the single, finely granular, intravacuolar protein body. Scale bar = 1 µm.
The secretory epidermis possesses few stomata. The outer tangential wall lacks ectodesmata and is covered with a thin reticulate cuticle (Fig. 2D). SEM observations did not reveal pores or cracks through which nectar could exude. However, the cuticle has characteristic swellings 2–7 µm high (Fig. 3A) and these are usually found at points coinciding with the middle lamella between adjoining epidermal cells (Figs 2A and 3B and C). The swellings occur exclusively on the surface of the nectary protuberance and are absent from the epidermal cells of the nectar reservoir.
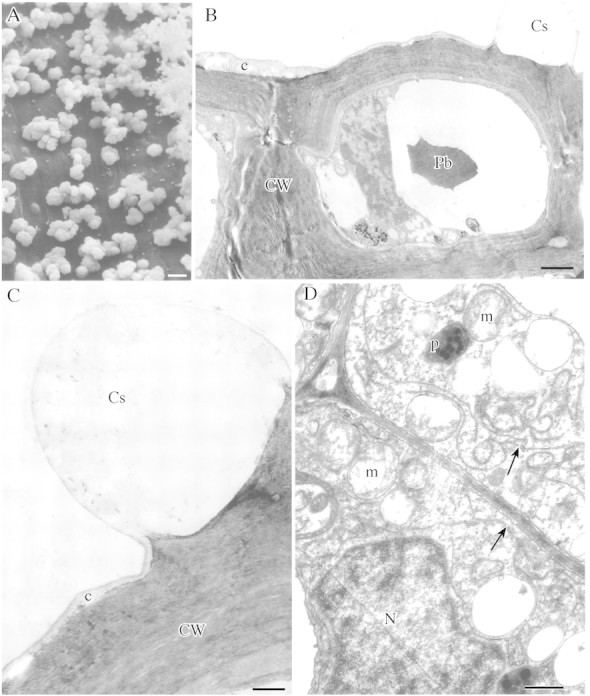
Fig. 3. A, SEM showing cuticular swellings on surface of nectary. Scale bar = 10 µm. B, Epidermal cell wall with cuticular swelling coinciding with junction between adjoining epidermal cells. Scale bar = 3 µm. C, Epidermal cell wall with cuticular swelling. Scale bar = 1 µm. D, Subsecretory parenchyma with thin cell walls and dense cytoplasm containing numerous ER profiles (arrows) and mitochondria. Scale bar = 5 µm.
Subsecretory parenchyma cells have distinctly thinner cell walls (0·5 µm, range 0·47–0·52 µm) with abundant plasmodesmata. Their cytoplasm contains numerous mitochondria, rough ER, dictyosomes and vesicles (Fig. 3D) but fewer plastids than secretory cells.
DISCUSSION
Floral morphology and ornithophily in orchids
The flowers of M. coccinea fulfil many of the criteria that characterize ornithophilous flowers, namely they show diurnal anthesis, they are weakly zygomorphic, possess a backwardly curved labellum, have scarlet flowers, produce abundant nectar and lack nectar guides. Furthermore, the tissues are tough and can thus withstand contact with a hard beak. Finally, a strong fold in the labellum partially closes the floral tube at the level of the anther and stigma and this would perhaps tend to force the bird to push its beak against the column to gain entry (van der Pijl and Dodson, 1969). However, M. coccinea differs from many bird‐pollinated orchids in that, whereas ornithophilous flowers tend to lack odour, a sweet honey‐like scent was occasionally detected in this species.
Over the years, a large number of orchid species have been presumed to be pollinated by hummingbirds solely on the basis of flower colour, presence of abundant nectar or absence of odour, but, although hummingbirds have on occasion been observed visiting such flowers, unequivocal evidence of hummingbird pollination (i.e. the transfer of pollinia) is rare. Many orchids presumed to be pollinated by hummingbirds have flowers that are various shades of red, orange or pink [e.g. Cochlioda rosea (Lindl.) Benth., C. vulcanica (Rchb.f.) Benth., Comparettia falcata Poepp. & Endl., Cyrtochilum mystacinum Lindl., C. retusum (Lindl.) Kraenzl., Elleanthus aurantiacus (Lindl.) Rchb.f., Epidendrum ibaguense H.B.K., E. pseudepidendrum Rchb.f., Isochilus linearis (Jacq.) R.Br. var. carnosiflorus (Lindl.) Correll, Laelia milleri Blumensch., Masdevallia rosea Lindl., Rodriguezia secunda H.B.K.], whereas others are rose‐purple to purple [e.g. Elleanthus capitatus (Poepp. & Endl.) Rchb.f., Epidendrum cnemidophorum Lindl., E. pfavii Rolfe, E. secundum Jacq., Sobralia amabilis (Rchb.f.) L.O. Williams] or even yellow [e.g. Elleanthus aureus (Poepp. & Endl.) Rchb.f.]. Observations by C. H. Dodson and G. P. Frymire (all cited in van der Pijl and Dodson, 1969) of hummingbirds visiting Elleanthus arpophyllostachys (Rchb.f.) Rchb.f., E. hallii (Rchb.f.) Rchb.f., E. hymenophorus Rchb.f., E. rosea Schltr., Epidendrum ardens Kraenzl. and E. scabrum Ruiz & Pav., do not in themselves prove that these orchids are pollinated by hummingbirds. More recently, however, Singer and Sazima (2000) have reported hummingbirds both visiting and pollinating Stenorrhynchos lanceolatus (Aubl.) L.C. Rich. Of the above species, Comparettia falcata, Elleanthus hymenophorus, Epidendrum cnemidophorum, E. pfavii and Isochilus linearis var. carnosiflorus were visited by the rufous‐tailed hummingbird (Amazilia tzacatl), Epidendrum secundum by an unidentified species of hummingbird (Amazilia sp.), Elleanthus arpophyllostachys by the booted racquet‐tail (Ocreatus underwoodii), and Sobralia amabilis and an unidentified species of Maxillaria with pink tubular flowers by the fiery‐throated hummingbird (Panterpe insignis). Singer (2003) has also reported the pollination of Elleanthus brasiliensis Rchb.f. by the hermit (Phaethornis petrei). A number of other species are also possible candidates for hummingbird pollination, but whether they are actually pollinated by hummingbirds cannot at present be corroborated due to a lack of field data.
Structure of the nectary and nectar secretion
In M. coccinea, the nectary is represented by a prominent protuberance on the base of the column. The secretory tissue of the nectary has a number of unusual features. The cells here are collenchymatous in that they have thick walls. Such walls have not been described for nectaries of other plant species. In M. coccinea, they are probably involved, especially in the absence of cutinized layers that would impede the flow of nectar, with the movement of this substance within the nectary. Numerous plasmodesmata would perhaps indicate that, in addition to apoplastic transport, symplastic transport of nectar occurs in this species. Models for the movement of pre‐nectar along the apoplast or symplast within secretory tissue and the subsequent secretion of nectar have been proposed by several researchers (e.g. Gunning and Hughes, 1976; Sawidis et al., 1987, 1989; Robards and Stark, 1988; Kronestedt‐Robards and Robards, 1991; Sawidis, 1991, 1998; Zellnig et al., 1991; Fahn, 2000). Some of these studies used radiolabelling to follow the path taken by sugars within the nectary (Fahn and Rachmilevitz, 1975; Heinrich, 1975; Meyberg and Kirsten, 1981; Sawidis, 1989; Stpiczyñska, 2003). Moreover, it had been proposed that both pathways of sucrose transport may operate simultaneously even in the same plant and that this is largely dependent on the developmental stage of the organ or tissue under investigation (Bush, 1999; Delrot et al., 2000; Lemoine, 2000; Williams et al., 2000). Sucrose may be imported directly from the apoplast into sink cells either by sucrose transporters or monosaccharide transporters following hydrolysis of sucrose to glucose and fructose by cell wall invertase (β‐d‐fructofuranoside fructohydrolase, EC 3.2.1.26). The nectar undergoes a final modification and is eventually secreted into the space between the plasmalemma and cell wall. However, since secretory vesicles were not observed in nectary cells, even during the nectar‐secretory stage, yet mitochondria were present in large numbers, then it is probable that here, as in other species (Fahn, 2000), sugars are actively transported across the plasmalemma.
Another remarkable feature is the uninterrupted layer of reticulate cuticle covering the outer tangential walls of the secretory epidermis. This, seemingly, does not impede nectar secretion. Having transversed the outer tangential wall, the nectar accumulates beneath the cuticle which, in turn, stretches and forms swellings. Usually, these swellings occur at points coinciding with the middle lamella between adjoining epidermal cells and this would perhaps indicate that within the nectary, nectar passes along the apoplast. Eventually, the nectar passes through the stretched and distended cuticle. The cuticle covering the nectary cells may be completely permeable, not only to secretory products, as in the glandular hairs of Leonotis (Ascensão and Pais, 1998) and Rosmarinus (Bottega and Corsi, 2000), but also to those substances that are resorbed, as in nectaries of Platanthera chlorantha (Stpiczyñska, 2003). However, in some plants, nectar may pass through a disrupted cuticle as in Limodorum abortivum (Pais and Figueiredo, 1994) or through cuticular pores as in the nectary hairs of Abutilon (Findlay and Mercer, 1971), the osmophores of Restrepia muscifera (Pridgeon and Stern, 1983) or the capitate hairs of Majorana syriaca (Werker et al., 1985).
The cell wall here is noteworthy in that not only does it appear to function in nectar transport but its collenchymatous nature would suggest that it may also have a supportive and/or protective function, preventing damage to the delicate secretory tissue by the hard beaks of visiting hummingbirds.
The nectary cells of M. coccinea are also remarkable in that they contain intravacuolar protein bodies similar to those found in the floral and extra‐floral nectaries of Passiflora (Durkee et al., 1981; Durkee, 1982), the extra‐floral nectaries of Vigna (Kuo and Pate, 1985) and the epidermal pseudopollen‐forming hairs of Maxillaria sanderiana (Davies et al., 2000). Such intravacuolar bodies are not common and, although their function in M. sanderiana is probably primarily storage, their role in nectary cells remains unclear and requires further investigation.
Subsecretory cells of both M. coccinea and those occurring in the floral or extra‐floral nectaries of other species (Durkee, 1982; Stpiczyñska, 1995) often contain raphides of calcium oxalate and these, according to Elias and Gelband (1977), may be involved with phloem metabolism and the active transport of sucrose. However, Davies has reported the presence of raphides in leaf (Davies, 1999) and floral tissue (Davies et al., 2000) for a number of Maxillaria spp. and has suggested that they may simply be excretory products and may perhaps discourage herbivory by invertebrates.
Nectary cells of M. coccinea, in contrast to those of other plants studied, contain no amyloplasts. The absence of amyloplasts is noteworthy since these organelles usually play an important role in nectar production. Starch stored within amyloplasts at the pre‐secretory stage can be utilized both as a source of energy for highly metabolic processes and as a source of sugars for nectar synthesis. During successive stages of secretory activity, plastids generally become elongated or develop an irregular profile and this is usually associated with a depletion in starch content (Durkee et al., 1981; Sawidis et al., 1989; Nepi et al., 1996; Sawidis, 1998). Unfortunately, since the nectaries of M. coccinea were studied only at the secretory stage, we were unable to ascertain whether starch accumulates during the pre‐secretory stage. Nevertheless, plastids devoid of starch, similar to those present in M. coccinea, and containing numerous plastoglobuli but few internal lamellae, have been observed, on occasion, in the nectary cells of Gymnadenia conopsea (Stpiczyñska and Matusiewicz, 2001). These closely resembled leucoplasts engaged in terpenoid synthesis (Gleizes et al., 1983; Cheniclet and Carde, 1985; Heinrich and Schultze, 1985; Turner et al., 1999) and the plastids which occur within the labellar papillae of Maxillaria cf. notylioglossa (Davies et al., 2003a). Thus, in the absence of starch, it is possible that sugars secreted in the nectar of M. coccinea are delivered in the phloem sap and then modified in the secretory cells before finally being secreted.
Comparative morphology
A protuberance on the ventral surface of the column, not unlike that found in M. coccinea, also occurs in M. parviflora (Poepp. & Endl.) Garay. This species, however, differs from M. coccinea in that its flowers are much smaller (5 mm diameter), are white with a yellow mid‐lobe to the labellum and the angle between the labellum and column is greater. Furthermore, the mid‐lobe of the lip is horizontal, not vertical and reflexed. Such protuberances have also been noted by others (Bennett and Christenson, 1993; M. McIllmurray, pers. comm., 2003) for M. parviflora but Bennett and Christenson (1993) interpreted this protuberance as a tabula infrastigmatica.
The tabula infrastigmatica, is best known from several species of Oncidiinae where the labellum is well developed at the expense of the other perianth parts, and is vertical. Situated on the column, the tabula occurs as a pad or plate which is often distinctive in colour or texture from the rest of the column and affords purchase for bees (mostly Centris) that alight on the vertical lip. By grasping the tabula with their mandibles, these insects can freely use their legs to gather oil droplets (Dressler, 1993). Moreover, orchids possessing a tabula tend not to produce nectar, whereas nectar‐producing flowers such as those of entomophilous Maxillaria spp. tend to have a more or less horizontal lip and lack a tabula.
Maxillariaparviflora, however, is rather peculiar in that it produces nectar, has a horizontal lip and a protuberance which can, on morphological grounds, be interpreted as either a nectary (as in M.coccinea) or a tabula. R. B. Singer (pers. comm., 2003) has observed droplets of nectar collecting in ‘a conch‐like cavity of the lip’ in this species. These droplets are licked by stingless bees (Meliponini) and Ponerinae ants, the pollinarium adhering to the frons or clypeus (Singer, 2003). Singer also reports that flowers of similar morphology occur in M. brevilabia Ames & Correll, M. concavilabia Ames & Correll and M. horichii Senghas (R. B. Singer, pers. comm., 2003). A search through published illustrations of floral dissections revealed that identical protuberances also occur in M. aggregata (H.B.K.) Lindl., M. fulgens (Rchb.f.) L.O. Williams, M. nubigena (Rchb.f.) C. Schweinf., M. ruberrima (Lindl.) Garay and M. sophronitis (Rchb.f.) Garay, all formerly assigned to Ornithidium Salisb. (Dunsterville and Garay, 1979; Bennett and Christenson, 1993). At first glance then, it would appear that the protuberance in M. parviflora is also a nectary rather than a tabula and that the ‘faucet and sink’ type of morphology found in M. coccinea is not unique to ornithophilous species but is, rather, universal, occurring in entomophilous species also. However, this conclusion appears to be erroneous since despite its similar appearance to the protuberance of M. coccinea, our semi‐thin sections through the column of M. parviflora failed to reveal secretory tissue, thus indicating that Singer’s proposal (R. B. Singer, pers. comm., 2003) that nectar is ‘secreted at the lip surface inside the cavity where it is offered to pollinators’ may well be correct. As a result, the true role of the protuberance in M. parviflora remains a mystery and serves to remind us that morphologically similar structures may indeed differ in their functions.
Finally, despite the absence of direct evidence, it would appear that M. coccinea, based on the unique combination of the floral features it possesses, is ornithophilous. However, ornithophily is the exception rather than the rule amongst Maxillaria spp. and as such, this species is atypical for the genus as a whole. Nevertheless, to the best of our knowledge, this is the only detailed study of the nectary of any Maxillaria species to date, and it thus affords a useful baseline against which other species can be compared. Given the enormity of the genus and the morphological diversity of its members, it is predicted that this diversity will also be reflected in both the position and structure of the nectary. Further studies of this kind would enable us to better understand the reproductive strategies and pollination biology of Maxillaria.
ACKNOWLEDGEMENTS
We are grateful to the Friends of the City of Swansea Botanical Complex, UK for their generosity in helping to fund this work, to Mgr Janusz Matusiewicz of CLA AR, Lublin, Poland for making electron microscopy facilities available to us and to Arek Derecki, Sudio De‐Pro, Lubartów, Poland for help with photography.
Supplementary Material
Received: 22 July 2003; Returned for revision: 1 September 2003; Accepted: 22 September 2003 Published electronically: 20 November 2003
References
- AckermanJD.1984. Pollination of tropical and temperate orchids. In Tan KW, ed. Proceedings of the Eleventh World Orchid Conference. Miami, Florida: American Orchid Society, 98–101. [Google Scholar]
- AckermanJD, del Castillo Mayda M.1992.The orchids of Puerto Rico and the Virgin Islands San Juan, Puerto Rico: University of Puerto Rico Press, 70–71. [Google Scholar]
- ArdittiJ.1992.Fundamentals of orchid biology. New York: John Wiley & Sons. [Google Scholar]
- AscensãoL, Pais MS.1998. The leaf capitate trichomes of Leonotis leonurus: histochemistry, ultrastructure and secretion. Annals of Botany 81: 263–271. [Google Scholar]
- BechtelH, Cribb P, Launert E.1981.The manual of cultivated orchid species. Poole, Dorset: Blandford Press. [Google Scholar]
- BennettDEJr Christenson EA. 1993. Icones Orchidacearum Peruviarum. Privately published by A. Pastorelli de Bennett, plates 109, 111 [Google Scholar]
- BottegaS, Corsi G.2000. Structure, secretion and possible functions of calyx glandular hairs of Rosmarinus officinalis L. (Labiatae). Botanical Journal of Linnean Society 132: 325–335. [Google Scholar]
- BrummittRK, Powell CE.1992.Authors of plant names. Royal Botanic Gardens, Kew. [Google Scholar]
- BushDR.1999. Sugar transporters in plant biology. Current Opinion in Plant Biology 2: 187–191. [DOI] [PubMed] [Google Scholar]
- ChenicletC, Carde J‐P.1985. Presence of leucoplasts in secretory cells and of monoterpenes in the essential oil: a correlative study. Israel Journal of Botany 34: 219–238. [Google Scholar]
- DaviesKL.1999. A preliminary survey of foliar anatomy in Maxillaria Lindleyana 14: 126–135. [Google Scholar]
- DaviesKL, Winters C.1998. Ultrastructure of the labellar epidermis in selected Maxillaria species (Orchidaceae). Botanical Journal of the Linnean Society 126: 349–361. [Google Scholar]
- DaviesKL, Roberts DL, Turner MP.2002. Pseudopollen and food‐hair diversity in Polystachya Hook. (Orchidaceae) Annals of Botany 90: 477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaviesKL, Turner MP, Gregg A.2003a. Lipoidal labellar secretions in Maxillaria Ruiz & Pav. (Orchidaceae). Annals of Botany 91: 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaviesKL, Turner MP, Gregg A.2003b. Atypical pseudopollen‐forming hairs in Maxillaria Ruiz & Pav. (Orchidaceae). Botanical Journal of the Linnean Society 143: 151–158. [Google Scholar]
- DaviesKL, Winters C, Turner MP.2000. Pseudopollen: its structure and development in Maxillaria (Orchidaceae). Annals of Botany 85: 887–895. [Google Scholar]
- DelrotS, Atanassova R, Maurousset L.2000. Regulation of sugar, amino acid and peptide plant membrane transporters. Biochemica et Biophysica Acta 1465: 281–306. [DOI] [PubMed] [Google Scholar]
- DodsonCH.1962. The importance of pollination in the evolution of the orchids of tropical America. American Orchid Society Bulletin 31: 525–534, 641,–649, 731–735. [Google Scholar]
- DodsonCH, Frymire GP.1961. Natural pollination of orchids. Missouri Botanical Garden Bulletin 49: 133–139. [Google Scholar]
- DresslerRL.1993.Phylogeny and classification of the orchid family. Cambridge Massachusetts: Dioscorides Press. [Google Scholar]
- DunstervilleGCK, Garay LA.1979.Orchids of Venezuela – an illustrated field guide. Botanical Museum, Harvard University, 490, 506, 512, 535, 548, 552. [Google Scholar]
- DurkeeLT.1982. The floral and extra‐floral nectaries of Passiflora II. The extra‐floral nectary. American Journal of Botany 69: 1420–1428. [Google Scholar]
- DurkeeLT, Gall DJ, Reisner WH.1981. The floral and extra‐floral nectaries of Passiflora I. The floral nectary. American Journal of Botany 68: 453–462. [Google Scholar]
- EliasTS, Gelband H.1977. Morphology, anatomy and relationship of extrafloral nectaries and hydathodes in two species of Impatiens (Balsaminaceae). Botanical Gazette 138: 206–217. [Google Scholar]
- FahnA.2000. Structure and function of secretory cells. Advances in Botanical Research 31: 37–75. [Google Scholar]
- FahnA, Rachmilevitz T.1975. An autoradiographical study of nectar secretion in Lonicera japonica Thunb. Annals of Botany 39: 975–976. [Google Scholar]
- FigueiredoACS, Pais MS.1992. Ultrastructural aspects of the nectary spur of Limodorum abortivum (L.) Sw. (Orchidaceae). Annals of Botany 70: 325–331. [Google Scholar]
- FindlayN, Mercer FV.1971. Nectar production in Abutilon I. Movement of nectar through the cuticle. Australian Journal of Biological Sciences 24: 647–656. [Google Scholar]
- FisherDB.1968. Protein staining of ribboned epon sections for light microscopy. Histochemie 16: 92–96. [DOI] [PubMed] [Google Scholar]
- GleizesM, Pauly G, Carde J‐P, Marpeau A, Bernard‐Dagan C.1983. Monoterpene hydrocarbon biosynthesis by isolated leucoplasts of Citrofortunella mitis Planta 159: 373–381. [DOI] [PubMed] [Google Scholar]
- GunningBES, Hughes IE.1976. Quantative assessment of symplastic transport of pre‐nectar into trichomes of Abutilon nectaries. Australian Journal of Plant Physiology 3: 619–637. [Google Scholar]
- HeinrichG.1975. Über den Glucose‐Metabolismus in Nektarien zweier Aloë‐Arten und über den Mechanismus der Pronektar‐Secretion. Protoplasma 85: 351–371. [Google Scholar]
- HeinrichG, Schultze W.1985. Composition and site of biosynthesis of the essential oil in fruits of Phellodendron amurense Rupr. (Rutaceae). Israel Journal of Botany 34: 205–217. [Google Scholar]
- JensenDA.1962.Botanical histochemistry. Principle and practice. San Francisco: Freeman. [Google Scholar]
- KocyanA, Endress PK.2001. Floral structure and development of Apostasia and Neuwiedia (Apostasioideae) and their relationships to other Orchidaceae. International Journal of Plant Sciences 162: 847–867. [Google Scholar]
- Kronestedt‐RobardsE, Robards AW.1991. Exocytosis in gland cells. In: Hawes CR, Coleman JOD, Coleman DE, eds. Endocytosis, exocytosis and vesicle traffic in plants Cambridge: Cambridge University Press, 199–232. [Google Scholar]
- KuoJ, Pate JP.1985. The extrafloral nectaries of cowpea (Vigna unguiculata (L.) Walp.). I. Morphology, anatomy and fine structure. Planta 166: 15–27. [DOI] [PubMed] [Google Scholar]
- LemoineR.2000. Sucrose transporters in plants: update on function and structure. Biochemica et Biophysica Acta 1465: 246–262. [DOI] [PubMed] [Google Scholar]
- MeybergM, Kirsten U.1981. The nectaries of Aptenia cordifolia – ultrastructure, translocation of 14C‐labelled sugars, and possible pathway of secretion. Zeitschrift für Pflanzenphysiology 104: 139–147. [Google Scholar]
- NeilandMR, Wilcock CC.1998. Fruit set, nectar reward and rarity in the Orchidaceae. American Journal of Botany 85: 1657–1671. [PubMed] [Google Scholar]
- NepiM, Ciampolini F, Pacini E.1996. Development and ultrastructure of Cucurbita pepo nectaries of male flowers. Annals of Botany 78: 95–104. [Google Scholar]
- O’BrienTP, Feder N, McCully ME.1965. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 49: 367–373. [Google Scholar]
- PaisMS, Figueiredo ACS.1994. Floral nectaries from Limodorum abortivum (L.) Sw. and Epipactis atropurpurea Rafin. (Orchidaceae); ultrastructural changes in plastids during the secretory process. Apidologie 25: 615–626. [Google Scholar]
- PorschO.1908. Neuere Untersuchungen über die Insektenan lockungsmittel der Orchideenblüte. Mittelungen Naturwissen schaftlichen Vereines für Steiermark 45: 346–370. [Google Scholar]
- PridgeonAM, Stern WL.1983. Ultrastructure of osmophores in Restrepia (Orchidaceae). American Journal of Botany 70: 1233–1243. [Google Scholar]
- RobardsA.W, Stark M.1988. Nectar secretion in Abutilon: a new model. Protoplasma 142: 79–91. [Google Scholar]
- RoubikDW.2000. Deceptive orchids with Meliponini as pollinators. Plant Systematics and Evolution 222: 271–279. [Google Scholar]
- SawidisT.1989. Autoradiographical study of the incorporation of tritium‐labelled glucose (D‐glucose‐6‐H3) in floral nectaries of Abutilon striatum (Dicks.). Bios 1: 211–219. [Google Scholar]
- SawidisT.1991. A histochemical study of nectaries of Hibiscus rosa‐sinensis Journal of Experimental Botany 42: 1477–1487. [Google Scholar]
- SawidisT.1998. The subglandular tissue of Hibiscus rosa‐sinensis nectaries. Flora 193: 327–335. [Google Scholar]
- SawidisT, Eleftheriou EP, Tsekos I.1987. The floral nectaries of Hibiscus rosa‐sinensis L. II. Plasmodesmatal frequencies. Phyton 27: 155–164. [Google Scholar]
- SawidisT, Eleftheriou EP, Tsekos I.1989. The floral nectaries of Hibiscus rosa‐sinensis III. A morphometric and ultrastructural approach. Nordic Journal of Botany 9: 63–71. [Google Scholar]
- SingerRB.2003. Orchid pollination: recent developments from Brazil. Lankesteriana 7: 111–114. [Google Scholar]
- SingerRB, Koehler S.2003. Toward a phylogeny of Maxillariinae orchids: multidisciplinary studies with emphasis on Brazilian species. Lankesteriana 7: 57–60. [Google Scholar]
- SingerRB, Sazima M.2000. The pollination of Stenorrhynchos lanceolatus (Aublet) L.C. Rich (Orchidaceae: Spiranthinae) by hummingbirds in south‐eastern Brazil. Plant Systematics and Evolution 223: 221–227. [Google Scholar]
- StpiczyñskaM.1995. The structure of floral nectaries of some species of Vicia L. (Papilionaceae). Acta Societatis Botanicorum Poloniae 64: 327–334. [Google Scholar]
- StpiczyñskaM.1997. The structure of the nectary of Platanthera bifolia L. (Orchidaceae). Acta Societatis Botanicorum Poloniae 66: 5–11. [Google Scholar]
- StpiczyñskaM.2003. Nectar resorption in the spur of Platanthera chlorantha Custer (Rchb.) Orchidaceae – structural and microautoradiographic study. Plant Systematics and Evolution 238: 119–126. [Google Scholar]
- StpiczyñskaM, Matusiewicz J.2001. Anatomy and ultrastructure of the spur nectary of Gymnadenia conopsea L. (Orchidaceae). Acta Societatis Botanicorum Poloniae 70: 267–272. [Google Scholar]
- TurnerG, Gershenzon J, Nielson EE, Froehlich JE, Croteau R.1999. Limonene synthase, the enzyme responsible for monoterpene biosynthesis in peppermint, is localized to leucoplasts of oil gland secretory cells. Plant Physiology 120: 879–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vanderPijlL, Dodson CH.1969.Orchid flowers: their pollination and evolution. Coral Gables, Florida: University of Miami Press. [Google Scholar]
- VaughnKC.1987.CRC handbook of plant cytochemistry. Boca Raton, FL: CRC Press. [Google Scholar]
- WerkerE, Ravid U, Putievsky E.1985. Structure of glandular hairs and identification of the main components of their secreted material in some species of Labiatae. Israel Journal of Botany 34: 31–45. [Google Scholar]
- WilliamsLE, Lemoine R, Sauer N.2000. Sugar transporters in higher plants – a diversity of roles and complex regulation. Trends in Plant Sciences 5: 283–290. [DOI] [PubMed] [Google Scholar]
- ZellnigG, Kronestedt‐Robards E, Robards AW.1991. Intercellular permeability in Abutilon nectary trichomes. Protoplasma 161: 150–159. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


