Abstract
• Background and Aims There is strong support for the monophyly of the orchid subtribe Maxillariinae s.l., yet generic boundaries within it are unsatisfactory and need re‐evaluation. In an effort to assemble sets of morphological characters to distinguish major clades within this subtribe, the pollinarium morphology and floral rewards of representative Brazilian species of this subtribe were studied.
• Methods The study was based on fresh material from 60 species and seven genera obtained from cultivated specimens. Variation of pollinarium structure and floral rewards was assessed using a stereomicroscope and by SEM analysis.
• Key Results Four morphological types of pollinaria are described. Type 1 appears to be the most widespread and is characterized by a well‐developed tegula. Type 2 lacks a stipe and the pollinia are attached directly to the viscidium. Type 3 also lacks a stipe, and the viscidium is rigid and dark. In Type 4, the stipe consists of the whole median rostelar portion and, so far, is known only from Maxillaria uncata. Nectar, trichomes, wax‐like and resin‐like secretions are described as flower rewards for different groups of species within the genus Maxillaria. Data on the biomechanics and pollination biology are also discussed and illustrated. In Maxillariinae flowers with arcuate viscidia, the pollinaria are deposited on the scuttellum of their Hymenopteran pollinators. In contrast, some flowers with rounded to rectangular, pad‐like viscidia fix their pollinaria on the face of their pollinators.
• Conclusions Pollinarium morphology and floral features related to pollination in Brazilian Maxillariinae are more diverse than previously suggested. It is hoped that the data presented herein, together with other data sources such as vegetative traits and molecular tools, will be helpful in redefining and diagnosing clades within the subtribe Maxillariinae.
Key words: Bifrenaria, flower morphology, Hylaeorchis, Maxillaria, Maxillariinae, Mormolyca, Orchidaceae, phylogeny, pollinarium, Scuticaria, Trigonidium, Xylobium
INTRODUCTION
The orchid subtribe Maxillariinae has recently been enlarged to include the species that were formerly assigned to the subtribes Lycastinae and Bifrenariinae (Dressler, 1993; Ryan et al., 2000; Whitten et al., 2000; Koehler et al., 2002). As currently circumscribed, the subtribe Maxillariinae is a species‐rich assemblage of approx. 600 neotropical species that are vegetatively diverse and exhibit a great variety of growth patterns. Within this subtribe there are pseudobulbous and pseudobulbless species with plicate, conduplicate or psigmoid leaves. Pseudobulbs, when present, are compressed, cylindrical to pyramidal, sometimes sulcate, always terminating the shoot and composed of a single internode (Dressler, 1993; Atwood and Mora de Retana, 1999). Despite the great variation in the vegetative architecture, the flower groundplan is rather conservative (Dressler, 1993; Atwood and Mora de Retana, 1999; Ryan et al., 2000; Whitten et al., 2000; Koehler et al. 2002). The inflorescence is a scape, mostly single‐flowered, sometimes presented in clusters. Flowers may be variously coloured and vary from spreading to campanulate (Atwood and Mora de Retana, 1999) or are, sometimes, held erect, such as in the genus Trigonidium Lindl. (Singer, 2002). They may also either be rewardless or offer disparate kinds of flower rewards to pollinators, such as nectar, trichomes and resin‐to‐wax‐like secretions (Dressler, 1993; Davies and Winters, 1998; Singer and Cocucci, 1999; Singer, 2002, 2003; Singer and Koehler, 2003). Sepals and petals are usually similar in form and free, but the lateral sepals may be connate to different degrees, forming a spur. The labellum is fixed or, as in most cases, hinged and articulated at the base of the column. The column is usually cylindric, thick and arcuate. All Maxillariinae genera have an incumbent anther and four pollinia, which are displayed in two pairs, each one generally composed of a larger pollinium clasping a smaller one.
In this orchid group, accessory structures are involved in the transfer of the pollinia. All Maxillariinae species present a well‐developed viscidium, which is a detachable, adhesive portion of the rostellum (Dressler, 1989). All Maxillariinae orchids have hyaline, elastic caudiculae conecting the pollinia to the viscidium or other pollinarium stalk (Dressler, 1993). Most species also have a stipe, which is a non‐sticky stalk of columnar origin that connects the pollinia to the viscidium (Dressler, 1993). Rasmussen (1986) has demonstrated that the term stipe corresponds to anatomically different structures. The tegular stipe, or tegula, generally has a single layer of epidermal cells and it leaves a distinct scar in the rostellum when removed (Rasmussen, 1986). The hamular stipe, or hamuli, is formed by a terminal, curved extension of the rostellum that connects the pollinia with the viscidium (Dressler, 1993). So far, only tegular stipes have been observed in the Maxillariinae (Dressler, 1993). Wasp, bee and ant pollinators have been recorded for a number of genera and species within the subtribe Maxillariinae (van der Pijl and Dodson, 1966; Dressler, 1993 and literature therein; Singer and Cocucci, 1999; Singer, 2002, 2003).
Strong molecular and non‐molecular evidence supports the subtribe Maxillariinae s.l. as a monophyletic assemblage of species (Dressler, 1993; Holtzmeier et al., 1998; Whitten et al., 2000). Yet generic boundaries within this taxonomically difficult group, especially of the highly diverse and clearly polyphyletic genus Maxillaria Ruiz et Pavón, are unsatisfactory due to the conflicting, arbitrary and unsupported generic concepts produced by various classification systems (e.g. Pabst and Dungs, 1977; Butzin and Senghas, 1996). To attempt a molecular and morphological phylogenetic revision of this complex subtribe, a collaboration among researchers of different countries has been established with the purpose of studying the phylogenetic relationships, systematics and pollination systems of Maxillariinae. A preliminary phylogeny by N. Williams and M. Whitten based on sequence data from ITS nrDNA is already available at www.flmnh.ufl.edu/natsci/herbarium/max/phylogenetics/phylogenetics.htm. The present contribution is part of this multidisciplinary project. This research is being performed to obtain sets of characters (based on flower and vegetative morphology, chemistry of flower rewards, breeding systems and pollination biology) that may help to identify and support clades defined by multiple molecular data sets. This procedure is being followed as a logical and necessary step prior to the proposal of nomenclatural revisions.
There are some interesting recent examples of massive nomenclatural changes in other orchid subtribes based solely on molecular data without an attempt to provide diagnostic morphological characters (van den Berg and Chase, 2000; Pridgeon and Chase, 2002). Some aspects of these rearrangements have been received without enthusiasm by the public or been rejected by other researchers (Chiron and Vitorino, 2002; Luer, 2002). This is regrettable since, in most cases, these works showed logical and clear patterns of relationships that might have been widely accepted by supporting the molecular findings with non‐molecular data. For instance, most plants transferred from Laelia Lindl. to Sophronitis Lindl. by van den Berg and Chase (2000) are an assemblage of easily identifiable, pseudobulbous, unifoliate plants, with eight pollinia. The type species of Laelia belongs to a monophyletic, unrelated, Mexican–Mesoamerican clade (van den Berg and Chase, 2000). Thus, the name Laelia was clearly misapplied to the Brazilian plants. The results of van den Berg et al. (2000) were also coherent from a biogeographic point of view, since they demonstrated that species from south‐eastern to north‐eastern Brazil are a distinct, monophyletic group. These findings could have easily been sustained with non‐molecular data and their taxonomic proposals should have been widely understood and accepted. Unfortunately, Chiron and Vitorino (2002) perceived morphological patterns behind the molecular trees of van den Berg et al. (2000) and proposed several nomenclatural changes, some of which are not necessarily well supported by the published molecular data. This is an example to demonstrate how molecular data need to be evaluated and supplemented by non‐molecular diagnostic characters to produce definitive and widely agreed taxonomic rearrangements.
The aims of the present study are to describe and illustrate pollinarium morphology and flower rewards of representative species of Brazilian Maxillariinae. The results are discussed with emphasis where the obtained data sets are consistent with other data sources, such as vegetative features and preliminary molecular data. It is hoped that the information provided here will be helpful in determining generic boundaries within the subtribe Maxillariinae.
MATERIALS AND METHODS
Throughout this paper the classification system of Pabst and Dungs (1977), which constitutes the most recent and complete one for the Brazilian Maxillariinae, will be used. In this classification system, species of Maxillariinae were designated to ‘alliances’ mainly according to gross vegetative characters. Although Ornithidium R. Br. is accepted by Pabst and Dungs (1977) as a distinct genus of the subtribe Maxillariinae, recent taxonomic treatments for this subtribe merged Ornithidium with Maxillaria (Dressler, 1993; Atwood and Retana, 1999). The recently described genus Hylaeorchis (Carnevali and Romero, 2000) is refered to in the system of Pabst and Dungs (1977) as Maxillaria rudolfii Hoehne. Morphological and molecular data indicate that Hylaeorchis is a distinct genus that is closely related to other genera of the Maxillariinae, such as Bifrenaria and Scuticaria (Carnevali and Romero, 2000; Koehler et al., 2002). The concept of the subtribe Maxillariinae follows Whitten et al. (2000). Unless indicated otherwise, the general taxonomic and morphological concepts of Dressler (1993) were followed.
Collection of flower material
Data were gathered from fresh flowers of plants currently in cultivation at the Instituto de Botânica de São Paulo (São Paulo, São Paulo State), at the Escola Superior de Agronomia Luiz de Queiroz (Piracicaba, São Paulo State), and at the Universidade Estadual de Campinas (Campinas, São Paulo State). Voucher specimens of all 60 species sampled were deposited at ESA, SP and UEC (Table 1). Dealing exclusively with fresh flowers was crucial for a correct interpretation of flower morphology, since delicate flower structures may be seriously damaged when specimens are pressed and dried. Also, flower rewards can be partially or completely removed, or even altered in form and composition, when using inappropriate fixation methods, such as 70 % ethanol or 70 % FAA (formalin‐acetic acid‐50% alcohol). Flower rewards were defined as any structure or secretion of the labellum that can be gathered or consumed by pollinators.
Table 1.
Distribution of pollinarium morphology and flower rewards in the studied taxa according to the alliances of Pabst and Dungs (1977)
| Taxa sampled | Voucher/source information | Pollinarium morphology | Flower reward |
| Hylaeorchis petiolaris Carnevali and G.A. Romero | 15847 (SP) | Type 2 | None |
| Maxillaria schunkeana Campacci & Kaustky | Koehler sn (UEC) | Type 1, av/tt | None |
| Mormolyca cf. galeata Lindl. | 16981 (SP) | Type 1, av/tt | None |
| Scuticaria hadwenii Hort. ex Hook. | 11996D (SP) | Type 1, av/tt | None |
| ‘Bifrenaria harrisoniae’ alliance | |||
| B. harrrisoniae (Hook) Rchb. f. | Simões et al. sn (UEC) | Type 1, rv/lt | None |
| B. tyrianthina (Lodd.) Rchb. f. | 5100 (SP) | Type 1, rv/lt | None |
| ‘Bifrenaria racemosa’ alliance | |||
| B. aureofulva (Hook) Lindl. | Simões et al sn (UEC); 15682 (SP) | Type 1, rv/lt | None |
| ‘Maxillaria alba’ alliance | |||
| M. alba (Hook) Lindl. | Koehler 184 (UEC); ESA 12 (ESA) | Type 3 | None |
| M. jenischiana (Rchb. f) C. Schweinf. | Breier 294 (UEC); 15679 (SP);1382 (SP) | Type 1, pv/st | None |
| ‘Maxillaria camaridii’ alliance | |||
| M. camaridii Rchb. f. | Koehler 215 (UEC) | Type 1, slightly av/st | Trichomes |
| M. pendens Pabst | Koehler sn (UEC); 29479D (ESA) | Type 1, pv/st | Nectar |
| M. rigida Barb. Rodr. | Breier 215 (UEC) | Type 1, pv/st | Nectar |
| ‘Maxillaria desvauxiana’ alliance | |||
| M. desvauxiana Rchb. f. | 2432 (SP); 702 (SP); 704 (SP) | Type 1, av/tt | None |
| ‘Maxillaria discolor’ alliance | |||
| M. brasiliensis Brieger & Illg | Breier 216 (UEC); Breier 159 (UEC) | Type 1, av/st | Trichomes |
| M. discolor (G. Lodd. ex. Lindl) Rchb. f. | 18826 (ESA), 19582 (ESA) | Type 1, av/st | Trichomes |
| M. nasuta Rchb. f. | 13234 (ESA) | Type 1, av/st | Resin‐like secretion |
| M. superflua Rchb. f. | 19498 (ESA) | Type 1, av/st | Resin‐like secretion |
| M. villosa (Barb. Rodr.) Cogn. | 14561 (SP) | Type 1, av/st | Trichomes |
| M. violaceopunctata Rchb. f. | 10111 (SP) | Type 1, av/st | Viscous secretion |
| ‘Maxillaria gracilis’ alliance | |||
| M. barbosae Loefgr. ex Porto | Faria sn (UEC); 16516 (SP), 1370 (SP) | Type 2 | None |
| M. gracilis Lodd. | 5153 (SP) | Type 2 | None |
| M. kautskyi Pabst | Koehler 261 (UEC) | Type 2 | None |
| ‘Maxillaria lactea’ alliance | |||
| M. friedrichstahlii Rchb. f. | 15196 (SP) | Type 1, sv/st | Resin‐like secretion |
| ‘Maxillaria lindleyana’ alliance | |||
| M. lindleyana Schltr. | Faria 122 (UEC) | Type 3 | Trichomes |
| ‘Maxillaria madida’ alliance | |||
| M. cogniauxiana Hoehne | 12253; 17069 (SP) | Type 1 | None |
| M. madida Lindl. | 5252 (SP) | Type 1, av/tt | None |
| ‘Maxillaria marginata’ alliance | |||
| M. chrysantha Barb. Rodr. | Koehler 181 (UEC); 33127 (ESA) | Type 1 | None |
| M. marginata Fenzl | Faria sn (UEC) | Type 2 | None |
| ‘Maxillaria multiflora’ alliance | |||
| M. leucaimata Barb. Rodr. | Breier 183 (UEC); 16766 (SP) | Type 3 | None |
| M. parkeri Hook. | 15044 (SP) | Type 3 | None |
| M. robusta Barb. Rodr. | Koehler 218 (UEC) | Type 3 | None |
| ‘Maxillaria picta’ alliance | |||
| M. consanguinea Klotzsch | Koehler 183 (UEC); 1952 (ESA) | Type 2 | None |
| M. phoenicanthera Barb. Rodr. | 4103 (SP); 33108 (ESA) | Type 2 | None |
| M. picta Hook. | Koehler 196 (UEC); Faria sn (UEC) | Type 2 | None |
| M. porphyrostele Rchb. f. | 11376 (SP); Koehler 197 (UEC); 12172 (SP) | Type 2 | None |
| M. ubatubana Hoehne | Koehler 216 (UEC); 23601 (ESA) | Type 2 | None |
| ‘Maxillaria pumila’ alliance | |||
| M. ferdinandiana Barb. Rodr | 1128 (SP) | Type 1, av/tt | None |
| M. pumila Hook. | 12070; 4056 (SP) | Type 1, av/tt | None |
| ‘Maxillaria rufescens’ alliance | |||
| M. cf. acutifolia Lindl. | Koehler 210 (UEC); 9301 (SP) | Type 1, av/tt | Trichomes |
| M. rufescens Lindl. | Breier 213 (UEC) | Type 1, av/tt | Trichomes |
| Maxillaria sp. | 953 (SP) | Type 1, av/tt | Trichomes |
| ‘Maxillaria splendens’ alliance | |||
| M. bradei Schltr. ex Hoehne | Koehler 225; 15665 (SP) | Type 3 | Trichomes |
| M. ochroleuca Lodd. ex Lindl. | Pansarin sn (UEC) | Type 3 | Trichomes |
| M. splendens Poepp. and Endl. | 15240 (SP) | Type 3 | Trichomes |
| ‘Maxillaria subulata’ alliance | |||
| M. acicularis Herb. ex Lindl. | 10423 (SP) | Type 1, av/st | None |
| M. juergensii Schltr. | 1003 (SP) | Type 1, av/st | None |
| M. vitelliniflora Barb. Rodr. | 8629 (SP) | Type 1, pv/st | None |
| ‘Maxillaria uncata’ alliance | |||
| M. cerifera Barb. Rodr. | 4527 (SP) | Type 1, pv/st | Wax‐like secretion |
| M. johannis Pabst | 8452 (SP) | Type 1, av/st | None |
| M. notylioglossa Rchb. f. | Breier 214 (UEC) | Type 1, pv/st | Resin‐like secretion |
| M. uncata Lindl. | Koehler 129 (UEC); 15485 (SP) | Type 4 | None |
| ‘Maxillaria valenzuelana’ alliance | |||
| M. equitans (Schltr.) Garay | Koehler 191 (UEC) | Type 1, av/st | Resin‐like secretion |
| M. valenzuelana (A. Rich.) Nash | 3179 (SP) | Type 1, av/st | Trichomes |
| ‘Ornithidium’ (sensu Pabst & Dungs, 1977) | |||
| Maxillaria parviflora (Poepp. & Endl.) Garay | Singer sn (UEC), 911 (SP) | Type 1, pv/st | Nectar |
| ‘Trigonidium latifolium’ alliance | |||
| T. obtusum Lindl. | Breier 253 (UEC); 11111 (ESA) | Type 2 | None |
| T. cf. turbinatum Rchb.f. | 15982 (SP) | Type 2 | None |
| ‘Trigonidium tenuis’ alliance | |||
| T. acuminatum Batem. ex Lindl. | Koehler 134 (UEC); 86441D (SP); 17796 (ESA) | Type 1, av/tt | None |
| ‘Xylobium colleyii’ alliance | |||
| X. colleyii (Batem. ex Lindl.) Rolfe | Koehler 105 (UEC) | Type 1, av/tt | None |
| ‘Xylobium variegatum’ alliance | |||
| X. foveatum (Lindl.) G. Nicholson | Koehler 200 (UEC) | Type 1, av/tt | None |
| X. variegatum (Ruiz & Pav.) Garay & Dunsterv. | Singer sn (UEC); A336 (SP) | Type 1, av/tt | None |
Plants deposited at UEC, SP and ESA are, respectively, in cultivation at Universidade Estadual de Campinas, Instituto de Botânica de São Paulo and Escola Superior de Agronomia Luiz de Queiroz.
rv, Truncate to rounded viscidium; av, arcuate viscidium; sv, sagittate viscidium; pv, pad‐like viscidium; lt, liguliform tegula; st, strap‐like tegula; tt, triangular tegula.
Morphological studies
Variation of pollinarium structure and distribution of floral rewards was studied using a Nikon SMZ‐U binocular stereomicroscope with a Nikon FD‐X 35mm camera attached. In some cases flower rewards were also observed using a scanning electron microscope (SEM, JEOL 5800LV). To avoid the elimination and/or alteration of the rewards, samples for SEM were dried using silica gel and sputter coated (gold) or observed fresh through low‐vaccuum scanning (Davies et al., 2003). The chemical composition of the rewards is currently under analysis by A. Marsaioli and collaborators (Instituto de Química, Universidade Estadual de Campinas) (Flach et al., 2003).
Pollination observations
Casual pollination observations of some species in cultivation were possible at the campus of the Universidade Estadual de Campinas (Campinas, São Paulo State), as well as at the Instituto de Botânica de São Paulo, which is located in the Fontes do Ipiranga State Park (São Paulo, São Paulo State). At both places, several Maxillariinae plants are cultivated in semi‐open conditions, which makes possible the observation of insects visiting and pollinating flowers. These casual observations are herein reported and discussed in a biological context since they are important for understanding not only the flower biomechanics, but also how pollinators exploit flower rewards in this group of plants.
RESULTS AND DISCUSSION
The observations given here indicate that pollinarium structure and flower rewards in the subtribe Maxillariinae are much more diverse than previously reported (see Hoehne, 1953; Pabst and Dungs, 1977; Dressler, 1993; Sprunger et al., 1996). The morphological variation of the pollinarium, as well as the nature of flower rewards described herein, are in agreement with many of the ‘alliances’ proposed by Pabst and Dungs (1977) (Table 1).
Pollinarium structure
Most of the species studied have a pollinarium composed by four superimposed, obovate to rotund, unequal (two larger, two smaller) pollinia. Some species, however, are exceptions. Maxillaria parviflora (Poepp. & Endl.) Garay has globose pollinia and, in the bifoliate species of Trigonidium Lindl. studied, T. obtusum Lindl. and T. cf. turbinatum Rchb.f., the pollinia have a peculiar linear to oblong format. They are displayed side‐by‐side, and not superimposed as in the other species of the subtribe. Also, contrary to other Maxillariinae species, these Trigonidium species have pollinia of similar size.
It was possible to recognize four distinct types of pollinarium structure within the sampled species. Each type is described and characterized below.
Type 1.
This pollinarium type consists of the four pollinia, a tegular stipe and a viscidium (Figs 1A and 2A–F). It is the most common among the species studied and it is suspected that it is also very common among other subtribes of Maxillarieae, since it has been reported for species of the Oncidiinae, Zygopetalinae and Stanhopeinae (Dressler, 1993). It was found in all the species of Bifrenaria Lindl. studied (Fig. 2A), Mormolyca Fenzl, Scuticaria Lindl., Xylobium Lindl., as well as in Trigonidium acuminatum Batem. ex Lindl. Within the species of Maxillaria studied, this type of pollinarium was found in all the species of the alliances ‘M. camaridii’, ‘M. desvauxiana’ studied (Fig. 2B and C), ‘M. discolor’ (Fig. 2D), ‘M. madida’, ‘M. pumila’, ‘M. subulata’, ‘M. rufescens’, ‘M. valenzuelana’, ‘M. uncata’ (except for M. uncata itself, Fig. 2E), M. chrysantha Barb. Rodr. (‘M. marginata’ alliance) as well as in the species M. schunkeana Campacci and Kautsky., M. jenischiana (Rchb. f.) C. Schweinf. (‘M. alba’ alliance), M. friedrichstahlii Rchb. f. (‘M. lactea’ alliance, Fig. 2F) and M. parviflora.
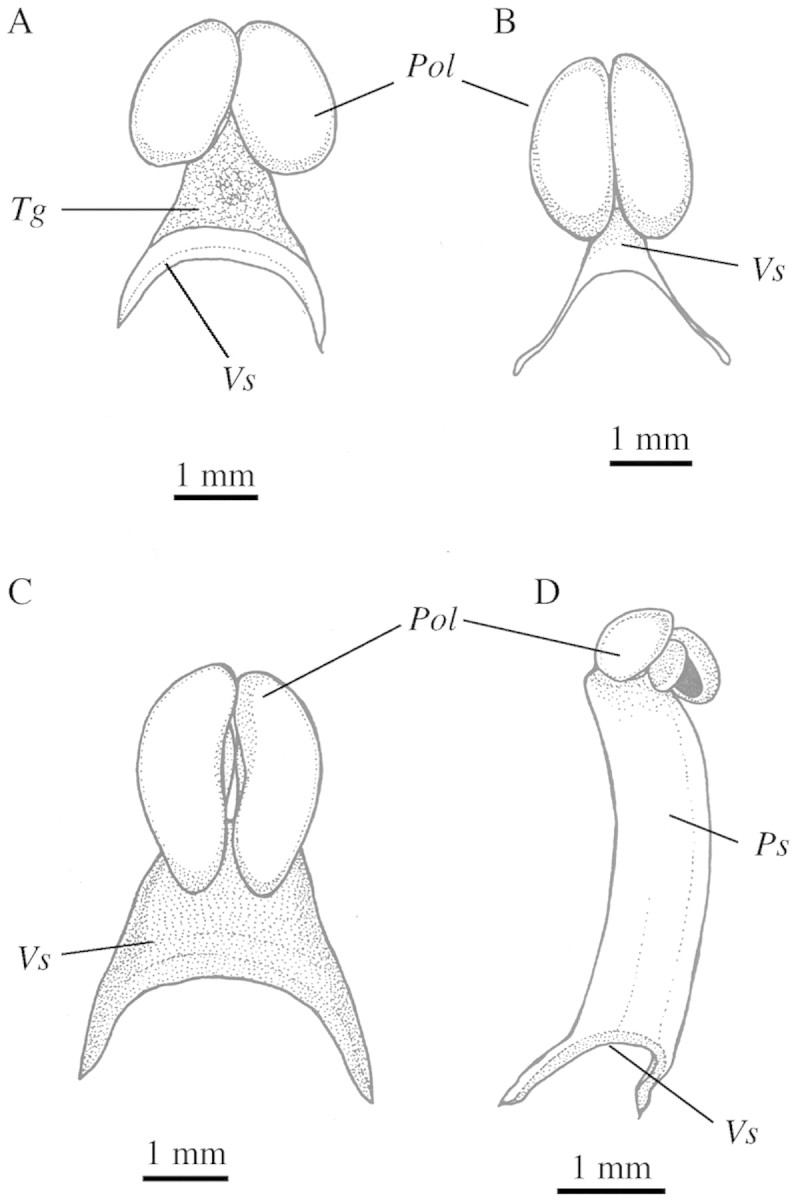
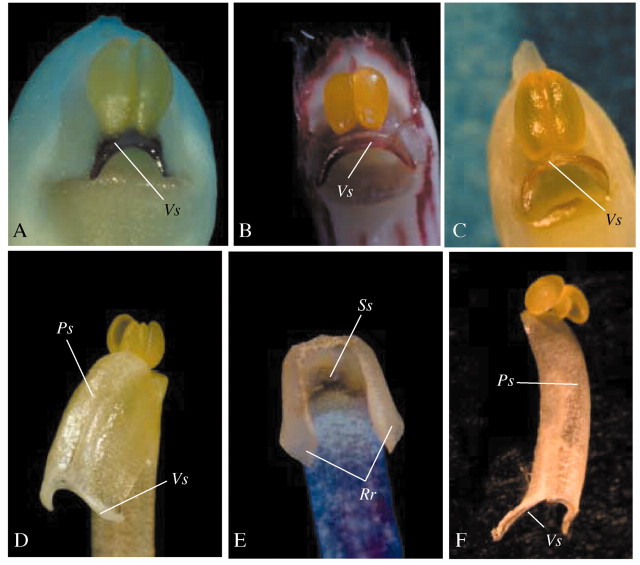
Fig. 1. Representative examples of pollinarium types: A, Maxillaria desvauxiana, Type 1 (with a well‐defined tegula); B, Maxillaria picta, Type 2 (devoid of tegula and with a soft, slender viscidium); C, Maxillaria bradei, Type 3 (devoid of tegula and with a rigid viscidium); D, Maxillaria uncata, Type 4 (with a pollinium stalk made up by the whole median portion of the rostellum). Pol, pollinia; Ps, pollinium stalk; Tg, tegula; Vs, viscidium.
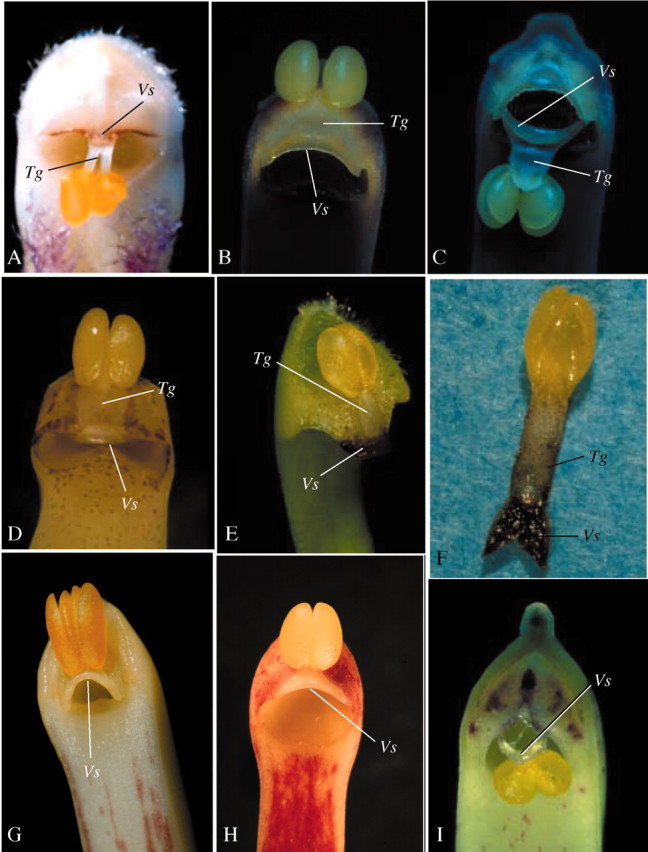
Fig. 2. A–F, Columns of species of the subtribe Maxillariinae with Type 1 pollinarium: A, Bifrenaria tyrianthina; B and C, Maxillaria desvauxiana; D, M. violaceopunctata; E, M. cerifera; F, M. friedrichstahlii (only the pollinarium). G–I, Columns of species of the subtribe Maxillariinae with Type 2 pollinarium: G, Trigonidium obtusum; H, M. marginata; I, M. porphyrostele. Tg, tegula; Vs, viscidium.
A considerable degree of variation in size, shape and colour of the pollinarium has been observed. Most Bifrenaria Lindl. species (sensu Koehler et al., 2002) have a bifurcate liguliform tegula (Fig. 2A), in which each stalk holds a pair of pollinia, and mostly a truncate to rounded viscidia. A bifurcate, although very short, tegula is also known for Rudolfiella aurantiaca (Lindl.) Hoehne and Scuticaria hadwenii Hort. ex Hook, which are phylogenetically closely related to the genus Bifrenaria (Koehler et al., 2002). This latter species is an exception within this group, having an arcuate viscidium. The viscidia in the alliances ‘M. uncata’ (Fig. 2E) (except for M. uncata Lindl. and M. johannis Pabst) and ‘M. camaridii’ (except for M. camaridii Rchb. f.), as well as in M. jenischiana (Rchb. f) C. Schweinf. (‘M. alba’ alliance), M. vitelliniflora Barb. Rodr. (‘M. subulata’ alliance) and in M. parviflora and are pad‐like in shape. It is noteworthy that all these species with pad‐like viscidia also display strap‐like, broader than longer, tegulae (Fig. 2E). The pollinaria of these species quite often resemble those of subtribe Oncidiinae (Dressler, 1993). Maxillaria johannis (‘M. uncata’ alliance) also has a strap‐like tegula, but the viscidium is arcuate. In M. camaridii (‘M. camaridii’ alliance) the tegula is also strap‐like in shape, but it is adnate to a linear or slightly arcuate, slender viscidium. Although M. friedrichstahlii Rchb. f. (‘M. lactea’ alliance) also has a strap‐like tegula, it has a very distinctive viscidium that is remarkable for its sagittate format and rigid texture (Fig. 2F). The genera Mormolyca Fenzl and Xylobium Lindl., as well as Trigonidium acuminatum Batem. ex Lindl., have slender and arcuate viscidia and a subtriangular tegula which sometimes is not clearly distinctive from the viscidium. Within the genus Maxillaria, pollinaria Type 1 with this overall morphology were found in all the species of the following alliances studied: ‘M. madida’, ‘M. pumila’, ‘M. subulata’ (except for M. vitelliniflora) and ‘M. rufescens.’, as well as for M. desvauxiana Rchb. f. (Fig. 2B and C), and M. schunkeana Campacci and Kautsky. Most species of the alliances ‘M. discolor’ and ‘M. valenzuelana’ have broad, strap‐like tegula and arcuate viscidia. In M. valenzuelana (A. Rich) Nash the tegula is very reduced and inconspicuous.
Type 2.
This type of pollinarium lacks a stipe, and the pollinia are directly connected to a broad, soft, arcuate viscidium (Figs 1B and 2G–I), which readily dehydrates and collapses after pollinarium removal. It was found in all the studied bifoliate Trigonidium species (Fig. 2G). The pollinia of these species are also noteworthy for being linear to oblong, equally long and parallel to each other, not superposed, obovate to rotund and heterogeneous in size (Fig. 2G; Singer, 2002). Pollinaria of Type 2 were also found in the Maxillaria species of the ‘M. picta’, ‘M. gracilis’ and ‘M, marginata’ alliances (Fig. 2H and I), except for M. chrysantha, and Hylaeorchis petiolaris Carnevali and G.A. Romero.
Type 3.
This pollinarium type is similar to Type 2, in that it also lacks a stipe and is composed of a broad, arcuate viscidium (Figs 1C and 3A–C). The viscidium, however, is dark brown and very rigid, with its dorsal surface often thickened in the middle region (Fig. 3A–C). In contrast to Type 2, the rigid viscidium of pollinarium Type 3 does not collapse when dehydrated after pollinarium removal. This kind of viscidium was found in all the species of the ‘M. multiflora’ studied (Fig. 3A and B), ‘M. splendens’ (Fig. 3C), and ‘M. lindleyana’ alliances, as well as in M. alba (Hook) Lindl. of the ‘M. alba’ alliance. Interestingly, plants of the three aforementioned alliances also have laterally compressed, unifoliate pseudobulbs and very long and erect pedicels. Although M. alba also has laterally compressed, unifoliate pseudobulbs, they are separated by a long rhizome covered by superimposed, sheathing coriaceous bracts – a vegetative feature that suggests its affinity with the Central American M. tenuifolia. Clearly, more research is needed to clarify whether this is a monophyletic assemblage of species.
Fig. 3. Columns of species of the subtribe Maxillariinae with Type 3 pollinarium: A, Maxillaria leucaimata; B, M. robusta; C, M. ochroleuca; D and E, column of M. uncata with pollinarium Type 4; F, pollinarium Type 4 of M. uncata. Ps, pollinium stalk; Rr, rostellar remnant; Ss, stigmatic surface; Vs, viscidium.
Type 4.
Pollinarium Type 4 was recorded only for the Amazonian Maxillaria uncata (‘M. uncata’ alliance, Figs 1D and 3D–F). Certainly, this is the most distinctive of the pollinaria types described. It consists of the whole median portion of the rostellum, which is entirely detached when removed, leaving no remaining tissue as in the typical tegular stipe. Pollinarium removal leaves two lateral rostelar remains (Fig. 3E) that become more divergent after pollinarium removal. The viscidium is arcuate, as in Types 3 and 4, but very thin and translucid (Fig. 3D and F). To the best of our knowledge, the pollinarium Type 4 does not fit the definition of tegula or hamuli, as previously described by Rasmussen (1986).
Flower rewards
No apparent flower reward was observed for the studied specimens of Bifrenaria, Mormolyca, Trigonidium or Xylobium. The flowers of these genera are most likely pollinated through deceit, sensu van der Pijl and Dodson (1966) and Dressler (1993). The pollination of Trigonidium obtusum was recently documented as a case of pseudocopulation (Singer, 2002). Within the genus Maxillaria, rewardless flowers were found in all the species of the ‘M. desvauxiana’, ‘M. multiflora’, ‘M. picta’, ‘M. gracilis’, ‘M. marginata’, ‘M. madida’, ‘M. pumila’ and ‘M. subulata’ alliances studied. Rewardless flowers were also registered for M. uncata and M. johannis (both from the ‘M. uncata’ alliance), M. alba (‘M. alba’ alliance) and M. schunkeana. The rewardless condition seems to be widespread in the subtribe Maxillariinae.
Trichomes.
Clusters of tightly packed, short to long, unicellular, claviform, yellowish trichomes were observed on the lip surface, mainly in the middle lobe region, of M. rufescens Lindl. (Figs 4A and 5A), Maxillaria sp. (‘M. rufescens’ alliance), M. camaridii (‘M. camaridii’ alliance), (Fig. 4G) as well as in all studied species of the ‘M. splendens’ and ‘M. lindleyana’ alliances. Such trichomes were first observed and described in the subtribe Maxillariinae by Porsch (1905). Similar, although extremely short, cushion‐like trichomes were also observed for the species M. brasiliensis Brieger & Illg., M. discolor (G. Lodd. ex Lindl.) Rchb. f., M. villosa (Barb. Rodr.) Cogn. (‘M. discolor’ alliance) (Fig. 4B) and M. valenzuelana (‘M. valenzuelana’ alliance).

Fig. 4. Flower rewards of species of the subtribe Maxillariinae: A and B, clusters of unicellular trichomes on the lip (A, Maxillaria rufescen; B, M. brasiliensis); C and D, resin‐like secretions on the lip (C, M. violaceopunctata; D, M. equitans); E, M. friedrichstahlii; F, wax‐like secretions on the lip of M. cerifera; G, lip trichomes of M. camaridii; H, nectar droplets on the lip of M. parviflora.
In the ‘M. splendens’ and ‘M. lindleyana’ alliances, the trichomes are apparently gathered by bee pollinators (Davies et al., 2000). Trigona bees were seen at the university campus in Campinas collecting the labelar trichomes of M. ochroleuca Lodd. ex Lindl., but without performing pollination. The peculiar trichomes of M. camaridii suggest that these structures may also be harvested by pollinators, although chemical analysis and pollination observations are needed to clarify this matter.
None of the Brazilian species studied presented pluricelular, moniliform, detachable trichomes, often called ‘pseudopollen’ owing to its pollen‐like superficial appearance (Davies et al., 2000). Davies et al. (2000) have reported the presence of pseudopollen in several Ecuadorian Maxillaria species of the ‘M. grandiflora’ alliance. Recently, Davies and collaborators (Davies et al., 2003) also considered the unicellular trichomes of the lip, such as these described here for species of the ‘M. discolor’ and ‘M. valenzuelana’ alliances, as ‘pseudopollen’. At least in the Ecuadorian species, multicellular trichomes are rich in starch, lipids and protein (Davies and Winters, 1998) and, therefore, they can be used by pollinators, e.g. to feed larvae or as nest‐building material. This leads us to suggest that the term ‘pseudopollen’ (‘pseudo’ means false) should be abandoned, since it suggests that pollinators may collect these trichomes by mistake, as if they were pollen. Observations on M. brasiliensis (‘M. discolor’ alliance) indicate that bee pollinators systematically visit (and pollinate) flowers of this species during the entire blooming period in order to collect their trichomes. This behaviour clearly indicates that the trichomes are somehow used during the pollinator’s life cycle. True deceitful orchids are usually pollinated over short periods of time and usually display low fruit set (van der Pijl and Dodson, 1966; Neiland and Wilcock, 1998), in agreement with the pollinator’s ability to recognize and avoid the flowers after a short period of interaction.
Secretions.
The species M. superflua Rchb. f., M. nasuta and M. violaceopunctata (all of the ‘M. discolor’ alliance) and M. equitans (Schltr.) Garay, from the ‘M. valenzuelana’ alliance, display viscous, resin‐like, amorphous secretions on the surface of the lip. Lip secretions were also recorded in most species of the ‘M. uncata’ alliance studied, which are either presented as wax‐like flakes, as in M. cerifera Barb. Rodr. (Figs 4E and 5C) or as resin‐like secretions, as in M. notylioglossa Rchb. f. Recently, Davies et al. (2003) have demonstrated through histochemical studies that the lip secretions of M. cerifera and M. notylioglossa are largely composed of lipids and protein. Resin‐like secretions were also recorded on the lip of M. friedrichstahlii (‘M. lactea’ alliance). Nectar was recorded only in a few scattered species of the genus Maxillaria, namely M. parviflora (Fig. 4H; Singer, 2003; Singer and Koehler, 2003), M. pendens Pabst and M. rigida Barb. Rodr. (both from the ‘M. camaridii’ alliance). In M. parviflora, droplets of nectar are concealed in a shallow depression of the lip (Fig. 4H). In M. pendens and M. rigida nectar can be found as small droplets at the base of the column.
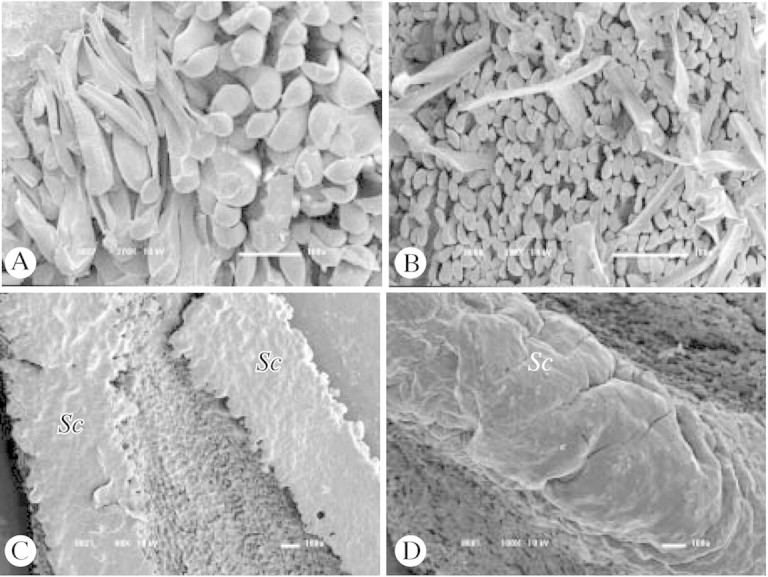
Fig. 5. SEM of lip surface: A, unicellular trichomes in M. rufescens (low‐vacuum); B, long and short unicellular trichomes of M. ochroleuca; C, wax‐like secretion of M. cerifera; D, resin‐like secretion of M. equitans. Sc, secretion. Bars = 100 µm.
Flower biomechanics and pollination biology
With the exception of M. parviflora, all species studied showed the labellum articulated at the base of the column. In M. parviflora, the lip is continuous, but not articulated, with the base of the column. The lip–column junction is very flexible, but the lip cannot move as freely as in the other species studied.
All the Maxillariinae species studied could be divided into two categories of trap flowers and open flowers, according to flower morphology and the way in which pollinators interact with them. Trap flowers were observed for all the species of the genus Trigonidium studied. These flowers correspond to enclosed, funnel‐like, upright structures, in which pollinators get trapped for brief periods of time after falling into the flower tube (Singer, 2002). In attempting to escape, the pollinators have the pollinarium fixed on their scutellum as they leave the flower. A pollinarium‐laden insect that gets trapped in consecutive flower visits will deposit the pollinia into the concave stigmatic surface (Singer, 2002). In the bifoliate Trigonidium species studied it has been noticed that the pollinia have to dehydrate considerably to fit the stigmatic cavity (Singer, 2002). This is probably a flower feature that hinders self‐pollination and promotes cross‐pollination as well. So far, pollination by sexually deceived drones of Plebeia droryana (Meliponini) has been demonstrated only in T. obtusum. Pollinators attempt copulation either with the sepals or with the tip of the lateral petals and slip into the flower, subsequently performing the aforementioned pollination steps (Singer, 2002).
The second category, open flowers, includes all the remaining studied Maxillariinae species. Flowers of this category allow the pollinators to arrive and leave the flower freely. The pollination process was documented for several of these species. Invariably, orchids with arcuate viscidia have their pollinaria fixed on the scutellum of the bee. Queens of Bombus brasiliensis (Apidae: Bombini; Fig. 6A) and males of Eufriesea violacea (Apidae: Euglossini) have been caught with pollinaria of Bifrenaria harrisoniae (Hook) Rchb.f. on their scutellum. Although species of this genus have a rounded to rectangular viscidium, the pollinarium of B. harrisoniae is also attached in the scuttellum of the pollinators. Workers of Trigona sp. (Apidae: Meliponini) have been recorded pollinating the rewardless flowers of Maxillaria leucaimata (‘M. multiflora’ alliance; Fig. 6B), M. picta Hook (Fig. 6C; Singer and Cocucci, 1999), M. porphyrostele Rchb. f. (both from the ‘M. picta’ alliance) and M. marginata Fenzl (‘M. marginata’ alliance). Vespidae wasps have also been observed pollinating the flowers of M. gracilis Lodd. (‘M. gracilis’ alliance; Fig. 6D). Trigona workers have also been recorded pollinating the flowers of M. brasiliensis (‘M. discolor’ alliance) while collecting the trichomes on the lip surface (Fig. 6E). In all these species, the broad arcuate viscidium firmly embraces the pollinator scutellum when the insect leaves the flower (Fig. 6A–E). The deposition of pollinaria on the scutellum of bees clearly enhances the chances of cross‐pollination, since it is very difficult for these insects to groom and remove the pollinaria from this region (Singer and Cocucci, 1999).

Fig. 6. Flower biomechanics. A–E, Flowers with broad, slender, arcuate viscidia that are fixed on the scutellum of hymenopteran pollinators: A, Bombus brasiliensis with pollinarium of Bifrenaria harrisoniae; B, Trigona worker dislodging a pollinarium of M. leucaimata; C, Trigona worker removing a pollinarium of M. picta; D, vespidae wasp with two pollinaria of M. gracilis; E, Trigona worker with pollinarium of M. brasiliensis. F, Ponerinae ant with two pollinaria of M. parviflora, a species with rounded viscidium.
Among the Maxillaria species with rounded to rectangular viscidia, plants of M. parviflora could only be observed briefly. These tiny flowers offer nectar in a shallow median depression on the lip surface. This nectar is licked by small Hymenoptera, such as workers of Plebeia and Tetragonisca (both Apidae: Meliponini), as well as Ponerinae ants (Fig. 6F). These latter insects were caught with pollinaria on their heads, deposited just below the antennae (Fig. 6F; Singer, 2003). When documenting the pollination biology of the Amazonian M. pendens, which is another species with rounded viscidium, Braga (1977) also recorded pollinarium fixation on the head of wasp pollinators, specifically on the surface of the composite eyes. More fieldwork is necessary to understand the pollination mechanisms in this group of species.
Taxonomic distribution of flower features
Genus Bifrenaria.
Recent phylogenetic molecular analyses have demonstrated that the genus Bifrenaria comprises a monophyletic group including Adipe Raf. and Cydoniorchis K. Senghas (Koehler et al., 2002). This genus has a pollinarium of Type 1, mostly with a rounded to rectangular viscidium and a bifurcate tegula. Yet species formerly assigned to Cydoniorchis display an entire liguliform tegula. The bifurcate tegula, however, is not an unique feature of this assemblage of species, since it is also present in Scuticaria hadwenii and Rudolfiela aurantiaca (Koehler et al., 2002). The genus Bifrenaria s.l. can be diagnosed mainly by vegetative features, such as the pyramidal unifoliate pseudobulbs with coriaceous plicate leaves.
Genus Xylobium.
There is strong molecular support for the genus Xylobium as a monophyletic group (Whitten et al., 2000). The species X. colleyii (Batem. ex Lindl.) Rolfe is very peculiar due to characters such as unifoliate pseudobulbs and large flowers, whereas the remaining species of this genus have small flowers and pseudobulbs of two or three leaves. Yet many flower features are consistent in the whole genus. All the species of Xylobium studied have plicate leaves and pseudobulbs round in section, multiflowered inflorescences with rewardless flowers, and Type 1 pollinaria with arcuate viscidia.
Genus Trigonidium.
The genus Trigonidium has traditionally been recognized by its upright, funnel‐like trap flowers. Even so, the species of this genus can be divided into two main groups according to flower as well as vegetative features. In the Brazilian bifoliate Trigonidium species, the linear‐oblong pollinia need to dehydrate to fit the stigmatic surface. Also all these species have Type 2 pollinaria, which are devoid of stipes (Fig. 2G). The only unifoliate Brazilian Trigonidium species we have studied so far, T. acuminatum, presents a pollinarium with a well‐defined tegular stipe (Type 1) and its pollinia, which have an oblong format, fit the stigmatic surface with no requirement of previous dehydration.
Genus Maxillaria.
Studied plants of the alliances ‘M. picta’ and ‘M. marginata’ have rewardless flowers with Type 2 pollinarium (Type 1 in M. chrysantha), which is devoid of tegula and with a broad, slender and arcuate viscidium (Fig. 2H and I). Also, all these plants have bifoliate aggregate pseudobulbs, flowers with a similar morphology, and are geographically restricted to South America, thriving from extreme north‐eastern Argentina (Misiones) to north‐eastern Brazil. These morphological features, as well as the geographical pattern of distribution, are also observed for the species of the ‘M. gracilis’ alliance, which it is believed are closely related to the former two alliances studied here.
The M. madida complex, including the ‘M. madida’, ‘M. pumila’, ‘M. subulata’ and ‘M paulistana’ alliances, also constitutes a morphologically coherent assemblage of Maxillariinae species restricted to South America, occurring from Argentina to Bahia, in north‐eastern Brazil. These species are easily recognizable small plants having coriaceous leaves and short inflorescences bearing shiny vinaceous to yellowish rewardless flowers with Type 1 pollinarium (bearing tegular stipes). Ongoing molecular research by S. Koehler suggests M. uncata to be closely related to the M. madida complex, but vegetative architecture, flower morphology and, especially, pollinarium structure of this species are very different when compared with the M. madida complex. Taxonomic studies considering M. uncata and its putative related species are necessary to clarify the phylogenetic position of this complex species as well as to understand the evolution of its peculiar pollinarium structure.
The species Maxillaria schunkeana, later described by Campacci and Kautsky (1993), poses an interesting problem. The vegetative architecture of this species resembles that of the ‘M. gracilis’ alliance, as this species has linear to lanceolate leaves. Yet flower morphology is extremely similar to the M. madida complex, as M. schunkeana flowers are dark vinaceous, with a shining rewardless lip and pollinarium of Type 1 (with tegular stipes). Preliminary molecular data suggest M. schunkeana is closely related to M. picta and M. marginata, but further morphological and molecular data are needed to clarify the phylogenetic position of this species.
The reward‐offering species M. cerifera, M. notylioglossa (both from the ‘M. uncata’ alliance) and M. friedrichstahlii (‘M. lactea’ alliance) also seem to form a cohesive morphological assemblage. They both have bifoliate pseudobulbs that are separated by a long rhizome covered by coriaceous bracts, a pollinarium of Type 1 composed of a liguliform tegula, with conspicuous resin or wax‐like rewards at the lip surface. Preliminary molecular data suggests this assemblage to be a monophyletic group (N. Williams, pers. comm.).
Another morphologically cohesive Maxillariinae assemblage of rewarding species includes the ‘M. discolor’ and ‘M. valenzuelana’ alliances. At a first glance, this group seems to be extremely heterogeneous, since it includes pseudobulbless plants, such as M. valenzuelana, and plants with laterally compressed, unifoliate pseudobulbs surrounded by several foliaceous bracts, such as M. villosa and allies (Illg, 1977a, b). Flower features, however, are quite consistent among theses species, since all of them have a pollinarium of Type 1 (Fig. 2D) and flower rewards varying from trichomes to resin‐like secretions (Fig. 4B–D). Similar vegetative shifts, concerning plants with unifoliate pseudobulbous to pseudobulbless plants, have already been reported for the genus Erycina (subtribe Oncidiinae), which today includes the fan‐leaved, pseudobulbless plants formerly assigned to Psygmorchis, together with the pseudobulbous, unifoliate species that comprised the genus Erycina s.str. (Williams et al., 2001). It is possible that the pseudobulbless species of Maxillaria from this group, such as M. valenzuelana and M. equitans, could have retained their seedling vegetative architecture due to neoteny, such as already suggested for several twig‐epiphyte Oncidiinae orchids (Chase, 1986). Recently, Barros (2002) proposed new combinations for some of the Brazilian species of the ‘M. discolor’ alliance under the genus Heterotaxys, formerly considered a section of the genus Maxillaria (Illg, 1977a, b). The acceptance of Heterotaxys as a separate genus of the Maxillariinae, however, needs to be considered with great caution, now that the phylogenetic relationships within this group are being studied (Singer and Koehler, 2003). The morphological data presented here as well as preliminary molecular data (N. Williams, pers. comm.) suggest that the reward‐offering species of the ‘M. discolor’ and ‘M. valenzuelana’ alliances, including species with and without pseudobulbs, are closely related, but further phylogenetic studies are necessary to corroborate this hypothesis, as well as to understand the relationships among species within these alliances and, ultimately, the evolution of the vegetative architeture within this group of species.
CONCLUSIONS
It is clear that the analysis of the flower morphology from representative species of Brazilian Maxillariinae will only partially fulfill the need for gathering non‐molecular sets of characters to define major clades within this subtribe. Some non‐ornamental genera restricted to the Andean region, such as Cryptocentrum Bentham, Anhtosiphon Schltr, Crhysocynis Linden & Reichenbach and Cyrtidiorchis Rauschert, are restricted to almost inaccessible places. As a result, vouchers and cultivated specimens from these genera are seldom available, nor are clear illustrations of their flower features (Brieger, 1977; Carnevali, 2001). Therefore, it will take a great amount of time and collaborative effort until the variation of flower features within the subtribe Maxillariinae are completely understood. It is hoped that this contribution will encourage others to initiate comparable research in different regions of the Neotropics. Information on the obscure Andean genera previously cited would be particularly valuable.
The idea that taxonomic changes in Maxillariinae orchids, or any other group of plants, should be made based solely on a few characters, such as flower features, is not supported. It is unlikely that restricted data sources, such as flower morphology and single DNA regions, will accurately reflect the evolutionary history of a group of species. It is believed emphatically that generic rearrangements should only be made after sets of diagnostic features, obtained from multiple data sources, are clearly determined for unequivocally monophyletic groups. The increasing acceptance of molecular tools to elucidate phylogenies and resolve taxonomic problems as well gives a unique opportunity, not only to clarify taxonomic matters but also to understand character evolution. Many traditional classifications have historically relied on the subjective importance that taxonomists gave to some a priori selected characters (e.g. Szlachetko, 1995). Molecular tools are, to date, the most powerful and objective way to produce large data sets. It is expected that once well‐supported molecular phylogenies are obtained, plotting non‐molecular characters (morphological, anatomical, chemical data) onto the obtained molecular trees will not only allow widely agreed generic rearrangements, but will also help in obtaining reliable evolutionary scenarios. If so, the evolution of vegetative architecture, flower features and pollination‐related traits, such as pollination strategies, will be better understood.
ACKNOWLEDGEMENTS
This study is part of a post‐doctoral research project by R.B.S. currently under development at the Botany Department of the Universidade Estadual de Campinas (Unicamp). This work was developed with the help of the Instituto de Botânica de São Paulo, the Escola de Agronomia Luiz de Queiroz, the Graduate Program in Plant Biology (Curso de Pós‐graduação em Biologia Vegetal) of Unicamp. We greatly acknowledge the Fundação de Amparo à Pesquisa do Estado de São Paulo for granting post‐doctoral and doctoral scholarships, respectively, to R.B.S. (FAPESP 01/ 08958‐1) and S.K. (FAPESP 02/02161‐7). We thank the staff of the Botany Department and of Unicamp for allowing us the use of facilities and for encouragement, Antonia Lima and Adriane Sprogis, for all the help with the SEM analysis, as well as the staff of the nurseries visited, Fabio de Barros and all his team from the Instituto de Botânica de São Paulo, and Elizabeth Ann Veasey and Josué Pontes from the Escola de Agronomia Luiz de Queiroz, for allowing us to collect material for study. We also thank Alessandra Barbosa, for taking care of the plants in cultivation at Unicamp, and Kevin Davies and Alan Gregg for providing important literature, Mark Whitten and Norris Williams for kindly sharing preliminary molecular data, and Robert Dressler, Ken Cameron and an anonymous reviewer for valuable suggestions and comments on this manuscript.
Supplementary Material
Received: 11 June 2003; Returned for revision: 15 September 2003; Accepted: 3 October 2003 Published electronically: 26 November 2003
References
- AtwoodJT, Mora de Retana DE.1999. Orchidaceae: tribe Maxillarieae: subtribes maxillariinae and Oncidiinae. Fieldiana 40: 1–182. [Google Scholar]
- BarrosF.2002. Notas taxonômicas para espécies brasileiras dos gêneros Epidendrum e Heterotaxis (Orchidaceae). Hoehnea 29: 109–113. [Google Scholar]
- BragaPIS.1977. Aspectos biológicos das Orchidaceae de uma campina da Amazônia Central. Acta Amazonica 2 suppl.: 1–89. [Google Scholar]
- BriegerFG.1977. On the Maxillariinae (Orchidaceae) with sepaline spur. Botanische Jahrbucher 97: 548–574. [Google Scholar]
- ButzinF, Senghas K.1996. Subtribus: Maxillariinae, Erster Band, Teil B. In: Brieger FG, Maatsch R, Senghas K, eds. Rudolf Schlechter, die Orchideen. Berlin, Wien: Blackwell Wissenschafts‐Verlag, 1727–1792. [Google Scholar]
- CampacciMA, Kautsky RA.1993. O gênero Maxillaria no Espírito Santo: uma nova espécie. Orquidário 7: 136–137. [Google Scholar]
- CarnevaliG.2001. A synoptical view of the classification of Cryptocentrum (Orchidaceae), new taxa, and a key to the genus. Harvards Papers in Botany 5: 467–486. [Google Scholar]
- CarnevaliG, Romero GA.2000.Orchids of Venezuela, a field guide. Caracas: Armitano. [Google Scholar]
- ChironGR, Vitorino CPN.2002. Revision des espéces Bresiliénnes du genre Laelia Lindl. Richardiana 2: 63–73. [Google Scholar]
- ChaseMW.1986. A reppraisal of the Oncidioid Orchids. Systematic Botany 11: 447–491. [Google Scholar]
- DaviesKL, Winters C.1998. Ultrastructure of the labellar epidermis in selected Maxillaria species (Orchidaceae). Botanical Journal of the Linnean Society 126: 349–361. [Google Scholar]
- DaviesKL, Winters C, Turner MP.2000. Pseudopollen: its structure and development in Maxillaria (Orchidaceae). Annals of Botany 85: 887–895. [Google Scholar]
- DaviesKL, Turner MP, Gregg A.2003. Lipoidal labellar secretions in Maxillaria Ruiz et Pav. (Orchidaceae). Annals of Botany 91: 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DresslerRL.1989. Rostellum and viscidium: divergent definitions. Lindleyana 1: 48–49. [Google Scholar]
- DresslerRL.1993.Phylogeny and classification of the Orchid family. Portland: Dioscorides Press. [Google Scholar]
- FlachA, Dondon RC, Singer RB, Koehler S, Amaral MCE, Marsaioli A.2003. The chemistry of pollination in selected Brazilian Maxillariinae orchids. Journal of Chemical Ecology (in press) [DOI] [PubMed] [Google Scholar]
- HoehneFC.1953. Orchidaceas. In: Hoehne FC, ed. Flora Brasilica, fasc 10, vol. 12(7). São Paulo: Secretaria da Agricultura. [Google Scholar]
- HoltzmeierMA, Stern WL, Judd WS.1998. Comparative anatomy and systematics of Sengha’s cushion species of Maxillaria (Orchid aceae). Botanical Journal of the Linnean Society 127: 43–82. [Google Scholar]
- IllgRD.1977a. Sobre a reprodução de Maxillaria brasiliensis Brieg. et Illg e M. cleistogama Brieg. et Illg. (Orchidaceae). Revista Brasileira de Biologia 37: 267–279. [Google Scholar]
- IllgRD.1977b. Revisão taxonômica da seção Heterotaxys (Lindl.) Brieger do gênero Maxillaria Ruiz et Pavón. Revista Brasileira de Biologia 37: 281–290. [Google Scholar]
- KoehlerS, Williams NH, Whitten WM, Amaral MCE.2002. Phylogeny of the Bifrenaria (Orchidaceae) complex based on morphology and sequence data from nuclear rDNA internal transcribed spacers (ITS) and chloroplast trnL‐trnF region. International Journal of Plant Sciences 163: 1055–1066. [Google Scholar]
- LuerCA.2002. A systematic method of classification of the Pleurothallidinae versus a strictly phylogenetic method. Selbyana 23: 57–110. [Google Scholar]
- NeilandMR, Wilcock CC.1998. Fruit set, nectar reward and rarity in the Orchidaceae. American Journal of Botany 85: 1657–1671. [PubMed] [Google Scholar]
- PabstGF, Dungs F.1977.Orchidaceae Brasilienses II. Hildesheim: Brucke‐Verlag. [Google Scholar]
- PorschO 1905. Beitrage zur histologischen Blutenbiologie I. Österreichische Botanische Zeitschrift 55: 165–173. [Google Scholar]
- PridgeonA, Chase MW 2001. A phylogenetic reclassification of Pleurothallidinae (Orchidaceae). Lindleyana 16: 235–271. [Google Scholar]
- PridgeonAM, Solano R, Chase MW.2001. Phylogenetic relationships in Pleurothallidinae (Orchidaceae): combined evidence from nuclear and plastid DNA sequences. American Journal of Botany 88: 2286–2308. [PubMed] [Google Scholar]
- RasmussenFN.1986. On the various contributions by which pollinia are attached to viscidia. Lindleyana 1: 21–32. [Google Scholar]
- RyanA, Whitten WM, Johnson MAT, Chase MW.2000. A phylogenetic reassesment of Lycaste and Anguloa (Orchidaceae: Maxillarieae). Lindleyana 15: 33–45. [Google Scholar]
- SingerRB.2002. The pollination mechanism in Trigonidium obtusum Lindl (Orchidaceae: Maxillariinae): Sexual mimicry and Trap‐flowers. Annals of Botany 89: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SingerRB.2003. Orchid pollination: recent developments from Brazil. Lankesteriana 7: 111–114. [Google Scholar]
- SingerRB, Cocucci AA.1999. Pollination mechanisms in four sympatric southern Brazilian Epidendroideae orchids. Lindleyana 148: 47–56. [Google Scholar]
- SingerRB, Koehler S.2003. Toward a phylogeny of Maxillariinae orchids: multidisciplinary studies with emphasis on Brazilian species. Lankesteriana 7: 57–60. [Google Scholar]
- SzlachetkoDL.1995. Systema Orchidalium. Fragmenta Floristica et Geobotanica suppl. 3: 1–152. [Google Scholar]
- SprungerS, Cribb P, Toscano de Brito A.1996.João Barbosa Rodrigues Iconographie des orchidées du Brésil. Basle: Friedrich Reinhardt Verlag. [Google Scholar]
- van den BergC, Chase MW.2000. Nomenclatural notes on Laeliinae I. Lindleyana 15: 115–119. [Google Scholar]
- van den BergC, Higgins WE, Dressler RL, Whitten WM, Sto‐Arenas MA, Culham A, Chase MW.2000. A phylogenetic analysis of Laeliinae (Orchidaceae) based on sequence data from internal transcribeb spacers (ITS) of nuclear ribosomal DNA. Lindleyana 15: 96–114. [Google Scholar]
- van der PijlL, Dodson CH.1966.Orchid flowers – their pollination and evolution. Coral Gables: University of Miami Press. [Google Scholar]
- WhittenWM, Williams NH, Chase MW.2000. Subtribal and generic relationships of Maxillarieae (Orchidaceae) with emphasis in Stanhopeinae. American Journal of Botany 87: 1842–1856. [PubMed] [Google Scholar]
- WilliamsNH, Chase MW, Fulcher T, Whitten MW.2001. Molecular systematics of the Oncidiinae based on evidence from four DNA sequence regions: expanded circumscriptions of Cyrtochilum, Erycina, Otoglossum and Trichocentrum and a new genus (Orchidaceae). Lindleyana 16: 113–139. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.