Abstract
• Background and Aims The use of grafted plants in vegetable crop production is now being expanded greatly. However, few data are available on the formation of graft unions in vegetables. In this work, the structural development of the graft union formation in tomato plants is studied, together with the possible relationship with activities of peroxidases and catalases.
• Methods Tomato (Lycopersicon esculentum Mill.) seedlings of cultivar Fanny were grafted on the rootstock of cultivar AR‐9704 using the ‘tongue approach grafting’ method, and were grown in a crop chamber. A study of the structural development of the graft union and the involvement of peroxidases and catalases in the process of graft formation was carried out during the first stages of the graft union (4, 8 and 15 d after grafting).
• Key Results Observation of the structure of the graft union showed formation of xylem and phloem vessels through the graft union 8 d after grafting. In addition, root hydraulic conductance, L0, indicate that the graft union is fully functional 8 d after grafting, which coincided with an increase of peroxidase and catalase activities.
• Conclusions These results suggest that increased peroxidase and catalase activities might be implicated in graft development in tomato plants.
Key words: Catalase, graft, Lycopersicon esculentum, peroxidase, tomato
INTRODUCTION
The use of grafted plants in vegetable crop production is still rare compared with the use of grafting for tree crops. However, this technique is now being expanded greatly to reduce infections caused by pathogens (Biles et al., 1989; Padgett and Morrison, 1990), to increase the resistance to drought (White and Castillo, 1989) and to enhance nutrient uptake (Ruiz‐Sifre et al., 1997). Few data are available on the formation of graft unions in vegetables.
The investigation of water relations by measurement of parameters such as root hydraulic conductance (L0) could indicate the nutrient and water uptake status of grafted plants (Turquois and Malone, 1996). In a previous investigation (Fernandez‐García et al., 2002), it was observed that measurements carried out above and below the graft union gave similar L0 values, suggesting that the graft union cannot be considered a barrier to water flow. These results are in agreement with those reported by Turquois and Malone (1996), who used a non‐destructive measurement of functional hydraulic connections within the intact plant, providing a useful indication of the progress of graft development. They showed that the graft union acts like a continuous unit with respect to water movement.
In grafted plants, vascular regeneration can re‐establish the continuity of the transport system by means of a complex developmental process involving structural and physiological differentiation of parenchyma into xylem and phloem elements (Jeffree and Yeoman, 1983). Lignin is present in all vascular plants. Apart from cellulose, lignin is the most abundant organic natural product known. Deposition of lignin in plants is normally located in the sclerenchyma of the ground tissues, as well as in tracheary elements and fibres of the vascular tissues. Lignin is synthesized mainly in cells to become part of the transport system. The last catalytic step in the synthesis of lignin is the oxidation of cinnamyl alcohols, and this step is catalysed by a peroxidase (Whetten et al., 1998). Peroxidases catalyse oxyreduction between H2O2 and various reductants. The range of electron donors is wide and includes phenols, amines and alcohols. Catalases are similar to peroxidases but, as in the case of catalase H2O2, can act as both electron donor and acceptor. Catalase is implicated in destruction of harmful H2O2 generated in excess by different subcellular processes, and by biotic and abiotic stresses.
In the present work, the structural development of the graft union formation is studied in tomato (Lycopersicon esculentum Mill.) plants together with the possible relationship with peroxidase and catalase activities.
MATERIALS AND METHODS
Plant material and culture conditions
Tomato seedlings, cultivars Fanny and AR‐9704 (rootstock), were germinated for 3–4 d in vermiculite moistened with 0·5 mm CaSO4 in a germination chamber at 30 °C. After this, plants were put in a controlled environment chamber at a day/night temperature of 25/20 °C, a day/night relative humidity of 65/85 % and a 16‐h photoperiod. Photon flux density was adjusted to 400 µmol m–2 s–1. Light was provided by a combination of fluorescent tubes (Philips TLD 36 W/83, Sylvania F36 W/GRO) and metal halide lamps (Osram HQL. T 400 W). After 9 d under these conditions, plants were grown hydroponically in aerated Hoagland nutrient solution: KNO3 (6 mm), Ca(NO3)2 (4 mm), NH4H2PO4 (1 mm), MgSO4 (1 mm), KCl (50 mm), H3BO3 (25 mm), MnSO4 (2 mm), ZnSO4 (2 mm), CuSO4 (0·5 mm), (NH4)2MoO4 (0·5 mm), Fe‐EDDHHA (20 mm).
Grafting procedure
Twenty‐five days after germination, cultivar Fanny (the scion) was grafted onto cultivar AR‐9704 (the rootstock) using the procedure of ‘cleft grafting’ described by Lee (1994). Grafted plants were covered with a transparent plastic bag for 5 d to increase the relative humidity and avoid leaf dehydration by water loss. Measurements and samples were taken 4, 8 and 15 d after grafting.
Root hydraulic conductance
Hydraulic conductance (L0) was measured by natural exudation of the roots. This method was based on volume flow through detached root systems. The aerial parts of a plant were removed, leaving a cylinder of leaf bases, and this was sealed with silicone grease into a tapered glass tube. After 2 h, the exuded xylem sap was collected using a Pasteur pipette and transferred to an Eppendorf tube. The sap was weighed and the roots removed and weighed. Sap flow was expressed as mg (g root f. wt)–1 h–1. Samples of sap (100 µL) were placed in Eppendorf tubes and the osmotic potentials of the sap and the root nutrient solution were measured using an osmometer (Digital Osmometer, Roebling, Berlin, Germany). The osmotic potential difference between the xylem sap and the external solution, ΔψΠ, was calculated from their osmolarity values. The hydraul ic conductance, L0, which has units mg (g root f. wt)–1 h–1 MPa–1, was determined as: L0 = Jv/Δψ Π (Fernandez‐García et al., 2002). Six replicate plants per treatment were sampled at each time point (4, 8 and 15 d).
Enzyme activities
Stems (1·0 g f. wt) were homogenized in 50 mm potassium phosphate buffer containing 1 % (w/v) polyvinyl pyrrolidone (pH 7·2) at 4 °C. The homogenate was filtered through a nylon net and centrifuged at 12000 g for 10 min. The supernatant was used to determine peroxidase and catalase activities. Five plants per treatment were analysed at each sampling time (4, 8 and 15 d).
Peroxidase activity was determined spectrophotometrically by following the increase in absorbance at 470 nm. The reaction mixture contained 50 mm potassium phosphate (pH 6·8), 10 mm hydrogen peroxide, 9 mm guaiacol and enzyme extract in a total volume of 3 mL, as described by Olmos et al. (1997).
Catalase was assayed according to Aebi (1984). The reaction mixture contained 50 mm potassium phosphate (pH 7·8), 10 mm H2O2 and the enzyme extract. Activity was determined by following the decomposition of H2O2 at 240 nm. The extinction coefficent was 36 mm–1 cm–1.
Tissue‐printing of peroxidases
Nitrocellulose membranes were soaked in distilled H2O and blotted dry with a tissue. Stem sections were placed on the membrane and lightly pressed on to it. The sections were removed carefully and the membrane rinsed with distilled water. Membranes were soaked in a 10 mm hydrogen peroxide/9 mm guaiacol solution as described above. Membranes were rinsed as soon as colour development was optimal (Spruce et al., 1987). Eight plants, grafted and ungrafted, were sampled at each time point (4, 8 and 15 d).
H2O2 determination
Total H2O2 was measured in fresh material by a peroxidase‐coupled assay, using 4‐aminoantipipyrine and phenol as substrate donors (Frew et al., 1983). Five plants, grafted and ungrafted, were sampled at each time point (4, 8 and 15 d).
Histochemical stain for lignin
Lignin was detected using the method described by Ros‐Barcelo (1998). The middle of the graft union was determined using a stereomicroscope and sectioned with a scalpel. Fresh cut sections (100–200 µm thick) were obtained using a hand‐microtome and were then soaked in 1·0 % (w/v) phloroglucinol in 25^:^75 (v/v) HCl–ethanol for 10–15 min. The stained sections were observed and photo graphed under a stereomicroscope (MZ8, Leica). A full section of the graft union was difficult to obtain at day 4 due to the limited development of the graft. Ten plants, grafted and ungrafted, were sampled at each time point (4, 8 and 15 d).
Scanning electron microscopy
Stems were frozen in liquid N2 and fractured with a pre‐cooled knife. The samples were then freeze‐dried before mounting and coating with gold. Samples were studied using a JEOL JSM‐6100 scanning electron microscope. The acceleration voltage used was 15 kV (Olmos and Hellin, 1998). Ten plants, grafted and ungrafted, were sampled at each time point (4, 8 and 15 d).
RESULTS
Root hydraulic conductance
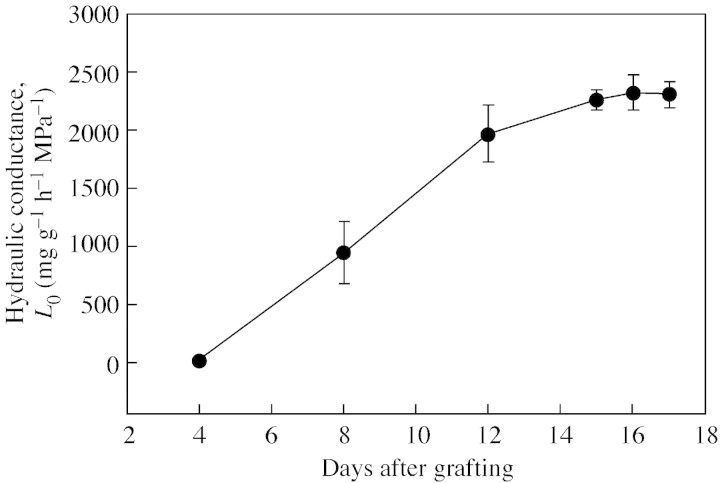
Root hydraulic conductance, L0, was measured at 4, 8, 12 and 15 d after grafting (Fig. 1). L0 increased linearly from 4 to 12 d, starting from zero, and attained a constant value between 12 and 15 d.
Fig. 1. Values of root hydraulic conductance (L0). Measurements were made using natural exudation (see text for details). Data are means ± s.e. (n = 6).
Scanning electron microscopy
Figure 2 shows the morphological differentiation of grafted tomato plants. Figure 2A shows the stem cross‐section from control plants before grafting. The graft union at day 4 is presented in Fig. 2B, where a necrotic layer can be observed. Figure 2C (scion cross‐section at the graft level) shows detail of hypertrophic cells in the graft union at 4 d. At day 8, in many grafted plants, the parenchymatic cells of the central pith were dead, leaving a central hole in which the surface was covered by a necrotic layer (Fig. 2D, scion cross‐section at the level of the graft union and Fig. 2E, stem longitudinal section at the level of the graft union). However, the vascular system was not damaged and, with new xylem strands, connected the rootstock and scion. At day 15, the graft union was fully developed (Fig. 2F, stem longitudinal section at the level of the graft union and Fig. 2H, surface of the graft union). Xylem vessels connecting the rootstock and scion could be observed in the graft union (Fig. 2G, detail of xylem vessels of the graft union).
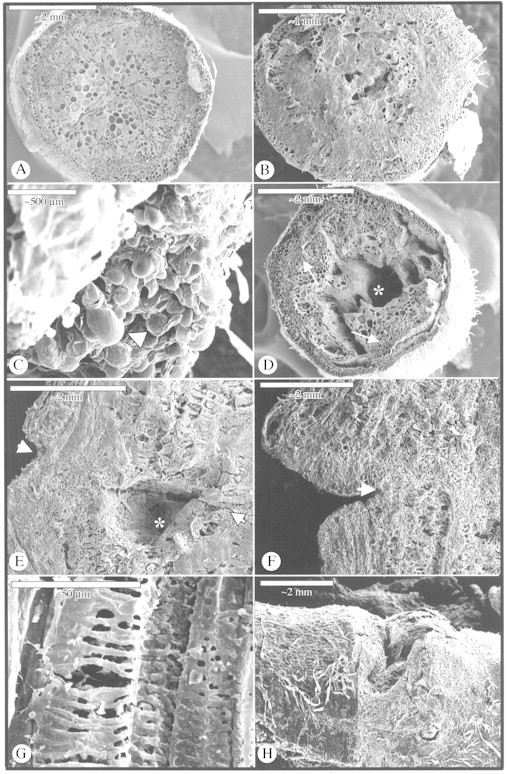
Fig. 2. Scanning electron microscopy of grafted plants. (A) Stem cross‐section from control plants before grafting. Bar = 2 mm. (B) Scion cross‐section from graft union at day 4, showing a necrotic layer at the graft interface. Bar = 1 mm. (C) Scion cross‐section, detail of callus cells in a graft union at 4 d, showing hypertrophic cells (arrow). Bar = 500 µm. (D) Transverse section of a scion at 8 d showing the pith totally destroyed, although the vascular system was unaltered (arrows). Bar = 2 mm. (E) Longitudinal section of the graft at 8 d showing the graft union (arrow). Bar = 2 mm. (F) Longitudinal section of the graft at 15 d (arrow = graft union). Bar = 2 mm. (G) Detail of xylem vessels from the graft union. Bar = 50 µm. (H) An external view of the graft union at 15 d after grafting. Bar = 2 mm. * Cavity of the pith.
Lignin localization (Wiesner test)
The Wiesner test is based on the reaction of phloroglucinol with the aromatic aldehyde fraction contained in lignin, the reaction yielding a pink stain. Figure 3 shows the progress of lignification during graft development.
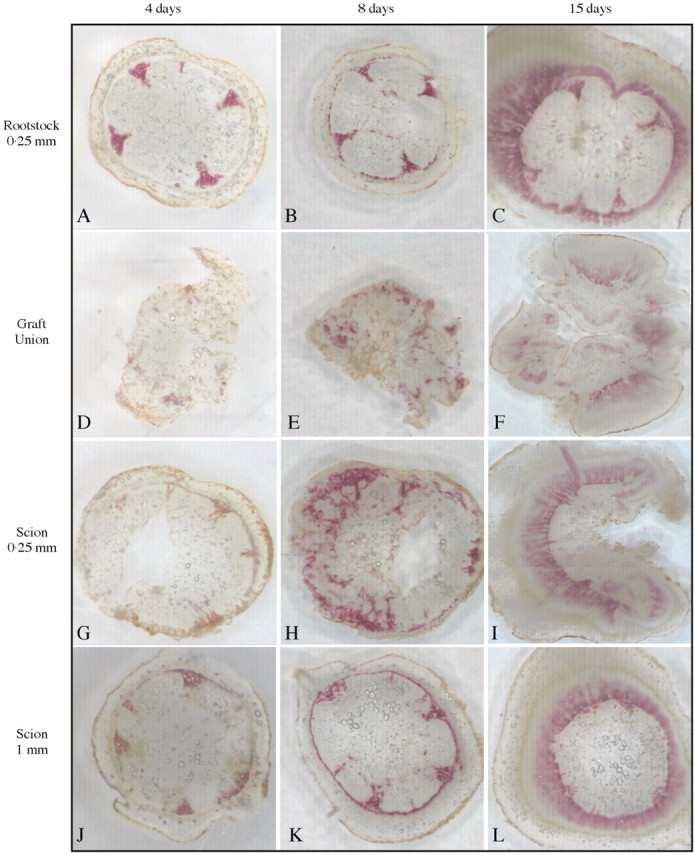
Fig. 3. Wiesner stain during graft development at day 4 (A, D, G and J), day 8 (B, E, H and K) and day 15 (C, F, I and L). Positive staining is shown as a pink colour. (A–C) Sections of the rootstock at 250 µm below the graft union. (D–F) Sections of the graft union. (G–I) Sections of the scion 250 µm above the graft union. (J–L) Sections of the scion at 1 mm above the graft union.
Four days after grafting, the stain was mainly localized in the xylem of the rootstock (Fig. 3A) and scion (Fig. 3J). In the graft union (Fig. 3D), small spots of pink stain could be observed. Similarly, the section 250 µm above the graft union showed a pink stain near the vascular strand of the scion (Fig. 3G).
At 8 d, a significant increase in staining was observed in the vascular system in both the rootstock (Fig. 3B) and in the scion (Fig. 3K). The graft union (Fig. 3E) showed a notable increase in staining compared with day 4. The section 250 µm above the union showed a large increase in staining (Fig. 3H) compared with day 4. It can also be observed that the lignifying cells exhibited a radial distribution.
At 15 d, the vascular systems of the rootstock (Fig. 3C) and scion (Fig. 3L) showed an intense pink colour. The graft union (Fig. 3F) showed a well‐developed, stained xylem. Similarly, the section 250 µm above the graft showed an intense stain in the vascular system (Fig. 3I).
Peroxidase and catalase activities
The activities of peroxidase and catalase were analysed in both parts of the graft (scion and rootstock) to differentiate between the involvement of each in graft formation.
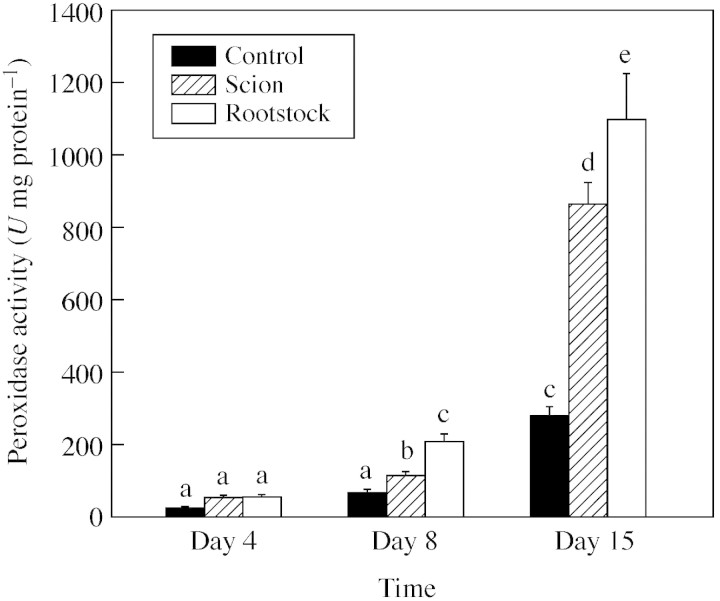
The increase of peroxidase activity is shown in Fig. 4. Peroxidase activity increased during plant development in both control and grafted plants. However, it was always higher in grafted than in control plants. At 8 and 15 d, a significant difference between rootstock and scion was observed, with activities being higher in the rootstock than in the scion.
Fig. 4. Peroxidase activity during graft development. Data are means ± s.e. (n = 5). Columns with the same letters are not significantly different for each parameter (P < 0·05, Tukey test).
Peroxidase tissue‐printing (Fig. 5) showed an increase in peroxidase activity as samples were taken nearer the region of the graft union. Four days after grafting (Fig. 5A), peroxidase activity was mainly localised in the graft union between the rootstock and scion, and 250 µm above the graft union (in the scion) (Fig. 5A, iii and iv). Peroxidase activity was much lower when measured more than 2 mm above or below the graft union (Fig. 5A, i and v). The control plants showed very slight peroxidase activity, similar to that found more than 2 mm from the graft union (Fig. 5A, vi). At 8 d after grafting, peroxidase activity was higher than at 4 d (Fig. 5B). However, the results were similar to those at 4 d, in that peroxidase was localized mainly in the graft union (Fig. 5B, iii). At this stage, peroxidase activity was absent in the central parenchyma, which correlates with the dead region observed by microscopy (Figs 2E and 3H). In the vascular region more than 2 mm above the graft union, intense points of peroxidase activity were observed. At 15 d after grafting (Fig. 5C), peroxidase activity was higher than at 8 d. Peroxidase was also mainly distributed at the graft union (Fig. 5C, iii). A high activity in the new vascular region of the graft union was also observed (Fig. 5C). In common with previous days, peroxidase activity was lower above and below the graft union.
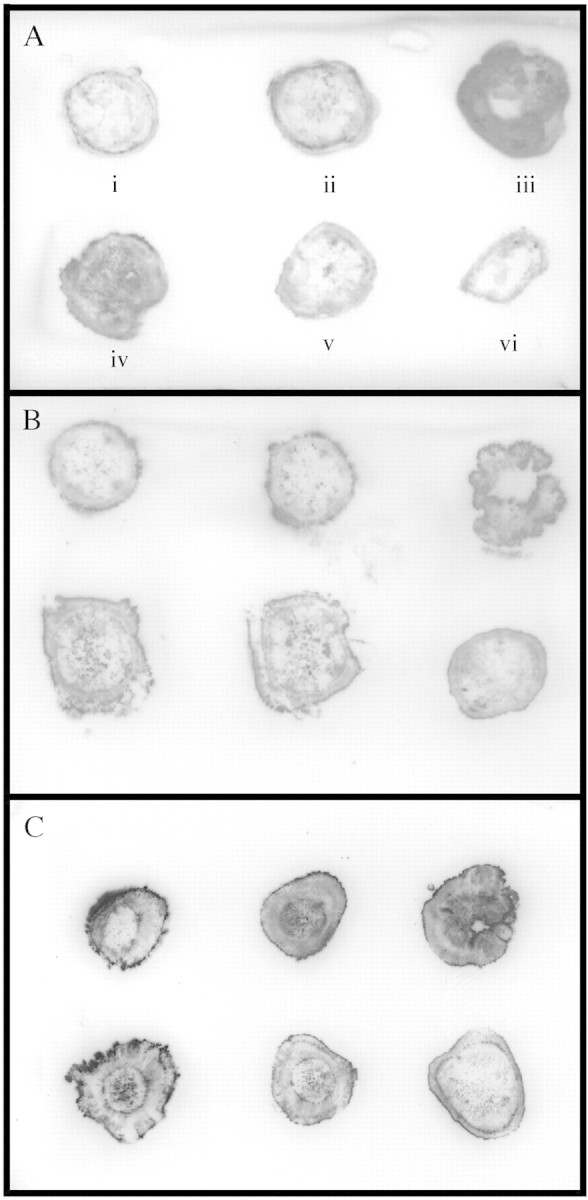
Fig. 5. Peroxidase tissue‐printing of graft development at (A) day 4, (B) day 8 and (C) day 15. Sections for tissue‐printing were taken sequentially at (i) 1 mm and (ii) 0·25 mm from the graft union in the rootstock, (iii) at the graft union, (iv) 0·25 mm and (v) 1 mm above the graft union in the scion and (vi) control sections from ungrafted plant.
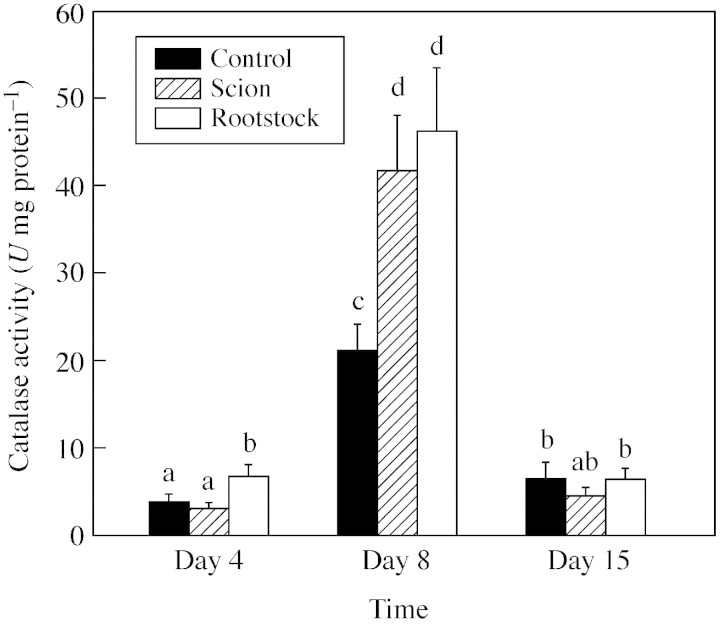
Figure 6 shows catalase activity during graft development. Catalase, an efficient scavenger of H2O2, showed some small variation during development of control plants. At 4 d after grafting, grafted plants showed the same catalase activity as control plants. However, activity was greatly increased at day 8, being about three times greater than for control plants. At day15, catalase activity was the same in control and grafted plants. No differences in catalase activity between the rootstock and scion were observed.
Fig. 6. Catalase activity during graft development. Data are means ± s.e. (n = 5). Columns with the same letters are not significantly different for each parameter (P < 0·05, Tukey test).
Table 1 shows quantification of H2O2 in scion and rootstock. Total H2O2 increased during plant development in both control and grafted plants. However, there was a significant increase in the amount of H2O2 at 8 d in grafted plants.
Table 1.
Quantification of H2O2 (nmol H2O2 per g f. wt) during plant development of control and grafted plants
| Day | Control | Graft union |
| 4 | 15·2 ± 3·2a | 19·4 ± 3·4a |
| 8 | 23·5 ± 3·9b | 64·6 ± 2·8d |
| 15 | 29·4 ± 5·1bc | 33·4 ± 4·7c |
Data are means ± s.e. (n = 5).
Data with the same letters are not significantly different for each parameter (P < 0·05, Tukey test).
DISCUSSION
A number of developmental stages can be recognized in the formation of a graft union. The early stage begins within 4 d, and is characterized by the death of cell layers at the graft interface as a wound reaction (Moore, 1984; Tiedemann, 1989), and by generation of a parenchymatous wound callus that fills the gap between the two graft components. In grafted tomato plants, the callus is formed by all living, undamaged cells at the graft union, probably from cambial, ray parenchyma and phloem parenchyma cells. Living cells from the surface quickly began to grow in size (hypertrophic cells) and to divide. Similar results have been obtained previously in auto‐grafted tomato plants (Jeffree and Yeoman, 1983). It was observed that, in many of the grafted plants, the pith of the rootstock exhibited a cavity of about four or five cell layers, although the vascular system was unaltered. This could indicate a slight incompatibility between scion and rootstock, or a wound response of the scion during the first days of grafting. Yeoman et al. (1978) found that the necrotic layer was produced in both compatible and incompatible Solanaceous grafts, which supports the latter hypothesis.
The differentiation of callus parenchyma to form new cambial initials and the subsequent union of the newly formed vascular strand with the original vascular bundle in both rootstock and scion begins between days 4 and 8 and is fully developed after 15 d. Stoddard and McCully (1979) showed mature xylem and phloem connections in pea root auto‐grafts 8 d after grafting. Most authors consider a graft union to be successful and complete when several phloem and xylem connections cross the graft interface. Turquois and Malone (1996) observed that the major hydraulic connections within the graft union of tomato became functional 5 d after grafting. The observations described are consistent with these results, the gradual increase of L0 4–8 d after grafting indicating the establishment of new connections in the vascular bundle in the graft union.
After the graft assemblage between the cells of the rootstock and scion was developed, differentiation of the new vascular system began. For this to occur, xylem differentiation and lignification are necessary. Many studies have suggested that peroxidases play a role in lignification (Whetten et al., 1998; Quiroga et al., 2000). During growth development of the stem of herbaceous plants, xylem cells are highly lignified. The results given here show that total peroxidase activity increased during development of control and grafted plants. However, grafted plants showed more activity than controls. Tissue printing of the grafted region demonstrated that peroxidase activity was located mainly in the graft union (Fig. 5A, iii, B, iii and C, iii). These results are in accordance with the increase of lignification observed in the graft union. Histochemical analysis in the grafted region was carried out to analyse lignin synthesis. Weisner staining can be used for specific detection of the hydroxycinnamyl aldehyde end units contained in lignin, which are assembled during the early stages of xylem cell wall lignification (Pomar et al., 2002). It has been observed that xylem differentiation in the graft begins as small areas of lignification 4 d after grafting. At the same stage, peroxidase activity was also increased. These results suggest that active biosynthesis of lignin occurs in the cells in the graft region a few days after grafting. At day 8, a high level of synthesis of lignin was observed in the scion. These new xylem strands in the scion exhibit a radial pattern with a high degree of lignification. This was correlated with a large increase of peroxidase activity in the graft.
Lignification is a process that requires H2O2 and cell wall peroxidases to bring about polymerization of lignin. In addition, H2O2 may serve as an immediate mechanism for disease resistance in response to pathogens and may play an important role in wound‐response and cell apoptosis (Bestwick et al., 1997; Orozco‐Cardenas and Ryan, 1999; Pellinen et al., 2002). Grafted tomato plants showed a significant increase in H2O2 at day 8, and catalase activity increased in parallel. Thus it is considered that catalase could mainly be involved in cellular defence against the high level of production of H2O2 observed at this stage. This H2O2 may have originated from the high level of lignification observed in the graft union during this stage, as xylem cells are actively lignifying their cell walls. A high concentration of H2O2 could be toxic for the parenchymatic cells, inducing the cellular death observed. Guan et al. (2000) have recently observed, in maize plants, that H2O2 mediated catalase gene expression in response to wounding.
Finally, it is considered that the results suggest that peroxidase and catalase activities are spatially and temporally coordinated. However, further study would be required to appreciate the full implications of this for graft development in tomato plants.
Supplementary Material
Received: 2 June 2003; Returned for revision: 12 September 2003; Accepted: 3 October 2003 Published electronically: 20 November 2003
References
- AebiM.1984. Catalase in vitro Methods in Enzymology 105: 121–126. [DOI] [PubMed] [Google Scholar]
- BestwickCS, Brown IR, Bennett MHR, Mansfield JW 1997. Localization of hydrogen peroxide accumulation during the hypersensitive reaction of lettuce cells to Pseudomonas syringae pv phaseolicola Plant Cell 9: 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BilesCL, Martyn RD, Wilson HD.1989. Isozymes and general proteins from various watermelon cultivars and tissue types. Hortscience 24: 810–812. [Google Scholar]
- Fernandez‐GarcíaN, Martinez V, Cerda A, Carvajal M.2002. Water and nutrient uptake of grafted tomato plants grown under saline conditions. Journal of Plant Physiology 159: 899–905. [Google Scholar]
- FrewJE, Jones P, Scholes G.1983. Spectrophotometric determinations of hydrogen peroxide and organic hydroperoxides at low concentrations in aqueous solutions. Analytica Chimica Acta 155: 139–150. [Google Scholar]
- GuanLM, Scandalios JG.2000. Hydrogen peroxide‐mediated catalase gene expression in response to wounding. Free Radical Biology and Medicine 28: 1182–1190. [DOI] [PubMed] [Google Scholar]
- HiragaS, Sasaki K., Ito H, Ohashi Y, Matsui H.2001. A large family of class III plant peroxidases. Plant and Cell Physiology 42: 462–468. [DOI] [PubMed] [Google Scholar]
- JeffreeCE, Yeoman MM.1983. Development of intercellular connections between opposing cells in a graft union. New Phytologist 93: 491–509. [Google Scholar]
- LeeJM.1994. Cultivation of grafted vegetables. 1. Current status, grafting methods, and benefits. Hortscience 29: 235–239. [Google Scholar]
- MooreR.1984. A model for graft compatibility–incompatibility in higher plants. American Journal of Botany 71: 752–758. [Google Scholar]
- OlmosE, Hellin E.1998. Ultrastructural differences of hyperhydric and normal leaves from regenerated carnation plants. Scientia Horticulturae 75: 91–101. [Google Scholar]
- OlmosE, Piqueras A, Martinez‐Solano JR, Hellin E.1997. The subcellular localization of peroxidase and the implication of oxidative stress in hyperhydrated leaves of regenerated carnation plants. Plant Science 130: 97–105. [Google Scholar]
- Orozco‐CardenasM, Ryan CA.1999. Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway. Proceedings of the National Academy of Science USA 96: 6553–6557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PadgettM, Morrison JC.1990. Changes in grape berry exudates during fruit‐development and their effect on mycelial growth of Botrytis cinerea Journal of the American Society for Horticultural Science 115: 269–273. [Google Scholar]
- PellinenRI, Korhonen MS, Tauriainen AA, Palva ET, Kangasjarvi J.2002. Hydrogen peroxide activates cell death and defense gene expression in birch. Plant Physiology 130: 549–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PomarE, Merino F, RosBarcelo A.2002. O‐4‐linked coniferyl and sinapyl aldehydes in lignifying cell walls are the main targets of the Wiesner (phloroglucinol‐HCl) reaction. Protoplasma 220: 17–28. [DOI] [PubMed] [Google Scholar]
- QuirogaM, Guerrero C, Botella MA, Barcelo A, Amaya I, Medina MI, Alonso FJ, deForchetti SM, Tigier H, Valpuesta V.2000. A tomato peroxidase involved in the synthesis of lignin and suberin. Plant Physiology 122: 1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ros‐BarceloA.1998. The generation of H2O2 in the xylem of Zinnia elegans is mediated by an NADPH‐oxidase‐like enzyme. Planta 207: 207–216. [Google Scholar]
- Ruiz‐SifreG, Santiago‐Santos LR, Ramirez‐Ramos LV.1997. Bioregulators and poinsettia plant quality. Journal of Agriculture of the University of Puerto Rico 81: 53–61. [Google Scholar]
- SpruceJ, Mayer AM, Osborne DJ.1987. A simple histochemical method for locating enzymes in plant tissue using nitrocellulose blotting. Phytochemistry 26: 2901–2903. [Google Scholar]
- StoddardFL, McCully ME.1979. Histology of the development of the graft union in pea roots. Canadian Journal of Botany 57: 1486–1501. [Google Scholar]
- TiedemannR.1989. Graft union development and symplastic phloem contact in the heterograft Cucumis sativus on Cucurbita ficifolia Journal of Plant Physiology 134: 427–440. [Google Scholar]
- TurquoisN, Malone M.1996. Non‐destructive assessment of developing hydraulic connections in the graft union of tomato. Journal of Experimental Botany 47: 701–707. [Google Scholar]
- WhettenRW, MacKay JJ, Sederoff RR.1998. Recent advances in understanding lignin biosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 49: 585–609. [DOI] [PubMed] [Google Scholar]
- WhiteJW, Castillo JA.1989. Relative effect of root and shoot genotype in yield of common bean under drought stress. Crop Science 29: 360–362. [Google Scholar]
- YeomanMM, Kilpatrick DC, Miedzybrodzka MB, Gould AR.1978. Cellular interactions during graft formation in plants, are they cognition phenomenon? Symposia of the Society for Experimental Biology 32: 139–160. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.