Abstract
• Background and Aims Trees with a partial leaf-shedding pattern and other morphological features a priori considered intermediate between those of the deciduous Nothofagus antarctica (G. Forster) Oersted and the evergreen N. dombeyi (Mirb.) Oersted (Nothofagaceae) were found in natural stands. The hybridization between a deciduous and an evergreen species of Nothofagus has not been reported so far in natural communities.
• Methods The putative hybrids and the two presumed parental species were compared using 14 enzyme systems as well as shoot, leaf and reproductive morphology.
• Key Results Six enzyme systems showed good resolution (MDH-B, IDH, SKDH, 6-PGDH, GOT and PGI) and in four of them (PGI, MDH-B, SKDH and 6-PGDH) the putative hybrids showed intermediate zymogram patterns between N. antarctica and N. dombeyi. Both principal coordinates analysis on isozyme data and principal components analysis (PCA) on quantitative morphological traits of shoots and leaves separated both parental species and located the putative hybrids closer to N. antarctica than to N. dombeyi. In the PCA, the number of basal cataphylls and the length : width ratio of leaves were the variables most discriminating among shoots of the three entities. The putative hybrids were intermediate between both species regarding leaf vernation, outline and venation, variation in leaf shape (length/width) with position on the parent shoot and in staminate inflorescence and cupule morphology. For other morphological traits, the putative hybrids resembled one of the parental species or differed from both species (e.g. valve morphology).
• Conclusions Isoenzymatic and morphological data sets support the idea of the hybrid nature (probably F1 generation) of the semi-deciduous trees found. Nothofagus antarctica and N. dombeyi are probably more closely related than previously assumed. The relevance of pollen type in revealing evolutionary relationships between Nothofagus species is supported, and that of leaf-shedding pattern is rejected.
Key words: Nothofagus antarctica, Nothofagus dombeyi, hybridization, semi-deciduous, isoenzymes, leaf morphology, reproductive morphology, Patagonia
INTRODUCTION
The genus Nothofagus Blume has a major ecological and economical importance for the temperate forests of the Southern Hemisphere (e.g. McQueen, 1976). Because of its systematic and biogeographical position, this genus is also relevant for the understanding of plant evolution (Hill, 1992; Manos et al., 2001). Nine species of Nothofagus are present in South America (from 36°30′S to 56°S) on both sides of the Andes. In this region, natural hybrids of Nothofagus have been reported between the following pairs of deciduous species: N. obliqua–N. nervosa (Donoso et al., 1990; Gallo et al., 1997), N. obliqua–N. glauca (formerly named N. leoni; van Steenis, 1953; Donoso and Landrum, 1979), and N. antarctica–N. pumilio (van Steenis, 1953; Quiroga et al., 2001), and between the evergreen species N. dombeyi–N. betuloides, N. dombeyi–N. nitida and N. nitida–N. betuloides (Donoso and Atienza, 1983; Premoli, 1996). In Nothofagus, hybridization seems to occur only between species sharing the same type of pollen. The significance of the traditional pollen type grouping in the systematics of Nothofagus [initially proposed in Praglowski (1982) and modified in Dettmann et al. (1990)], was reconciled some years later by the congruent infrageneric classification proposed by Hill and Read (1991) and by the cladistic analysis performed by Hill and Jordan (1993). The hybridization between a deciduous and an evergreen species of Nothofagus has not been reported so far in natural communities, even though N. obliqua (a deciduous species from South America) and N. menziesii (an evergreen species from New Zealand) were found to hybridize under cultivation in England (Wingston, 1979).
Recent studies suggest that hybrid Nothofagus trees may have good potential as timber producers. For instance, height growth has been reported to be superior for individuals resulting from the hybridization between N. nervosa and N. obliqua than for the parental species (Gallo et al., 1997), although fitness of natural hybrids might be reduced by post-zygotic barriers (e.g. sensibility to frost; Gallo, 2002). In addition, genetic diversity in N. nervosa has been found to be higher in populations with more frequent hybridization (Marchelli and Gallo, 2001, 2002). Recently, the importance of natural hybridization in maintaining intra-generic genetic variation in Nothofagus has been discussed and a hybridization model for two of its species has been suggested (Gallo, 2002).
Study species and putative hybrids
Nothofagus antarctica (G. Forster) Oersted (Nothofagaceae), an autumn–winter deciduous species found from 37°S to 56°S, may reach 15 m in height though frequently develops into 2–3 m tall bushes (e.g. Dimitri, 1972). It is considered the South American Nothofagus species with the highest levels of morphological variation (e.g. Romero, 1980) and ecological tolerance (Donoso, 1993) and inhabits relatively dry areas, on hydromorphic soils, subalpine communities and cool valley bottoms (Dimitri, 1972). Nothofagus dombeyi (Mirb.) Oersted, an evergreen species, may be found between 39°S and 46°S and grows as a tree up to 50 m high (Dimitri, 1972). It is a major component of several forest types and inhabits well-drained sites at relatively low altitude and with precipitation levels relatively high for northern Patagonia (>700 mm annually; Veblen et al., 1996).
Nothofagus antarctica and N. dombeyi co-occur in valleys usually below 1000 m in elevation, frequently close to rivers or lakes (McQueen, 1976). However, truly mixed N. antarctica–N. dombeyi forests are uncommon. Although both species are clearly distinguished morphologically (Dimitri, 1972; Correa, 1984), they share many developmental features. For instance, young trees of both species have a similar pattern of differentiation among axis types (i.e. trunk, main branches, secondary branches and short branches; Barthélémy et al., 1999; Stecconi et al., 2001) and a similar pattern of shoot morphology variation with axis age (Barthélémy et al., 1997). In addition, both species belong to the same pollen type (Nothofagus fusca type b; Dettmann et al., 1990).
In the present study, Nothofagus antarctica, N. dombeyi and putative hybrid individuals between these species found in sympatric areas were compared by means of both genetic and morphological traits.
MATERIALS AND METHODS
Location and characteristics of putative hybrids
Ten trees with morphological features a priori considered intermediate between those of N. antarctica and N. dombeyi were found between July and August 2001 (winter). By then these trees had shed only part of their foliage and were bearing both green and yellow or red leaves (Fig. 1). They inhabited two sympatric areas within the Nahuel Huapi National Park, Argentina: Cerro Otto (three individuals), within the township of San Carlos de Bariloche (41°08′S, 71°20′W, 900 m a.s.l.); and Estancia La Primavera (seven individuals), near the town of Villa Traful (40°41′S, 71°16′W, 850 m a.s.l.). At Cerro Otto, the putative hybrids occur in a N. antarctica–Austrocedrus chilensis (D. Don) Pic.-Serm. et Biz. forest affected by human activities (tree-felling, fires); individuals of N. dombeyi are present about 2000 m away from the putative hybrids. At Estancia La Primavera, the putative hybrids were found in a valley bottom within an open woodland dominated by N. antarctica and A. chilensis and affected by livestock grazing; N. dombeyi trees are present at about 300 m distance from the putative hybrids. At both sites, the putative hybrids were up to 50 m apart from each other, scattered among N. antarctica trees and shrubs. The putative hybrids ranged between 2·1 and 13·5 m in height, between 2·6 and 28·3 cm in dbh (diameter at breast height) and between 15 and 31 years in age. Two of these trees (both from La Primavera) developed staminate and pistillate inflorescences during the 2001–2002 and 2002–2003 growth periods.
Fig. 1.
Putative hybrid trees with yellow leaves at Estancia La Primavera in winter (centre). Part of a leafless individual of N. antarctica and evergreen individuals of Austrocedrus chilensis (Cupressaceae) may be seen in the left foreground.
The putative hybrids were compared with the presumed parental species, N. antarctica and N. dombeyi, by using: (a) vegetative morphology, (b) reproductive morphology and (c) isoenzymatic traits.
Vegetative morphology
For 12 individuals of N. dombeyi and N. antarctica and eight putative hybrids (two of them were not included because of their small size), the most distal (last produced) shoot of three to ten main branches was sampled at the end of the 2001–2002 growing season. The sampled N. dombeyi trees were located within 2-km radius from the putative hybrids whereas the sampled N. antarctica trees were located in proximities of the putative hybrids, at Cerro Otto and La Primavera. For the sake of shoot size homogeneity, only shoots with more than nine green leaves and lacking sylleptic branches were selected (see Puntieri et al., 1998; Barthélémy et al., 1999). Stem length (to the nearest 1 mm with a ruler) and proximal-end diameter (to the nearest 0·1 mm with digital callipers), number of cataphylls (counted from scars on the stem) and green leaves, and the length, maximum width and one-side surface area of each lamina (hereafter referred to as leaf area; obtained with Scion Image Beta 4·02 after shoot scanning) were recorded for each shoot. The relationship between length and width was computed for each lamina as a measure of leaf shape.
The nodes of each shoot were numbered correlatively starting with one for the most proximal node. Leaf area and length : width ratio were averaged for each position and each biological entity (N. dombeyi, N. antarctica and the putative hybrids), considering separately shoots with up to 15 nodes and shoots with more than 15 nodes (shoots with a different number of nodes may also differ in average leaf size and shape; Puntieri et al., 2001). The presence of a cataphyll in a node was computed as a zero for the surface area of each leaf, and as a missing value for the length/width relationship. [Cataphylls of Nothofagus are leaves which lack a lamina or have a tiny, short-lasting lamina (Puntieri et al., 1998; Barthélémy et al., 1999).] Data concerning fallen or damaged leaves were considered missing.
Principal components analysis (PCA) was performed for all shoots sampled for the three entities (N. dombeyi, 74 shoots; N. antarctica, 90 shoots; putative hybrids, 66 shoots), including the following quantitative variables: (1) stem diameter, (2) stem length : diameter ratio, (3) stem length : number of nodes ratio, (4) number of cataphylls, (5) area of the leaf in position 8 (the leaf in position 7 or 9 was used when leaf 8 was missing), (6) length : width ratio of the leaf in position 8, (7) position of the leaf of maximum leaf area relative to the number of nodes of the shoot and (8) position of the leaf of maximum length : width ratio relative to the number of nodes of the shoot. These variables were selected among those that, according to previous studies on Nothofagus, are useful descriptors of shoot morphology (Puntieri et al., 2001, 2003). Other variables highly correlated with variables (1) to (8) were excluded from this analysis. Variables (1) to (8) were compared with analysis of covariance (ANCOVA) with two factors and one covariable (Sokal and Rohlf, 1981). The factors included in these analyses were biological entity and tree of origin of the shoot (eight trees per entity, nested within each entity); the number of nodes of the shoot was the covariable.
The following leaf attributes of qualitative assessment, employed in other studies to differentiate N. dombeyi and N. antarctica (e.g. Philipson and Philipson, 1988; Hill and Read, 1991; Gandolfo and Romero, 1992), were observed for leaves of the three entities: vernation, time of persistence on the stem, outline, bilateral symmetry, base form, margin, dentation and texture of the leaf lamina; the relative thickness, orientation and termination of secondary veins at the margin; and the presence and type of inter-secondary veins (following Hickey, 1973). Leaf vernation was observed in at least ten buds per entity, manually dissected under a stereomicroscope. These data were obtained from the shoots sampled for the quantitative assessment of leaf morphological attributes, and complemented with other observations of fresh material along the year.
Reproductive morphology
The number of flowers per dichasium of staminate flowers, and the total length, outline and number of lamellae of fully lignified cupule valves of female inflorescences were compared among the three entities (N. dombeyi, six individuals; N. antarctica, six individuals; putative hybrids, two individuals). The material studied had been collected at the sites mentioned in Table 1. For scanning electron microscopy, staminate inflorescences and mature cupules were fixed in FAA at the site of collection, dehydrated in increasing concentrations of ethanol and critical-point dried using a Balzers SCD 030. The samples were mounted onto aluminium stubs, coated with gold and examined using a Jeol JSMII scanning electron microscope. Fertile specimens examined are deposited at the herbaria of Universidad de Buenos Aires (BAFC Picca 6, 27, 54, 63–65, 69, 86–88, 110, 143, Argentina).
Table 1.
Geographic location of the collection sites for the genetic and reproductive morphological characterization of the parental species
| Genetic |
Latitude |
Longitude |
Reproductive |
Latitude |
Longitude |
|---|---|---|---|---|---|
| Villarino | 40°27′ | 71°32′ | Currhué Grande | 39°50′ | 71°31′ |
| Correntoso | 40°38′ | 71°39′ | Lolog | 40°01′ | 71°20′ |
| Espejo | 40°41′ | 71°41′ | San Martín de los Andes | 40°09′ | 71°21′ |
| La Primavera | 40°42′ | 71°16′ | Chapelco | 40°14′ | 71°16′ |
| Guillelmo | 41°22′ | 71°30′ | Meliquina | 40°19′ | 71°23′ |
| La Primavera | 40°42′ | 71°16′ | |||
| Culebras | 41°15′ | 71°23′ | |||
| Roca | 41°22′ | 71°45′ |
Isoenzymatic traits
To characterize the parental species and compare the putative hybrids with them, samples from five different locations where both species coexist in sympatry were taken (Table 1). In each site, buds from 20–29 individuals of each species were collected and kept at −20 °C until electrophoretical analysis was carried out. A total of 106 individuals of N. dombeyi and 123 of N. antarctica were screened. Buds from the ten putative hybrids were also obtained and treated in the same way.
Bud tissue was homogenized and proteins extracted with the vegetative extraction buffer I from Cheliak and Pitel (1984). Wicks of 10 × 3 mm were imbibed and conserved at −20 °C until electrophoresis was carried out. Isozymes were separated by horizontal starch gel electrophoresis following the same conditions described in Marchelli and Gallo (2000). Two buffer systems were employed: (1) electrode 0·13 m Tris–0·04 m citric acid pH 7; gel diluted electrode buffer (1 : 2·5) for 4 h at 180 mA; (2) electrode 0·3 m boric acid–0·06 m NaOH pH 8·2 (Poulik, 1959); gel 0·07 m Tris–0·008 m citric acid pH 8·7 for 5 h at 65 mA. Fourteen enzyme systems were scored: ADH (EC 1.1.1.1), FUM (EC 4.2.1.2), IDH (EC 1.1.1.42), MDH (EC 1.1.1.37), 6-PGDH (EC 1.1.1.44) and SKDH (EC 1.1.1.25) with buffer system (1); DIA (EC 1.6.4.3), GDH (EC 1.4.1.3), GOT (EC 2.6.1.1), NADHDH (EC 1.6.99.3), PER (EC 1.11.1.7), PGM (EC 2.7.5.1) and PGI (EC 5.3.1.9) with buffer system (2) and MR (EC 1.6.99.2) with both systems. Staining solutions were prepared according to Cheliak and Pitel (1984) with slight modifications.
Zymograms were divided into zones within which the observed phenotypic variation was supposed to be controlled by a one-gene locus. The banding patterns were compared with those of related species for which genetic analyses were carried out in previous studies (e.g. N. nervosa, Marchelli and Gallo, 2000; N. obliqua, M. M. Azpilicueta and L. Gallo, INTA, Bariloche, Argentina, unpubl. res.) and also with putative loci scored in N. dombeyi (Premoli, 1997). These comparisons showed a consistent pattern since the same zones were detected in the zymograms for the same tissue among the compared species. For example, the PGI system displayed two zones with similar pattern of variation (zone A monomorphic and zone B polymorphic) in all species compared (N. nervosa, N. obliqua, N. dombeyi and the three entities analysed in this study). According to this, the observed zymogram variation was considered to be under strong genetic control and treated as genetic markers. Each variation zone within a zymogram was considered as a putative locus and therefore the observed bands within each zone were considered as putative alleles. Enzyme systems were designated with their conventional abbreviation and zones in decreasing order of relative mobility, both in capital letters, and putative alleles with numbers in decreasing order of relative mobility.
Allele frequencies, proportion of polymorphic loci (P), considering polymorphic those loci with at least two variants, regardless of its frequency (Berg and Hamrick, 1997), mean number of alleles per locus (AL), genetic diversity (ν = effective number of alleles; Gregorius, 1978), observed and expected heterozygosities (Ho and He respectively; Nei, 1973) and Gregorius' genetic distances (d0; Gregorius, 1974) were calculated for both parental species and the putative hybrids.
Principal coordinates analysis (PCoA) was performed including the individuals of the three entities (N. dombeyi, 106 individuals; N. antarctica, 122 individuals; putative hybrids, 10 individuals) taking into account 21 qualitative variables. These variables were scored as presence or absence of the 21 alleles from the six more informative isozyme loci: MDH-B (four alleles), IDH (two alleles), SKDH (four alleles), GOT-B (two alleles), GOT-C (three alleles) and PGI (six alleles). Besides, after testing homogeneity in allelic frequencies among collection sites for each parental species with a chi-squared goodness-of-fit test, cluster analysis (UPGMA method) using Gregorius' genetic distance was performed for the different collection sites and the putative hybrids. Differences in genetic distance were tested (α = 0·05) using the program GDA-NT (Genetic Data Analysis and Numerical Tests, with 500 permutations; B. Degen, Institute for Forest Genetics, Grosshansdorf, Germany, unpubl. res.). Two computational programs were employed for the calculation of the different genetic parameters: GSED (Genetic Structures from Electrophoresis Data; Gillet, 1994) and POPGENE (Yeh and Boyle, 1997). An error probability (α) of 0·05 was used in all comparisons unless otherwise specified.
RESULTS
Vegetative morphology
In shoots of all three entities, leaf area increased from the proximal end of the shoot towards intermediate positions and decreased from intermediate to distal positions (Fig. 2A and B). For N. antarctica, shoots with more than 15 nodes had larger leaves than those with fewer nodes; the incidence of shoot size on leaf area was less notable for the other two entities (Fig. 2A and B). The largest leaf per shoot had a lower area and a more distal position for the putative hybrids than for N. dombeyi and N. antarctica (Fig. 2A and B; Table 2). Leaf area for leaves in intermediate and distal positions tended to be higher for N. antarctica than for N. dombeyi and the putative hybrids (Fig. 2A and B).
Fig. 2.
Variations in surface area (A and B) and length : width ratio (C and D) of the lamina as related to leaf position on the shoot (starting at the most proximal node). Data corresponding to shoots with up to 15 nodes (A and C) and shoots with more than 15 nodes (B and D) of N. antarctica (white squares), N. dombeyi (black circles) and putative hybrids between these species (grey triangles) are shown separately.
Table 2.
Mean (±s.e.) for quantitative morphological traits of shoots of N. antarctica, N. dombeyi and their putative hybrids and Fisher's F for the effects of entity, sampled tree (factors) and number of nodes (covariable)
| Mean ± s.e. |
Effects of factors and covariable |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
N. antarctica |
Putative hybrids |
N. dombeyi |
Entity |
Tree |
No. of nodes |
|||||
| Stem diameter (mm) | 2·6±0·04 | 2·1±0·05 | 2·8±0·05 | 103·4 | 4·5 | 102·7 | ||||
| Stem length/diameter | 5·7±0·12 | 5·9±0·19 | 6·4±0·17 | 13·5 | 4·9 | 156·0 | ||||
| Stem length/number of nodes | 0·8±0·01 | 0·8±0·02 | 1·0±0·02 | 105·4 | 6·2 | 65·3 | ||||
| Number of cataphylls | 3·8±0·05 | 2·9±0·09 | 2·1±0·04 | 105·2 | 5·9 | 10·6 | ||||
| Area of 8th leaf (cm2) | 4·3±0·17 | 2·3±0·08 | 3·1±0·10 | 55·4 | 18·3 | 8·1 | ||||
| Length : width ratio of 8th leaf | 1·6±0·02 | 1·7±0·02 | 1·9±0·04 | 100·5 | 26·3 | 13·9 | ||||
| Position max. leaf area | 0·4±0·01 | 0·5±0·02 | 0·4±0·01 | 12·3 | 2·2 | 6·6 | ||||
| Position max. length/width | 0·7±0·02 | 0·8±0·02 | 0·8±0·02 | 5·6 | 2·5 | 7·2 | ||||
The mean size (diameter), thickness (length/diameter), length of internodes (length/number of nodes) and number of cataphylls of the shoots; the size (leaf area) and form (length/width) of the leaf on position 8 and the position (relative to the number of nodes of the shoot) of the largest leaf (with highest leaf area and length/width) on the shoot are shown. n = 176 shoots for each comparison. All F values are significant (P ≤ 0·05).
In all three entities, the leaf length : width ratio tended to increase from proximal to intermediate leaves and to remain relatively constant for intermediate and distal leaves (Fig. 2C and D). For both shoot sizes considered, the length : width ratio differed between entities. The maximum value for this relationship per shoot was notably higher for N. dombeyi than for the putative hybrids and N. antarctica (Fig. 2C and D; Table 2).
The first two axes of PCA explained, respectively, 30 % (eigenvalue = 2·4) and 24 % (eigenvalue = 1·9) of the variation among shoots. The factors with the highest absolute coefficient for the first axis were: the length : number of nodes ratio, the length : diameter ratio and the stem diameter; and those for the second axis were: the area and the length : width ratio of the leaf in position 8 and the number of cataphylls (Fig. 3A). Shoots corresponding to the three entities could be differentiated by plotting first vs. second axis, mostly due to the variations in the number of cataphylls (N. antarctica > putative hybrids > N. dombeyi) and the length : width ratio of the leaf in position 8 (N. dombeyi > putative hybrids > N. antarctica). The means of all variables included in the PCA (even those with lower absolute coefficients in this analysis) differed significantly among entities (Table 2). Stems of N. dombeyi shoots were thicker and had higher length : diameter and length : number of nodes ratios than those of the other two entities. The leaf in position 8 was larger for N. antarctica, intermediate for N. dombeyi and smaller for the putative hybrids. The leaf of maximum area had a more distal position in the putative hybrids than in either of the two parental species, whereas the leaf of maximum length : width ratio was more proximal in shoots of N. antarctica than in those of the other two entities (Table 2). The effect of tree of origin of the shoot was significant for all variables, especially (as indicated by the value of F) the size and form of the leaf in position 8. The number of nodes of the shoot also had a significant effect on the variation of all variables, in particular stem diameter and length : diameter ratio (Table 2).
Fig. 3.

Ordination of shoots of N. dombeyi (black circles), N. antarctica (white squares) and the putative hybrids between these species (grey triangles) along the first two axes of PCA on morphological traits (A) and PCoA on genetic traits (B; alleles from isozyme loci MDH-B, IDH, SKDH, GOT-B, GOT-C and PGI). The weights of the most discriminating variables on each PCA axis are shown with arrows (‘p.rel Amax’ and ‘p.rel L/W max’ = position of the leaf with maximum area or length : width ratio, respectively, relative to the number of nodes of the shoot; ‘area P8’ and ‘length/width P8’ = area or length : width ratio, respectively, of the leaf in position 8). Due to the high homogeneity among N. antarctica individuals (n = 122) and also among N. dombeyi (n = 106) in B, points are overlapped.
Data concerning qualitative leaf morphology are synthesized in Fig. 4 and Table 3. In N. antarctica trees all leaves are shed in autumn. In N. dombeyi most leaves produced in one spring–summer period stand at least until the following growing season. The putative hybrids shed many leaves in autumn and retain fresh-looking green and yellow or red leaves until after bud opening in the following spring (Fig. 1). This leaf-shedding pattern may be qualified as semi-deciduous.
Fig. 4.
Representative shoots, leaves, cross-section of a bud (two leaf primordia, central shoot axis and glands) and leaf margin (details) of N. dombeyi, N. antarctica and a putative hybrid tree. The arrows indicate the proximal end of each shoot. For the sake of clarity, leaves have been forced with their apex away from the stem. S.V., secondary vein.
Table 3.
Qualitative comparison of morphological and isoenzymatic traits between N. antarctica, N. dombeyi and putative hybrids
|
N. antarctica |
Putative hybrids |
N. dombeyi |
||||
|---|---|---|---|---|---|---|
| Vernation |
Notably folded |
Partially folded |
Plane |
|||
| Lamina | ||||||
| Outline | Ovate | Ovate to lanceolate | Ovate to lanceolate | |||
| Bilateral symmetry | Notably asymmetrical (symmetrical) | Asymmetrical (symmetrical) | Asymmetrical (symmetrical) | |||
| Base | Cordate to straight | Obtuse to cuneate | Obtuse to cuneate | |||
| Dentation | Composed (simple) | Simple (composed) | Simple (composed) | |||
| Margin | Crenate-dentate (serrate) | Serrate | Serrate | |||
| Types of teeth | A1 (B1) | B1 (A1) | B1 (A1) | |||
| Texture |
Chartaceous |
Chartaceous to coriaceous |
Coriaceous |
|||
| Secondary veins | ||||||
| Thickness | Thick | Intermediate | Thin | |||
| Trajectory | Uniformely curved | Straight | Straight to abruptly curved | |||
| Angle variation | Dimishing to the apex | Uniform | Increasing to the apex | |||
| Division | At some distance from the margin | Close to the margin | Close to the margin | |||
| Inter-secondary veins | Absent | Present: simple | Present: composed | |||
| Staminate dichasia |
One-flowered |
One- to three-flowered |
Three-flowered |
|||
| Cupule | ||||||
| Valve outline | Narrowly ovate | Ovate to lanceolate, constricted | Narrowly lanceolate | |||
| Lamellae outline |
Widely ovate |
Ovate |
Lanceolate |
|||
| Isoenzymatic traits | ||||||
| MDH-B* | 2 | 2 and 3 | 3 | |||
| MDH-C* | 1 | 1 | 1 | |||
| IDH† | 1 | 1 | 2 | |||
| SKDH* | 2 | 2 and 3 | 3 | |||
| PGI* | 2 | 2 and 3 | 3 | |||
| GOT-B* | 2 | 2 | 2 | |||
| GOT-C* | 2 | 2 | 2 | |||
| 6-PGDH‡ | 2 and 3 | 1 and 3 | 1 and 2 | |||
Leaf traits are described following Hickey (1973). Conditions in parenthesis are less common than those preceding them.
Most frequent allele.
Monomorphic loci with different banding pattern.
Most frequent banding patterns.
In the putative hybrids leaf primordia folds decrease in depth from the margins to the mid-vein, whereas in N. antarctica, leaf primordia folds are always deep. Putative hybrids may be considered to have a vernation pattern intermediate between those of N. dombeyi and N. antarctica (Table 3; Fig. 4).
Reproductive morphology
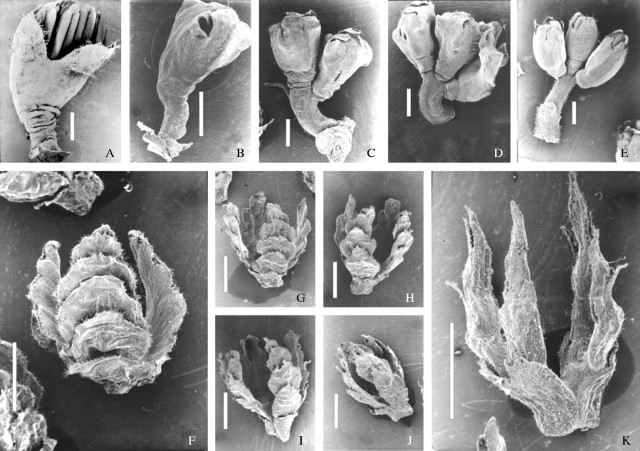
The staminate inflorescences of N. antarctica are one-flowered and those of N. dombeyi are three-flowered in the material studied (Fig. 5A and E; Table 3). In the putative hybrid trees, staminate inflorescences have one to three flowers (Fig. 5B–D). In all three entities, a group of three nuts is subtended by a four-valved lignified cupule. Each valve has four or five longitudinally aligned dorsal lamellae (Fig. 5F–K). Only cupule length and valve shape show differences among the three taxa. In the putative hybrids cupule length varies from 4·9 to 7·2 mm. In the case of N. antarctica and N. dombeyi this value ranges between 4·0 and 5·0 mm and between 4·5 and 5·2 mm, respectively. Variation in valve shape was found within each of the two fertile putative hybrids (Table 3), although all of these cupules tended to have valves and lamellae somewhat wider than those of N. dombeyi and narrower than those of N. antarctica (Fig. 5F–K). Besides, notable constrictions at the level of lamellae attachment are evident in each valve only in the putative hybrids (Fig. 5G–J; Table 3).
Fig. 5.
SEM micrographs of staminate inflorescences (A–E) and cupules (F–K) of N. antarctica (A and F), N. dombeyi (E and K), and putative hybrid trees (B–D and G–J). Bars: A–E = 1 mm; F–K = 2 mm.
Isoenzymatic traits
Six of the enzyme systems analysed showed good resolution in both species and the putative hybrids (MDH, IDH, SKDH, 6-PGDH, GOT and PGI), allowing the screening of eight putative loci. Given the complexity of 6-PGDH zymograms and the lack of genetic analysis of the observed phenotypic variation, this enzyme was only considered at a qualitative level.
Some differences among the banding patterns of the three entities were found (Table 3). The putative hybrids were intermediate between the parental species in four loci: PGI, MDH-B, SKDH and 6-PGDH. The most diagnostic enzymes were PGI and MDH-B, with a different most-frequent allele in each species and heterozygous patterns in the putative hybrids. SKDH showed the same alleles in both species with differences in their frequencies; all the putative hybrids had a heterozygous banding pattern. In the case of GOT-B, the same two alleles were present in similar frequencies both in N. antarctica and N. dombeyi, but greater heterozygosity was observed in the putative hybrids (Table 4). MDH-C was monomorphic for the same allele both in N. antarctica and the putative hybrids, but a rare allele appeared in N. dombeyi. Finally, IDH was monomorphic in the three entities, but with different banding patterns; the putative hybrids had a zymogram similar to that of N. antarctica.
Table 4.
Gene pool genetic diversity and genetic distances in N. antarctica, N. dombeyi and their putative hybrids
|
d0* |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Species |
N |
P |
AL |
ν |
Ho |
He |
Putative hybrids |
N. dombeyi |
|
| N. antarctica | 103·9 | 71·4 | 2·29 | 1·142 | 0·115 | 0·125 | 0·157 | 0·467 | |
| Putative hybrids | 8·7 | 57·1 | 2·00 | 1·359 | 0·400 | 0·264 | – | – | |
| N. dombeyi | 92·6 | 87·7 | 3·00 | 1·209 | 0·118 | 0·173 | 0·364 | – | |
N, mean number of individuals analysed; P, percentage of polymorphic loci; AL, mean number of alleles per locus; ν, genetic diversity (Gregorius, 1978); Ho and He, observed and expected heterozygosities, respectively; d0, Gregorius' genetic distance.
All pairwise genetic distances are significantly different (P ≤ 0·05).
The first two axes of the PCoA explain 69 % (eigenvalue = 40·0) and 9 % (eigenvalue = 5·0) of the variation, respectively. The highly discriminant power of the first coordinate axis clearly separated N. dombeyi from the group formed by N. antarctica and the putative hybrids, with the latter two entities overlapped (Fig. 3B). A higher degree of overlapping was found among N. antarctica than among N. dombeyi individuals. The second axis did not discriminate the putative hybrids from N. antarctica.
In agreement with the PCoA, allele frequencies were homogeneous among sites for each parental species and for six of the seven loci analysed (locus SKDH was the exception), thus allowing the treatment of the five sites as a pool for comparing the parental species and the putative hybrids. The latter showed a lower percentage of polymorphic loci than N. antarctica and N. dombeyi and a lower mean number of alleles per locus, but presented a higher genetic diversity and a considerably higher heterozygosity, both observed and expected, than the parental species (Table 4).
Genetic distance was high between N. antarctica and N. dombeyi, intermediate between N. dombeyi and the putative hybrids and low between N. antarctica and the putative hybrids (Table 4). Notwithstanding, all pairwise genetic distances were significantly different. The cluster analysis showed a clear separation between the two species, and a position of the putative hybrids closer to N. antarctica than to N. dombeyi, also in agreement with the PCoA (Fig. 6).
Fig. 6.
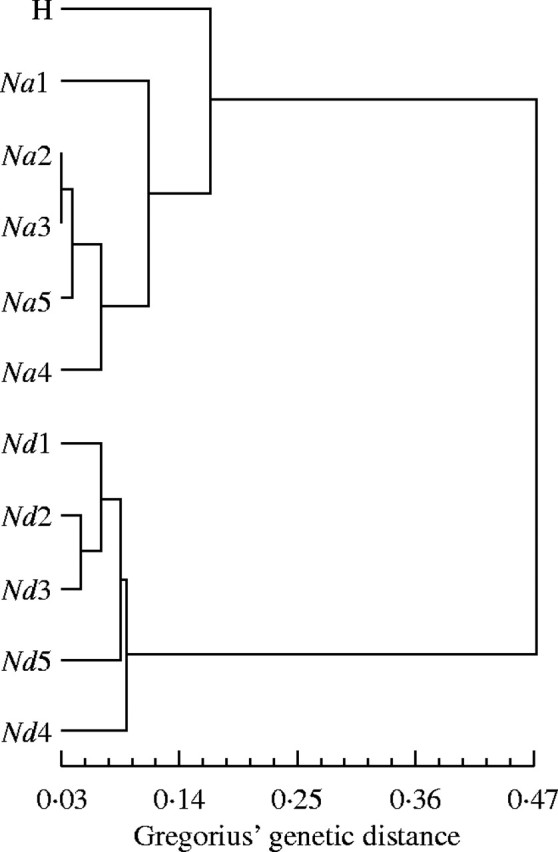
Cluster diagram of five sampled populations of each species and the putative hybrids according to the UPGMA method using Gregorius' genetic distances. Na, Nothofagus antarctica; Nd, N. dombeyi; H, putative hybrids. The numbers correspond to collection sites: 1, Correntoso; 2, Villarino; 3, Traful; 4, Espejo; 5, Guillelmo.
DISCUSSION
Hybridization between N. antarctica and N. dombeyi
Morphological traits are normally considered to be controlled by few or many loci. The identification of hybrids by means of morphological traits is unreliable because of the environmental, dominant and epistatic effects on these oligogenic or polygenic characters. However, they provide very useful evidence of the role of hybridization in the origin of evolutionary novelties (Rieseberg and Carney, 1998). On the other hand, genetic markers can provide undoubted proofs of hybridization, but usually lack information on the adaptive features of hybrids. Therefore, the combined analysis of both kinds of traits should be the best strategy to identify and understand hybridization.
The availability of species-specific genetic markers is the uttermost condition to prove the existence of hybridization and to monitor it with precision (Gallo et al., 1997; Gallo, 2002). Among the eight allozymes analysed, two showed diagnostic alleles: MDH-B and PGI. These enzymes had different alleles in each parental species in frequencies close to fixation while the putative hybrids were heterozygous. This evidence strongly supports the hybrid condition of the intermediate individuals, which are very likely F1 hybrids. Therefore we will refer to them as hybrids between N. antarctica and N. dombeyi. Seventeen out of 18 vegetative morphological features compared were informative since they showed differences among the entities (bilateral symmetry of the lamina exhibited much overlapping); in nine of them the hybrids were intermediate between the parental species. This is in agreement with most F1 hybrids that usually present a mosaic of parental and intermediate morphological characters, due to the effect of dominance (Rieseberg and Ellstrand, 1993).
Morphological and genetic traits of hybrids and parental species
According to taxonomic studies, N. antarctica and N. dombeyi trees differ in leaf-shedding pattern, leaf, flower and cupule morphology and tree habit (e.g. Dimitri, 1972; Correa, 1984). The most evident morphological trait to characterize hybrids between these species is leaf lifespan. In the hybrid trees observed, leaves at different stages of abscission, as well as green leaves, were observed throughout the year, and the proportion and position of autumn-shed leaves varied both within- and between-trees. The fact that these trees shed some of their senescent leaves in autumn, while retaining leaves with different pigmentation, may suggest alterations in the genetic factors related to leaf abscission (see Kozlowski and Pallardy, 1997, and references therein). These features shared by the ten Nothofagus hybrid trees described here are unusual for temperate trees (see Kozlowski, 1971), and would enhance the value of these trees as ornamentals.
In Nothofagus spp., leaf vernation is plicate in deciduous species and plane in evergreen species (Philipson and Philipson, 1979; Tanai, 1986). The intermediate vernation reported here for the hybrids agrees with their semi-deciduous character. This correspondence between vernation and leaf lifespan could be related to the positive relationships among leaf primordia thickness, their tendency not to be folded within the bud and the time of persistence of the resulting leaf.
Morphological features such as leaf size and form, usually employed to differentiate Nothofagus species (e.g. Dimitri, 1972; Correa, 1984), vary according to endogenous species-specific gradients. The gradients of variation in leaf area and shape with leaf position on the parent shoot were, in general terms, similar for all three entities compared. The hybrids exhibited leaf area values more similar to those of N. dombeyi than to those of N. antarctica for each position on the shoot, but tended to be intermediate between both species in leaf shape for each position (though more similar in this respect to those of N. antarctica), thickness and trajectory of secondary veins and types of teeth. Hybrids resembled N. dombeyi concerning the ending of secondary veins and presence of inter-secondary veins. The present data agreed with those of previous studies regarding leaf-architecture differences between N. antarctica and N. dombeyi, although more intra-specific variation was found than in the previous studies (Romero, 1980; Gandolfo and Romero, 1992). The endogenous gradients of variation in leaf architecture would need more detailed studies. The use of average values for morphological traits and herbarium specimens (which usually represent an unknown proportion of a species' morphological variation) has reduced the discriminating power of morphological traits in previous comparative studies within and between species of Nothofagus. Multivariate analyses including habit, leaf, flower and fruit information have been applied, in combination with genetic analyses, to differentiate botanical entities (e.g. Manos, 1997; Bartish et al., 2002; Olson, 2002; Rumpunen and Bartish, 2002). Nevertheless, most of these studies (and certainly all of those about Nothofagus) do not explore the discriminating power of either shoot morphology or the endogenous gradients of variation in leaf morphology. In the present study, the number of cataphylls per shoot, the size and shape of the leaf in a given position and, to a lesser extent, stem diameter, slenderness and internode length, enabled all three Nothofagus entities to be distinguished and explained some of the morphological variation among shoots. In this respect, the higher morphological variation among N. dombeyi shoots and the clearer distinction between this species and both N. antarctica and the hybrids are consistent with the results of genetic data. The possible adaptive relevance of shoot morphological features assessed in the present study has been mentioned in a recent comparative study (Puntieri et al., 2003).
Some reproductive morphological traits, for which N. dombeyi and N. antarctica are clearly different, exhibit, for the hybrids, a wide range of variation which overlaps at each end with one of the parental species. Such is the case for the morphology of cupules and staminate inflorescences. In the latter case, the staminate dichasia are one-flowered in N. antarctica and three-flowered (exceptionally one- or two-flowered) in N. dombeyi (Picca, 1998), whereas the two flowering hybrid trees observed show a singular variation from one- to three-flowered. The hybrids would be differentiated clearly from the parental species by notable constrictions at the level of lamellae attachment to the valve that are only present in the hybrids. Cupule valves of these hybrids vary considerably in length but, except for isolated cases, are longer than those of N. dombeyi and N. antarctica. The hybrids and the parental species do not differ significantly in some important qualitative taxonomic characters broadly employed in Nothofagus systematics, such as the number of bracts of the cupule, the number of nuts per cupule, the number of female flowers per dichasium and the presence of glands in the lamellae, which reflects the close affinities among these three taxa (Hill and Read, 1991; Manos et al., 2001).
Isoenzymatic traits analysed either with PCoA or UPGMA clustering separated the two parental species and clustered the hybrids in a position closer to N. antarctica than to N. dombeyi. This grouping pattern was more notable than that resulting from PCA on morphological traits. The two analysed loci which clearly differentiated the three entities (MDH-B and PGI, see above) presented species-specific alleles or nearly diagnostic alleles among other hybridizing Nothofagus spp. (Gallo et al., 1997; Premoli, 1997; Marchelli and Gallo, 2000). The plausible link between these enzymes and adaptive traits could suggest that different directional selection forces may be acting upon each species under its particular set of environmental conditions. The higher levels of gene pool heterozygosity and genetic diversity observed among the intermediate individuals are also indicative of their hybrid origin, as in the case of other Nothofagus species in which a heterosis effect was argued (Gallo et al., 1997; Gallo, 2002).
Hybrids of Nothofagus and evolutionary relationships
The existence of hybrids between South American Nothofagus species has been highlighted in a number of studies (e.g. Tuley, 1980; Donoso et al., 1990; Gallo et al., 1997). None of these studies indicated the possibility of hybridization between the evergreen N. dombeyi and the deciduous N. antarctica. All individuals described here as hybrids were found in plant communities frequently visited by people and affected by livestock, tree logging and fires for a long time (Dimitri, 1972), where N. antarctica was among the dominant tree species and N. dombeyi trees were at least 200 m away. After the data analyses for the present study had been finished, six more individuals with features similar to those of the hybrids were observed in disturbed N. antarctica–A. chilensis woodlands. The limited capacity of dispersal of Nothofagus seeds (Donoso, 1993) suggests that the hybrids could have resulted from the pollination of N. antarctica pistillate flowers with pollen from N. dombeyi trees.
Hybridization is mentioned as one of the causes of speciation in plants through the reinforcement of pre-zygotic and post-zygotic reproductive barriers between two species. This is theoretically difficult since it requires the development of reproductive isolation in sympatry (Rieseberg, 1997). The occurrence of hybrids between N. antarctica and N. dombeyi suggests that pre-zygotic barriers are not strong enough or can be surpassed in certain cases. Moreover, the development of flowers and the production of fruits by two of the hybrids indicate that post-zygotic barriers are also weak. A certain equilibrium in the generation and reproductive success of natural hybrids could be maintained indefinitely as a potential intra-generic genetic variation, as suggested by Gallo (2002) for another Nothofagus hybridization system. Future studies on seed development and viability would shed light on this issue.
The close evolutionary affinity of N. antarctica, N. pumilio and N. dombeyi has been supported by a number of studies on Nothofagus (e.g. Jordan and Hill, 1999; Manos, 1997). The former two species share some features easily detectable by the human eye, such as the deciduous habit, leaf outline and dentation, and thickness of secondary leaf veins. This, together with the knowledge of occurrence of hybrids between them (van Steenis, 1953; Quiroga et al., 2001), would suggest a closer relationship between N. antarctica and N. pumilio than between any of them and N. dombeyi (e.g. Hill and Dettmann, 1996; Manos, 1997). Cladograms based on both morphological and genetic data or on either of these two data sets support this view (e.g. Hill and Dettmann, 1996; Manos, 1997; Jordan and Hill, 1999). On the other hand, some morphological and architectural features unaccounted for in previous studies on sub-generic affinities, are shared by N. antarctica and N. dombeyi, but are very uncommon in N. pumilio, e.g. presence of vertical shoots with spirally-arranged leaves, sylleptic branches and indeterminate growth (Barthélémy et al., 1999). The close relationship between N. dombeyi and N. antarctica is now supported by the occurrence of natural hybrids between them (present study). Studies concerning the evolutionary relationships among these, as well as other plant species, should consider the variability of both morphological and genetic traits within each of the species involved. This could lead to the pondering of each of the traits to be employed in the computation of between-species distances. The present study supports the idea that pollen type is a key factor in the crossing between Nothofagus species, and is, therefore, likely to be closely linked with the evolution of this genus (Hill and Read, 1991; Manos et al., 2001), whereas the leaf-shedding pattern should not be considered in the search for evolutionary connections among Nothofagus, in coincidence with Hill and Read (1991).
Supplementary Material
Acknowledgments
We are grateful to the personnel of Estancia La Primavera for allowing us access to one of the sampling sites, and to Alfredo Passo, Laura D'Atri and Pablo de Brito for their help in field work. This study was supported by the Universidad Nacional del Comahue (Project B094) and the Consejo Nacional de Investigaciones Científicas y Técnicas (PEI 0800/99). The laboratory analyses were supported partially with funds of the ‘Nothofagus’-Project (BID-FONTAGRO).
LITERATURE CITED
- Barthélémy D, Caraglio Y, Costes E. 1997. Architecture, gradients morphogénétiques et âge physiologique chez les végétaux. In: Bouchon P, Reffye P de, Barthélémy D, eds. Modélisation et simulation de l'architecture des plantes. Paris: INRA Editions, Science Update, 89–136. [Google Scholar]
- Barthélémy D, Puntieri J, Brion C, Raffaele C, Marino J, Martinez P. 1999. Características morfológicas y arquitecturales de las especies de Nothofagus Blume (Fagaceae) del Norte de la Patagonia Argentina. Boletín de la Sociedad Argentina de Botánica 34: 29–38. [Google Scholar]
- Bartish IV, Jeppsson N, Nybom H, Swenson U. 2002. Phylogeny of Hippophae (Elaeagnaceae) inferred from parsimony analysis of chloroplast DNA and morphology. Systematic Botany 27: 41–54. [Google Scholar]
- Berg EE, Hamrick JL. 1997. Quantification of genetic diversity at allozyme loci. Canadian Journal of Forest Research 27: 415–424. [Google Scholar]
- Cheliak WM, Pitel JA. 1984.Techniques for starch gel electrophoresis of enzymes from forest tree species. Information Report PI-X-42. Petawawa National Forestry Institute. Canadian Forestry Service. Agriculture Canada. [Google Scholar]
- Correa MN. 1984. Fagaceae. In: Correa MN, ed. Flora Patagónica VIII (IV). Buenos Aires: INTA, 4–11. [Google Scholar]
- Dettmann ME, Pooknall DT, Romero EJ, Zamaloa M del C. 1990.Nothofagidites ex Potonic 1960; a catalogue of species with notes on the paleogeographic distribution of Nothofagus Bl. (Southern Beech). New Zealand Geological Survey Paleontological Bulletin 60: 1–79. [Google Scholar]
- Dimitri MJ. 1972.La Región de los Bosques Andino-Patagónicos. Sinopsis General. Colección Científica X. Buenos Aires: INTA. [Google Scholar]
- Donoso C. 1993.Bosques templados de Chile y Argentina. Santiago de Chile: Editorial Universitaria. [Google Scholar]
- Donoso C, Atienza JA. 1983. Hibridación natural entre especies de Nothofagus siempreverdes en Chile. Bosque 5: 21–34. [Google Scholar]
- Donoso C, Landrum LR. 1979.Nothofagus leoni, a natural hybrid between Nothofagus obliqua and Nothofagus glauca New Zealand Journal of Botany 1: 353–360. [Google Scholar]
- Donoso C, Morales J, Romero M. 1990. Hibridación natural entre roble (Nothofagus obliqua (Mirb.) Oerst.) y raulí (N. alpina (Poepp. & Endl.) Oerst.), en bosques del sur de Chile. Revista Chilena de Historia Natural 63: 49–60. [Google Scholar]
- Gallo L. 2002. Conceptual and experimental elements to model natural inter-specific hybridization between two mountain southern beeches (Nothofagus spp). In: Degen B, Loveless MD, Kremer A, eds. Modelling and experimental research on genetic processes in tropical and temperate forests. Kourou, French Guyane: Embrapa-Silvolab. [Google Scholar]
- Gallo L, Marchelli P, Breitembücher A. 1997. Morphological and allozymic evidence of natural hybridization between two southern beeches (Nothofagus spp.) and its relation to heterozygosity and height growth. Forest Genetics 4: 15–23. [Google Scholar]
- Gandolfo MA, Romero EJ. 1992. Leaf morphology and a key to species of Nothofagus Bl. Bulletin of the Torrey Botanical Club 119: 152–166. [Google Scholar]
- Gillet E. 1994.Genetic Structures from Electrophoresis Data. version 1·0 University of Göttingen, http://www.uni-forst.gwdg.de/forst/fg/index.htm. [Google Scholar]
- Gregorius HR. 1974. On the concept of genetic distance between populations based on gene frequencies. In: Proceedings Joint IUFRO Meeting, S.02.04.1–3, Stockholm, Session I, 17–26. [Google Scholar]
- Gregorius HR. 1978. The concept of genetic diversity and its formal relationship to heterozygosity and genetic distance. Mathematical Biosciences 41: 253–271. [Google Scholar]
- Hickey LJ. 1973. Classification of the architecture of Dicotyledon leaves. American Journal of Botany 60: 17–33. [Google Scholar]
- Hill RS. 1992.Nothofagus: evolution from a southern perspective. Trends in Ecology and Evolution 7: 190–194. [DOI] [PubMed] [Google Scholar]
- Hill RS, Dettmann ME. 1996. Origin and diversification of the genus Nothofagus In: Veblen TT, Hill RS, Read J, eds. The ecology and biogeography of Nothofagus forests. Yale: Yale University Press, 11–24 [Google Scholar]
- Hill RS, Jordan GJ. 1993. The evolutionary history of Nothofagus (Nothofagaceae). Australian Systematic Botany 6: 111–126. [Google Scholar]
- Hill RS, Read J. 1991. A revised infrageneric classification of Nothofagus (Fagaceae). Botanical Journal of the Linnean Society 105: 37–72. [Google Scholar]
- Jordan G, Hill RS. 1999. The phylogenetic affinities of Nothofagus (Nothofagaceae) leaf fossils based on combined molecular and morphological data. International Journal of Plant Sciences 160: 1177–1188. [DOI] [PubMed] [Google Scholar]
- Kozlowski TT. 1971.Growth and development of trees. Vol. I. Seed germination, ontogeny and shoot growth. New York: Academic Press. [Google Scholar]
- Kozlowski TT, Pallardy SG. 1997.Physiology of woody plants, 2nd edn. San Diego: Academic Press. [Google Scholar]
- McQueen DR. 1976. The ecology of Nothofagus and associated vegetation in South America. Tuatara 22: 38–68. [Google Scholar]
- Manos PS. 1997. Systematics of Nothofagus (Nothofagaceae) based on rDNA spacer sequences (ITS): taxonomic congruence with morphology and plastid sequences. American Journal of Botany 84: 1137–1155. [PubMed] [Google Scholar]
- Manos PS, Zhou Z, Cannon CH. 2001. Systematics of Fagaceae: phylogenetic test of reproductive trait evolution. International Journal of Plant Sciences 162: 1361–1379. [Google Scholar]
- Marchelli P, Gallo L. 2000. Genetic analysis of isozyme variants in open pollinated families of Southern beech Nothofagus nervosa (Phil.) Dim. et Mil. Silvae Genetica 49: 90–98. [Google Scholar]
- Marchelli P, Gallo L. 2001. Genetic diversity and differentiation in a southern beech subjected to introgressive hybridization. Heredity 87: 284–293. [DOI] [PubMed] [Google Scholar]
- Marchelli P, Gallo L. 2002. Genetic differentiation among Argentinean populations of southern beech Nothofagus nervosa In: Degen B, Loveless MD, Kremer A, eds. Modelling and experimental research on genetic processes in tropical and temperate forests. Kourou, French Guyane: Embrapa-Silvolab. [Google Scholar]
- Nei M. 1973. Analysis of gene diversity in subdivided populations. Proceedings of the National Academy of Sciences of the USA 70: 3321–3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson M. 2002. Combining data from DNA sequences and morphology for a phylogeny of Moringaceae (Brassicales). Systematic Botany 27: 55–73. [Google Scholar]
- Philipson WR, Philipson MN. 1979. Leaf vernation in Nothofagus New Zealand Journal of Botany 17: 417–421. [Google Scholar]
- Philipson WR, Philipson MN. 1988. A classification of the genus Nothofagus (Fagaceae). Botanical Journal of the Linnean Society 98: 27–36. [Google Scholar]
- Picca PI. 1998.Estudio exomorfológico y anatómico de las inflorescencias de las especies austroamericanas del género Nothofagus Bl. (Fagaceae). PhD Thesis, Universidad de Buenos Aires, Argentina. [Google Scholar]
- Poulik MD. 1959. Starch gel electrophoresis in a discontinuous system of buffers. Nature 180: 1477–1479. [DOI] [PubMed] [Google Scholar]
- Praglowski, J. 1982. Fagaceae L.: Fagoideae. World Pollen and Spore Flora 11: 1–28. [Google Scholar]
- Premoli AC. 1996. Allozyme polymorphisms, outcrossing rates, and hybridization of South American Nothofagus Genetica 97: 55–64. [Google Scholar]
- Premoli AC. 1997. Genetic variation in a geographically restricted and two widespread species of South American Nothofagus Journal of Biogeography 24: 883–892. [Google Scholar]
- Puntieri J, Barthélémy D, Martinez P, Raffaele E, Brion C. 1998. Annual shoot growth and branching patterns in Nothofagus dombeyi (Fagaceae). Canadian Journal of Botany 76: 673–685. [Google Scholar]
- Puntieri J, Damascos MA, Souza MS. 2001. Tendencias ontogenéticas en el tamaño y la forma de las hojas de Nothofagus pumilio (Poepp. et Endl.) Krasser (Fagaceae). Ecología Austral 11: 105–114. [Google Scholar]
- Puntieri J, Souza MS, Barthélémy D, Mazzini C, Brion C. 2003. Axis differentiation in two South American Nothofagus species (Nothofagaceae). Annals of Botany 92: 589–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga MP, Vidal Russell R, Premoli AC. 2001.Posible hibridación entre Nothofagus pumilio y N. antarctica: evidencia morfológica e isoenzimática. I Reunión Binacional de Ecología Argentina-Chile, Bariloche, Argentina. [Google Scholar]
- Rieseberg LH. 1997. Hybrid origins of plant species. Annual Review of Ecology and Systematics 28: 359–389. [Google Scholar]
- Rieseberg LH, Carney SE. 1998. Plant hybridization. Tansley Review No. 102. New Phytologist 140: 599–624. [DOI] [PubMed] [Google Scholar]
- Rieseberg LH, Ellstrand NC. 1993. What can morphological and molecular markers tell us about plant hybridization? Critical Reviews in Plant Sciences 12: 213–241. [Google Scholar]
- Romero EJ. 1980. Arquitectura foliar de las especies Sudamericanas de Nothofagus Boletín de la Sociedad Argentina de Botánica 19: 289–308. [Google Scholar]
- Rumpunen K, Bartish IV. 2002. Comparison of different estimates based on morphometric and molecular data, exemplified by various leaf shape descriptors and RAPDs in the genus Chaenomeles (Rosaceae). Taxon 51: 69–82. [Google Scholar]
- Sokal RR, Rohlf FJ. 1981.Biometry, 2nd edn. New York: Freeman and Co. [Google Scholar]
- Stecconi M, Puntieri J, Barthélémy D. 2001. Secuencia de desarrollo de individuos de Nothofagus antarctica y N. pumilio (Fagaceae) desde una perspectiva arquitectural. Boletín de la Sociedad Argentina de Botánica 36 (Suppl.): 30. [Google Scholar]
- Tanai T. 1986. Phytogeographic and phylogenetic history of the genus Nothofagus Bl. (Fagaceae) in the southern hemisphere. Journal of the Faculty of Science, Hokkaido University 21: 505–582. [Google Scholar]
- Tuley G. 1980.Nothofagus in Britain. Forestry Commission Forest Record 122: 1–26. [Google Scholar]
- van Steenis CGGJ. 1953. Results of the Archbold expeditions Papuan Nothofagus Journal of the Arnold Arboretum 34: 300–375. [Google Scholar]
- Veblen TT, Donoso C, Kitzberger T, Rebertus AJ. 1996. Ecology of southern Chilean and Argentinean Nothofagus forests. In: Veblen TT, Hill RS, Read J, eds. The ecology and biogeography of Nothofagus forests. Yale: Yale University Press, 293–353. [Google Scholar]
- Wingston DL. 1979.Nothofagus (Blume) in Britain. Watsonia 12: 344–345. [Google Scholar]
- Yeh F, Boyle T. 1997. POPGENE Microsoft Window-based Software for Population Genetics Analysis, Version 1.2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.