Abstract
• Background and Aims Common buckwheat (Fagopyrum esculentum) is a dimorphic self-incompatible plant with either pin or thrum flowers. The S supergene is thought to govern self-incompatibility, flower morphology and pollen size in buckwheat. Two major types of self-fertile lines have been reported. One is a type with long-homostyle flowers, Kyukei SC2 (KSC2), and the other is a type with short-homostyle flowers, Pennline 10. To clarify whether the locus controlling flower morphology and self-fertility of Pennline 10 is the same as that of KSC2, pollen tube tests and genetic analysis have been performed.
• Methods Pollen tube growth was assessed in the styles and flower morphology of KSC2, Pennline 10, F1 and F2 plants that were produced by the crosses between plants with pin or thrum and Pennline 10.
• Key Results Pollen tubes of Pennline 10 reached ovules of all flower types. The flower morphology of F1 plants produced by the cross between thrum and Pennline 10 were thrum or pin, and when pin plants were used as maternal plants, all the F1 plants were pin. Both plants with pin or short-pin flowers, whose ratio of style length to anther height was smaller than that of pin, appeared in F2 populations of thrum × Pennline 10 as well as in those of pin × Pennline 10.
• Conclusion The results suggest that Pennline 10 possesses the s allele as pin does, not an allele produced by the recombination in the S supergene, and that the short style length of Pennline 10 is controlled by multiple genes outside the S supergene.
Key words: Self-incompatibility, heteromorphic flowers, modifier genes, pollen tube growth test, genetic analysis, Fagopyrum esculentum
INTRODUCTION
Self-incompatibility (SI) is a genetic mechanism to prevent self-fertilization after pollination. Most species with heteromorphic flowers have di-allelic SI. Distylous incompatibility encompasses two types of floral architecture: thrum, having short styles and high anthers; and pin, having long styles and low anthers. This characteristic is controlled by a single gene complex that segregates as a simple Mendelian factor, with one dominant allele (S) found only in thrum plants and one recessive allele (s) present in the heterozygous state in thrum plants and in the homozygous state in pin plants (Garber and Quisenberry, 1927). Self-incompatibility is primarily a reaction between haploid pollen tubes and a diploid style, but thrum pollen, despite segregation of S and s, behaves as if it were all S type. This effect is because of the sporophytic determination of the pollen reaction and S being dominant over s.
Common buckwheat (Fagopyrum esculentum) has typical distylous sporophytic self-incompatibility. Sharma and Boyes (1961) considered the S locus of common buckwheat to be similar to the S supergene proposed to occur in Primula (Dowrick, 1956). They postulated that the S supergene of buckwheat consists of five genes: G, style length; IS, stylar incompatibility; IP, pollen incompatibility; P, pollen size; and A, anther height. Pin has small pollen grains, and thrum has larger pollen grains. Pin-linked characters are recessive, and thrum-linked characters are dominant, and therefore the genotype of pin is giSiPpa/giSiPpa and that of thrum is GISIPPA/giSiPpa, although the nature and correct order of these five genes are unknown.
Self-fertile common buckwheat lines have been obtained by spontaneous or artificial mutation (Schoch-Bodmer, 1934; Tatebe, 1953; Sharma and Boyes, 1961; Marshall, 1969). Marshall (1970) developed a self-fertile buckwheat line derived from a mutant of common buckwheat, and named it Pennline 10. In 1991, self-compatible wild buckwheat, Fagopyrum homotropicum, which is very similar to F. esculentum ssp. ancestralis except for long-homostylous flowers and self-compatibility, was discovered in Yunnan province, China (Ohnishi, 1998). Self-compatible common buckwheat lines have been produced by interspecific crosses between F. esculentum and F. homotropicum with embryo rescue (Campbell, 1995; Aii et al., 1998; Woo et al., 1999; Matsui et al., 2003b). The flower morphology of the self-compatible lines is long homostyle and is controlled by a single gene (Campbell, 1995; Aii et al., 1998; Woo et al., 1999; Matsui et al., 2003a, b). The allele controlling homomorphic flowers was designated as Sh, and the dominance relationship of Sh with S and s was found to be S > Sh > s (Woo et al., 1999). Matsui et al. (2003b) suggested that self-compatibility, flower morphology, and the dominance relationship are due to the genotype of giSIPPA/giSIPPA caused by the recombination in the S supergene. However, the self-fertilization of Pennline 10 has not been investigated in detail. In the present study, it is inferred that genes outside the S supergene control functions of the S locus in Pennline 10.
MATERIALS AND METHODS
Plant materials
Two self-fertile buckwheat lines were used—Pennline 10 (kindly provided by National Seed Storage Laboratory USDA-ARS) and Kyukei SC2 (KSC2), produced by a cross between Fagopyrum esculentum and F. homotropicum (Matsui et al., 2003b)—and also two self-incompatible cultivars, Botansoba and Shinano 1. Pennline 10 has short-homostylous flowers (reduced style) (Marshall, 1969), and KSC2 has long-homostylous flowers (Matsui et al., 2003b). F1 plants were produced by hand pollination between either Botansoba or Shinano 1 and Pennline 10. F1 plants produced by the cross between Shinano 1 and Pennline 10 with pin and thrum flowers were designated as F1P and F1T, respectively. F2 populations were obtained by self-pollination of the F1 plants.
Observation of pollen tube growth
To identify cross-compatibility and -incompatibility of Pennline 10, pollen tube growth was evaluated. Incompatibility reactions were evaluated based on pollen tube growth rather than seed sets because seed sets were easily influenced by enviromental conditions. Branches with buds and flowers were collected and stood in bottles with water in a dark room at 20 °C. The next morning, the flowers which were just starting to open were detached from the branch, emasculated, put on 0·8 % agar plates, and cross-pollinated. A check was not made for self-pollen grains on the stigmas with a lens because it had been confirmed that few self-pollen grains pollinated in this test. At 6 or 24 h later, the styles were collected and fixed with acetic acid : ethanol (3 : 7). After being rinsed with distilled water for 15 min, the styles were treated with 1 n sodium hydroxide for 120 min at 60 °C, briefly washed with distilled water, and then stained with 0·1 % aniline blue for 60 min at 60 °C. After a brief rinse with distilled water, the pollen germination and pollen tube growth were examined by fluorescence microscopy (Leica, Wetzlar, Germany).
RESULTS
Cross-compatibility of Pennline 10
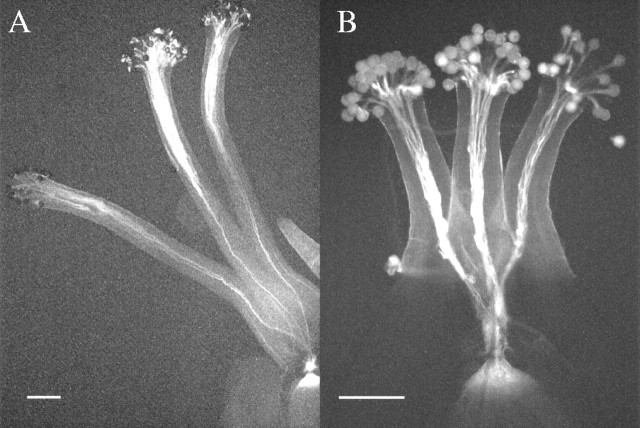
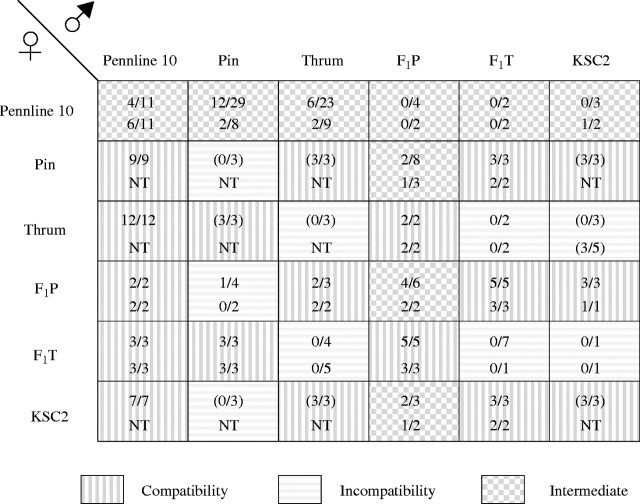
When pollen grains of Pennline 10 were crossed with the pistils of pin, thrum and long-homostyle flowers, the pollen tubes reached into the ovules (Figs 1A and B and 2). Therefore, the pollen grains of Pennline 10 were compatible with all style types, suggesting that pollen grains of Pennline 10 have lost the S function. In the crosses on the pistils of Pennline 10 of pollen from other plants with different flower morphology, pollen tube growth was unstable (Fig. 2). In addition, pollen tube growth of the crosses between pin and F1P, between F1P and F1P, and between KSC2 and F1P was unstable. However, pollen–style interactions in other cross combinations were distinct (Fig. 2).
Fig. 1.
Pollen tube growth of Pennline 10 on the styles of self-incompatible plants. Pollen tubes reach the base of the pin style (A) and the thrum style (B). Bars = 0·2 mm.
Fig. 2.
Pollen tube growth in each cross combination. Data are presented as number of styles with pollen tubes reaching the ovule/number of pistils pollinated. Upper values are findings at 6 h after pollination, and lower values are those at 24 h after pollination. Values in parentheses are from Matsui et al. (2003b). NT, not tested.
Flower morphology of F1 and F2
To clarify whether the loss of S function of pollen is caused by the deletion of IP in the S supergene or controlled by genes outside the S supergene, the flower morphology of F1 and F2 plants was evaluated. Twenty-five F1 seeds, obtained by using Pennline 10 pollen, were grown in a glasshouse or a growth chamber. When thrum plants were used as maternal plants, the flower morphology of F1 plants were thrum or pin, and when pin plants were used as maternal plants, all the F1 plants were pin (Table 1). All the F1 plants produced by the cross between Botansoba and Pennline 10 set selfed seeds, but plants produced by the cross between Shinano 1 and Pennline 10 set no or few F2 seeds. These results suggest that the self-compatibility of Pennline 10 is influenced by the genetic background. Plants having pin flowers appeared in all eight F2 populations (02AL10 to 02AL17) derived from the cross between Botansoba with either pin or thrum flowers and Pennline 10, including those derived from F1 plants having thrum flowers, F1T (Table 2). Two populations, 02AL10 and 02AL13, had no short-homostyle plant, and an intermediate flower phenotype, short-pin, whose ratio of style length to anther height is smaller than that of pin and larger than that of Pennline 10, was observed.
Table 1.
Flower morphology of F1 plants
| Cross combination |
Flower morphology of F1 plants |
||||
|---|---|---|---|---|---|
| Female |
Male |
Thrum |
Pin |
||
| Botansoba (T) | Pennline 10 (SH) | 3 | 2 | ||
| Botansoba (P) | Pennline 10 (SH) | 0 | 4 | ||
| Shinano 1 (T) | Pennline 10 (SH) | 6 | 3 | ||
| Shinano 1 (P) | Pennline 10 (SH) | 0 | 7 | ||
P, pin; T, thrum; SH, short homostyle.
Table 2.
Flower morphology of F2 plants
| Flower morphology |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| F2 |
|||||||||
| Line |
F1 |
Thrum |
Pin |
Short pin |
Short homostyle |
||||
| 02AL10 | Thrum | 6 | 2 | 3 | 0 | ||||
| 02AL11 | Thrum | 19 | 7 | 9 | 2 | ||||
| 02AL12 | Pin | 0 | 48 | 27 | 1 | ||||
| 02AL13 | Thrum | 33 | 21 | 3 | 0 | ||||
| 02AL14 | Pin | 0 | 24 | 7 | 3 | ||||
| 02AL15 | Pin | 0 | 17 | 1 | 1 | ||||
| 02AL16 | Pin | 0 | 18 | 14 | 7 | ||||
| 02AL17 | Pin | 0 | 25 | 4 | 2 | ||||
All lines were produced by the cross between Botansoba and Pennline 10. Lines from 02AL10 to 02AL13 were produced by the cross between thrum plants and Pennline 10, and from 02AL14 to 02AL17 were produced by the cross between pin plants and Pennline 10.
DISCUSSION
Distylous self-incompatibility such as that of buckwheat and Primula is mainly controlled by the S supergene (Dowrick, 1956; Sharma and Boyes, 1961; Matsui et al., 2003b). Self-compatible variants probably resulting from recombination in the S allele have been reported. Wedderburn and Richards (1992) reported that homostyly in some homostyle species had arisen secondarily by recombination within the S complex linkage group in Primula. Matsui et al. (2003b) reported that a self-compatible allele, Sh, derived from F. homotropicum had arisen by recombination in the S supergene. If the short-homostyle trait of Pennline 10 had arisen by recombination in the S supergene, its genotype would be considered to be GIsipa/GIsipa (Fig. 3). If this model is correct the pollen tubes of the short-homostylous plants should be compatible with the styles of thrum plants but incompatible with the styles of pin plants and the styles of the short-homostylous plants should be incompatible with thrum pollen but compatible with pin pollen. In addition, the pollen tubes of short-homostylous plants should be incompatible with the style of long-homostylous plants, and the reciprocal cross also should be incompatible, because the genotype of long homostyle is giSIPPA/giSIPPA (Fig. 3). Furthermore, the flower morphology of F1 plants produced by the cross between thrum and Pennline 10 should be thrum or short homostyle (Fig. 4), and only short-homostylous plants should be produced by the cross between pin and Pennline 10 (Fig. 4).
Fig. 3.
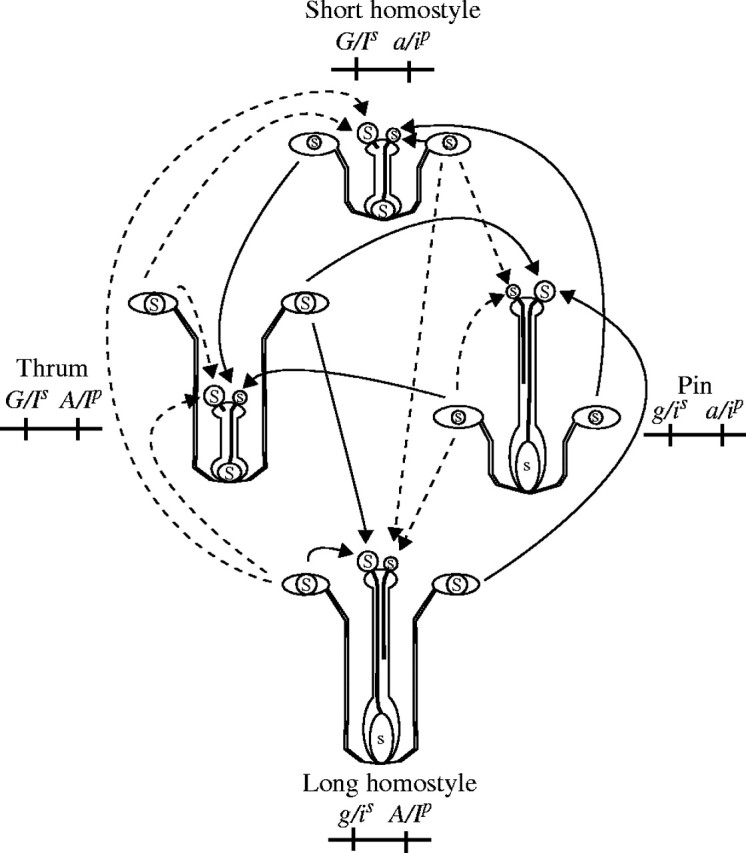
Expected compatibility interactions among a pin, thrum, long-homostyle and short-homostyle plants. Crosses shown by arrows are compatible cross and arrows with broken lines are incompatible cross.
Fig. 4.
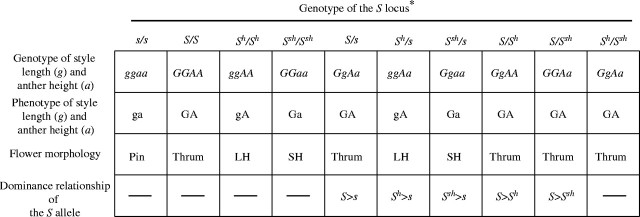
Expected dominance relationships among s, S, Sh and Ssh alleles based on the dominance relationships of the style length, g, and anther height, a, genes. Ssh, tentatively designated here as short homostyle, occurs by recombination in the S supergene. *Genotypes of S/s is normal type for thrum under a natural environment. Genotypes of S/S and Sh/Ssh plants are tentatively designated here because these plants should not be produced by self-incompatibility action.
However, in the present study, pollen of Pennline 10 was compatible with all the flower types. In addition, short-homostylous plants were not obtained, and pin plants appeared in the F1 plants produced by the cross between thrum and short homostyle. Furthermore, short-homostyle plants were not found and only pin plants appeared among the F1 plants of the pin × short-homostyle cross. These results indicate that short homostyle of Pennline 10 was not generated through recombination in the S supergene.
If the self-compatible gene of Pennline 10 is due to deletions of IP and A, F2 plants derived from the self-pollination of the F1 thrum plant that arose from the cross between thrum and Pennline 10 should segregate thrum and short homostyle in a 3 : 1 mono-factorial ratio. In addition, F2 plants derived from the self-pollination of F1 pin plants that arose from the cross between either thrum or pin and Pennline 10 should segregate short homostyle and pin in a 3 : 1 mono-factorial ratio. However, the F2 plants derived from the F1 thrum plants included pin plants, and thrum plants were not observed among the F2 plants derived from the F1 pin plants. Furthermore, plants with short-pin flowers occurred in both F2 populations. These results suggest that the S locus of Pennline 10 is ss genotype same as pin and that modifier genes affect self-compatibility and style length.
Polygenes or major genes outside the S locus responsible for breakdown of self-incompatibility have been reported to occur in many plants, e.g. alsike clover (Townsend, 1969), Brassica (Thompson and Taylor, 1966; Nasrallah and Wallace, 1968; Hinata et al., 1983) and Petunia (Tsukamoto et al., 2003). Mather (1950) reported a mutant that has a gene that shortens the length of pin stigmas in P. sinensis. Kurian and Richards (1997) reported that there are at least two loci with additive effect on the genes on the style length, stigma papilla length and style cell length. In the present study, flower morphology in the F1 population was pin or thrum, indicating that the modifier genes did not have an effect because of their heterozygosity. However, pollen tube growth was unstable in the pin × F1P, F1P × F1P, and KSC2 × F1P crosses, suggesting that the genes for self-compatibility might show partial dominance or operate a late-acting system sensitive to environmental conditions. An intermediate flower phenotype, short-pin, recognized in the F2 population was probably due to homozygosity of some of the modifier genes, and short homostyles are probably produced when all of the polygenes are in their homozygous forms in a plant.
The compatibility or incompatibility of Pennline 10 was not clarified when it was used as the style parent. The reason pollen tubes did not reach the ovule by self-pollination of Pennline 10 may be the influence of various environmental factors on the expression of polygenes. High seed fertility of Pennline 10 might be caused not by self-compatibility but by genes controlling intensity of self-incompatibility. Further study is needed to clarify the compatibility or incompatibility of the style of Pennline 10.
Many reports demonstrate that polygenes control the intensity of self-incompatibility (Nasrallah and Wallace, 1968; Crowe, 1971; Richards and Thurling, 1973), and the self-fertilization of Pennline 10 is likely to be due to such genes. Seed production of buckwheat is influenced by day length and temperature, suggesting that the expression of the involved genes is influenced by various environmental conditions. There is no report of QTL analysis of the intensity of self-incompatibility with molecular maps in buckwheat. QTL analysis would give further information on heteromorphic self-incompatibility.
Acknowledgments
We thank Y. Asai and J. Tsurumoto for growing the plants and Y. Kiryu, R. Tajiri-Yamai and N. Matsui for flower bagging and sampling. We also thank E. Fukai and R. Komiya for their technical advice regarding the pollen tube growth test.
LITERATURE CITED
- Aii J, Nagano M, Penner GA, Campbell CG, Adachi T. 1998. Identification of RAPD markers linked to the homostylar (Ho) gene in buckwheat. Breeding Science 48: 59–62. [Google Scholar]
- Campbell C. 1995. Inter-specific hybridization in the genus Fagopyrum In: Proceedings of the 6th International Symposium on Buckwheat, Japan, 255–263. [Google Scholar]
- Crowe LK. 1971. Polygenic control of outbreeding in Borago officinalis Heredity 27: 111–118. [Google Scholar]
- Dowrick VPJ. 1956. Heterostyly and homostyly in Primula obconica Heredity 10: 219–236. [Google Scholar]
- Garber RJ, Quisenberry KS. 1927. The inheritance of length of style in buckwheat. Journal of Agricultural Research 34: 181–183. [Google Scholar]
- Hinata K, Okazaki K, Nishio T. 1983. Gene analysis of self-compatibility in Brassica campestris var. Yellow Sarson (a case of recessive epistatic modifier). In: Proceedings of the 6th International Rapeseed Conference, Paris, Vol. 1, 354–359. [Google Scholar]
- Kurian V, Richards AJ. 1997. A new recombinant in the heteromorphy ‘S’ supergene in Primula Heredity 78: 383–390. [Google Scholar]
- Marshall HG. 1969. Isolation of self-fertile, homomorphic forms in buckwheat, Fagopyrum sagittatum Gilib. Crop Science 9: 651–653. [Google Scholar]
- Marshall HG. 1970. Registration of ‘Pennline 10’ buckwheat. Crop Science 10: 726. [Google Scholar]
- Mather K. 1950. The genetical architecture of heterostyly in Primula sinensis Evolution 4: 340–352. [Google Scholar]
- Matsui K, Tetsuka T, Hara T. 2003. Two independent gene loci controlling non-brittle pedicels in buckwheat. Euphytica 134: 203–208. [Google Scholar]
- Matsui K, Tetsuka T, Nishio T, Hara T. 2003. Heteromorphic incompatibility retained in self-compatible plants produced by a cross between common and wild buckwheat. New Phytologist 159: 701–708. [DOI] [PubMed] [Google Scholar]
- Nasrallah ME, Wallace DH. 1968. The influence of modifier genes on the intensity and stability of self-incompatibility in cabbage. Euphytica 17: 495–503. [Google Scholar]
- Ohnishi O. 1998. Search for the wild ancestor of buckwheat. I. Description of new Fagopyrum (Polygonaceae) species and their distribution in China and Himalayan hills. Fagopyrum 15: 18–28. [Google Scholar]
- Richards RA, Thurling N. 1973. The genetics of self-incompatibility in Brassica campestris L. ssp. oleifera Metzg. Genotypic and environmental modification of S-locus control. Genetica 44: 439–453. [Google Scholar]
- Schoch-Bodmer H. 1934. Zum Heterostylieproblem: Griffelbeschaffenheit und Pollenschlauchwachstum bei Fagopyrum esculentum Planta 22: 149–152. [Google Scholar]
- Sharma KD, Boyes JW. 1961. Modified incompatibility of buckwheat following irradiation. Canadian Journal of Botany 39: 1241–1246. [Google Scholar]
- Tatebe T. 1953. Physiological research on the fertility of the buckwheat. III. On the self-fertile, long-styled plants. Japanese Journal of Breeding 2: 240–244 [in Japanese with English summary]. [Google Scholar]
- Thompson KF, Taylor JP. 1966. The breakdown of self-incompatibility in cultivars of Brassica oleracea Heredity 21: 637–648. [Google Scholar]
- Townsend CE. 1969. Self-compatibility studies with diploid alsike clover, Trifolium hybridum L. IV. Inheritance of type II self-compatibility response to temperature and the segregation of S-alleles in diploid alsike clover. Crop Science 9: 443–446. [Google Scholar]
- Tsukamoto T, Ando T, Kokubun K, Watanabe H, Sato T, Masada M, Marchesi E, Kao T. 2003. Breakdown of self-incompatibility in a natural population of Petunia axillaries caused by a modifier locus that suppresses the expression of an S-RNase gene. Sexual Plant Reproduction 15: 255–264. [Google Scholar]
- Wedderburn FM, Richards AJ, 1992. Secondary homostyly in Primula L.: evidence for the model of the S supergene. New Phytologist 121: 649–655. [Google Scholar]
- Woo SH, Adachi T, Jong SK, Campbell CG. 1999. Inheritance of self-compatibility and flower morphology in an inter-specific buckwheat hybrid. Canadian Journal of Plant Science 79: 483–490. [Google Scholar]