Abstract
• Background and Aims Hymenaea courbaril (Leguminosae-Caesalpinioideae) is a tree species with wide distribution through all of the Neotropics. It has large seeds (approx. 5 g) with non-photosynthetic storage cotyledons rich (40 %) in a cell wall polysaccharide (xyloglucan) as a carbon reserve. Because it is found in the understorey of tropical forests, it has been considered as a shade-tolerant, late-secondary species. However, the physiological mechanisms involved in seedling establishment, especially regarding the interplay between storage and light intensity, are not understood. In this work, the ecophysiological role of this carbon cotyledon reserve (xyloglucan) is characterized, emphasizing its effects on seedling growth and development during the transition from heterotrophy to autotrophy under different light conditions.
• Methods Seedlings of H. courbaril were grown in environments with different light intensities, and with or without cotyledons detached before xyloglucan mobilization. Development, growth, photosynthesis and carbon partitioning (dry mass and [14C]sucrose) were analysed in each treatment.
• Key Results The detachment of cotyledons was not important for seedling survival, but resulted in a strong restriction (50 % less) of shoot growth, which was the main sink for the cotyledon carbon reserves. Carbon restriction promoted an early maturation of the photosynthetic apparatus without changes in the net CO2 fixation per unit area. The reduced surface area of the first leaves in seedlings without cotyledons was evidence of limited growth and development of seedlings in low light conditions (22 µmol m−2 s−1 photon flux).
• Conclusions There is an increase in the importance of storage xyloglucan in cotyledons for H. courbaril seedling development as light intensity decreases, confirming that this polymer plays a key role in the adaptation of this species to establish successfully in the shadowed understorey of the forest.
Key words: Carbon partitioning, forest, light, growth, cell wall, Hymenaea courbaril, photosynthesis, seedling, xyloglucan, storage
INTRODUCTION
Seedlings are an especially sensitive stage in the plant life cycle and initial growth and survival can be strongly influenced by the reserves that the seeds have available. In this way, large seed size is generally associated with a greater ability of seedlings to cope with loss of photosynthetic tissue (e.g. through herbivory) and/or circumstances in which seedlings are likely to experience carbon deficit early in development, such as in the shaded environments of rain forests (Westoby et al., 1992; Armstrong and Westoby, 1993; Leishman and Westoby, 1994; Bonfil, 1998). The presence of seed reserves is thought to enable seedlings to tolerate shade for longer by providing carbon reserves to support respiration while the plant is in net carbon deficit. Usually, the amount of seed resources is important for the development of leaf area and other organs necessary for the uptake of external resources that ultimately enable seedlings to maintain autotrophic growth (Wulf, 1986; Paz and Martínez-Ramos, 2003).
The transition from dependency on seed reserves to dependency on external resources occurs gradually and the length of the transition is determined by the species and by the environment (Kitajima, 1996). Species with leaf-like photosynthetic cotyledons will start to depend on light availability earlier than those with non-photosynthetic storage cotyledons, which have to wait for the development of the first leaves. These differences are also related to the plasticity of biomass allocation in response to limiting resources, and have been applied to help understand the plant's growth ability and/or performance in shaded forest understoreys or in open land (Whitmore, 1996; Cao and Ohkubo, 1998). In general within a species, shade-grown individuals allocate relatively more biomass to shoots than to roots (lower root : shoot ratio, R : S) and have greater ratios of total leaf area per unit area (Kitajima, 1994). These are considered to be adaptive phenotypic responses to shading because they increase the ratio of photosynthesis to respiration at the whole-plant level and contribute to the maintenance of a positive carbon budget and maximization of growth in the shade (Givnish, 1988).
According to Lee and Langenheim (1975), the genus Hymenaea originated in Africa some 65 million years ago. It spread and adapted very well in the Neotropical regions, generating many different species. Within the genus, H. courbaril is considered one of the most successful species, with 17 different varieties in tropical forests ranging from North America (Mexico) through Central America to almost all tropical countries in South America. It has been shown as a potential dominant in rain forests principally due to its shade (Souza and Válio, 1999) and drought (Gerhardt, 1993) tolerances. However, until now the strategies adopted by the seedlings of H. courbaril to survive under a closed canopy have not been fully understood.
Hymenaea courbaril has large seeds with non-photosynthetic globoid cotyledons, rich in a storage cell-wall polysaccharide (xyloglucan) that corresponds to about 40 % of the dry mass of the seed (Buckeridge and Dietrich, 1990). The germination of seeds of H. courbaril is epigeal (Flores and Benavides, 1990) and the initial development is mainly supported by mobilization of storage xyloglucan over a period of approx. 2 months after germination (Tiné et al., 2000). The mobilization of this reserve has been shown to be quite complex, involving the co-ordinated action of four enzymes that completely degrade xyloglucan and produce a drastic decline in the dry mass of cotyledons, with a concomitant increase in transitory starch and embryo growth (Buckeridge et al., 2000b; Tiné et al., 2000). Santos et al. (2004) demonstrated the existence of a communication system between the growing top shoot of the seedling and its cotyledons during storage mobilization. Auxin is produced in developing leaves and transported downwards, and is thus probably the principal messenger involved in the interaction between storage xyloglucan mobilization and seedling growth and establishment.
Although physiological and biochemical studies on xyloglucan mobilization suggest that this storage compound can be advantageous to the establishment of seedlings of H. courbaril under shade conditions, no experiments have been designed to test this hypothesis. The goal of this work was to characterize the ecophysiological role of this cotyledon carbon reserve (xyloglucan), emphasizing its effects on H. courbaril seedling growth and development during the period of transition between heterotrophy and autotrophy under different light conditions.
MATERIALS AND METHODS
Seeds of Hymenaea courbaril L. were obtained from trees growing in a gallery forest at São João da Boa Vista county (22°00′S; 47°18′W), São Paulo, Brazil. Seeds were stored for 4 years in dry (RH 35 %) and cold (8 °C) conditions. They were divided according to the fresh mass of dormant seeds (water content 6·5 %) and the group corresponding to the statistical mode (4·6–5·3 g) was selected for experiments. Seeds were scarified with sand paper at the lateral position in relation to the embryo, surface-sterilized for 15 min in tenfold-diluted commercial hypochlorite bleach, rinsed, and placed on trays between two sheets of wet paper at 30 °C until germination was evident (0·5 cm of radicle visible). The time in all experiments was recorded in days in relation to the beginning of imbibition (i.e. days after imbibition). Germinated seeds were placed in pots (2 L) with a mixture of organic soil and vermiculite (2 : 1, v : v) and the pots were placed in three different environments, as described below.
The environments were chosen principally to represent differences in light intensity and quality (Table 1). (1) Growth room. Pots were arranged randomly on shelves illuminated with ten fluorescent lamps (60 W) and four incandescent lamps (40 W). The photoperiod throughout the experiment was 12 h. The temperature was regulated with the help of an air conditioning system with continuous air change. The purpose of using these conditions was to maintain plants under relatively constant temperature, humidity and intermediate light intensity (200 μmol m−2 s−1). (2) Greenhouse. These experimental conditions had the purpose of obtaining higher levels of radiation and temperature. Pots were also randomly arranged in the available area. (3) Forest. A set of conditions were chosen in the forest that are very similar to those where young H. courbaril seedlings are found growing naturally. Pots were randomly placed in the forest understorey with natural conditions of radiation, temperature and red : far red ratio (Table 1). In all experiments the seedlings were watered once daily.
Table 1.
Characteristics of the three different environmental conditions where the seedlings of H. courbaril were grown
| Environment |
R : FR* |
Air humidity (%) |
PPFD† (µmol m−2 s−1) |
Air temperature (°C) |
|---|---|---|---|---|
| Greenhouse | 1·2 | 59 ± 19 | 670 | 28 ± 6 |
| Growth room | 1·4 | 61 ± 18 | 194 | 23 ± 2 |
| Forest | 0·5 | 76 ± 10 | 22‡ | 22 ± 2 |
Red-to-far red ratio measured at the same time as PPFD.
PPFD (photosynthetically active photon flux density) measured at about 1200 h.
The forest environment also showed sunflecks within the range 200–700 μmol m−2 s−1 PPFD.
The red : far red ratios (R : FR, 655–665 : 725–735 nm ratio) and photosynthetically active photon flux density (PPFD, 400–700 nm) were recorded on a horizontal surface with SKR 110 and SKP 210 sensors (Skye Instruments Ltd, Llandrindod Wells, UK), respectively. A hygrothermograph (Oakton 08368-60, Cole-Parmer Instrument Co., Chicago, USA) was used to continuously record the temperature and air humidity at each site. The environments in which the experiments were performed differed principally in PPFD (22–670 μmol m−2 s−1) and R : FR ratios (0·5–1·4), although some variation in average humidity and average temperature was also observed between environments (Table 1). There was an inverse relationship between temperature and humidity in the greenhouse (r = −0·83) and forest (r = −0·79), whereas in the growth room the humidity varied independently of temperature (r = −0·07), due to the control of temperature by air conditioning. In the greenhouse and growth room, the light quality was similar to an open environment (R : FR = 1·2 and 1·4, respectively), while in the forest a shaded condition was present (R : FR = 0·5).
Under growth room conditions, Tiné et al. (2000) found that xyloglucan mobilization occurred between 35 and 55 d after seedling emergence, beginning when the eophyll (first leaf) was visible between the cotyledons. Under our experimental conditions, eophyll development occurred around 30 d after germination and was followed by a drastic decrease in cotyledon dry mass. In all experiments, to test the effect of cotyledon detachment during xyloglucan mobilization, cotyledons were removed when the eophyll became visible (approx. 2 mm outside the cotyledons).
During growth, five plants per treatment (with and without cotyledons) in each environment were harvested on day 24 (at cotyledon detachment), and 36 and 49 d after the beginning of water imbibition by the seeds. At each harvest the following were determined: dry mass of the cotyledons and seedling parts (shoot and root); height of the seedling; and leaf area. Leaf area was determined before drying using a portable leaf area meter (LI-3000A, LI-COR, Inc., Lincoln, USA). In addition, leaf emergence was recorded daily.
All mass values were determined after drying at 70 °C for 72 h. On the basis of the results for leaf area and total mass, leaf area ratio (LAR, total leaf area divided by the total seedling mass) was calculated. For each harvesting date, the relative growth rate of the seedlings (RGR) and the relative mobilization rate of reserves (RMR) were calculated as follows:
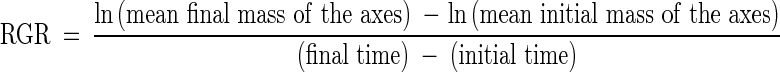 |
and
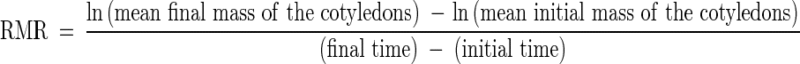 |
The axes in the calculation of RGR correspond to the whole seedling mass without the mass of the cotyledons. To obtain the average initial mass of cotyledons and axes employed in the calculation of RGR and RMR (0–24 days), 50 dormant seeds (4·6–5·3 g) were manually separated into seed coat, cotyledons and axes, and then dried (70 °C for 72 h) and weighed individually.
Differences between treatments (presence or absence of cotyledons in three environments) for each sample date were subjected to analysis of variance (SANEST, Zonta and Machado, 1991). When the F-value was significant at P < 0·05, the multiple-comparison Tukey test (P < 0·05) was performed.
To follow photosynthesis during the heterotrophy–autotrophy transition, another set of 40 plants (20 had their cotyledon removed, as described above) was grown under the same conditions as in the growth room. Evaluation of biochemical and photochemical performance was confined to the eophyll, which reached full expansion during the reserve-dependent period. Measurements were made once a week from the beginning of eophyll expansion until dehiscence of the cotyledons.
Net photosynthesis (A) was determined following measurements of leaf gas exchange rates at different light intensities and CO2 concentrations using a portable infrared gas analyser LI-6400 (LI-COR, Inc., Lincoln, USA) equipped with a LED-source chamber (LI-6400-02B) and a CO2-mixer (LI-6400-01). Light curves were obtained by holding leaf temperature (25 °C) and CO2 concentration (340 μmol mol−1) constant and exposing the leaves to a PPFD range from 500 to 0 μmol m−2 s−1. Using the same fixed temperature, but with saturating PPFD (200 μmol m−2 s−1, determined from the light curves), CO2 curves were obtained within a range from 0–1000 μmol mol−1. In addition, the photochemical performance of eophylls was assessed by measurement of variable fluorescence : maximum fluorescence ratio (Fv : Fm) of chlorophyll from photosystem II (PSII) using a modulated fluorometer (OS5-FL, Opti-Sciences Inc., Tyngsboro, USA). Measurements were performed in dark-adapted leaves (over 20 min) that received a saturating flash (0·8 s) of red light (7000 μmol m−2 s−1).
Carbon distribution was assessed in nine 35-day-old seedlings grown in the growth room. Uniformly labelled [14C]sucrose solution (5 µl, 18520 Bq) was applied, with a Hamilton syringe (25 µl), into the middle section of one cotyledon in each plant. Two non-treated seedlings were kept as a control. Feeding of [14C]sucrose occurred between 0900 h and 1000 h. Seventy-two hours after injection of the cotyledons, seedlings were harvested and separated into the following parts: treated cotyledon, untreated cotyledon, roots, hypocotyl, eophyll, first metaphyll (true leaf) and top of the shoot (last internode including the apical part and the second metaphyll with initial development). Seedling parts were dried at 70 °C for 72 h and, after determination of dry mass, they were ground to a fine powder. Samples of each seedling part, ranging from 20–60 mg, were suspended in 5 mL of scintillation fluid (Ultima Gold™, Packard BioScience B.V., The Netherlands) and radioactivity was counted in a liquid scintillation counter (Tri-Carb 2100 TR, Packard BioScience B.V., The Netherlands). An external standard ratio using non-radioactive material from similar seedling parts was used to correct for quenching. Results were converted to percentages of the total recovered activity. The relative sink strength of the seedling parts was calculated by dividing the percentage of radioactivity by the dry mass of the respective part (Palit, 1985). To follow radioactivity in the structural compounds, 50 mg of root, hypocotyl and the first metaphyll were each submitted to an alcohol extraction (four times in ethanol 80 %, 80 °C). After centrifugation (10 000 g, 10 min), each alcohol-insoluble material was dried, powdered, suspended in 5 mL of scintillation fluid and counted as described above. The counts obtained were subtracted from the total counts of the whole material, giving an estimate of the radioactivity incorporated in the structural compounds of the tissues.
RESULTS
Seedling development
Development was slower for seedlings growing in the forest and in the growth room than in the greenhouse (Fig. 1). Although this effect increased throughout the period of observation, the differences first appeared at the time of seedling emergence, and this was possibly related to the warmer temperatures. Relative to the eophylls of seedlings growing in the greenhouse, development of seedlings in the growth room and forest were delayed by 2·5 and 5 d, respectively (Fig. 1). These delays were taken into consideration when scheduling cotyledon detachment, so that it was performed at 22 d (greenhouse), 24 d (growth room) and 27 d (forest).
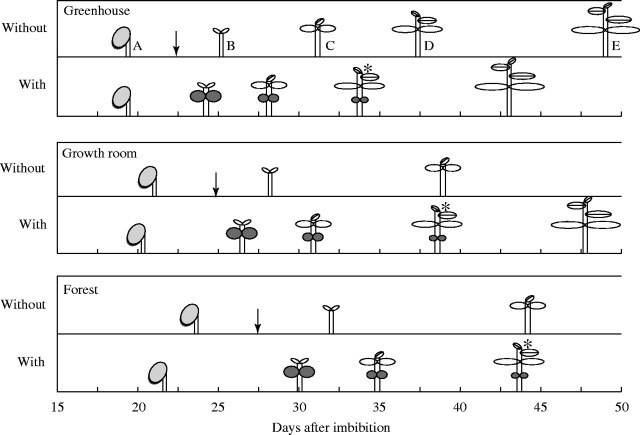
Fig. 1.
Schematic representation of development of seedlings of H. courbaril growing in greenhouse, growth room and forest environments. Data represent the mean emergence dates of (A) seedlings, (B) eophylls, and (C) first, (D) second and (E) third metaphylls of seedlings with and without cotyledons. The detachment of cotyledons was performed at 22, 24 and 27 days after the beginning of imbibition (black arrows). Asterisks mark the date by which 50 % of cotyledons had dropped.
Cotyledon detachment slowed production of the first metaphyll (in relation to non-detached control seedlings) by 7 d in the forest and 5·9 d in the growth room. These delays were greater than those observed in the greenhouse, where there was little difference (approx. 2·5 days) in relation to non-detached seedlings (Fig. 1).
In all environments, 50 % of the seedlings lost their cotyledons when the second metaphyll started to expand (asterisks in Fig. 1). Thus, the times for which cotyledons were present were 34 ± 2, 38 ± 2·5 and 43 ± 3·4 d for seedlings growing in the greenhouse, growth room and forest, respectively. The average remaining mass of the cotyledons was 278 ± 48 mg, with no significant differences between treatments (Fig. 2A). By using the observed times for which the cotyledons persisted for each treatment and discounting the time that seeds took to start germinating (up to 15 d after the beginning of imbibition in all treatments), calculations showed a total period of dependence on cotyledon reserves of approx. 19, 23 and 28 d for greenhouse, growth room and forest, respectively.
Fig. 2.
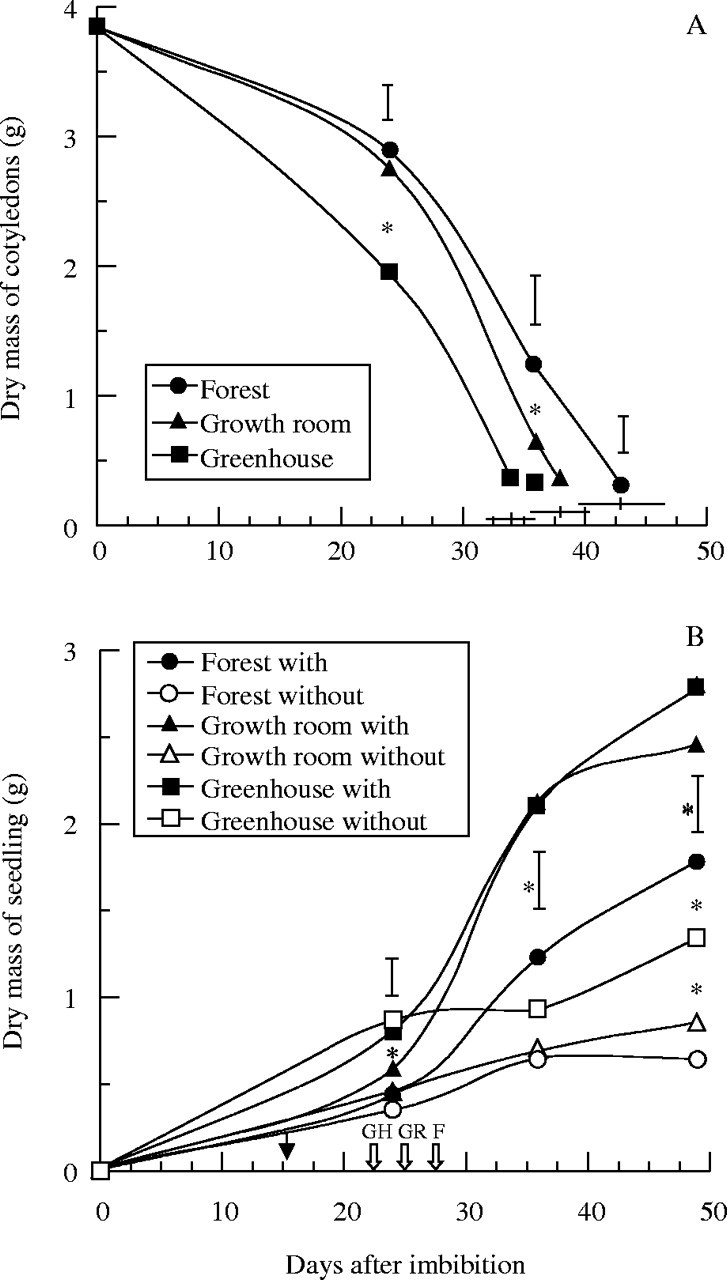
Changes in dry mass of (A) cotyledons and (B) total seedlings of H. courbaril. The seedlings were grown either in a greenhouse (GH), growth room (GR) or forest (F) with or without cotyledons; the that which these were detached is indicated by arrows. The black arrow indicates the date of germination (15 d after imbibition in all environments). The horizontal bars in (A) represent the mean of the date (± s.d.) by which cotyledons had dropped. Vertical bars represent results of a Tukey test (n = 5, P < 0·05). Asterisks indicate significant differences between treatments.
Seedling growth and carbon distribution
A direct relationship between storage mobilization and initial seedling growth (r = −0·99, −0·99 and −0·96 for forest, growth room and greenhouse, respectively) can be seen in Fig. 2A and B. However, under the light conditions in the forest, mobilization of the storage compounds from the cotyledons to the axis took longer than in the other environments. In comparison with seedlings growing in the growth room and greenhouse, which showed higher relative mobilization rates (RMR, Table 2; note that a larger absolute value means a higher rate of mobilization) between 24 and 36 d, the seedlings growing in the forest showed a peak of RMR only in the period between 36 and 49 d after imbibition.
Table 2.
Relative mobilization rate (RMR) of the cotyledon reserves and relative growth rate (RGR) of seedlings of H. courbaril grown in forest, growth room and greenhouse conditions with and without attached cotyledons
| Treatments |
Period after imbibition of seeds (d) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable |
Environment |
Cotyledons |
0–24 |
24–36 |
36–49 |
0–49 |
||||
| RMR (mg g−1 day−1) | Greenhouse | With | −28 | −142* | −6† | −68‡ | ||||
| Growth room | With | −14 | −122 | −45† | −62‡ | |||||
| Forest | With | −12 | −70 | −114† | −59‡ | |||||
| RGR (mg g−1 day−1) | Greenhouse | With | 161 | 80 | 22 | 107 | ||||
| Greenhouse | Without | 169 | 4 | 27 | 92 | |||||
| Growth room | With | 151 | 106 | 9 | 105 | |||||
| Growth room | Without | 142 | 33 | 15 | 83 | |||||
| Forest | With | 140 | 85 | 27 | 98 | |||||
| Forest | Without | 129 | 53 | 1 | 77 | |||||
Calculated using the difference in dry mass of cotyledons over the 24–34 period.
Calculated using the difference in dry mass of cotyledons over the periods 34–36, 36–38 and 36–49 for greenhouse, growth room and forest, respectively.
Calculated using the differences in dry mass of cotyledons for the intervals of 0–36, 0–38 and 0–49 days for greenhouse, growth room and forest respectively.
Despite the fact that absolute dry mass of whole seedlings increased slowly up to 24 d in all environmental conditions (Fig. 2B), relative growth rate (RGR) was higher during the same period (Table 2). These higher RGR values at early stages of development were probably due to a small initial size of embryos in dormant seeds of H. courbaril (0·015 g), which grew quickly during this early period. Furthermore, after the period of xyloglucan mobilization, seedling growth tended to slow down, as can be seen by the lower RGR observed between 36 and 49 d (Table 2).
The effect of cotyledon detachment on growth was clearly observed by a decrease in RGR at the end of the period of storage mobilization (0–49 d, Table 2). In all cases, the results were negative and different between environments (−14·0 %, −20·9 % and −21·4 % for greenhouse, growth room and forest, respectively). The effect of cotyledon detachment on RGR was stronger when seedlings were growing in the greenhouse in the period between 24 and 36 d. However, it was not so different, in terms of the magnitude of the difference from intact seedlings, from its effect on seedlings growing in the growth room during the same period (Table 2). On the other hand, the effect of the absence of cotyledons was relatively smaller when seedlings were growing in the forest than when growing in the other environments (Table 2). The fact that seedlings grew more slowly in the forest than in the greenhouse or growth room (Figs 1 and 2B), may possibly explain the effect of cotyledon removal on seedling growth during the period of observation.
In all environments, the seedlings whose cotyledons were detached were smaller than those with cotyledons (Fig. 3A–C). The height of seedlings without cotyledons growing in the forest and growth room increased up to 8 and 11 d after detachment, respectively, and then growth nearly ceased (Fig. 3A, B). However, seedlings without cotyledons growing in the greenhouse maintained a slow but continuous increase of height throughout the experiment (Fig. 3C). This might be related to the photosynthetic contribution of the leaves present. It is interesting to note that even when growing under rather low light conditions (22 µmol m−2 s−1), seedlings of H. courbaril did not etiolate.
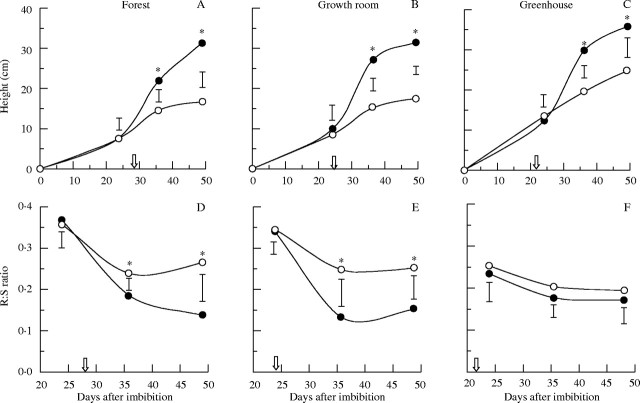
Fig. 3.
Height (A–C) and root-to-shoot ratio (D–F) of seedlings of H. courbaril growing either with (closed symbols) or without (open symbols) cotyledons, in forest (A, D), growth room (B, E) or greenhouse (C, F). The arrows indicate the date at which the detachment of cotyledons was performed. Vertical bars represent results of a Tukey test (n = 5, P < 0·05). Asterisks indicate significant differences between treatments.
Regarding carbon distribution, detachment of cotyledons strongly affected the investment in shoot development, except for seedlings growing in the greenhouse. During the period of storage mobilization, seedlings without cotyledons growing in the forest and growth room did not reduce R : S ratios, as has been observed for intact seedlings (Fig. 3D, E). This highlights the importance of the storage compounds as a source of carbon for shoot development when seedlings were growing in the light conditions of the forest and the growth room. Comparing the results after the period of xyloglucan mobilization (day 49), seedlings growing in the greenhouse maintained a R : S ratio around 0·2, i.e. investing approx. 80 % of their biomass in the shoot, both with and without cotyledons (Fig. 3F). On the other hand, seedlings with cotyledons growing in the forest and growth room invested approx. 85 % in the shoots, whereas seedlings without cotyledons decreased investment in shoots to approx. 75 % (Fig. 3D, E).
The carbon allocation pattern of seedlings with cotyledon reserves was confirmed by injection of [14C]sucrose in one of the cotyledons at the point of maximal RMR in the growth room (35 d) and determination of radioactivity distribution after 3 d (Table 3). Only 30 % of the total radioactivity injected remained in the cotyledon whereas almost 60 % of the radioactivity was present in the shoots of the seedling (hypocotyl, eophyll, first metaphyll and top shoots) and less than 10 % was present in the roots (Table 3). It is interesting to note that no radioactivity was detected in the untreated cotyledon, characterizing the absence of carbon flux from the embryonic axis into the cotyledons, and from one of the cotyledons to the other.
Table 3.
Relative distribution of [14C]sucrose between different tissues of 35-d-old seedlings of H. courbaril grown in a growth room
| Relative radioactivity (%)* |
|||||
|---|---|---|---|---|---|
| Tissue |
Counts per tissue |
Sink strength† |
Alcohol-insoluble‡ |
||
| Top shoot§ | 15 ± 2·9 | 41 ± 7·3 | – | ||
| First metaphyll | 24 ± 6·8 | 22 ± 8·7 | 36 ± 5·3 | ||
| Eophyll | 6 ± 2·9 | 2 ± 1·3 | – | ||
| Hypocotyl | 14 ± 4·5 | 7 ± 3·7 | 23 ± 6·9 | ||
| Untreated cotyledon | 0 ± 0·0 | 0 ± 0·0 | – | ||
| Treated cotyledon | 30 ± 8·3 | 20 ± 5·1 | – | ||
| Roots | 10 ± 6·2 | 8 ± 4·2 | 17 ± 3·8 | ||
The radioactive sucrose was injected (5 μL, 18520 Bq) in one cotyledon (treated) and the 14C-scintillation was read after 72 h.
The numbers represent the average of nine replicates (seedlings) ± s.d.
Percentage of total counts per g of dry mass.
Dry tissues of root, hypocotyl and first metaphyll were subjected to analcohol extraction (ethanol 80 %, 80 °C) and the 14C-scintillation of insoluble material was determined.
Last internode including the apical part and the second metaphyll withinitial development.
An estimate of the relative sink intensity was obtained by calculating the percentage of radioactivity per dry mass in every seedling part. Development of the shoots was associated with stronger sink intensity as compared with the roots (Table 3). Furthermore, 17, 23 and 36 % of the radioactivity were found in the insoluble fractions of roots, hypocotyl and first metaphyll, respectively (Table 3), indicating a higher carbon incorporation in structural compounds of leaves relative to other parts of the seedling.
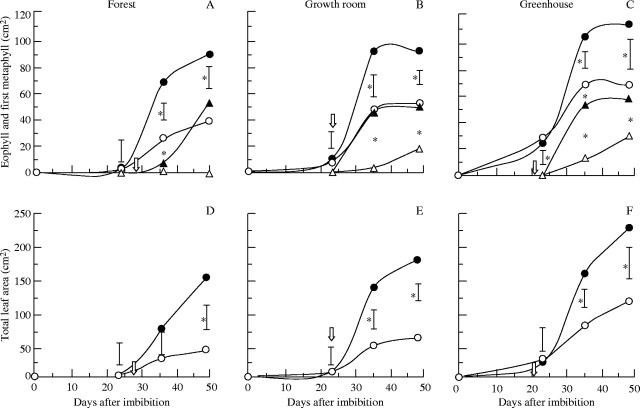
For 50-d-old seedlings it was observed that eophyll development was strongly affected by cotyledon detachment, limiting leaf area by 37, 45 and 60 % (Fig. 4A–C) for greenhouse, growth room and forest light conditions, respectively. The development of the first metaphyll was affected in a similar fashion as the eophyll, but in this case the reduction was stronger, reaching 100 % in the forest. The strongest effect of cotyledon detachment was observed on seedlings growing in the forest, where the reduction of total leaf area was approx. 77 %. For the other environmental conditions, where light intensity was considerably higher (Table 1), the reduction was progressively smaller (60 % for growth room and 50 % for greenhouse).
Fig. 4.
Changes in leaf area of seedlings of H. courbaril growing with (closed symbols) or without (open symbols) cotyledons in forest (A, D), growth room (B, E) and greenhouse (C, F). (A–C) Area of eophylls (circles) and of first metaphyll (triangles), (D–F) summation of area of all leaves present per seedlings in each treatment. The arrows indicate the date on which the detachment of cotyledons was performed. Vertical bars represent results of a Tukey test (n = 5, P < 0·05). Asterisks indicate significant differences between treatments.
LAR was only significantly affected by the presence or absence of cotyledons in the forest (Fig. 5A). Despite the lower rate of development in the forest, 50-d-old seedlings with cotyledons presented levels of LAR that were higher than in the growth room and similar to those in the greenhouse environments. In this regard, storage xyloglucan present in cotyledons of H. courbaril appeared to be more important under lower levels of irradiation, as observed in the forest.
Fig. 5.
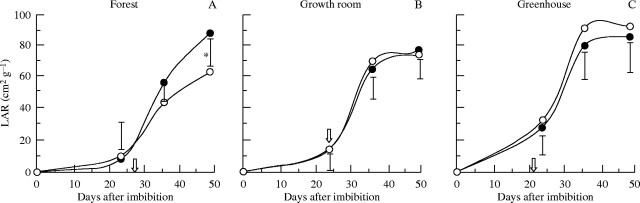
Leaf area ratio (LAR) of seedlings of H. courbaril growing with (closed symbols) or without (open symbols) cotyledons in (A) forest, (B) growth room, and (C) greenhouse. The arrows indicate the date on which the detachment of cotyledons was performed. Vertical bars represent results of a Tukey test (n = 5, P < 0·05). Asterisks indicate significant differences between treatments.
Heterotrophy–autotrophy transition
At the end of the period of storage mobilization (average 38 d), the seedlings started to depend exclusively on photosynthesis and light availability for growth so that, except for seedlings growing without cotyledons in the forest, RGRs became similar among seedlings growing with or without cotyledons (Table 2). Seedlings without cotyledons maintained lower rates of growth and development in the growth room (average 194 µmol m−2 s−1) and in the greenhouse (average 670 µmol m−2 s−1). This was related to the photosynthetic establishment and performance of the eophylls, which were able to achieve a bigger expansion in area during the period of xyloglucan mobilization (Fig. 4A–C).
The eophylls from seedlings that had their cotyledons detached attained maximum net assimilation rate approx. 5 d before those in which cotyledons were kept intact. This can be seen by following the changes in light compensation point, maximum net assimilation rate (Amax) and Fv : Fm ratio reached during expansion of the eophylls (Fig. 6). Photosynthetic establishment in seedlings was clearly correlated with changes in the colour of leaves. Leaves of seedlings that had their cotyledons detached before xyloglucan mobilization did not show the typical red colour (related to the presence of anthocyanin—M. Martins, M. Aidar and M. Buckeridge, unpubl. obs.) during the first steps of expansion.
Fig. 6.
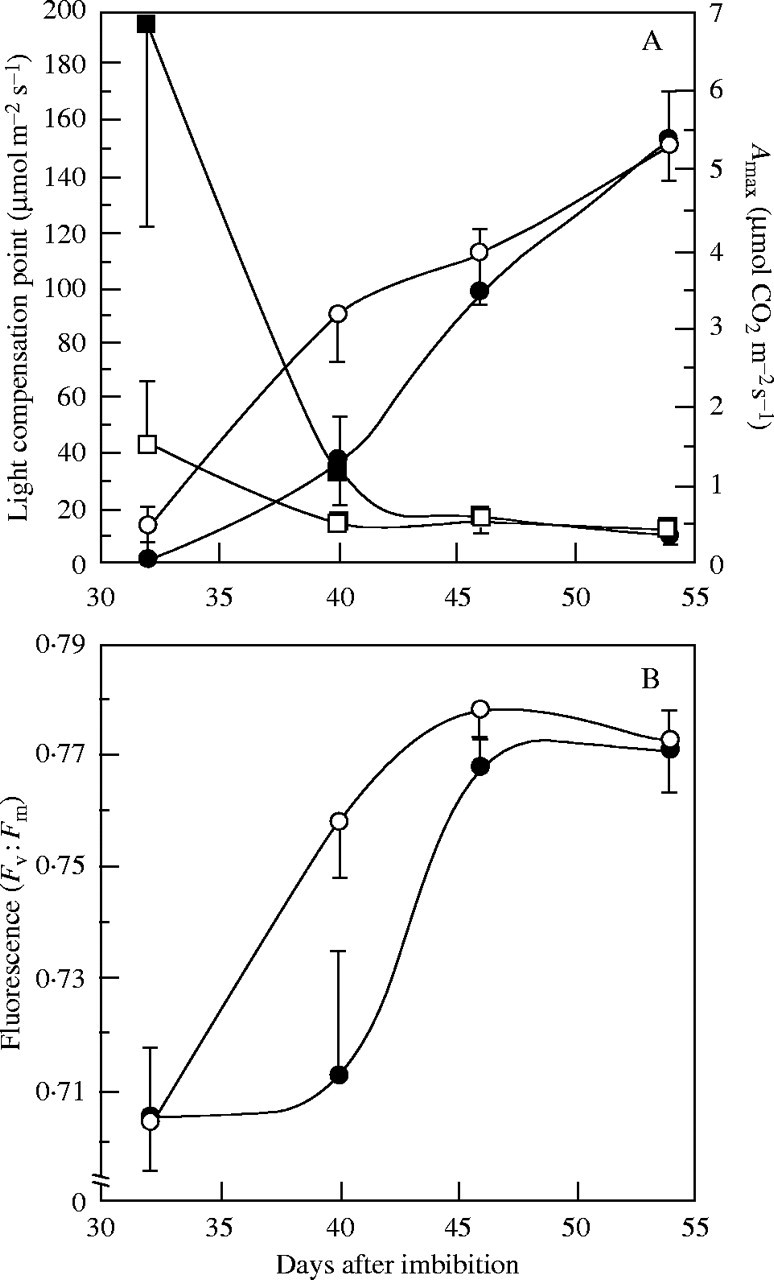
Changes in (A) light compensation point (squares) and maximum net photosynthesis (Amax, circles), and (B) fluorescence of 32-, 40-, 46- and 54-d-old eophylls of seedlings of H. courbaril growing with (closed symbols) or without (open symbols) cotyledons in a growth room. The maximum net photosynthesis was measured using 200 μmol m−2 s−1 of PPFD and 350 μmol mol−1 of CO2. Vertical bars represent the standard deviation (n = 20).
The Fv : Fm ratio, which evaluates the performance of photosystem II, increased linearly from 32 to 46 d (from 0·70 to 0·77) in developing eophylls of seedlings without cotyledons. However, in eophylls of intact seedlings, the performance of photosystem II increased only after 40 d (Fig. 6B). Thus, eophylls of H. courbaril established their light-harvesting systems as well as CO2 assimilation concurrently with the storage mobilization process, and the overlap period between storage mobilization and photosynthesis establishment (heterotrophy to autotrophy transition) could be characterized as a transition period of approx. 15 d, i.e. from 30 to 45 d.
After complete expansion, eophylls of seedlings of H. courbaril with and without cotyledons showed a light saturation at about 200 µmol m−2 s−1 (a similar PPFD level to that observed in the growth room), reaching around 6 µmol CO2 m−2 s−1 as a maximum assimilation rate with fixed CO2 concentration (Fig. 7A). When CO2 concentration was increased to almost three times the current environmental concentration (approx. 800 µmol CO2 mol−1), the expanded eophylls (54-d-old) reached only 30 % more (2 µmol CO2 m−2 s−1) in both seedlings with and without cotyledons (Fig. 7B). Growth and development observed for seedlings without cotyledons growing in the greenhouse and in the growth room were directly related to the photosynthetic performance of the eophylls and to the light conditions, which were similar to or above light saturation level in these environments. On the other hand, after the period of xyloglucan mobilization in seedlings grown in the forest, only those in which the cotyledons were kept produced a photosynthetic leaf area capable of supporting autotrophic growth under the light conditions found in the understorey of the forest (see RGR at 36–49 d, Table 2).
Fig. 7.
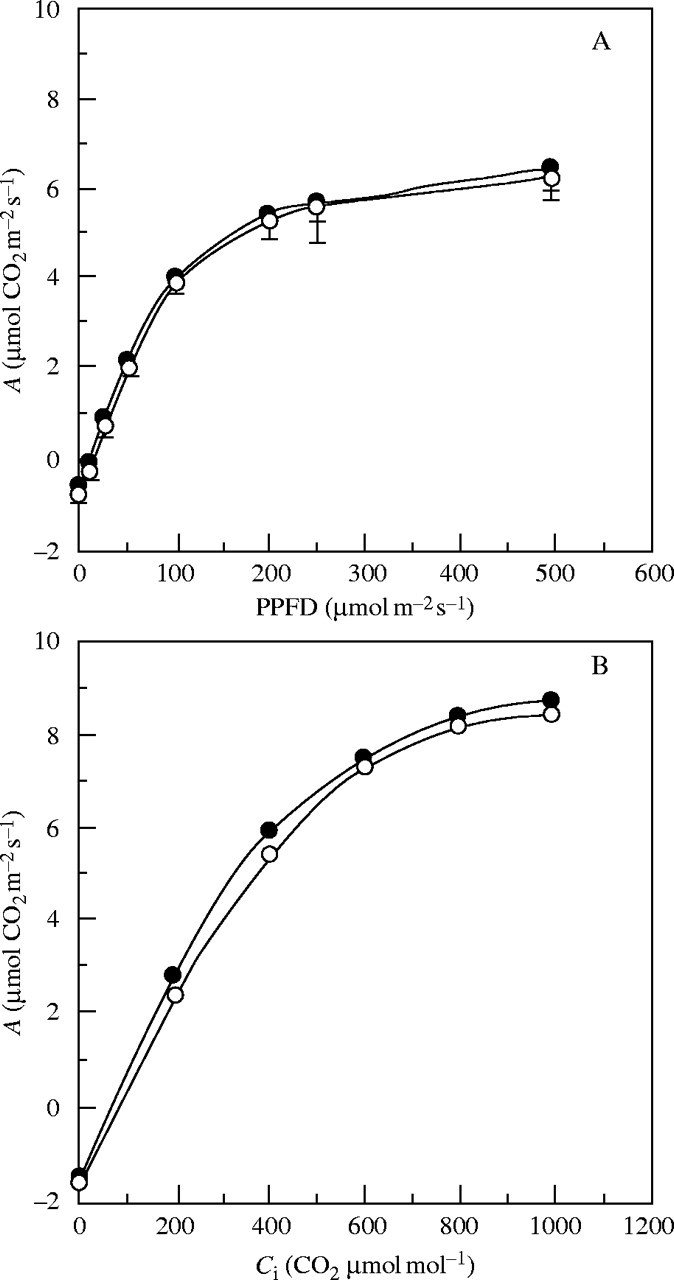
Response of net photosynthesis (A) to (A) photosynthetic photon flux density (PPFD), and (B) internal concentration of CO2 (Ci) by 54-d-old eophylls of seedlings of H. courbaril growing with (closed symbols) or without (open symbols) cotyledons in a growth room. The curves in (A) were obtained with constant CO2 at 340 μmol mol−1, and in (B) with constant PPFD at 200 μmol m−2 s−1. Vertical bars represent the standard deviation (n = 20).
DISCUSSION
Storage mobilization and seedling development are synchronized
Our results confirm and extend the observations that carbon reserves (mainly xyloglucan) in cotyledons of H. courbaril are mobilized after germination and their degradation is concurrent with the development of the seedling (Tiné et al., 2000). We have shown that even when seedlings develop under contrasting environmental conditions, xyloglucan mobilization takes place after emergence of the eophylls, and the pace of mobilization is synchronized with the expansion of the eophylls and the first metaphyll as well as with seedling development. This interdependence between cotyledons and shoots has been consistently characterized by the observation that xyloglucan catabolism is strictly controlled by auxin produced in the shoots and transported downwards (Santos et al., 2004).
The environmental factors influencing these synchronized phenomena probably change depending on the developmental stage. Thus, during germination and until the stage at which the eophylls become photosynthetically active, one of the main environmental factors affecting the physiological and biochemical processes appears to be temperature. Furthermore, during this first phase, the raffinose series oligosaccharides are the principal carbon reserve in H. courbaril (Tiné et al., 2000) and the temperature may be co-ordinating this mobilization to the developing seedling axes and determining the moment of radicle protrusion.
The second phase follows when both processes, storage mobilization in the cotyledon and photosynthesis in the developing eophylls, are active at the same time. Our results suggest that the start and duration of this overlapping period depend on the growth rate of the seedling, which was influenced by temperature and, in particular, light in the three environments. Under the conditions found in the forest, the presence of the cotyledons was crucial in maintaining a minimal level of leaf expansion to enable the seedlings to tolerate a low-light environment. On the other hand, under conditions where light intensity was higher (greenhouse and growth room), photosynthesis appears to have had a more important role in development, resulting in seedlings that were taller and with greater total leaf area than those in the forest. This has to be analysed in view of the events occurring during the course of seedling development. Whereas 50-d-old seedlings (with cotyledons) grown in the greenhouse produced four leaves, in the forest only the eophyll and the first metaphyll were fully expanded. These observations can be compared with results obtained with Desmodium paniculatum, where growth was directly related to the availability of light and seed reserves (Wulf, 1986). Furthermore, our previous results also indicate that storage mobilization in the cotyledons of H. courbaril is able to buffer the effect of incubation of developing seedlings under high atmospheric CO2 concentration (Aidar et al., 2002). Therefore, at this stage, the two metabolic pathways of carbon in the seedling appear to compete or act antagonistically when the availability of light was maximized experimentally.
After the overlapping storage mobilization–photosynthesis period, a third phase follows during which the shrunken cotyledons fall and the seedling becomes dependent exclusively on photosynthesis. In this last phase, light and light-harvesting capacity become relatively more important influences on seedling development (Leishman and Westoby, 1994; Welander and Ottosson, 1998). This probably explains why only the seedlings with cotyledons growing in the forest maintained high RGR, whereas in the greenhouse and growth room growth rates were maintained independently of the presence of the cotyledons.
In terms of light-harvesting capacity, the present work shows that the total leaf area of seedlings that developed with cotyledons is approximately twice that of those that grew without them. This suggests that this species, which is thought to be well adapted to shade conditions, uses xyloglucan as part of a strategy to produce a larger leaf area. This probably provides means to avoid a negative carbon budget, as suggested by Foster (1986) and DeLucia et al. (1998). The synchronism between storage mobilization and construction of the photosynthetic apparatus is clearly indicated by the results presented for evaluation of the light-harvesting capacity of the leaves (eophylls), as well as the CO2 assimilation capacity. The fact that light saturation occurred at 200 µmol m−2 s−1 with a rather low light compensation point (11·8 µmol m−2 s−1) indicates that H. courbaril indeed presents a high degree of tolerance to shade. These findings agree with those of Whitmore (1996) and Kitajima (1994) who found similar performance in photosynthetic systems of several tropical shade-tolerant climax trees.
Paulilo and Felippe (1998) and Souza and Válio (1999) studied plantlets of H. courbaril and concluded that this species can be considered to be shade tolerant. However, no work has been performed with the aim of understanding the mechanisms that allow this species to become established in the understorey of tropical rain forests. Among the reasons why seedlings of H. courbaril can be classified as shade tolerant is the fact that the species has large seeds (approx. 5 g per seed) which, from an ecophysiology perspective, is considered to be associated with the capacity to adapt to shaded environments (Foster, 1986; Westoby et al., 1992; Leishman and Westoby, 1994). Seed size has also been associated with the functional type of the cotyledons (Kitajima, 1996). Seedlings that develop under very low light intensities usually contain larger quantities of reserves so that the amount of carbon stored is, after mobilization to developing leaves, sufficient to guarantee that the new individual will be capable of performing photosynthesis (Wulf, 1986; Paz and Martínez-Ramos, 2003). This is the case of H. courbaril, which has non-CO2-assimilating cotyledons that store large amounts of xyloglucan (Buckeridge and Dietrich, 1990; Santos et al., 2004). Such behaviour can be contrasted with species capable of developing quickly under higher light intensities, such as Schizolobium parahybum (Malavasi and Malavasi, 2001). In the Leguminosae, some species have leaf-like photosynthetic cotyledons and the proportion of carbon reserves is either very low (e.g. in Piptadenia gonoacantha and Anadenanthera falcata) or quite high—but in the latter case the storage compounds can be present as endospermic cell-wall polysaccharides (galactomannan; see Buckeridge et al., 2000a for a review).
Several authors have shown that the R : S ratio decreases with development of seedlings adapted to shaded environments, which corresponds to an increase in leaf area ratio (Foster, 1986; Westoby et al., 1992; Kitajima, 1996; DeLucia et al., 1998; Huante and Rincón, 1998; Lei and Lechowicz, 1998). We have demonstrated that in H. courbaril the shoots are the main carbon sink tissues, since in experiments where radioactive sucrose was injected into one of the cotyledons during storage mobilization, radioactivity was directed mainly (approx. 70 %) to the shoots. It is interesting to note that after the storage mobilization period (third phase), this pattern of carbon partitioning seems to invert. Souza and Válio (1999) found that radioactive sucrose injected into the leaves of 140-d-old seedlings of H. courbaril (i.e. long after mobilization) was directed mainly to the lower parts of the seedling. This probably explains the fact that under lower light intensity (i.e. in the forest and growth room) the R : S ratio decreased during the storage mobilization period whereas no difference was observed for seedlings growing under higher light intensity in the greenhouse. On the basis of these results, it can be suggested that during the initial phases of seedling establishment in shade conditions the carbon reserves are used to build the photosynthetic shoots, as previously observed for other species (Wulf, 1986; Leishman and Westoby, 1994). However, after becoming capable of producing sugars, the leaves of seedlings of H. courbaril start to direct carbon towards the root system.
The role of storage compounds of cotyledons in seedling establishment
From a general perspective, our results suggest that the storage compounds (mainly xyloglucan) found in the cotyledons appear to play an important role as an ecological advantage for the establishment of seedlings of H. courbaril. Furthermore, the growth and development of seedlings with and without cotyledons tended to have an analogous behaviour, as previously reported for species with seed of different sizes (Wulf, 1986; Westoby et al., 1992; Armstrong and Westoby, 1993; Leishman and Westoby, 1994; Bonfil, 1998; Paz and Martínez-Ramos, 2003).
Westoby et al. (2002) identified four leading dimensions of ecological variation among species that can help in understanding plant ecological strategies. One of these dimensions is the leaf mass per area-leaf life span (LMA-LL), which is involved in the maintenance of a positive budget during plant growth. According to Kikuzawa's theory (1995), for leaf life span during the initial period of leaf development, there is a period of ‘investment’ in which leaf tissues are being built and need carbon and energy from other plant parts. In the case of seedlings, this period can be dependent on an immediate establishment of photosynthesis, so that development of the shoot will depend mainly on cotyledon photosynthetic capacity and stored nitrogen (storage proteins). For this strategy to reach maximal performance, adequate light intensity is crucial, so that the establishment of a seedling of this type would be expected in more open areas in the forest. On the other hand, for seedlings that establish under less illuminated conditions, such as the understorey of rain forests (in which we found PPFD at midday to be as low as 11 µmol m−2 s−1), such a strategy may be less adaptive since the photosynthetic potential to support early growth is very low. Thus, seedlings that establish in these conditions will have an enormous advantage if their seeds supply the largest amount of carbon reserves possible in order to maximize LMA-LL.
The carbon reserves of cotyledons may be playing yet another ecophysiological role during the establishment of H. courbaril seedlings in the forest. This is related to resistance or tolerance of seedlings to herbivory, since the absence of cotyledons may reduce levels of carbohydrates and thus the ability of seedlings to re-grow new leaves (Bonfil, 1998). We consistently observed the loss of the characteristic red colour of young leaves of seedlings growing without cotyledons. It is thought that the low levels of carbohydrates are also modulated by the expression of photosynthetic genes in the new leaves (Pego et al., 2000). The red colour of the leaves is a typical phenomenon of shade-tolerant species and is related to protection against herbivores during leaf expansion and the formation of chlorophyll (Lambers et al., 1998). This red colour in the foliage is classically attributed to anthocyanins, which are thought to protect leaf nutrient resorption during leaf senescence by shielding photosynthetic tissues from excess light (Hoch et al., 2003). This process has been related to a ‘sugar-sensing’ mechanism and is associated with levels of sucrose and hexoses (Smeekens, 2000). The presence or absence of intense red colour in young leaves of H. courbaril has been observed to be dependent on the presence or absence of the cotyledons, respectively. This appears to indicate the occurrence of storage carbohydrate mobilization somewhere in the plant and highlights the possible presence of an active carbohydrate signal mechanism associated with the link between the cotyledons and leaf development.
Our results help to indicate the extent to which storage compounds present in the cotyledons contribute to improving the performance of the seedlings throughout the three different phases that seedlings have to go through in order to become established. Hymenaea courbaril is known as one of the main species in a genus that is extremely plastic, occurring either in humid (gallery forests) or in arid (savannah and caatinga) environments (Lee and Langenheim, 1975). Our experiments, providing a range of environmental conditions and measuring the performance of the seedlings in a variety ways, have demonstrated the importance of the plasticity of the ecophysiological responses of seedlings of H. courbaril to the enormous success that this species has achieved in the Neotropical region.
Acknowledgments
This study was supported by Fundação de Amparo a Pesquisa do Estado de São Paulo (BIOTA-Fapesp 98/05124-8). The authors thank Mrs A. M. Baroni and L. I. V. Amaral for technical assistance with photosynthesis and radioactivity analyses. The authors also acknowledge the excellent suggestions made by Dr C. Bonfil.
LITERATURE CITED
- Aidar MPM, Martinez CA, Costa AC, Costa PMF, Dietrich SMC, Buckeridge MS. 2002. Effect of atmospheric CO2 enrichment on the establishment of seedlings of jatobá, Hymenaea courbaril L. (Leguminosae, Caesalpinioideae). Biota Neotropica 2:(http://www.biotaneotropica.org.br/v2n1/en/abstract?article+/BN01602012002). [Google Scholar]
- Armstrong DP, Westoby M. 1993. Seedlings from large seeds tolerate defoliation better: a test using phylogenetically independent contrasts Ecology 74: 1092–1100. [Google Scholar]
- Bonfil C. 1998. The effects of seed size, cotyledon reserves, and herbivory on seedling survival and growth in Quercus rugosa and Q. laurina (Fagaceae). American Journal of Botany 85: 79–87. [PubMed] [Google Scholar]
- Buckeridge MS, Dietrich SMC. 1990. Galactomannan from Brazilian legume seeds. Revista Brasileira de Botânica 13: 109–112. [Google Scholar]
- Buckeridge MS, Dietrich SMC, Lima DU. 2000. Galactomannans as the reserve carbohydrate in legume seeds. In: Gupta AK, Kaur N, eds. Carbohydrate reserves in plants—synthesis and regulation. Amsterdam: Elsevier, 283–316. [Google Scholar]
- Buckeridge MS, Santos HP, Tiné MAS. 2000. Mobilization of storage cell wall polysaccharides in seeds. Plant Physiology and Biochemistry 38: 141–156. [Google Scholar]
- Cao KF, Ohkubo T. 1998. Allometry, root/shoot ratio and root architecture in understory saplings of deciduous dicotyledoneous trees in central Japan. Ecological Research 13: 217–227. [Google Scholar]
- DeLucia EH, Sipe TW, Herrick J, Maherali H. 1998. Sapling biomass allocation and growth in the understory of deciduous hardwood forest. American Journal of Botany 85: 955–963. [PubMed] [Google Scholar]
- Flores EM, Benavides CE. 1990. Germinación y morfología de la plántula de Hymenaea courbaril L. (Caesalpinaceae). Revista de Biologia Tropical 38: 91–98. [Google Scholar]
- Foster SA. 1986. On the adaptive value of large seeds for tropical moist forest trees: a review and synthesis. The Botanical Reviews 52: 260–299. [Google Scholar]
- Gerhardt K. 1993. Tree seedling development in tropical dry abandoned pasture and secondary forest in Costa Rica. Journal of Vegetation Science 4: 95–102. [Google Scholar]
- Givnish TJ. 1988. Adaptation to sun and shade, a whole-plant perspective. Australian Journal of Plant Physiology 15: 63–92. [Google Scholar]
- Huante P, Rincón E. 1998. Responses to light changes in tropical deciduous woody seedlings with contrasting growth rates. Oecologia 113: 53–66. [DOI] [PubMed] [Google Scholar]
- Hoch WA, Singsaas EL, McCown BH. 2003. Resorption protection. Anthocyanins facilitate nutrient recovery in autumn by shielding leaves from potentially damaging light levels. Plant Physiology 133: 1296–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuzawa K. 1995. The basis for variation in leaf longevity in plants. Vegetation 121: 89–100. [Google Scholar]
- Kitajima K. 1994. Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia 98: 419–428. [DOI] [PubMed] [Google Scholar]
- Kitajima K. 1996. Cotyledon functional morphology, patterns of seed reserve utilization and regeneration niches of tropical tree seedlings. In: Swaine MD, ed. Ecology of tropical forest tree seedlings—man and the biosphere series, 17, France: UNESCO/Parthenon, 193–210. [Google Scholar]
- Lambers H, Chappin III FS, Pons TL. 1998. Life cycles: environmental influences and adaptations. In: Lambers H, Chappin III F S, Pons T L, eds. Plant physiological ecology. New York: Springer-Verlag, 352–375. [Google Scholar]
- Lee Y, Langenheim JH. 1975.Systematics of the genus Hymenaea L. (Leguminosae, Caesalpinioideae, Detarieae) Berkeley: University of California Publications in Botany, 69. [Google Scholar]
- Lei TT, Lechowicz MJ. 1998. Diverse responses of maple saplings to forest light regimes. Annals of Botany 82: 9–19. [Google Scholar]
- Leishman MR, Westoby M. 1994. The role of large seed size in shaded conditions: experimental evidence. Functional Ecology 8: 205–214. [Google Scholar]
- Malavasi UC, Malavasi MM. 2001. Leaf characteristics and chlorophyll concentration of Schyzolobium parahybum and Hymenaea stilbocarpa seedlings grown in different light regimes. Tree Physiology 21: 701–703. [DOI] [PubMed] [Google Scholar]
- Palit P. 1985. Translocation and distribution of 14C-labelled assimilate associated with growth of jute (Corchorus olitorius L.). Australian Journal of Plant Physiology 12: 527–534. [Google Scholar]
- Paulilo MTS, Felippe GM. 1998. Growth of the shrub-tree flora of the Brazilian cerrados: a review. Tropical Ecology 39: 165–174. [Google Scholar]
- Paz H, Martínez-Ramos M. 2003. Seed mass and seedling performance within eight species of Psychotria (Rubiaceae). Ecology 84: 439–450. [Google Scholar]
- Pego JV, Kortstee AJ, Huijser C, Smeekens SCM. 2000. Photosynthesis, sugars and the regulation of gene expression. Journal of Experimental Botany 51(special issue): 407–416. [DOI] [PubMed] [Google Scholar]
- Santos HP, Mercier H, Purgatto E, Buckeridge MS. 2004. The control of storage xyloglucan mobilization in cotyledons of Hymenaea courbaril L. Plant Physiology 135: 287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza RP, Válio IFM. 1999. Carbon translocation as affected by shade in saplings of shade tolerant and intolerant species. Biologia Plantarum 42: 631–636. [Google Scholar]
- Smeekens S. 2000. Sugar-induced signal transduction in plants. Annual Review of Plant Physiology and Plant Molecular Biology 51: 49–81. [DOI] [PubMed] [Google Scholar]
- Tiné MAS, Cortelazzo AL, Buckeridge MS. 2000. Xyloglucan mobilization in cotyledons of developing plantlets of Hymenaea courbaril L. (Leguminosae-Caesalpinoideae). Plant Science 154: 117–126. [DOI] [PubMed] [Google Scholar]
- Welander NT, Ottosson B. 1998. The influence of shading on growth and morphology in seedlings of Quercus robur L. and Fagus sylvatica L. Forest Ecology and Management 107: 117–126. [Google Scholar]
- Westoby M, Falster DS, Moles AT, Vesk PA, Wright IJ. 2002. Plant ecological strategies: some leading dimensions of variation between cspecies. Annual Review of Ecology and. Systematics 33: 125–59. [Google Scholar]
- Westoby M, Jurado E, Leishman M. 1992. Comparative evolutionary ecology of seed size. Tree 7: 368–372. [DOI] [PubMed] [Google Scholar]
- Whitmore TC. 1996. A review of some aspects of tropical rain forest seedling ecology with suggestions for further enquiry. In: Swaine MD, ed. Ecology of tropical forest tree seedlings—man and the biosphere series, 17, Paris: UNESCO/Parthenon, 3–39. [Google Scholar]
- Wulf R. 1986. Seed size variation in Desmodium paniculatum II. Effects on seedling growth and physiological performance. Journal of Ecology 74: 99–114. [Google Scholar]
- Zonta EP, Machado AA. 1991.Manual do SANEST: Sistema de análise estatística para microcomputadores. Pelotas: UFPel, 102pp. [Google Scholar]