Abstract
• Background and Aims The aim of this study was to determine the role of nitrogen- and storage-affected carbohydrate availability in rooting of pelargonium cuttings, focusing on the environmental conditions of stock plant cultivation at low latitudes, transport of cuttings, and rooting under the low light that prevails during the winter rooting period in Central European greenhouses.
• Methods Carbohydrate partitioning in high-light-adapted cuttings of the cultivar ‘Isabell’ was studied in relation to survival and adventitious root formation under low light. Effects of a graduated supply of mineral nitrogen to stock plants and of cutting storage were examined.
• Key Results Nitrogen deficiency raised starch levels in excised cuttings, whereas the concentrations of glucose and total sugars in leaves and the basal stem were positively correlated with internal total nitrogen (Nt). Storage reduced starch to trace levels in all leaves, but sugar levels were only reduced in tissues of non-nitrogen deficient cuttings. Sugars accumulated in the leaf lamina of stored cuttings during the rooting period, whereas carbohydrates were simultaneously exhausted in all other cutting parts including the petioles, thereby promoting leaf senescence. The positive correlation between initial Nt and root number disappeared after storage. Irrespectively of storage, higher pre-rooting leaf glucose promoted subsequent sugar accumulation in the basal stem and final root number. The positive relationships between initial sugar levels in the stems with cutting survival and in leaves with root formation under low light were confirmed in a sample survey with 21 cultivars provided from different sources at low latitudes.
• Conclusions The results indicate that adventitious rooting of pelargonium cuttings can be limited by the initial amount of nitrogen reserves. However, this relationship reveals only small plasticity and is superimposed by a predominant effect of carbohydrate availability that depends on the initial leaf sugar levels, when high-light adaptation and low current light conditions impair net carbon assimilation.
Key words: Adventitious rooting, senescence, nitrogen, sugars, carbohydrates, quality, storage, cuttings, geranium, Pelargonium × hortorum
INTRODUCTION
Carbohydrate distribution within a plant is affected by the nitrogen supply, which strongly influences the processes of carbon assimilation, allocation and partitioning (Kaiser, 1997). In general, decreased concentrations of total non-structural carbohydrates (TNC) are observed with an increasing nitrogen supply. However, the magnitude, and even the direction, of responses are highly specific for individual carbohydrates and different plant tissues, and further depend on other environmental factors (Reddy et al., 1996; Druege et al., 2000). Both the nitrogen and carbohydrate status have a great impact on the post-harvest performance of plants (Druege, 2000). Such relations are even more complicated not only when high survival and continuous development are expected, but also when the harvested product is a starting material that requires a particular subsequent response, such as the regeneration of adventitious roots. Adventitious root formation of cuttings, which is the most important regeneration process used in the propagation of ornamental plants, is substantially affected by the initial status of both nitrogen and carbohydrate (Blazich, 1988; Veierskov, 1988). Given the conflicting reports on these relationships (Hansen et al., 1978; Haissig, 1986; Henry et al., 1992; Leakey and Storeton-West, 1992; Pellicer et al., 2000), it appears that the question as to whether the initial nitrogen or carbohydrate status constitute a limiting factor for subsequent root formation cannot be answered in general terms, and the plant species and the environmental conditions during rooting have to be taken into consideration. It has previously been demonstrated that even very high nitrogen concentrations in chrysanthemum cuttings have a predominantly promotive influence on subsequent adventitious root formation under light conditions allowing for high current photosynthesis. This was irrespective of simultaneously lower carbohydrate concentrations, which were observed especially after additional cold storage (Druege et al., 2000). Both carbohydrate distribution and root formation were strongly dependent on internal total nitrogen (Nt) and to a lesser extent on the nitrate level in cuttings.
The production of young pelargonium plants in Europe relies on the worldwide cultivation of stock plants from, for example, Southern Europe, North Africa and Central America, and the subsequent storage, transport and rooting of excised cuttings in Central Europe. The survival and adventitious root formation of such cuttings are affected not only directly by the unfavourable conditions during storage and transport (Kadner et al., 2000), but also by insufficient adaptation of the cuttings, which are produced under the high light intensities of low latitudes and are thus not adapted to the prevailing low light conditions during the winter rooting period in Central European greenhouses (Forschner and Reuther, 1984). Relationships found between initial carbohydrates and subsequent rooting of unstored zonal pelargonium cuttings (Reuther and Roeber, 1980) and effects of pre-storage sugar application on post-storage rooting (Paton and Schwabe, 1987) have indicated that this plant species is sensitive to carbohydrate availability.
This paper focuses on the role of nitrogen- and storage-affected carbohydrate availability in cutting survival and adventitious root formation under low light conditions. A greenhouse experiment was carried out in order to examine the effects of a graduated nitrogen supply to the stock plants and cold storage of cuttings. The relationship between Nt, the partitioning of non-structural carbohydrates in high-light-adapted pelargonium cuttings and the rooting response was studied. This was followed by a sample survey of cuttings provided by propagators from different local sources at low latitudes.
MATERIALS AND METHODS
Plant material and treatments
A greenhouse experiment was carried out with Pelargonium × hortorum L.H. Bailey ‘Isabell’. A factorial design was used, with three nitrogen treatments of stock plants and two storage treatments of excised cuttings (unstored and stored), using three replicates. In January, fifty young plants for each replication plot (in total, nine plots = 450 stock plants) were planted in a thin layer (5 cm) of low-fertilized commercial peat (Einheitserde Typ P, Patzer Company, Germany) at a density of 14 plants m−2. Plants were pinched back to four leaves after 1 week and then were managed as stock plants. Water was supplied manually. In the same manner, all plants received the same basic nutrient supply with the application of about 12 L m−2 week−1 of nutrient solution, containing 0·2 to 0·8 g L−1 Flory Basis 2 (3 % nitrate-N + 15 % P2O5 + 35 % K2O + 5 % MgO + microelements; Euflor Company, Germany) and 0·24 g L−1 calcium nitrate (19 % Ca + 14·5 % nitrate-N + 1 % ammonium-N). According to the method of Druege et al. (2000), three nitrogen dosages (N-low, N-medium and N-high: 0·5, 1·5 and 4·0 g N m−2 week−1, respectively) were adjusted by combining the basic nutrient solution with different doses of ammonium nitrate (9 % nitrate-N + 9 % ammonium-N). Plants were shaded when natural radiation exceeded 630 µmol m−2 s−1. The means of continuously measured photosynthetic flux densities (PPFD) in the greenhouse were calculated over an average daylength of 16 h as 206 µmol m−2 s−1 (400 to 700 nm) during the whole cultivation period, and as 281 µmol m−2 s−1 (400 to 700 nm) during the period when the cuttings were harvested (from 15 to 23 weeks after planting). Heating/ventilation set points for temperature were 19/20 °C (day) and 17/18 °C (night), providing an average air temperature of 19·4 °C and a relative humidity of 57·0 %. Cuttings were harvested on three occasions (15, 20 and 23 weeks after planting the stock plants). Terminal cuttings with three leaves, of which at least one was fully developed, were removed with a sterilized knife, leaving the first two leaves of the axillary shoot on the stock plant. For each replication plot, 138 cuttings were divided into three lots. The first lot of 50 cuttings (L1) was used for analyses of Nt in the dry matter of whole cuttings. The second lot of 44 cuttings (L2) was used for immediate rooting to study the dry mass (DM) and carbohydrates during propagation (L2·1: 3 × 8 = 24 cuttings for days 1 (insertion), 6 and 13) and the rooting response (L2·2: 20 cuttings). The third lot (L3) of 44 cuttings (L3·1: 24 cuttings; L3·2: 20 cuttings) was first stored and then subsequently used in the same manner. For storage, the cuttings were immediately transferred into perforated polyethylene bags, packed in cardboard boxes and then kept in the dark at 10 °C for 1 week.
Following the experiment, a sample survey of cuttings supplied from different propagators was carried out. During a winter season (November to January), seven batches of cuttings of Pelargonium × hortorum consisting of 21 cultivars including the post-harvest-sensitive ‘Mitzou’ (Kadner et al. 2000) were delivered to our institute from four different local sources in low latitudes (Table 1). Cuttings were packed in perforated polyethylene bags at low temperatures and then transported via air in cardboard boxes. Selective recording of transport conditions revealed transport periods of 46 to 63 h and tremendous fluctuations in temperature, ranging from 3·2 °C to 18·0 °C.
Table 1.
External cutting samples of Pelargonium × hortorum transported from Mexico (M, four batches), France (F), Spain (Sp) and the Canary Islands (CI)
| Local source |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cultivar |
M1 |
M2 |
M3 |
M4 |
Fr |
Sp |
CI |
||||||
| ‘Colorado’ | S | – | – | – | – | – | – | ||||||
| ‘Rosecrystal’ | S | – | – | – | – | – | – | ||||||
| ‘Lovesong’ | S | – | – | – | – | – | – | ||||||
| ‘Serena’ | S | – | – | – | – | – | – | ||||||
| ‘Laura’ | S | – | – | – | – | – | – | ||||||
| ‘Sassa’ | S | – | – | – | – | – | – | ||||||
| ‘Glacis’ | S | – | – | – | – | – | – | ||||||
| ‘Lorca’ | S | – | – | – | – | – | – | ||||||
| ‘Areno’ | S | – | – | – | – | – | – | ||||||
| ‘Faro’ | S | S | S | – | – | – | – | ||||||
| ‘Sidonia’ | S | – | S | S | S | – | – | ||||||
| ‘Mitzou’ | S | S | S | S | S | – | – | ||||||
| ‘Lachsball’ | – | S | S | S | S | S | – | ||||||
| ‘Isabell’ | – | S | S | S | S | – | – | ||||||
| ‘Tamara’ | – | S | S | S | – | – | – | ||||||
| ‘Roseball’ | – | S | – | – | – | – | – | ||||||
| ‘Bergpalais’ | – | – | – | – | S | – | – | ||||||
| ‘Castelo’ | – | – | – | – | S | – | – | ||||||
| ‘Penve’ | – | – | – | – | – | S | – | ||||||
| ‘Goldenit’ | – | – | – | – | – | – | S | ||||||
| ‘Signal’ | – | – | – | – | – | – | S | ||||||
S = sampling of 9 + 9 +10 + 60 cuttings for analyses of Nt, carbohydrates, dry matter and rooting.
Determination of survival rate and adventitious rooting
Rooting was carried out in a greenhouse using perlite as a rooting substrate without application of fertilizer or plant hormones. Leaf damage, survival and adventitious rooting were studied on 20 cuttings per lot (L2·2, L3·2). To provide Central European winter rooting conditions during spring and summer, the cuttings were subjected to additional shade by a curtain. The average PPFD measured in the greenhouse was 22 ± 2 µmol m−2 s−1 (400 to 700 nm) per 16 h daylength for the different rooting periods. Adequate humidity was provided by intermittent misting, and heating/ventilation set points for day and night temperatures were 20/22 °C. The average, maximum and minimum air temperatures for the entire experiment were 22·1 °C, 38·7 °C and 19·4 °C, respectively. In the case of the sample survey, 60 cuttings from each delivered sample were rooted during the winter period (November to January) under similar conditions, but without additional shading (PPFD: 31 µmol m−2 s−1 (400 to 700 nm) per 16 h daylength). After 20 d, the percentage of decayed cuttings (almost all leaves and/or terminal buds destroyed) was calculated, and the number of roots per cutting and mean root length was determined for the remaining cuttings.
Chemical analysis
In the experiment, initial Nt was analysed in the whole cuttings of the L1 lots. In the sample survey, nine cuttings were used from each sample for the same analyses. Plant material was dried as described by Druege et al. (1998) and Nt was measured with a CHN-Rapid analyser using the Dumas method (Ehrenberger, 1991). In the experiment, eight of both unstored (L2·1) and stored cuttings (L3·1) were used for each sampling date (days 1, 6 and 13 of rooting) for determinations of carbohydrates (three cuttings) and dry matter concentrations (five cuttings) in the different cutting parts. Samples were always taken between 0700 h and 1100 h, and cuttings were separated into leaf lamina, petioles, upper stems and basal stems (1 cm). In the case of the sample survey, leaf lamina and whole stems of nine and ten cuttings were used from each sample for determinations of pre-rooting carbohydrate and dry matter concentrations, respectively, immediately after arrival. For the carbohydrate analyses, the plant material of each sample was cut into small pieces (≤25 mm3), immediately transferred to sealed polypropylene tubes containing cold aqueous ethanol (80 %, −20 °C) and then stored in a freezer below −20 °C until analysis. The dry matter of the parallel samples was determined gravimetrically after drying for 24 h at 105 °C. Sugars were extracted in 80 % ethanol (Druege et al., 1998), and glucose, fructose and sucrose concentrations were determined by an enzyme-coupled colorimetric reaction (Hendrix, 1993) using glucose-6-phosphate dehydrogenase (EC 1.1.1.49), hexokinase (EC 2.7.1.1), phosphoglucose isomerase (EC 5.3.1.9) and invertase (EC 3.2.1.26) (Sigma Chemical Company, Taufkirchen, Germany). Starch concentrations in the extraction residues were determined after digestion by amyloglucosidase (from Aspergillus niger, EC 3.2.1.3) via the glucose released as previously described (Druege et al., 2000). Corresponding to Nt, carbohydrate concentrations were related to dry matter.
Statistics
Data were analysed within the ANOVA/MANOVA and Multiple Regression modules of the Statistica software program (Statsoft, 1995). The effects of treatments on nitrogen, carbohydrate and rooting characteristics were tested by analyses of variance. Least significant differences were calculated and the comparison of mean values was carried out using the Newman–Keuls test with a significance level of at least P < 0·05. Linear regressions and correlations were calculated between concentrations of nitrogen, carbohydrate characteristics for the different cutting parts and the percentage of survival of cuttings, and the number of subsequently formed adventitious roots. In the case of the experiment, the replicates were analysed individually to cover the whole variation of data, while in the sample survey the individual plots represented the mean values from each delivered sample of cuttings.
RESULTS
Nitrogen response and pre-rooting carbohydrate distribution in cuttings
As reflected by nitrogen uptake and growth in relation to nitrogen availability, low nitrogen fertilization caused nitrogen deficiency, whereas the medium-N supply proved adequate to meet the nitrogen demand of the stock plants (Table 2). The highest supply resulted in a strong accumulation of nitrogen in the substrate, but only slightly increased Nt without significantly affecting number and fresh mass (FM) of harvested cuttings. The effects of nitrogen supply and cold storage on dry matter (DM) and carbohydrate concentrations in different cutting parts during propagation are presented in Figs. 1–4, where day 1 represents the initial condition at the beginning of the rooting period. Neither nitrogen nor storage had a significant influence on the initial DM of whole cuttings (Fig. 1A). With regard to the pre-rooting carbohydrate status, strong interactions were found between nitrogen supply and storage.
Table 2.
Effect of nitrogen supply to stock plants on the nitrogen concentration in the substrate, the nitrogen status of pelargonium cuttings at harvest and the yield of cuttings
| Nitrogen treatment |
||||||
|---|---|---|---|---|---|---|
| Character |
N-low |
N-medium |
N-high |
|||
| Substrate (n = 9) | Nitrate-N [mg L−1] | 6 ± 10 | 60 ± 32 | 342 ± 154 | ||
| Whole cutting1 | Nt [mg g−1 dry mass] | 20·1a | 36·6b | 38·7c | ||
| Cuttings2 | Number per stock plant | 14·7a | 25·5b | 24·3b | ||
| Cuttings1 | Average fresh mass [g] | 3·75a | 5·26b | 4·99b | ||
Mean and 2cumulative values of three harvest dates. Different superscripts indicate significant differences (P < 0·05) between nitrogen treatments.
Fig. 1.
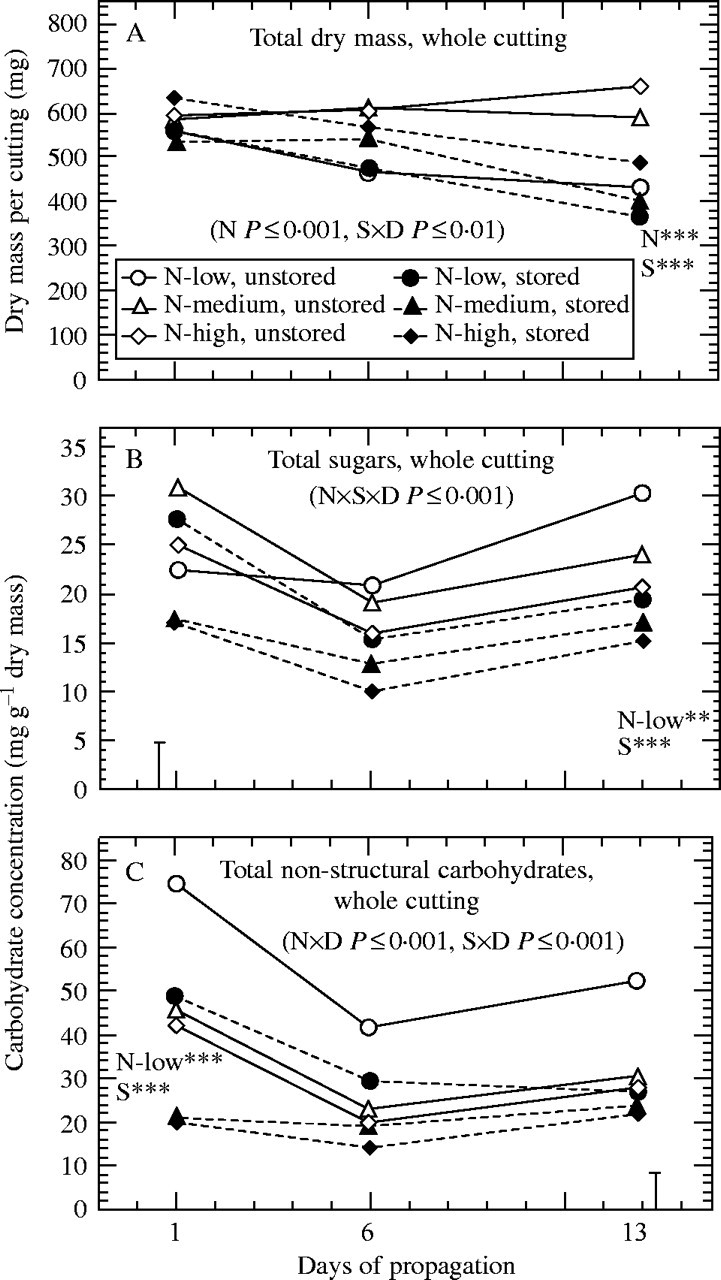
Effect of pre-harvest nitrogen supply and storage on (A) dry matter of whole cuttings, and concentrations of (B) total sugars, and (C) total non-structural carbohydrates in whole cuttings during propagation (n = 9). ×D indicates interaction with time. Where main effects are significant at days 1 and 13 (**P < 0·01, ***P < 0·001): N = significant effect of nitrogen (N-low < N-medium < N-high); S = significant effect of storage; N-low = N-low significantly different from other nitrogen treatments. In cases of significant interactions between nitrogen and storage, vertical bars represent LSD (P < 0·05).
Fig. 2.
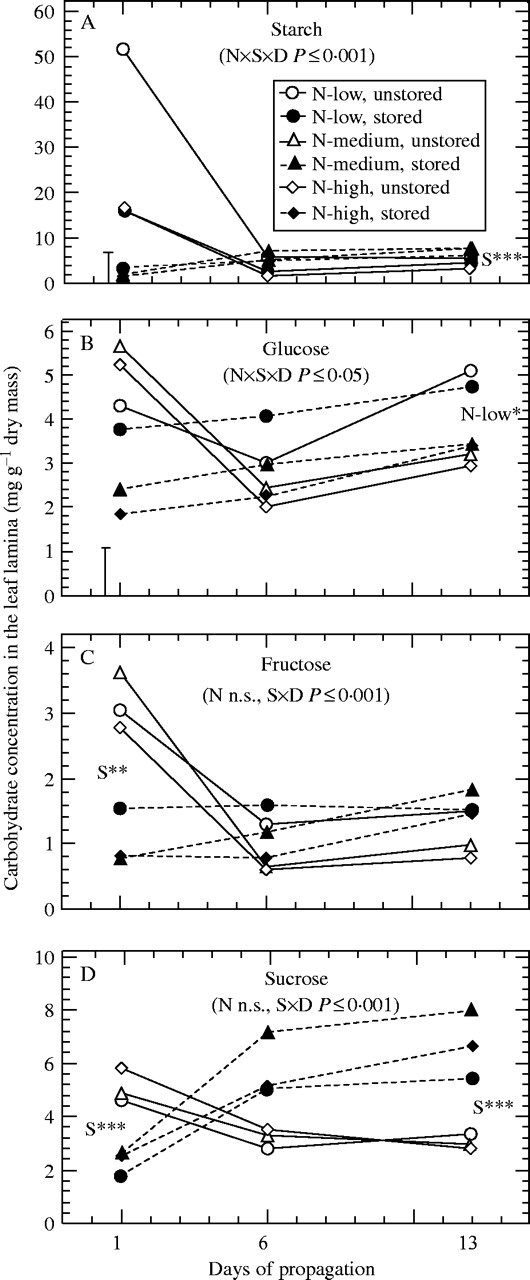
Effect of pre-harvest nitrogen supply and storage on concentrations of (A) starch, (B) glucose, (C) fructose, and (D) sucrose in the leaf lamina during propagation (n = 9). ×D indicates interaction with time. Where main effects are significant at days 1 and 13 (*P < 0·05, **P < 0·01, ***P < 0·001): N-low = N-low significantly different from other nitrogen treatments; S = significant effect of storage. In cases of significant interactions between nitrogen and storage, vertical bars represent LSD (P < 0·05).
Fig. 3.
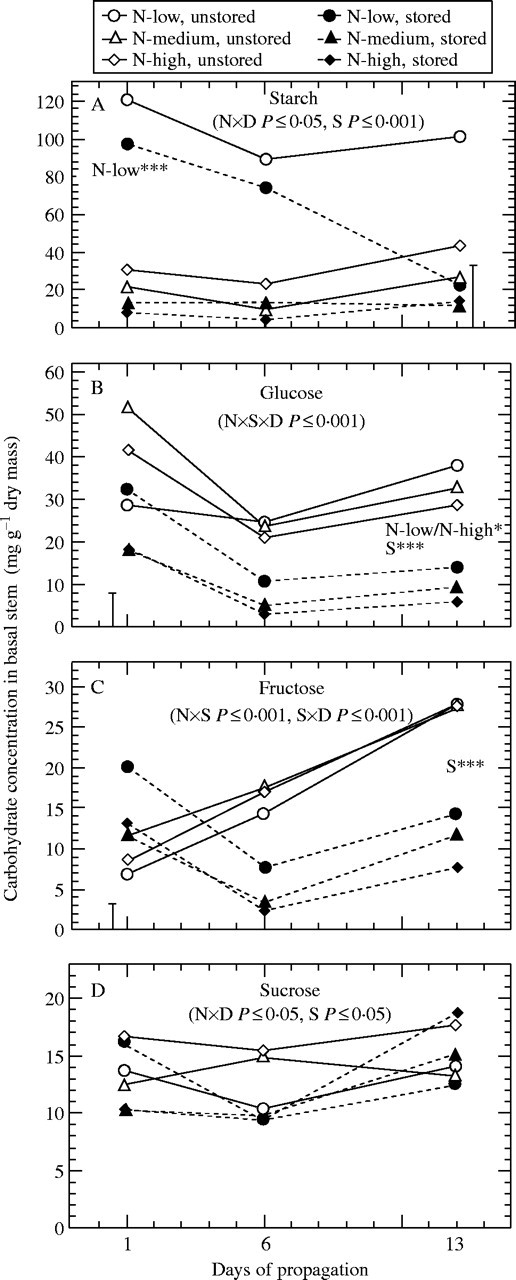
Effect of pre-harvest nitrogen supply and storage on concentrations of (A) starch, (B) glucose, (C) fructose, and (D) sucrose in the basal stem during propagation (n = 9). ×D indicates interaction with time. Where main effects are significant at days 1 and 13: N-low*** = N-low significantly different (P < 0·001) from other nitrogen treatments; N-low/N-high* = significant difference (P < 0·05) between low- and high-nitrogen; S*** = significant effect of storage at P < 0·001. In cases of significant interactions between nitrogen and storage, vertical bars represent LSD (P < 0·05).
Fig. 4.
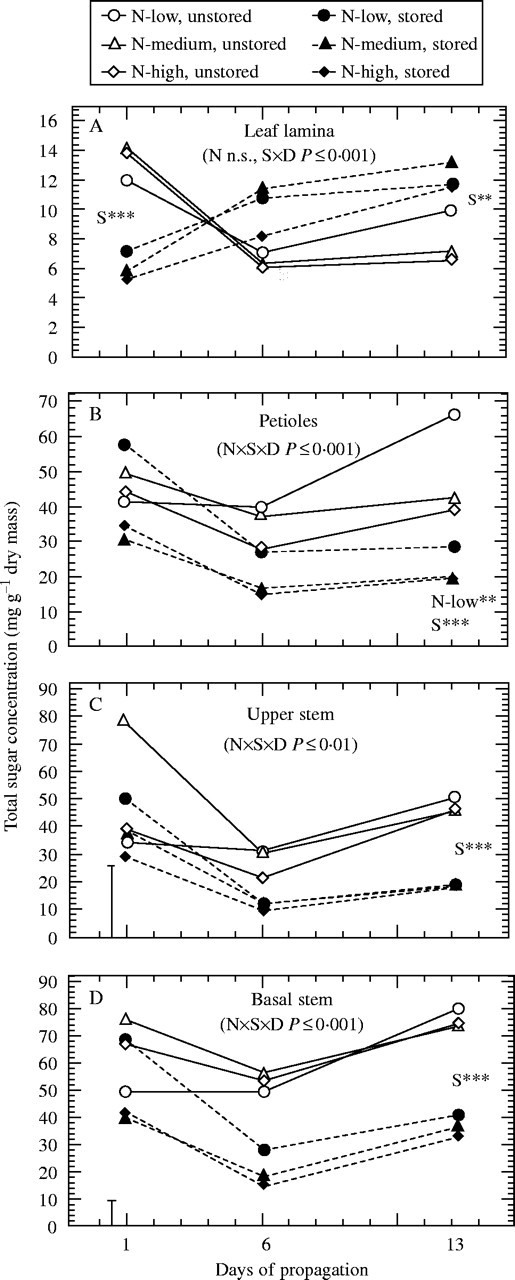
Effect of pre-harvest nitrogen supply and storage on concentrations of total sugars in (A) the leaf lamina, (B) petioles, (C) upper stem, and (D) basal stem during propagation (n = 9). ×D indicates interaction with time. Where main effects are significant at days 1 and 13: N-low** = N-low significantly different (P < 0·01) from other nitrogen treatments; S**(***) = storage significant at P < 0·01 (or P < 0·001). In cases of significant interactions between nitrogen and storage, vertical bars represent LSD (P < 0·05).
Nitrogen deficiency resulted in a strong increase of starch concentrations in the leaf lamina (Fig. 2A) and basal stems (Fig. 3A) of excised cuttings, also contributing to the highest concentrations of total non-structural carbohydrates (TNC) at the whole-cutting level (Fig. 1C). By contrast, basal stems of same cuttings contained the lowest levels of glucose and total sugars (TS) (Figs 3B, 4D). In most cases, highest sugar levels were measured when cuttings were excised from stock plants moderately supplied with nitrogen (Figs. 1–4). A further increase of nitrogen significantly decreased concentrations of glucose in the basal stem (Fig. 3B) and of TS in the upper stem (Fig. 4C) and at the whole-cutting level (Fig. 1B).
Storage decreased starch concentrations to trace amounts in the leaf lamina (Fig. 2A). This effect was less pronounced in the other cutting parts, as shown for basal stems (Fig. 3A). Whereas independently of nitrogen the levels of fructose, sucrose and TS in the leaf lamina (Figs 2C, D, 4A) and of TNC in whole cuttings (Fig. 1C) decreased during storage, the same treatment raised the concentrations of fructose and TS in basal stems of low-nitrogen cuttings (Figs 3C, 4D). Increasing the pre-harvest nitrogen supply promoted a storage-induced depletion of glucose in the leaf lamina and in basal stems (Figs 2B, 3B) and of the TS in the basal stem and at the whole-cutting level (Figs 4D, 1B). As a result, in most cases the highest initial sugar levels were found in the tissues of those stored cuttings that had been collected from the low-nitrogen treatment (Figs. 1–4).
With regard to the unstored cuttings, Nt was positively correlated with the levels of glucose in the leaf lamina and in basal stems, with the levels of fructose in basal stems, and with the levels of TS in all cutting parts and whole cuttings (Table 3). By contrast, starch and TNC concentrations were generally negatively correlated with nitrogen. Storage removed the correlations found for leaf carbohydrates at harvest, and in the case of sugars in the basal stem reversed the positive relationships into negative correlations, which also resulted in negative correlations between Nt and glucose, fructose and TS at the whole-cutting level. No correlation was observed for sugars when unstored and stored cuttings were considered in combination whereas, irrespectively of the cutting part, starch and TNC were negatively correlated with Nt (Table 3).
Table 3.
Correlation coefficients, calculated from different data pools, between Nt in whole pelargonium cuttings as independent variables and pre-rooting carbohydrate concentrations in different cutting parts as dependent variables
| Concentration of |
Tissue |
At harvest (n = 27) |
After storage (n = 27) |
All cuttings (n = 54) |
|---|---|---|---|---|
| Glucose | Leaf lamina | 0·44* | n.s. | n.s. |
| Basal stem | 0·53** | −0·68*** | n.s. | |
| Whole cutting | n.s. | −0·67*** | n.s. | |
| Fructose | Leaf lamina | n.s. | n.s. | n.s. |
| Basal stem | 0·49** | −0·49** | n.s. | |
| Whole cutting | n.s. | −0·60** | n.s. | |
| Sucrose | Leaf lamina | n.s. | n.s. | n.s. |
| Petioles | n.s. | n.s. | n.s. | |
| Whole cutting | n.s. | n.s. | n.s. | |
| Total sugars | Leaf lamina | 0·39* | n.s. | n.s. |
| Basal stem | 0·63*** | −0·66*** | n.s. | |
| Whole cuttings | 0·42* | −0·67*** | n.s. | |
| Starch | Leaf lamina | −0·73*** | n.s. | −0·42** |
| Basal stem | −0·65*** | −0·86*** | −0·72*** | |
| Whole cutting | −0·78*** | −0·86*** | −0·66*** | |
| Total non-structural carbohydrates | Leaf lamina | −0·60*** | n.s. | −0·32* |
| Basal stem | −0·58** | −0·85*** | −0·70*** | |
| Whole cutting | −0·64*** | −0·82*** | −0·60*** |
Total sugars = glucose + fructose + sucrose; total non-structural carbohydrates = total sugars + starch.
n.s., non-significant;
P < 0·001;
P < 0·01;
P < 0·05.
Dry matter and carbohydrate dynamics during root formation
After the first week of propagation no adventitious roots were visible, and also at day 13, independent of the treatment, 33 % of cuttings did not show any roots. The root mass of the remaining cuttings was very low (mean FM = 50 mg per cutting) and therefore was not included in the sampling. Pre-harvest nitrogen supply and storage influenced the course of variation in cutting dry matter during the first 2 weeks of propagation (Fig. 1A). Whereas DM remained at the same level when cuttings had been excised from the medium- or high-nitrogen fertilized stock plants, it decreased in the nitrogen-deficient cuttings to a significantly lower value when compared with the other nitrogen treatments (Fig. 1A). Storage of cuttings also promoted a subsequent loss of dry matter. Even though the two-way interaction between nitrogen and storage was not significant at day 13 (Fig. 1A), the storage effect was small and not statistically significant when low nitrogen cuttings were regarded individually.
Carbohydrate concentrations in the leaf lamina of unstored cuttings decreased after five days of propagation and then, in most cases, remained at similarly low levels until the last sampling date (Fig. 2). The decrease of leaf starch was most pronounced in the nitrogen-deficient cuttings, which showed the highest initial levels (Fig. 2A), whereas the significantly higher starch concentration in basal stems of the same cuttings was maintained during rooting (Fig. 3A). Concurrently, the glucose concentrations in the leaf lamina and basal stems of the low-nitrogen cuttings increased from initially being the lowest to being the highest among the nitrogen treatments on day 13 (Figs 2B, 3B). An obviously promoting effect of nitrogen deficiency on post-severance sugar availability was also indicated by the TS in the leaf lamina and petioles (Fig. 4A, B) and at the whole-cutting level (Fig. 1B). The same cuttings showed the highest TNC levels in whole-cuttings throughout the sampling period (Fig. 1C). Irrespective of the nitrogen treatments, a linear increase of fructose concentrations was observed for the basal stems of unstored cuttings (Fig. 3C), whereas no clear trend was found for sucrose (Fig. 3D).
When the cuttings had been stored before insertion, the initially lower concentrations of starch, glucose and particularly sucrose increased, especially over the first 5 d of propagation (Fig. 2). After 12 d, this amounted to slightly higher levels of starch and substantially higher concentrations of sucrose when compared with the unstored cuttings (Fig. 2A, D). The glucose concentrations in these leaves were not different from those of the unstored cuttings but were generally highest in case of nitrogen-deficiency (Fig. 2B). A different post-storage response of carbohydrates was observed in the basal stems (Fig. 3). The already decreased pre-rooting starch concentrations (day 1) of the medium- and high-nitrogen treatments remained at the same low levels during rooting, whereas the relatively high pre-rooting starch concentrations in the low-nitrogen cuttings showed a strong decrease to about 20 % of the level of the unstored cuttings (Fig. 3A). Even though the initial fructose concentrations in the basal stems were increased after storage, concentrations of both reducing sugars decreased during the subsequent rooting to significantly lower concentrations, with the low-nitrogen cuttings maintaining the highest relative level throughout (Fig. 3B, C). It can be seen from Fig. 4 that leaf sugar depletion during storage resulted in a post-storage accumulation of TS in these tissues, whereas simultaneously all other cutting parts, including the petioles, experienced a sugar shortage. This resulted in decreased TS at the whole-cutting level for all nitrogen treatments after 12 d (Fig. 1B). The observed relation between initial sugar concentration in leaves and subsequent sugar levels in the basal stems was highlighted by analyses of correlation. The concentration of TS measured in the basal stem at day 6 was less dependent on the initial sugar levels in this tissue (r = 0·42, P < 0·01, n = 54) but was strongly positively correlated with the initial sugar concentration in the leaf lamina (Fig. 5). In respect to individual sugars, initial glucose in leaves showed the best correlation, which also held true when the unstored cuttings were regarded separately (Fig. 5A). Including the other sugars had only a minor effect on the relationship overall, but resulted in a stronger correlation for the immediately rooted cuttings (Fig. 5B).
Fig. 5.

Linear regressions calculated between pre-rooting glucose (A) and total sugar (B) concentrations in the leaf lamina as independent variables, and the total sugar concentration in the basal stem at day 6 as the dependent variable (all cuttings, n = 54; unstored cuttings, n = 27).
Rooting response of cuttings as related to Nt and carbohydrate partitioning
During the first two weeks of rooting all leaves were retained by the unstored cuttings. The stored cuttings already showed leaf senescence and decay on day 13, which dramatically reduced the final survival rate, particularly in case of highest pre-harvest nitrogen supply (Table 4). According to the variation of DM observed during rooting (Fig. 1A), the total FM of non-stored cuttings at the end of the rooting period was significantly lower when cuttings had been collected from low-nitrogen stock plants, whereas storage reduced FM to the same level for all nitrogen treatments (Table 4). Increasing the pre-harvest nitrogen supply promoted subsequent biomass production of unstored cuttings (1·27, 1·54 and 2·13 g for N-low, N-medium and N-high; compare FM in Tables 2 and 4). However, irrespectively of the observed root growth, storage of the non-nitrogen deficient cuttings resulted in a negative balance of total FM until the end of rooting period (N-medium −0·33 g, N-high −0·07 g). Cuttings excised from moderately nitrogen-fertilized stock plants produced the highest number of roots, whereas nitrogen deficiency decreased root number and FM. The low rooting ability of these cuttings was not additionally affected by storage, but the same treatment decreased the root number in the non-nitrogen deficient cuttings (Table 4). The mean root length of 3·1 cm was not significantly affected by the treatments.
Table 4.
Survival of leaves and cuttings and average fresh mass and adventitious root formation of pelargonium cuttings as affected by pre-harvest nitrogen supply and storage of cuttings
| Losses of plant tissue (until day 13) |
Final rooting response (day 21) |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of leaves1 |
Number of whole cuttings1 |
Survival rate (%) |
Number of roots |
Fresh mass of roots (g) |
Total fresh mass (g) |
|||||||||||||||||
| Nitrogen treatment |
Unstored |
Stored |
Unstored |
Stored |
Unstored |
Stored |
Unstored |
Stored |
Unstored |
Stored |
Unstored |
Stored |
||||||||||
| N-low | 0·0a | 0·6a | 0·0a | 0·1ab | 80·0c | 60·0b | 7·4a | 8·4a | 0·29a | 0·38ab | 5·02a | 4·66a | ||||||||||
| N-medium | 0·0a | 1·3a | 0·0a | 0·1a | 77·8c | 53·3ab | 12·7c | 6·6a | 0·55b | 0·42ab | 6·80b | 4·93a | ||||||||||
| N-high | 0·3a | 2·8b | 0·0a | 0·4b | 77·8c | 43·3a | 10·8b | 7·7a | 0·52b | 0·51b | 7·12b | 4·92a | ||||||||||
Different superscripts indicate significant differences (P < 0·05) at specified days of propagation.
Per eight cuttings. Otherwise average of three harvest dates and three replication plots (n = 9).
Correlations, calculated with Nt and carbohydrate characteristics as independent variables and survival rate and root number as dependent variables, highlighted the interplay between nitrogen and carbohydrates in relation to the rooting response. No significant correlation was calculated between Nt in whole cuttings and the subsequent survival of cuttings (Table 5). Survival of the unstored cuttings and of all cuttings (unstored + stored) significantly increased with increasing pre-rooting concentrations of glucose, fructose and TS in the leaf lamina (Table 5). With regard to the basal stem, a high positive correlation between glucose and survival was found when the data from unstored and stored cuttings was pooled (Table 5). It can be seen from Fig. 6A that this relationship mainly resulted from the storage effect. The number of roots formed on unstored cuttings was positively correlated with Nt (Table 5). However, this relationship was only based on the effect of nitrogen deficiency and was completely removed by storage (Fig. 6B). The initial sugar concentrations in the leaf lamina and basal stems were positively correlated to the number of roots (Table 5). With respect to glucose in the leaves, positive regressions were calculated for all data pools irrespective of whether unstored cuttings, stored cuttings or combinations of both were considered (Table 5, Fig. 6C). Considering only the non-nitrogen deficient cuttings, thereby excluding the limiting influence of nitrogen (Fig. 6B), strengthened the positive influence of initial glucose (Fig. 6C). A strong dependency was found of root number on the sugar concentration determined at day 6 in the basal stem, a relationship that also was strengthened by exclusion of nitrogen deficiency (Fig. 6D). Calculating the carbohydrate concentrations at the whole-cutting level did not improve, but rather diminished the relationships (Table 5). In addition, the relationships became less strong when the initial carbohydrate content (mg per cutting) was calculated as independent variable. Using this parameter, the highest correlation was calculated for the glucose content (r = 0·44, P < 0·001, n = 54). This correlation was lower when compared to the relation found for the glucose concentration in leaves (Table 5) and became insignificant when unstored or stored cuttings were regarded individually.
Table 5.
Correlation coefficients, calculated from different data pools, between Nt in whole pelargonium cuttings and carbohydrate concentrations in different cutting parts as independent variables and survival and number of subsequently formed adventitious roots as dependent variables
| Survival rate (%) |
Number of roots |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration of |
Tissue |
At harvest (n = 27) |
After storage (n = 27) |
All cuttings (n = 54) |
At harvest (n = 27) |
After storage (n = 27) |
All cuttings (n = 54) |
||||
| Glucose | Leaf lamina | 0·61*** | n.s. | 0·62*** | 0·39* | 0·46* | 0·56*** | ||||
| Basal stem | n.s. | n.s. | 0·47*** | 0·45* | n.s. | 0·52*** | |||||
| Whole cutting | n.s. | n.s. | 0·38** | n.s. | 0·38* | 0·44* | |||||
| Fructose | Leaf lamina | 0·56** | n.s. | 0·56*** | n.s. | n.s. | 0·37** | ||||
| Basal stem | n.s. | n.s. | −0·28* | 0·52** | n.s. | n.s. | |||||
| Whole cutting | 0·43* | n.s. | n.s. | n.s. | n.s. | n.s. | |||||
| Sucrose | Leaf lamina | n.s. | n.s. | 0·38** | n.s. | n.s. | 0·30* | ||||
| Basal stem | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |||||
| Whole cutting | n.s. | n.s. | 0·42* | n.s. | n.s. | 0·33* | |||||
| Total sugars | Leaf lamina | 0·50** | n.s. | 0·60*** | n.s. | n.s. | 0·47*** | ||||
| Basal stem | n.s. | n.s. | 0·29* | 0·54** | n.s. | 0·43** | |||||
| Whole cutting | n.s. | n.s. | 0·40** | n.s. | n.s. | 0·44*** | |||||
| Starch | Leaf lamina | n.s. | n.s. | 0·45*** | −0·66*** | n.s. | n.s. | ||||
| Basal stem | n.s. | n.s. | n.s. | −0·59** | n.s. | n.s. | |||||
| Whole cutting | n.s. | n.s. | 0·34* | −0·68*** | n.s. | n.s. | |||||
| Total non-structural carbohydrates | Leaf lamina | n.s. | n.s. | 0·56*** | −0·55** | n.s. | n.s. | ||||
| Basal stem | n.s. | n.s. | n.s. | −0·53** | n.s. | n.s. | |||||
| Whole cutting | n.s. | n.s. | 0·45*** | −0·57** | n.s. | n.s. | |||||
| Nt | Whole cutting | n.s. | n.s. | n.s. | 0·73*** | n.s. | n.s. | ||||
Total sugars = glucose + fructose + sucrose; total non-structural carbohydrates = total sugars + starch.
n.s., non-significant;
P < 0·001;
P < 0·01;
P < 0·05.
Fig. 6.

Linear regressions calculated between pre-rooting glucose in the basal stem (A), pre-rooting Nt in whole cuttings (B), pre-rooting glucose in leaves (C), and total sugars in the basal stem at day 6 (D) as independent variables and the subsequent survival (A) and number of roots (B, C, D) as dependent variables. Encircled plots in (B) and plots with black (unstored cuttings) and white (stored cuttings) centres in (C, D) represent non-nitrogen-deficient cuttings (n = 36). All cuttings, n = 54; unstored cuttings, n = 27.
Sample survey of external material
The relevance of the nitrogen- and carbohydrate-rooting relationships found under the experimental conditions was evaluated for the practical propagation of pelargoniums during winter in Central Europe (Table 1). The Nt values in the external cutting samples revealed only a very small variability (mean ± s.d. = 40·3±3·9 mg g−1 DM, n = 39). In all cases, starch concentrations in the leaves were below 1 mg g−1 DM (data not shown). Sugar concentrations in these tissues and in the stems reached much higher levels and showed a large variability among samples. Therefore, correlation analyses were carried out using the initial sugar concentrations as independent and the final survival rate and root number as dependent variables. Contrary to the main experiment, no relationship could be established between the glucose level in leaves and the decay of cuttings. However, the survival rate proved to be positively correlated to the pre-rooting glucose level in the cutting stems, which corresponded to the relationship found for the basal stems in the experiment. It becomes apparent that there is no strong quantitative relationship (Fig. 7A), but rather that when a critical glucose level of 30 mg g−1 DM was exceeded, there was a greater probability for high cutting survival. The correlation became stronger by focussing on the pooled data of the repeated samples of the cultivars. In contrast to the experiment, the number of roots was not related to the level of any carbohydrate in the stems. But in accordance with the results of the main experiment, root number was positively correlated with the initial concentrations of glucose and fructose in the leaf lamina (r = 0·40 and r = 0·53, P < 0·05, n = 39). The highest correlation was calculated for the TS in leaves, which held true for different data pools including the individual data of the cultivars ‘Mitzou’ and ‘Lachsball’ (Fig. 7B). The same relations for ‘Isabell’ (r = 0·95, P < 0·09) and for ‘Sidonia’ (r = 0·61, P < 0·39) missed the significance level due to the low replication number. It becomes apparent from the non-specified cultivars that the relationship between leaf sugars and root number was also persistent when leaf senescence and decay was small (open symbols, Fig. 7A, B).
Fig. 7.
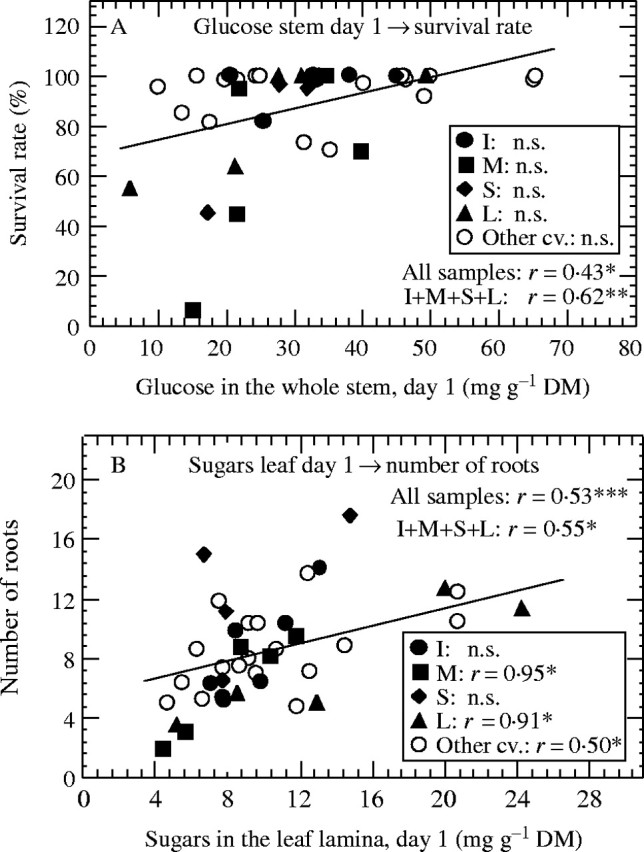
Relationship between pre-rooting concentrations of glucose in whole stems (A) and total sugars in the leaf lamina (B) as independent variables and the subsequent survival (A) and number of roots (B) as dependent variables. Data from the sample survey (see Methods). All samples, n = 39; M = ‘Mitzou’ (n = 5); L = ‘Lachsball’ (n = 5); I = ‘Isabell’ (n = 4); S = ‘Sidonia’ (n = 4); I + M + S + L, n = 18; other cv, n = 21.
DISCUSSION
With regard to the carbohydrate composition of the cuttings at harvest, which was after growth under a high light intensity, the negative relationship between Nt and starch levels and the contrasting positive nitrogen response of sugars even at the whole-cutting level correspond to similar relationships previously found for chrysanthemum (Druege et al., 2000). Such responses reflect the high interrelation between carbohydrate biosynthesis and N-assimilation, which are potentially competitive processes since both rely on inputs of reduced carbon and energy (Kaiser, 1997). In the case of chrysanthemum, higher sucrose concentrations were also measured in leaves with increasing nitrogen supply, which was explained by an increased export of assimilate towards the nitrogen-mediated sinks in the stock plant (Druege et al. 2000). The sugar response in pelargonium was mainly based on glucose, which is the sugar most readily available for covering local demands, but it can also be converted into sucrose for subsequent export. Related processes involve changed source/sink relations, which also respond to nitrogen form (Schortemeyer et al., 1997), as well as signalling mechanisms (Scheible et al., 1997). It appears that the ability of plants to report and regulate nitrogen uptake and metabolism involve sensing both inorganic (i.e. nitrate) and reduced or organic nitrogen (e.g. ammonium, glutamine) (Coruzzi and Zhou, 2001). These mechanisms may also explain the significant decrease of glucose levels in the basal stems after excessive nitrogen supply (day 1 in Fig. 3B), which were not simply related to changing Nt (Table 2). The same treatment of chrysanthemum resulted in a much stronger response of nitrate in cuttings when compared with Nt, but the carbohydrates were related to Nt and mainly not to nitrate (Druege et al., 2000). Apart from the internal nitrogen status, local effects from nitrate around the root (Stitt, 1999) as well as osmotic effects (Druege, 2000) also have to be considered.
The decrease in carbohydrate levels in the leaves, particularly of starch, during storage agrees with results on the same species after short-term storage at higher temperatures (Purer and Mayak, 1989, Arteca et al., 1996) and can be attributed to metabolic processes including respiration (Behrens, 1988). The high storage sensitivity of leaf starch was also observed in chrysanthemum cuttings (Druege et al., 2000) and is probably related to its transitory character. This includes starch mobilization in source leaves, not only in darkness, but also under low light conditions, and is assumed to involve a circadian component (Geiger et al., 2000). The generally stronger depletion of the initially higher sugars in the non-nitrogen-deficient cuttings during storage, and also during rooting under low light, indicates an after-effect of the nitrogen-mediated source/sink condition of the cuttings. This may be caused by the lower starch levels at harvest but may also reflect a different post-harvest carbohydrate requirement, possibly related to ongoing processes of nitrogen assimilation and transport. In intact plants, such processes have been shown to depend on diurnal changes responding to light conditions (Stitt et al., 2002).
Forschner and Reuther (1984) showed that photosynthesis of Pelargonium × hortorum cuttings is low unless adventitious roots are initially visible. When high-light-adapted cuttings were rooted at a PPFD of 20 µmol m2 s−1, net photosynthesis could not be detected. The time courses of variation in dry matter and of carbohydrate concentrations that were determined in our study during the first 12 d of rooting of the high-light-adapted cuttings under similar low light conditions confirm these findings, and clearly indicate that net assimilation of carbon did not occur during this period. The unstored nitrogen-deficient cuttings, on the one hand, experienced a loss of dry matter, but on the other hand maintained the relatively highest carbohydrate concentrations in the tissues. This indicated that the dry matter loss was not related to the general carbon availability but was rather caused by other effects of nitrogen deficiency on functional integrity (Marschner, 1995).
After storage, a loss of dry matter in the non-nitrogen-deficient cuttings resulted in lower DM after 12 d when compared to the unstored cuttings. The lower concentrations of carbohydrates in the respective tissues indicate that carbon deficiency was causally involved. The smaller storage response of the low-nitrogen cuttings can be explained by the fact that those cuttings already suffered from nitrogen deficiency and maintained a relatively higher availability of carbohydrates.
The observation that carbohydrate levels in the leaf lamina of unstored cuttings decreased during rooting whereas the basal stems returned to (or even exceeded) pre-rooting levels after a small transient decrease indicates a basipetal transport of carbon from the leaves to the stem base. The observed post-storage accumulation of sugars in the leaf lamina of stored cuttings was related to the previous depletion, which is known to up-regulate genes involved in photosynthesis, remobilization and carbohydrate export (Koch, 1996). However, the concurrent depletion in all other tissues including the petioles during the rooting period does not support a causal role of source activity, but rather points towards a disturbed export of carbohydrates from the leaves, which finally resulted in carbohydrate exhaustion of the whole cuttings. In particular, sucrose accumulated in the leaf lamina (Fig. 2D), which supports the hypothesis that a disturbed phloem loading was causally involved. Sucrose is loaded into minor veins from source-leaf apoplasms by an energy-dependent transport responding to alterations in the source/sink balance (Lalonde et al., 2003). A symplasmic component is also characteristic for most loading pathways, and this route seems to be particularly susceptible to low temperatures (Van Bel, 1993).
Considering the courses of DM and of TNC levels until day 13, the observed increase of total FM of unstored cuttings until the end of rooting period can be explained by the utilization of the initial carbohydrate reserves. The highest post-insertion total biomass production after excessive nitrogen supply, compared with the highest root growth already obtained after moderate nitrogen supply (Tables 2, 4), indicate that excessive nitrogen supply increased the sink strength of shoots (Scheible et al., 1997). Storage strongly promoted leaf senescence and reduced the survival of cuttings, and this was more pronounced after the highest nitrogen supply. Leaf senescence is influenced by a combination of internal factors, including plant hormones such as cytokinins, abscisic acid and ethylene (Smart, 1994). Results obtained by Kadner et al. (2000) did not support a predominating role of ethylene in storage-induced senescence of pelargonium cuttings. The positive relationship between pre-rooting glucose levels in stem tissues and the subsequent survival of ‘Isabell’, which was established for all treatments, also held true in principle in the sample survey. Even though the latter study revealed a crucial threshold value rather than a strong quantitative relationship, the pre-rooting sugar level in the stems of pelargonium cuttings seems to be of greater importance to subsequent leaf senescence than the initial leaf sugar concentration. Carbohydrate depletion has often been observed in senescing tissues (Smart, 1994; Ranwala et al., 2000), but it has also been shown that carbohydrate accumulation in leaves can trigger leaf senescence (Fischer et al., 1998, Paul and Foyer, 2001), counteracting the senescence-inhibiting influence of cytokinins (Wingler et al., 1998). The depletion of stem sugars during storage was followed by strong post-storage sugar accumulation in leaves, and leaf sucrose measured at day 13 was negatively correlated with the survival of cuttings (r = −0·31, P < 0·05, n = 54). This might indicate that sugar accumulation was also causally involved in post-storage leaf senescence.
With regard to adventitious root formation, the results reflect an interplay between nitrogen and carbon availability. The reduced number of adventitious roots formed in the unstored low-nitrogen cuttings indicates that adventitious root formation in pelargonium is sensitive to nitrogen deficiency. The mechanisms that might be causally involved include the provision of essential nitrogen compounds to the region of root regeneration (Blazich, 1988; Pellicer et al., 2000) as well as indirect effects (Druege et al., 2000). The gaseous compound nitric oxide may also be involved, as this has recently been shown to interact with auxin signalling in adventitious root formation (Pagnussat et al., 2002). However, the significant decrease in root numbers when excessive nitrogen was supplied to the stock plants and the uncoupling of the positive relationship between Nt and root number after storage makes it evident that the initial amount of nitrogen reserves is not a predominant characteristic determining the rooting capacity of pelargonium.
In contrast to the variable relationship with nitrogen, adventitious root formation was predominantly dependent on the initial reserves of sugars in the cuttings. The correlation analyses revealed that carbohydrate concentrations and contents at the whole-cutting level were less important than sugar concentrations in the leaves. In the case of leaf glucose, the positive correlations held true for all sources of variation in the experiment and were confirmed in the sample survey, even though for those cuttings the TS provided the best relationships. The strong positive inter-correlations found in the main experiment between initial leaf sugars, sugars in the stem base during rooting and the final number of adventitious roots indicates the dependency of adventitious root formation on the influx of leaf-derived carbohydrates, and the limitation of the latter by the initial leaf sugar levels in cases of minor contribution of current photosynthesis. Carbon allocation towards the basal stem and related influences on rooting may be driven by the sink strength of the lower stem, which may establish at very early stages of rooting (Pellicer et al., 2000), but may also be driven by the assimilate export of source leaves as already manifested in the stock plant (Druege et al., 2000). Adventitious root formation relies on an adequate supply of carbohydrates to the region of root regeneration, where they can promote root initiation and development by diverse mechanisms (Haissig, 1986; Veierskov, 1988). Recent studies using plant tissue culture techniques support the role of carbohydrates in providing the energy and carbon skeletons needed for root regeneration (Li and Leung, 2000; Calamar and de Klerk, 2002). However, in addition to these and also osmotic functions, carbohydrates modulate gene activity (Koch, 1996) and interact strongly with plant hormone signalling (Leon and Sheen, 2003). Supply of leaf-derived carbohydrates may also provide co-delivery of other components required for adventitious root formation (Druege et al., 2000), particularly with regard to the vascular transport of auxins (Baker, 2000).
It is concluded that growth and adventitious root formation of pelargonium cuttings is sensitive to nitrogen. However, where high-light-adaptation and low current light conditions impair net carbon assimilation, then cutting survival and root regeneration become predominantly determined by the initial sugar availability as affected by pre-harvest nitrogen supply and cutting storage. Further investigation is required regarding the dependency of the relationships we observed on varying light conditions during cutting establishment, particularly in relation to the possible contribution of current assimilates and to other physiological processes involved.
Acknowledgments
This work was supported by the Ministries of Nutrition, Agriculture and Forestry of the state of Brandenburg, the free state of Thuringia and the Federal Republic of Germany. We greatly appreciate the skilful and accurate technical assistance of Baerbel Broszies, Sabine Czekalla and Claudia Hoffmann. We thank the members of the Young Plant Group of the German Central Association of Horticulture for providing the cuttings used in the sample survey.
LITERATURE CITED
- Arteca RN, Arteca JM, Wang T-W, Schlagnhaufer CD. 1996. Physiological, biochemical, and molecular changes in Pelargonium cuttings subjected to short-term storage conditions. Journal of the American Society for Horticultural Science 121: 1063–1068. [Google Scholar]
- Baker D. 2000. Vascular transport of auxins and cytokinins in Ricinus Plant Growth Regulation 32: 157–160. [DOI] [PubMed] [Google Scholar]
- Behrens V. 1988. Storage of unrooted cuttings. In: Davis TD, Haissig BE, Sankhla N, eds. Adventitious root formation in cuttings. Portland, Oregon: Dioscorides Press, 235–247. [Google Scholar]
- Blazich FA. 1988. Mineral nutrition and adventitious rooting. In: Davis TD, Haissig BE, Sankhla N, eds. Adventitious root formation in cuttings. Portland, Oregon: Dioscorides Press, 61–69. [Google Scholar]
- Calamar A, de Klerk GJ. 2002. Effect of sucrose on adventitious root regeneration in apple. Plant Cell, Tissue and Organ Culture 70: 207–212. [Google Scholar]
- Coruzzi GM, Zhou L. 2001. Carbon and nitrogen sensing and signaling in plants: emerging ‘matrix effects’. Current Opinion in Plant Biology 4: 247–253. [DOI] [PubMed] [Google Scholar]
- Druege U. 2000. Influence of pre-harvest nitrogen supply on post-harvest behaviour of ornamentals: importance of carbohydrate status, photosynthesis and plant hormones. Gartenbauwissenschaft 65: 53–64. [Google Scholar]
- Druege U, Zerche S, Kadner R. 1998. Relation between nitrogen and soluble carbohydrate concentrations and subsequent rooting of Chrysanthemum cuttings. Advances in Horticultural Science 12: 78–84. [Google Scholar]
- Druege U, Zerche S, Kadner R, Ernst M. 2000. Relation between nitrogen status, carbohydrate distribution and subsequent rooting of chrysanthemum cuttings as affected by pre-harvest nitrogen supply and cold-storage. Annals of Botany 85: 687–701. [Google Scholar]
- Ehrenberger F. 1991.Quantitative organische Elementaranalyse. Weinheim: VCH. [Google Scholar]
- Fischer A, Brouquisse R, Raymond P. 1998. Influence of senescence and of carbohydrate levels on the pattern of leaf protease in purple nutsedge (Cyperus rotundus). Physiologia Plantarum 102: 385–395. [Google Scholar]
- Forschner W, Reuther G. 1984. Photosynthese und Wasserhaushalt von Pelargonium-Stecklingen während der Bewurzelung unter dem Einfluss verschiedener Licht- und CO2-Bedingungen. Gartenbauwissenschaft 49: 182–190. [Google Scholar]
- Geiger DR, Servaites JC, Fuchs MA. 2000. Role of starch in carbon translocation and partitioning at the plant level. Australian Journal of Plant Physiology 27: 571–582. [Google Scholar]
- Haissig BE. 1986. Metabolic processes in adventitious rooting of cuttings. In: Jackson MB, ed. New root formation in plants and cuttings. Dordrecht, Boston, Lancaster: Martinus Nijhoff Publishers, 141–189. [Google Scholar]
- Hansen J, Stroemquist LH, Ericsson A. 1978. Influence of the irradiance on carbohydrate content and rooting of cuttings of pine seedlings (Pinus sylvestris L.). Plant Physiology 61: 975–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix DL. 1993. Rapid extraction and analysis of nonstructural carbohydrates in plant tissues. Crop Science 33: 1306–1311. [Google Scholar]
- Henry PH, Blazich FA, Hinesley LE. 1992. Nitrogen nutrition of containerized eastern redcedar. II. Influence of stock plant fertility on adventitious rooting of stem cuttings. Journal of the American Society for Horticultural Science 117: 568–570. [Google Scholar]
- Kadner R, Druege U, Kuehnemann, F. 2000. Ethylenemission von Pelargonienstecklingen waehrend der Lagerung bei unterschiedlichen Temperaturen. Gartenbauwissenschaft 65: 272–279. [Google Scholar]
- Kaiser WM. 1997. Regulatory interaction of carbon- and nitrogen metabolism. In: Behnke HD, Luettge U, Esser K, Kadereit JW, Runge M, eds. Progress in botany. Vol. 58. Berlin, Heidelberg: Springer-Verlag, 150–163. [Google Scholar]
- Koch KE. 1996. Carbohydrate-modulated gene expression in plants. Annual Review of Plant Physiology and Plant Molecular Biology 47: 509–540. [DOI] [PubMed] [Google Scholar]
- Lalonde S, Tegeder M, Throne-Holst M, Frommer WB, Patrick JW. 2003. Phloem loading and unloading of sugars and amino acids. Plant Cell and Environment 26: 37–56. [Google Scholar]
- Leakey RRB, Storeton-West R. 1992. The rooting ability of Triplochiton scleroxylon cuttings: the interactions between stock plant irradiance, light quality and nutrients. Forest Ecology and Management 49: 133–150. [Google Scholar]
- Leon P, Sheen J. 2003. Sugar and hormone connections. Trends in Plant Science 8: 110–116. [DOI] [PubMed] [Google Scholar]
- Li M, Leung DWM. 2000. Starch accumulation is associated with adventitious root formation in hypocotyl cuttings of Pinus radiata Journal of Plant Growth Regulation 19: 423–428. [Google Scholar]
- Marschner H. 1995.Mineral nutrition of higher plants. 2nd Edition. London: Academic Press. [Google Scholar]
- Pagnussat G, Simontachi M, Puntarulo S, Lamattina L. 2002. Nitric oxide is required for root organogenesis. Plant Physiology 128: 954–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton F, Schwabe WW. 1987. Storage of cuttings of Pelargonium × hortorum Bailey. Journal of Horticultural Science 62: 79–87. [Google Scholar]
- Paul MJ, Foyer CH. 2001. Sink regulation of photosynthesis. Journal of Experimental Botany 52: 1383–1400. [DOI] [PubMed] [Google Scholar]
- Pellicer V, Guehl J-M, Daudet F-A, Cazet M, Riviere LM, Maillard P. 2000. Carbon and nitrogen mobilization in Larix × eurolepis leafy stem cuttings assessed by dual 13C and 15N labelling: relationships with rooting. Tree Physiology 20: 807–814. [DOI] [PubMed] [Google Scholar]
- Purer O, Mayak S. 1989. Pelargonium cuttings—effect of growth regulators. Acta Horticulturae 261: 347–354. [Google Scholar]
- Ranwala AP, Miller WB, Kirk TI, Hammer PA. 2000. Ancymidol drenches, reversed greenhouse temperatures, postgreenhouse cold storage, and hormone sprays affect postharvest leaf chlorosis in Easter lily. Journal of the American Society for Horticultural Science 125: 248–253. [Google Scholar]
- Reddy AR, Reddy KR, Padjung R, Hodges HF. 1996. Nitrogen nutrition and photosynthesis in leaves of Pima cotton Journal of Plant Nutrition 19: 755–770. [Google Scholar]
- Reuther G., Roeber R. 1980. Einfluss unterschiedlicher N-Versorgung auf Photosynthese und Ertrag von Pelargonienmutterpflanzen. Gartenbauwissenschaft 45: 21–29. [Google Scholar]
- Scheible, W-R, Lauerer M, Schulze E-D, Caboche M, Stitt M. 1997. Accumulation of nitrate in the shoot acts as a signal to regulate shoot-root allocation in tobacco. The Plant Journal 11: 671–691. [Google Scholar]
- Schortemeyer M, Stamp P, Feil B. 1997. Ammonium tolerance and carbohydrate status in Maize cultivars. Annals of Botany 79: 25–30. [Google Scholar]
- Smart C M. 1994. Gene expression during leaf senescence. Transley Review No. 64. New Phytologist 126: 419–448. [DOI] [PubMed] [Google Scholar]
- Statsoft, 1995.Statistica for Windows [Computer program manual]. Tulsa, OK: Statsoft, Inc., 2325 East 13th Street, Tulsa, OK 74104, USA. [Google Scholar]
- Stitt M. 1999. Nitrate regulation of metabolism and growth. Current Opinion in Plant Biology 2: 178–186. [DOI] [PubMed] [Google Scholar]
- Stitt M, Muller C, Matt P, Gibon Y, Carillo P, Morcuende R, Scheible WR, Krapp A. 2002. Steps towards an integrated view of nitrogen metabolism. Journal of Experimental Botany 53: 959–970. [DOI] [PubMed] [Google Scholar]
- Van Bel AJE. 1993. Strategies of phloem loading. Annual Review of Plant Physiology and Plant Molecular Biology 44: 253–281. [Google Scholar]
- Veierskov B. 1988. Relations between carbohydrates and adventitious root formation In: Davis TD, Haissig BE, Sankhla N, eds. Adventitious root formation in cuttings. Portland, Oregon: Dioscorides Press, 70–78. [Google Scholar]
- Wingler A, Von Schaewen A, Leegood RC, Lea PJ, Quick WP. 1998. Regulation of leaf senescence by cytokinin, sugars, and light. Plant Physiology 116: 329–335. [Google Scholar]


