Abstract
• Background and Aims Climate projections predict drier and warmer conditions in the Mediterranean basin in the next decades. The possibility of such climatic changes modifying the growth of two Mediterranean species, Erica multiflora and Globularia alypum, which are common components of Mediterranean shrublands, was assessed.
• Methods A field experiment was performed from March 1999 to March 2002 to prolong the drought period and to increase the night-time temperature in a Mediterranean shrubland, where E. multiflora and G. alypum are the dominant species. Annual growth in stem diameter and length of both species was measured and annual stem biomass production was estimated for 1999, 2000 and 2001. Plant seasonal growth was also assessed.
• Key Results On average, drought treatment reduced soil moisture 22 %, and warming increased temperature by 0·7–1·6 °C. Erica multiflora plants in the drought treatment showed a 46 % lower annual stem elongation than controls. The decrease in water availability also reduced by 31 % the annual stem diameter increment and by 43 % the annual stem elongation of G. alypum plants. New shoot growth of G. alypum was also strongly reduced. Allometrically estimated biomass production was decreased by drought in both species. Warming treatment produced contrasting effects on the growth patterns of these species. Warmer conditions increased, on average, the stem basal diameter growth of E. multiflora plants by 35 %, raising also their estimated stem biomass production. On the contrary, plants of G. alypum in the warming treatment showed a 14 % lower annual stem growth in basal diameter and shorter new shoots in spring compared with controls.
• Conclusions The results indicate changes in the annual productivity of these Mediterranean shrubs under near future drier and warmer conditions. They also point to alterations in their competitive abilities, which could lead to changes in the species composition of these ecosystems in the long term.
Key words: Biomass, climate change, drought, Erica multiflora, Globularia alypum, growth, Mediterranean species, warming
INTRODUCTION
Climate projections predict drier and warmer conditions in the Mediterranean basin in the next decades (Houghton et al., 2001). Responses to such climatic changes may differ among species and cannot be readily generalized, since contrasting effects of simulated climate changes on the ecophysiology of co-occurring species have often been reported (Chapin and Shaver, 1985; Chapin et al., 1995; Day et al., 1999). Hence, it is essential to improve our knowledge on the response of the different Mediterranean species to drier and warmer conditions to be able to predict the impact of climate change on Mediterranean ecosystems. Specifically, a proper understanding of how decreases in water availability and increases in temperature might affect the growth patterns of Mediterranean species will allow more reliable climate change scenarios of ecosystem functioning to be developed.
Water is the resource most often limiting plant growth in the Mediterranean-climate ecosystems (e.g. Mayor and Rodà, 1994; Borghetti et al., 1998; Royce and Barbour, 2001; Ogaya et al., 2003). Water scarcity may affect plant growth through effects on photosynthetic rates that can be direct, mostly as a consequence of stomatal closure (for reviews, see Chaves, 1991; Yordanov et al., 2000), or indirect, mostly because of reduced nutrient supply to the roots (Chapin, 1980). In addition, water stress may result in accelerated leaf senescence and, in consequence, after a period of water stress, even if gas exchange rates are re-established, whole-plant growth may be reduced due to a decrease in the leaf area per unit of dry weight (leaf area ratio, LAR) (Pereira, 1995).
Temperature may influence plant growth through direct effects on metabolic rates, including photosynthesis, respiration, nutrient and water uptake, and resource utilization. Temperature is also a key modifier of ontogenetic development rate (Morison and Lawlor, 1999) and thus, it has been found that warmer conditions accelerate organ initiation and expansion rates (Yoshie and Fukuda, 1994; Chapin and Shaver, 1996; Morison and Lawlor, 1999). Moreover, since temperature is an important factor controlling the phenology of plants, climatic warming is likely to affect the timing of the onset and cessation of growth (Kramer, 1995). Indeed, studies of phenological time series have showed a lengthening of the growing season over the last century in North America and Europe (e.g. Menzel and Fabian, 1999; Menzel, 2000; Schwartz and Reiter, 2000; Peñuelas and Filella, 2001), and particularly in the Mediterranean basin (Peñuelas et al., 2002), as spring events have advanced and autumn events have been delayed (Menzel, 2000; Peñuelas et al., 2002). In addition, plant growth might be affected indirectly by temperature effects on decomposition of soil organic matter, mineralization of soil nutrients and soil moisture (Chapin et al., 1995; Jarvis and Linder, 2000; Kirschbaum, 2000). Because evapotranspiration rates are positively related to temperature, increased temperatures are likely to be associated with increased rates of water loss (Kirschbaum, 2000; Lloyd and Fastie, 2002). Therefore, if temperature warms without a compensating increase in precipitation, plants may become increasingly water-stressed, which could lead to decreases in growth rate in water-limited ecosystems (Kirschbaum, 2000; Lloyd and Fastie, 2002).
Different responses of co-occurring species to climate change may alter their competitive interactions and lead to changes in the species composition of ecosystems. Hence, the present study deals with the effects of extended summer drought or night-time warming on the annual and seasonal growth of two co-occurring shrubs, Erica multiflora and Globularia alypum, which are common species of the Mediterranean shrublands. Evergreen shrubland is an important and widespread vegetation type throughout the Mediterranean region. The shrubs do provide substantial cover in these otherwise sparsely vegetated landscapes, thus controlling runoff and soil erosion, and have become important grazing grounds for local pastoral animals. Climatic changes may have particular strong effects on these ecosystems, which are already subjected to other stresses such as drought, intensive grazing or frequent fires. A previous study suggested a differential effect of drier conditions on the growth of these two Mediterranean species, since, although both species showed lower shoot water potentials, transpiration rates and stomatal conductances under our drought treatment, only E. multiflora plants had lower leaf net photosynthetic rates (Llorens et al., 2003b). Even though no significant effect of warmer conditions on the photosynthetic performance of these two species was found, this does not preclude an effect of warming on growth. It has been established that processes that are integrated at annual time steps (as is growth) provide insight into longer term responses of vegetation to environmental changes and thus, are more useful than instantaneous physiological measurements (e.g. gas exchange rates) in predicting decadal vegetation changes (Chapin and Shaver, 1996).
In the present study the aim was to assess whether near future drier or warmer conditions could (a) modify the annual or seasonal growth patterns of the two studied species, and (b) affect differently each species which, thus, could lead to a change in their competitive balance in these ecosystems. To address these issues, a field experiment was performed using a new non-intrusive technique to experimentally dry or warm a Mediterranean shrubland (Beier et al., 2004).
MATERIALS AND METHODS
The study site and species description
The study was carried out in a dry shrubland (Rosmarino-Ericion) at the Garraf Natural Park, Barcelona, noth-east Spain (41°18′N, 1°49′E), at 210 m a.s.l. and on a south-south-east slope (13°). The climate is typically Mediterranean. The site, which is located on terraces of abandoned vineyards, suffered big fires in the summers of 1982 and 1994. The soil is a petrocalcic calcixerept (Soil Survey Staff, 1998), thin (12–37 cm), with a loamy texture and abundant calcareous nodules. Currently the regenerating vegetation covers 50–60 % with a maximum height of 70 cm. The dominant species at the study site, Erica multiflora L. (Ericaceae) and Globularia alypum L. (Globulariaceae), are evergreen, sclerophyllous shrubs that typically occur on basic soils of the western Mediterranean Basin, where they are common components of the coastal shrubland. Both species resprout from underground organs after above-ground biomass removal. Vegetative growth occurs twice a year: in spring (from March to June) and autumn (from September to November). Flowering starts in August–September.
Experimental system
Two types of climatic manipulations were carried out on the study site, using automatically sliding curtains (approx. 20 cm above vegetation maximum height) (Beier et al., 2004):
Extended summer drought was induced by covering the natural vegetation and soil with transparent and waterproof plastic curtains during all rain events over the two growing seasons, from March–April to July–August and from September–October to December–January. During the period when the drought treatment was working, the rain sensors activated the curtains to cover plants and soil whenever it rained and removed the curtains when the rain stopped.
Passive night-time warming was achieved by covering the vegetation and soil during the night by aluminium curtains. Solar energy is accumulated in the ecosystem during the day and a fraction of the energy is re-radiated back to the atmosphere at night as long-wave infrared radiation. The covering of the ecosystem with the curtains at night reduced the loss of infrared radiation, simulating the mechanism of global warming due to the accumulation of greenhouse gases. The coverage of the study plots was activated automatically according to preset light (<5 µmol m−2 s−1). This treatment was started on 16 March 1999 and continued throughout the study, except during three periods (August 1999, January 2000 and September–October 2001), when the treatment was stopped for calibration of system effects or due to mechanical problems. The curtains were automatically removed during night-time rain events to avoid excluding precipitation.
Nine plots of 20 m2 (4 × 5 m) were established in the study site: three untreated controls, three warming and three drought plots. Plots were organized in three blocks (each block with one control, one drought and one warming plot). Control plots had similar scaffolding to warming and drought plots, but with no curtain. All the study plots were open around the edges. The outer 0·5 m of each study plot was considered a buffer zone and all the measurements were conducted in the central 12 m2 area.
Environmental data
Precipitation was registered at the study site with a standard rain gauge. Soil moisture was measured every 1–2 weeks throughout the study period using Time Domain Reflectometry. At each sampling date, volumetric water content at 0–15 cm was estimated on three fixed sampling points per plot using a cable tester (1502B, Tektronix, Beaverton, OR, USA). Air (20 cm above ground) and soil temperatures (2 and 10 cm depth) were obtained by means of temperature sensors RTD Pt100 1/3 DIN (Desin Instruments, Barcelona, Spain) located in open areas of the three plots (control, drought and warming) of one block. Sensors were always protected against solar radiation. Temperatures were measured every 10 min, the average of three measurements of each sensor being recorded.
Plant annual growth
Before treatment applications, five to seven living stems on each of four or five plants of E. multiflora and G. alypum were tagged in each plot. In total, 235 stems of E. multiflora and 268 of G. alypum were labelled. Basal diameters and maximum lengths of the tagged stems were measured in winter each year from 1999 to 2002. To minimize the impact of noncircular stem cross-sections, stem diameter was calculated as the mean of two perpendicular measurements taken at the stem base with a digital caliper to the nearest 0·01 mm. To allow re-measuring, a line was painted with a permanent marker on the exact point of the stem where the diameter was measured. Maximum stem length was assessed by measuring the distance from the stem base to the more distant point at the end of the longest branch. Annual stem growth was calculated as the difference between consecutive winter measurements.
Plant seasonal growth
Whereas stem growth of E. multiflora is mainly apical, lateral shoot growth may account for a large proportion of stem growth of G. alypum. For this reason, different parameters were used to assess the seasonal stem growth of these two species. In E. multiflora, the maximum stem length was used to evaluate seasonal growth, whereas in G. alypum the number and length of all the new shoots of each tagged stem were measured. The total shoot growth per stem of G. alypum was calculated as the sum of the length of all the new shoots. Seasonal growth was calculated as the difference between consecutive non-growing season (winter and summer) measurements.
Shoot elongation rates
At the beginning of the experiment three shoots from three different stems of five plants of each species in each plot were labelled. Thus, 15 shoots of each species were tagged in each plot, 135 in total per species. At the beginning of May 2000 the spring length of these shoots was measured and the measurements after 1 month were repeated. The shoot elongation rate was calculated as: (final length − initial length)/number of days.
Biomass allometric relationships
Allometric relationships between stem biomass (dry weight) and stem diameter or length were calculated for both species, using plants collected from the surrounding area outside the plots. Total stem biomass was measured after drying the plant material in an oven at 70 °C until constant weight. Since multiple stepwise regression analysis showed that both stem diameter and stem length explained a significant part of the variability in stem biomass, allometric equations including both variables were used to estimate the annual stem biomass increments of the tagged stems of each species for 1999, 2000 and 2001.
Environmental influence on plant growth
The influence of soil moisture on annual stem diameter growth of both species in the different treatments was analysed by means of Pearson correlations using the annual means per plot of 2000 and 2001 (n = 6 for each treatment). Data from 1999 were not used because no soil moisture records were available until mid-March.
Leaf fall
The leaf fall of four to eight plants of E. multiflora and nine to 12 plants of G. alypum per plot was monitored during 2000. Plant litterfall was collected bimonthly by means of open collectors (4·4 cm of diameter) located under each selected plant. Samples were dried to constant weight and afterwards leaves were separated and weighted.
Statistical analyses
Statistical analyses were performed by means of repeated-measures analysis of variance (ANOVAR, GLM procedures in SPSS 10·0·6) with the initial stem diameter, length or biomass as a covariate. To compare annual stem growth between the two species or between years or seasons for each species, only control plants were used. In the analyses of treatment effects, the factor ‘block’ was included to assess whether treatment effects were homogeneous among the blocks. However, neither the effect of block nor the interaction between block and treatment were significant. To test the effect of treatments on seasonal growth parameters, ANOVARs were performed for each species for spring and autumn separately. Data on stem length and biomass of both species and on length, number and total growth of G. alypum shoots were log transformed to achieve normality. To analyse the effect of treatments on shoot relative growth rates and leaf fall of each species, ANOVAs with treatment and block as factors were performed. Drought and warming treatments were always compared with control treatment separately. In all the analyses, means per plant were used.
RESULTS
Environmental data
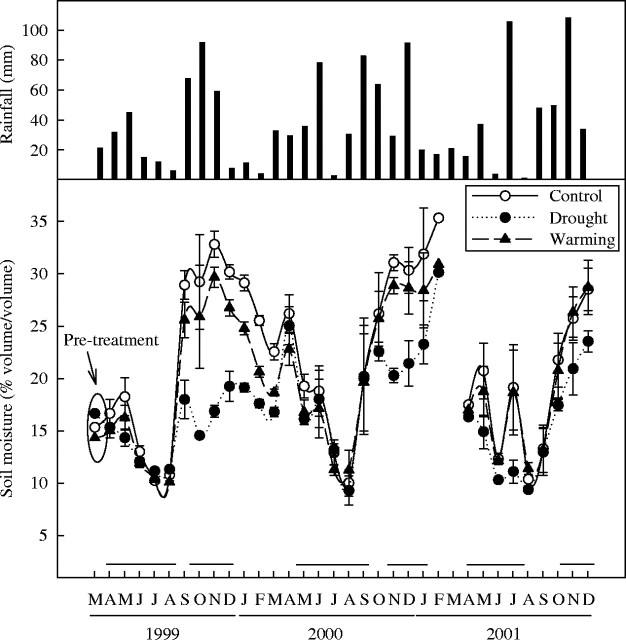
Total annual rainfall was 420, 489 and 458 mm in 1999, 2000 and 2001, respectively. Drought treatment reduced the soil moisture 22 % on average throughout the study period (Fig. 1). The maximum reductions occurred in autumn (up to 50 % in October and November 1999). Warming treatment had a slight effect on soil moisture, reducing it on average 8 % throughout the study period (Fig. 1).
Fig. 1.
Monthly rainfall (mm) and mean soil moisture (% volume/volume) of control, drought and warming treatments throughout the study period. Error bars indicate the standard error of the mean (n = 2–5 d; values are averages of three plots). Lines above the months indicate the periods when drought treatment was acting. Data partly derived from Llorens et al. (2003b).
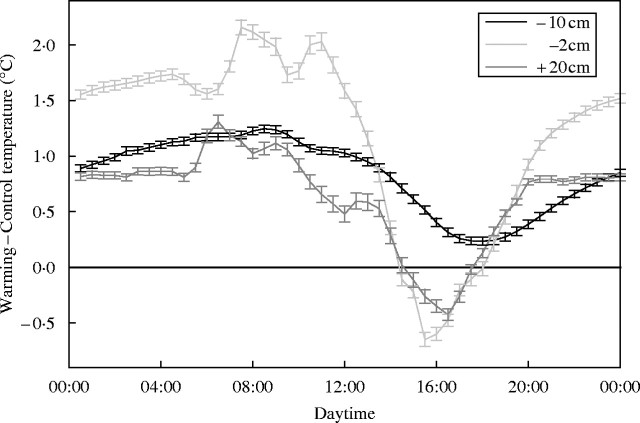
Mean annual air temperatures were slightly lower in 1999 and 2000 (13·8 °C both years) than in 2001 (14·0 °C). Warming treatment increased minimum temperatures on average 0·7 °C in the air, 1·6 °C at 2 cm depth and 1·1 °C at 10 cm depth. The reduced heat loss in the warming plots at night also increased the diurnal air and soil temperatures, with a maximum after sunrise and a minimum in the late afternoon (Fig. 2).
Fig. 2.
Annual averages of air (20 cm above ground) and soil (2 and 10 cm depth) temperature (°C) differences between warming and control plots (measured in one of the blocks from April 2000 to April 2001). Error bars indicate the standard error of the mean (n = 365). Data partly derived from Llorens et al. (2003b).
Annual stem growth in basal diameter and length
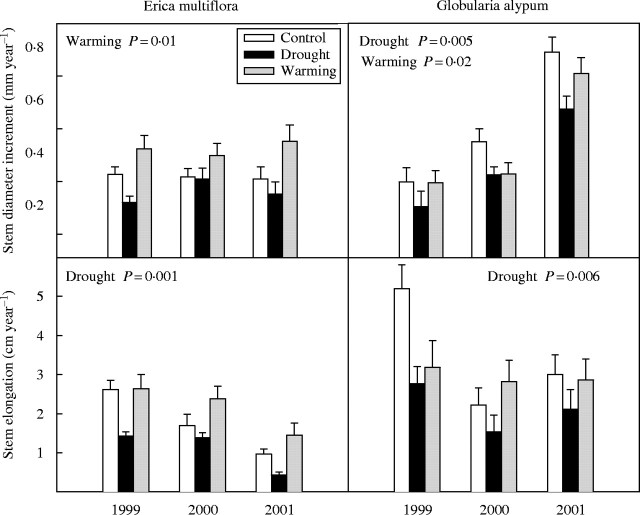
Control plants of E. multiflora showed significantly lower annual stem growth in basal diameter (F1,21 = 11·1, P = 0·003) and length (F1,22 = 15·8, P = 0·001) than control plants of G. alypum throughout the study period (Fig. 3).
Fig. 3.
Diameter and length increment of E. multiflora and G. alypum stems in 1999, 2000 and 2001 for control, drought and warming plants. Overall treatment effects are depicted when significant. Error bars indicate the standard error of the mean (n = 14–16).
Although drought did not affect significantly the overall annual stem diameter growth of E. multiflora, plants of this species had a 46 % lower annual stem elongation in the drought plots compared with controls throughout the study period (F1,22 = 13·5, P = 0·001). Globularia alypum plants showed overall reductions of 31 % and 43 % in their annual stem diameter growth (F1,21 = 9·7, P = 0·005) and elongation (F1,23 = 9·2, P = 0·006), respectively, under drier conditions (Fig. 3).
In the warming treatment, annual stem diameter growth was 35 % higher overall in E. multiflora plants (F1,22 = 7·1, P = 0·01) and a 14 % lower in G. alypum plants (F1,22 = 6·7, P = 0·02) compared with controls. Warming did not affect significantly the annual stem elongation of these species (Fig. 3).
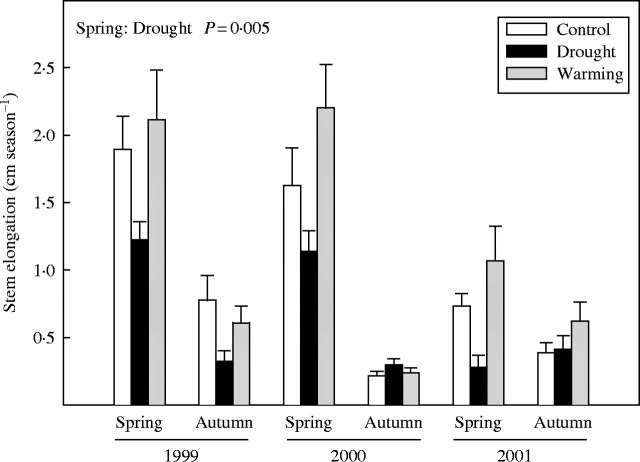
Seasonal stem elongation of E. multiflora plants
Control plants of E. multiflora showed higher stem elongation in spring than in autumn (F1,25 = 46·3, P < 0·001) (Fig. 4). Drought reduced significantly the stem elongation of E. multiflora plants in spring (F1,22 = 9·9, P = 0·005), but not in autumn. Warming had no significant effect on the seasonal stem elongation of E. multiflora (Fig. 4).
Fig. 4.
Stem elongation of E. multiflora in spring and autumn of 1999, 2000 and 2001 for control, drought and warming plants. Effects of treatments in spring and/or autumn are depicted when significant. Error bars indicate the standard error of the mean (n = 14–15).
Seasonal shoot growth of G. alypum plants
Mean length and number of new shoots per stem and, thus, total new shoot growth of G. alypum was also higher in spring than in autumn (P < 0·001 in all the analyses) (Fig. 5).
Fig. 5.
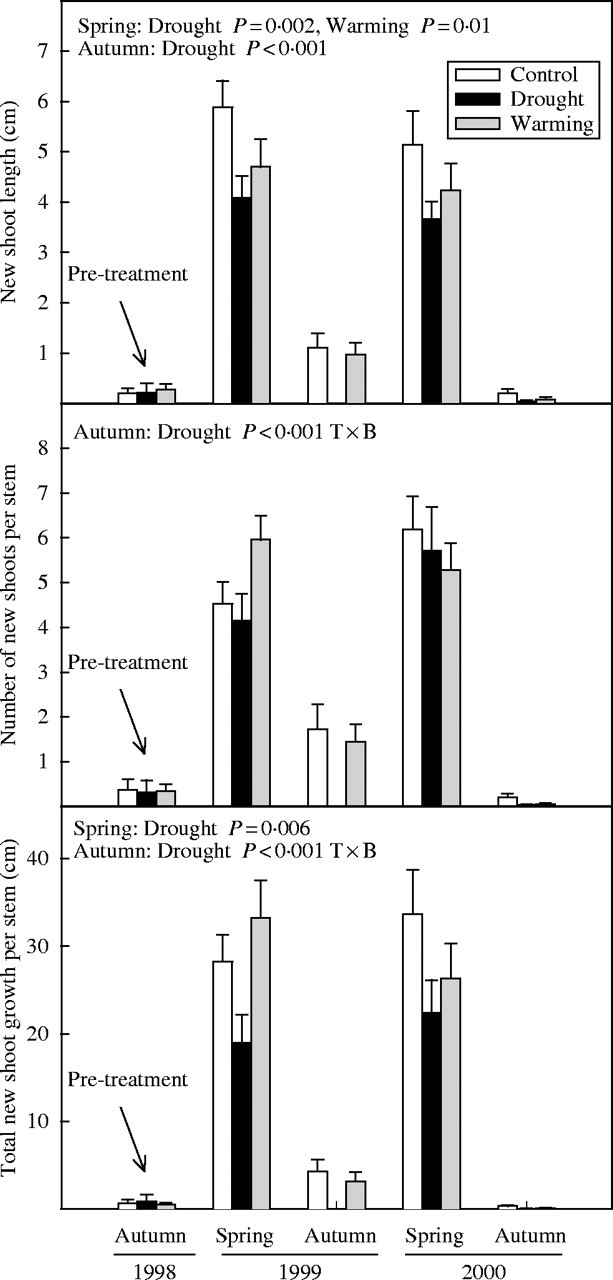
Mean shoot length, number of shoots and total shoot growth of G. alypum in autumn 1998 and spring and autumn of 1999 and 2000 for control, drought and warming plants. Effects of treatments in spring and autumn are depicted when significant. T×B indicates that the interaction between treatment and block was significant. Error bars indicate the standard error of the mean (n = 14–15).
Drought significantly reduced the mean length (F1,22 = 46·6, P < 0·001) and number (F1,22 = 14·9, P = 0·001) of G. alypum new shoots throughout the study period. However, whereas drought effects on the length of the new shoots of G. alypum were significant both in spring (F1,23 = 12·6, P = 0·002) and autumn (F1,22 = 28·4, P < 0·001), drought effects on the number of new shoots were significant only in autumn (F1,22 = 41·1, P < 0·001). Overall, this species showed a lower shoot growth per stem under drier conditions compared with controls (F1,22 = 33·4, P < 0·001), with significant effects in spring (F1,23 = 9·4, P = 0·006) and autumn (F1,22 = 43·7, P < 0·001) (Fig. 5).
In spring, shoots of G. alypum were shorter in the warming plots than in controls (F1,22 = 7·0, P = 0·01) (Fig. 5).
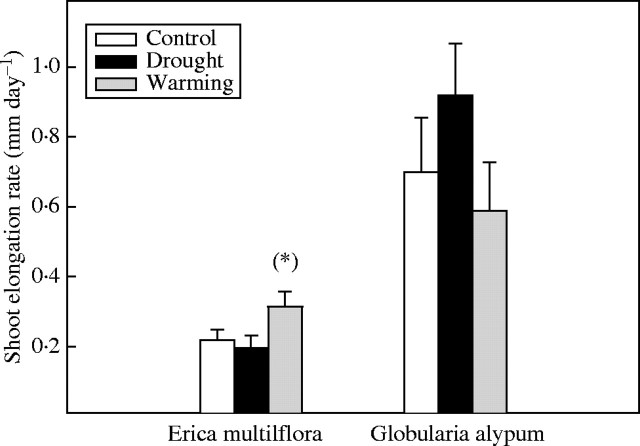
Shoot elongation rates in spring 2000
Drought did not affect significantly the elongation rates of E. multiflora and G. alypum new shoots. In contrast, shoot elongation rates of E. multiflora were slightly higher under warming treatment compared with controls (F1,24 = 3·2, P = 0·08) (Fig. 6).
Fig. 6.
Shoot elongation rate (mm day−1) of E. multiflora and G. alypum in spring 2000 for control, drought and warming plants. (*) indicates marginally significant (P < 0·1) differences between treatment and control plants. Error bars indicate the standard error of the mean (n = 15).
Annual stem biomass increments
Allometric relationships between stem biomass and stem diameter or length were highly significant; the correlation between stem biomass and diameter being stronger than between stem biomass and length (Table 1). As the allometric equations including both variables, stem diameter and length, had slightly stronger correlation coefficients, both variables were used to estimate stem biomass.
Table 1.
Allometric relationships between stem biomass (B) and stem diameter (D) and length (L) in E. multiflora (n = 40) and G. alypum (n = 100)
| Species |
Allometric relationship |
r2 |
P |
|
|---|---|---|---|---|
| Stem diameter | E. multiflora | log B = −0·506 + 2·324 log D | 0·947 | <0·001 |
| G. alypum | log B = −0·897 + 2·619 log D | 0·926 | <0·001 | |
| Stem length | E. multiflora | log B = −4·738 + 2·086 log L | 0·839 | <0·001 |
| G. alypum | log B = −5·408 + 2·280 log L | 0·640 | <0·001 | |
| Stem diameter + length | E. multiflora | log B = −1·73 + 1·79 log D + 0·57 log L | 0·961 | <0·001 |
| G. alypum | log B = −2·11 + 2·23 log D + 0·54 log L | 0·942 | <0·001 |
Plants of both species showed a lower production of annual stem biomass in the drought plots than in controls throughout the study period (F1,22 = 5·7, P = 0·03 and F1,22 = 5·3, P = 0·03 for E. multiflora and G. alypum, respectively; Fig. 7). Conversely, warming enhanced the annual stem biomass production of E. multiflora plants (F1,22 = 4·1, P = 0·05), whereas it did not significantly affect the estimated annual stem biomass production of G. alypum (Fig. 7).
Fig. 7.
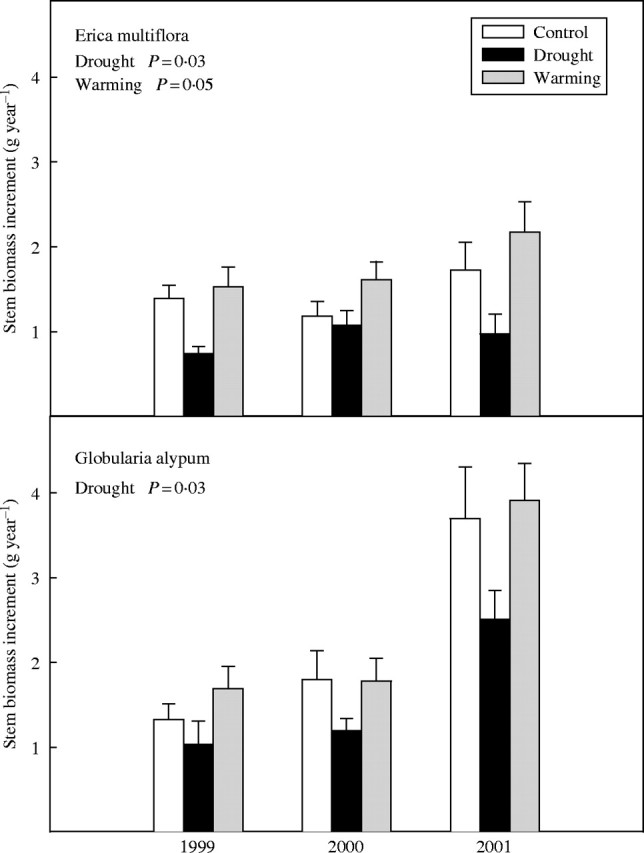
Stem biomass increment of E. multiflora and G. alypum in 1999, 2000 and 2001 for control, drought and warming plants. Overall treatment effects are depicted when significant. Error bars indicate the standard error of the mean (n = 14–16).
Environmental influence on annual stem diameter growth
Annual stem diameter increments of E. multiflora did not significantly correlate with soil moisture. Conversely, annual stem diameter increments of G. alypum plants showed positive significant correlations with mean soil moisture throughout the first six months of the year, and mainly with the mean soil moisture of February and March (Fig. 8).
Fig. 8.
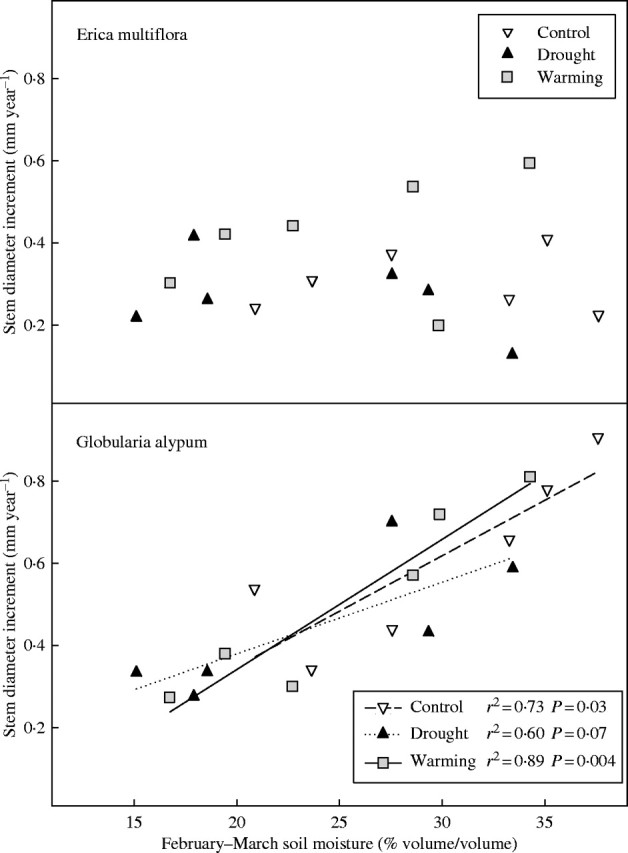
Annual diameter increment vs. mean soil moisture of February and March for control, drought and warming plots in 2000 and 2001 (n = 6 for each treatment).
Leaf fall
The treatments did not significantly affect the leaf fall of the two species studied (Table 2).
Table 2.
Annual leaf litter production (g m−2 year−1) below E. multiflora and G. alypum plants in 2000 for control, drought and warming treatments
| Control |
Drought |
Warming |
|
|---|---|---|---|
| E. multiflora (n = 19–23) | 141·8 ± 11·4 | 145·7 ± 14·6 | 118·6 ± 12·3 |
| G. alypum (n = 30–33) | 94·4 ± 8·6 | 93·7 ± 9·4 | 92·0 ± 5·1 |
Values are means ± standard errors.
DISCUSSION
Species comparison
The lower annual stem growth found in control plants of E. multiflora compared with control plants of G. alypum (Fig. 3) is in accordance with the lower photosynthetic rates found in the former compared with the latter species in a previous study (Llorens et al., 2003a). In this earlier study, E. multiflora was described as a water conservative species and G. alypum as a water spender species. Accordingly, in the present study, no significant dependence on soil moisture of annual stem diameter growth of E. multiflora was found, whereas stem diameter increments of G. alypum were significantly correlated with volumetric soil water content, the strongest correlations being with the average soil moisture of February and March (Fig. 8). This result suggests that E. multiflora probably relies more on the reserves stored during winter to supply seasonal growth in spring, whereas G. alypum depends more on concurrent carbon and nutrient uptake.
Seasonal growth comparison
Seasonal stem growth of the two studied species was higher in spring than in autumn (Figs 4 and 5), despite precipitation and photosynthetic rates of these species being lower in spring (Fig. 1; Llorens et al., 2003a). Lower growth in autumn might be a consequence of shorter days or might be due to reserve depletion in summer (Larcher and Thomaser-Thin, 1988), since these shrubs show null or very low net photosynthesis during the hottest season (Llorens et al., 2003a). In contrast, mild temperatures allow net photosynthesis and probably accumulation of carbon reserves during winter (Larcher and Thomaser-Thin, 1988; Llorens et al., 2003a) and these reserves are likely to support the comparatively strong stem and shoot development observed in spring, as it has also been suggested for other Mediterranean sclerophylls (Oliveira et al., 1994). Resource competition between growth and reproduction in autumn might also contribute to the higher growth observed in spring, since reproductive organs are usually strong sinks for carbohydrate and nitrogen reserves (Bloom et al., 1985).
Drought treatment effects
Drier conditions reduced the annual and seasonal growth of G. alypum, as it has been reported for other Mediterranean species (e.g. Borghetti et al., 1998; Lebourgeois et al., 1998; Ogaya et al., 2003). The imposed drought also reduced the annual stem elongation of E. multiflora plants, as a consequence of spring rather than autumn decreases (Figs 3 and 4). However, drought did not significantly affect the annual stem diameter growth of E. multiflora (Fig. 3), in accordance with the lower dependence on soil moisture of this species compared with G. alypum (Fig. 8; Llorens et al., 2003a). The observed reductions in stem growth in both species would have decreased their annual stem biomass production (Fig. 7), assuming that drier conditions did not modify the allometric relationship between stem biometry and biomass.
Reductions in growth under the imposed drier conditions might be explained by decreases in leaf net photosynthetic rates. Indeed, leaf net photosynthetic rates of E. multiflora plants were 34 % lower in the drought plots than in controls (Llorens et al., 2003b). In contrast, drier conditions did not affect significantly the leaf net photosynthetic rates of G. alypum plants (Llorens et al., 2003b). This apparent discrepancy between photosynthesis and growth reflects a complex relationship between these two processes. Besides the large difference in the time scales between instantaneous photosynthetic measurements and long-term growth, growth is controlled by several other processes in addition to carbon acquisition (Xiong et al., 2000). For instance, differences in plant leaf area may change growth rates without important changes in photosynthetic rates (Pereira, 1995). Since it has been reported that water stress may result in accelerated leaf senescence (Pereira, 1995), a greater leaf fall in G. alypum plants in the drought treatment, might result in a lower total leaf area and, thus, might explain the observed reduction in growth without any change in leaf net photosynthetic rates. However, no significant effect of the present drought treatment was found on the annual leaf fall of G. alypum plants in 2000 (Table 2). Drought-induced limitation in growth of G. alypum does not either seem to be due to reduced N or P acquisition associated with water shortage, since it had previously been found that N and P foliar concentrations tended to increase in G. alypum plants under drought treatment (Peñuelas et al., 2004). Alternately, a greater carbon allocation to roots in droughted plants of G. alypum might explain a lower carbon investment on above-ground growth in this species. Further more detailed studies on gas exchange, growth patterns and carbon allocation are warranted to disentangle this apparent contradiction between growth decrease and unchanged photosynthetic rates.
Warming treatment effects
Warming produced contrasting effects on the annual stem diameter growth of these species. While warming increased the annual stem diameter growth of E. multiflora by 35 % on average, it decreased the annual stem diameter growth of G. alypum by 14 % over the whole study period (Fig. 3). Assuming no changes in carbon allocation patterns, an approx. 1 °C increase in temperature would enhance annual stem biomass production of E. multiflora, but it would not affect G. alypum (Fig. 7). Other studies have also reported contrasting effects of warming on the growth patterns of co-occurring species (e.g. Chapin and Shaver, 1985, 1996; Day et al., 1999; Weltzin et al., 2000).
Positive growth responses to temperature increases have often been reported (e.g. Coleman and Bazzaz, 1992; Graglia et al., 1997; Arft et al., 1999; Jarvis and Linder, 2000; Ro et al., 2001; Theurillat and Guisan, 2001), although most of warming experiments have been performed in cool and moist regions. Enhanced diameter growth of E. multiflora plants in warming plots might result from higher relative growth rates (RGR) or a longer growing season. Indeed, it was found that plants of E. multiflora in the warming treatment showed slightly higher shoot elongation rates than controls (Fig. 6), as has also been reported for other shrub species in experimentally heated plots in a Rocky Mountain meadow (Harte and Shaw, 1995). Since there were no significant differences in leaf net photosynthetic rates or specific leaf area (SLA) between warming and control plants of E. multiflora (Llorens et al., 2003b), the observed higher shoot elongation rates in warming plants compared with controls could be the result of a greater biomass allocation to leaves (Xiong et al., 2000). However, warming did not modify significantly the relationship between stem length and diameter of this species (data not shown), which suggests that there were no significant changes in biomass allocation between leaves and woody tissues. A lengthening of the growing season might also have contributed to the observed higher annual stem growth of E. multiflora plants under warmer conditions. In fact, it is predicted that the onset of the spring growing season will advance by up to 6 d per 1 °C increase in winter air temperature (Menzel and Fabian, 1999), which is similar to the temperature rise achieved in the present warming treatment. Peñuelas et al. (2002) reported that leaves of deciduous species unfold on average 16 d earlier and fall 13 d later in a nearby area after a temperature increase of 1·4 °C over 49 years.
The reduction by warming of the annual diameter growth of G. alypum plants cannot be explained by changes in leaf gas exchange rates, SLA or leaf fall, since no significant effect of our warming treatment was found on these parameters (Llorens et al., 2003b; Table 2). Decreases in growth with increasing temperature have been related to increased heat damage or temperature-induced drought stress (Barber et al., 2000; Kirschbaum, 2000; Lloyd and Fastie, 2002). In our study, warming treatment reduced the soil moisture on average 8 % compared with control treatment. Particularly, soil moisture was significantly lower in the warming plots compared with controls during February and March 2000. Accordingly, the highest reduction in diameter increment of G. alypum plants under warming was observed in 2000. Thus, it is suggested that the higher sensitivity to soil moisture found in G. alypum compared to E. multiflora might explain the reduction in basal diameter growth of G. alypum in the warming plots. Although increases in dark respiration of G. alypum plants in the warming treatment might have contributed to their reduction in growth (Pereira, 1995), several studies have concluded that the ratio of respiration to photosynthesis does not deviate significantly from constancy over a broad range of temperatures (e.g. Gifford, 1994, 1995; Waring et al., 1998).
The present warming treatment also reduced the mean length of new shoots of G. alypum plants in spring (Fig. 5). Shoot length of G. alypum plants was, in general, more sensitive to our experimental changes of climatic conditions than the number of shoots. Pre-formation of buds in the previous spring could explain why the effect of treatments on the number of shoots was not evident until autumn 1999 or even spring 2000. The likely dependence of the number of shoots in a particular growing season on the biomass of shoots in the preceding growing seasons, would act as an important buffer against exceptionally severe growing seasons, as it was found for the number of leaves in Cassiope tetragona (Callaghan et al., 1989). Thus, similar shoot growth can be attained in different years by small numbers of long shoots or large numbers of short shoots, according to weather conditions. Such a mechanism, which buffers annual variation in growth, could be of considerable importance in the Mediterranean-climate areas, where there is a large unpredictability in the precipitation patterns. Thus, significant decreases in the number of shoots and in the total shoot growth per stem of G. alypum plants can be expected from consecutive adverse growing seasons.
Concluding remarks
The results of the present study point to changes in the annual productivity of these Mediterranean shrubs under near future drier and warmer conditions. Drier conditions would decrease the annual productivity of both species, but, in E. multiflora, the stimulation of growth by warmer conditions might compensate, at least partially, such a decrease. Thus, future drier and warmer conditions would probably have a stronger negative impact on the annual productivity of G. alypum than of E. multiflora. Alterations in the competitive interactions of these two species in response to the predicted climatic changes could lead, in the long-term, to variations in the species composition of these Mediterranean ecosystems.
Acknowledgments
This research was supported by the EU projects CLIMOOR (ENV4-CT97-0694) and VULCAN (EVK2-CT-2000-00094) and by MCYT-REN2001-0278/CLI and MCYT-REN2002-0003/GLO grants from the Spanish Government.
LITERATURE CITED
- Arft AM, Walker MD, Gurevitch J, Gurevitch J, Alatalo JM, Bret-Harte MS, Dale M, Diemer M, Gugerli F, Henry GHR, et al. 1999. Responses of tundra plants to experimental warming: meta-analysis of the international tundra experiment. Ecological Monographs 69: 491–511. [Google Scholar]
- Barber VA, Juday GP, Finney BP. 2000. Reduced growth of Alaskan white spruce in the twentieth century from temperature-induced drought stress. Nature 405: 668–673. [DOI] [PubMed] [Google Scholar]
- Beier C, Emmett B, Gundersen P, Tietema A, Peñuelas J, Estiarte M, Gordon C, Gorissen A, Llorens L, Rodà F, Williams D. 2004. Novel approaches to study climate change effects on terrestrial ecosystems in the field. Drought and passive night-time warming. Ecosystems 7: 583–597. [Google Scholar]
- Bloom AJ, Chapin III FS, Mooney HA. 1985. Resource limitation in plants—an economic analogy. Annual Review of Ecology and Systematics 16: 363–392. [Google Scholar]
- Borghetti M, Cinnirella S, Magnani F, Saracino A. 1998. Impact of long-term drought on xylem embolism and growth in Pinus halepensis Mill. Trees 12: 187–195. [Google Scholar]
- Callaghan TV, Carlsson BA, Tyler NJC. 1989. Historical records of climate-related growth in Cassiope tetragona from the Arctic. Journal of Ecology 77: 823–837. [Google Scholar]
- Chapin III FS. 1980. The mineral nutrition of wild plants. Annual Review of Ecology and Systematics 11: 233–260. [Google Scholar]
- Chapin III FS, Shaver GR. 1985. Individualistic growth response of tundra plant species to environmental manipulations in the field. Ecology 66: 564–576. [Google Scholar]
- Chapin III FS, Shaver GR, Giblin AE, Nadelhoffer KJ, Laundre JA. 1995. Responses of arctic tundra to experimental and observed changes in climate. Ecology 76: 694–711. [Google Scholar]
- Chapin III FS, Shaver GR. 1996. Physiological and growth responses of arctic plants to a field experiment simulating climatic change. Ecology 77: 822–840. [Google Scholar]
- Chaves MM. 1991. Effects of water deficits on carbon assimilation. Journal of Experimental Botany 42: 1–16. [Google Scholar]
- Coleman JS, Bazzaz FA. 1992. Effects of CO2 and temperature on growth and resource use of co-occurring C3 and C4 annuals. Ecology 73: 1244–1259. [Google Scholar]
- Day TA, Ruhland CT, Grobe CW, Xiong F. 1999. Growth and reproduction of Antarctic vascular plants in response to warming and UV radiation reductions in the field. Oecologia 119: 24–35. [DOI] [PubMed] [Google Scholar]
- Gifford RM. 1994. The global carbon cycle: a viewpoint on the missing sink. Australian Journal of Plant Physiology 21: 1–15. [Google Scholar]
- Gifford RM. 1995. Whole plant respiration and photosynthesis of wheat under increased CO2 concentration and temperature: long-term vs. short-term distinctions for modelling. Global Change Biology 1: 385–396. [Google Scholar]
- Graglia E, Jonasson S, Michelsen A, Schmidt IK. 1997. Effects of shading, nutrient application and warming on leaf growth and shoot densities of dwarf shrubs in two arctic-alpine plant communities. Ecoscience 4: 191–198. [Google Scholar]
- Harte J, Shaw R. 1995. Shifting dominance within a montane vegetation community: results of a climate-warming experiment. Science 267: 876–880. [DOI] [PubMed] [Google Scholar]
- Houghton JT, Ding Y, Griggs DJ, Noguer M, van der Linden PJ, Dai X, Maskell K, Johnson CA. 2001.Climate change 2001: the scientific basis. Third assessment report of Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press. [Google Scholar]
- Jarvis P, Linder S. 2000. Constraints to growth of boreal forests. Nature 405: 904–905. [DOI] [PubMed] [Google Scholar]
- Kirschbaum MUF. 2000. Forest growth and species distribution in a changing climate. Tree Physiology 20: 309–322. [DOI] [PubMed] [Google Scholar]
- Kramer K. 1995. Phenotypic plasticity of the phenology of seven European tree species in relation to climatic warming. Plant, Cell and Environment 18: 93–104. [Google Scholar]
- Larcher W, Thomaser-Thin W. 1988. Seasonal changes in energy content and storage patterns of Mediterranean sclerophylls in a northernmost habitat. Acta Oecologica 9: 271–283. [Google Scholar]
- Lebourgeois F, Lévy G, Aussenac G, Clerc B, Willm F. 1998. Influence of soil drying on leaf water potential, photosynthesis, stomatal conductance and growth in two black pine varieties. Annales des Sciences Forestieres 55: 287–299. [Google Scholar]
- Llorens L, Peñuelas J, Filella I. 2003. Diurnal and seasonal variations in the photosynthetic performance and water relations of two co-occurring Mediterranean shrubs, Erica multiflora and Globularia alypum Physiologia Plantarum 118: 84–95. [DOI] [PubMed] [Google Scholar]
- Llorens L, Peñuelas J, Estiarte M. 2003. Ecophysiological responses of two Mediterranean shrubs, Erica multiflora and Globularia alypum, to experimentally drier and warmer conditions. Physiologia Plantarum 119: 231–243. [DOI] [PubMed] [Google Scholar]
- Lloyd AH, Fastie CL. 2002. Spatial and temporal variability in the growth and climate response of treeline trees in Alaska. Climatic Change 52: 481–509. [Google Scholar]
- Mayor X, Rodà F. 1994. Effects of irrigation and fertilization on stem diameter growth in a Mediterranean holm oak. Forest Ecology and Management 68: 119–126. [Google Scholar]
- Menzel A, Fabian P. 1999. Growing season extended in Europe. Nature 397: 659. [Google Scholar]
- Menzel A. 2000. Trends in phenological phases in Europe between 1951 and 1996. International Journal of Biometeorology 44: 76–81. [DOI] [PubMed] [Google Scholar]
- Morison JIL, Lawlor DW. 1999. Interactions between increasing CO2 concentration and temperature on plant growth. Plant, Cell and Environment 22: 659–682. [Google Scholar]
- Ogaya R, Peñuelas J, Martínez-Vilalta J, Mangirón M. 2003. Effect of drought on diameter increment of Quercus ilex, Phillyrea latifolia, and Arbutus unedo in a holm oak forest of NE Spain. Forest Ecology and Management 180: 175–184. [Google Scholar]
- Oliveira G, Correia O, Martins-Louçao MA, Catarino FM. 1994. Phenological and growth patterns of the Mediterranean oak Quercus suber L. Trees 9: 41–46. [Google Scholar]
- Peñuelas J, Filella I. 2001. Responses to a warming world. Science 294: 793–795. [DOI] [PubMed] [Google Scholar]
- Peñuelas J, Filella I, Comas P. 2002. Changed plant and animal life cycles from 1952 to 2000 in the Mediterranean region. Global Change Biology 8: 531–544. [Google Scholar]
- Peñuelas J, Gordon C, Llorens L, Nielsen T, Tietema A, Beier C, Bruna P, Emmett B, Estiarte M, Gorissen A. 2004. Non-intrusive field experiments show different plant responses to warming and drought among sites, seasons and species in a North-South European gradient. Ecosystems 7: 598–612. [Google Scholar]
- Pereira JS. 1995. Gas exchange and growth. In: Schulze E-D, Caldwell MM, eds. Ecophysiology of photosynthesis. Berlin: Springer-Verlag,147–181. [Google Scholar]
- Ro H-M, Kim P-G, Lee I-B, Yiem M-S, Woo S-Y. 2001. Photosynthetic characteristics and growth responses of dwarf apple (Malus domestica Borkh. cv. Fuji) saplings after 3 years of exposure to elevated atmospheric carbon dioxide concentration and temperature. Trees 15: 195–203. [Google Scholar]
- Royce EB, Barbour MG. 2001. Mediterranean climate effects. II. Conifer growth phenology across a Sierra Nevada ecotone. American Journal of Botany 88: 919–932. [PubMed] [Google Scholar]
- Schwartz MD, Reiter BE. 2000. Changes in North American spring. International Journal of Climatology 20: 929–932. [Google Scholar]
- Soil Survey Staff. 1998. Keys to soil taxonomy. US Department of Agriculture–NRCS. [Google Scholar]
- Theurillat J-P, Guisan A. 2001. Potential impact of climate change on vegetation in the European Alps: a review. Climatic Change 50: 77–109. [Google Scholar]
- Waring RH, Landsberg JJ, Williams M. 1998. Net primary production of forests: a constant fraction of gross primary production? Tree Physiology 18: 129–134. [DOI] [PubMed] [Google Scholar]
- Weltzin JF, Pastor J, Harth C, Bridgham SD, Updegraff K, Chapin CT. 2000. Response of bog and fen plant communities to warming and water-table manipulations. Ecology 81: 3464–3478. [Google Scholar]
- Xiong FS, Mueller EC, Day TA. 2000. Photosynthetic and respiratory acclimation and growth response of Antarctic vascular plants to contrasting temperature regimes. American Journal of Botany 87: 700–710. [PubMed] [Google Scholar]
- Yordanov I, Velikova V, Tsonev T. 2000. Plant responses to drought, acclimation, and stress tolerance. Photosynthetica 38: 171–186. [Google Scholar]
- Yoshie F, Fukuda T. 1994. Effects of growth temperature and winter duration on leaf phenology of Erythronium japonicum, a forest spring geophyte. Oecologia 97: 366–368. [DOI] [PubMed] [Google Scholar]