Abstract
• Background and Aims Ultraviolet-B (UV-B) radiation effect on reproductive parts of the plants has received little attention. We studied the influence of UV-B radiation on flower and pollen morphology, pollen production and in vitro pollen germination and tube growth of six genotypes of soybean (Glycine max).
• Methods Soybean genotypes were investigated by growing them under four levels of biologically effective UV-B radiation of 0 (control), 5, 10 and 15 kJ m−2 d−1 in sunlit controlled-environment chambers.
• Key Results Reductions in lengths of flower, standard petal, and staminal column along with reduced pollen production, germination and tube growth were observed in all genotypes with increasing UV-B radiation. Combined response index (CRI), the sum of percentage relative responses in flower size, pollen production, pollen germination and tube growth due to UV-B radiation varied with UV-B dosage: −67 to −152 with 5 kJ m−2 d−1, −90 to −212 with 10 kJ m−2 d−1, and −118 to −248 with 15 kJ m−2 d−1 of UV-B compared to controls. Genotypes were classified based on the UV-B sensitivity index (USI) calculated as CRI per unit UV-B, where D 90-9216, DG 5630RR and D 88-5320 were classified as tolerant (USI > −7·43), and DP 4933RR, Stalwart III and PI 471938 were sensitive (USI < −7·43) in their response to UV-B radiation. Pollen grains produced in plants grown at 15 kJ m−2 d−1 UV-B radiation were shrivelled and lacked apertures compared to control and other UV-B treatments in both sensitive and tolerant genotypes, and the differences were more conspicuous in the sensitive genotype (PI 471938) than in the tolerant genotype (D 90-9216). The number of columellae heads of the exine was reduced with increasing UV-B radiation.
• Conclusions Soybean genotypes varied in their reproductive response to UV-B radiation. The identified UV-B tolerant genotypes could be used in future breeding programmes.
Key words: Floral morphology, Glycine max, pollen germination, pollen morphology, ultraviolet-B radiation
INTRODUCTION
Significant trends of reduction in the total column of ozone are apparent in both northern and southern hemispheres at both mid- and high latitudes (Herman et al., 1999). Recent projections suggest that ozone depletion will reach its maximum in the coming years and forecasted to recover slowly over the next several decades. Relative to the 1970s, present-day losses in stratospheric ozone are estimated to be 50 % in the spring over Antarctica and 5 % in the mid-latitudes during the year (Madronich et al., 1999). However, a number of uncertainties exist, including interactions with other projected changes in global climate such as global warming (Schindell et al., 1998; Madronich et al., 1999). The measurable attenuation of the stratospheric ozone layer and consequent increase in the terrestrial UV-B radiation showed a 6–14 % increase since 1970s (Kerr and McElroy, 1993; UNEP, 2002) which has raised interest in understanding the deleterious effects of UV-B radiation on higher plants (for reviews see Searles et al., 2001; Kakani et al., 2003a; Krupa, 2003). Global terrestrial UV-B radiation levels range between 2 and 12 kJ m−2 d−1 on a given day with near equator and mid-latitudes receiving higher doses (total ozone mapping spectrometer 2002, http://toms.gsfc.nasa.gov/ery-uv/euv.html). The UV-B levels under soybean growing conditions in the USA ranged from 4·2 to 8·7 kJ m−2 d−1 during June–August 2002 (http://uvb.nrel.colostate.edu/UVB/).
Many studies evaluating the impact of enhanced UV-B on crop yields have been carried out in both field and greenhouse conditions (Kakani et al., 2003a). Almost half of them showed that enhanced UV-B radiation decreased yield and the other half showed no effect on the yield. The UV-B supplied in these studies varied considerably (2·5–63 kJ m−2 d−1) and some studies conducted in the field on soybean (Glycine max) showed that UV-A was effective in mitigating the response to UV-B damage (Caldwell et al., 1994). Recent studies showed genotypic variation in the physiological responses of 20 soybean cultivars to UV-B radiation of about 5 kJ m−2 d−1 (Zu et al., 2003), which decreased grain yield by 15–92 % (Yuan et al., 2002; Ambasht et al., 2003). Understanding mechanisms and causes for the yield losses in crops when they are exposed to enhanced UV-B radiation is necessary.
Pollen release and viability were shown to be the major limiting factors for fruit set under several other environmental stresses such as high-temperature stress in tomato (Lycopersicon esculentum Mill.) (Peet et al., 1998) and groundnut (Arachis hypogaea L.) (Prasad et al., 1999). High temperatures have been reported to affect microsporogenesis more than megasporogenesis and post-anthesis reproductive developmental processes in tomato (Monterroso and Wien, 1990; Peet et al., 1998). Along with high temperatures, other stresses such as water-deficit (Shen and Webster, 1986) and low night temperatures (Mercado et al., 1997) reduced pollen germination, and tube lengths. Therefore, it is necessary to understand UV-B effects on pollen morphology, germination and tube growth.
Some studies, where pollen collected from healthy plants was directly exposed to UV-B by exposing the germination media to UV-B, showed that UV-B has reduced pollen germination (Torabinejad et al., 1998; Musil et al., 1999; Feng et al., 2000). In natural growth conditions, however, the plant itself will be exposed to UV-B radiation, and this may result in both direct morphological disturbances on pollen that may later reduce pollen germination and pollen tube growth. Therefore, studies on pollen collected from UV-B-irradiated plants are necessary to gain knowledge on the effect of increased UV-B radiation on pollen morphology along with germination and other related characteristics. The objectives were to examine the effects of UV-B radiation on flower and pollen morphology, pollen production, and in vitro pollen germination and tube growth of soybean genotypes, and also to understand the intraspecific variation of soybean in response to UV-B radiation.
MATERIALS AND METHODS
Soil–plant–atmosphere research chambers
The experiment was conducted at the R.R. Foil Plant Science Research Facility, Mississippi State University (33°28′N, 88°47′W), Mississippi State, Mississippi, USA, in 2003 using four soil–plant–atmosphere research (SPAR) chambers. The SPAR facility has the capability to precisely control temperature and CO2 concentration ([CO2]) at predetermined set points for plant growth studies under near ambient levels of photosynthetically active radiation (PAR). Details of the operation and control of SPAR chambers have been described by Reddy et al. (2001) and Zhao et al. (2003). Each SPAR unit consists of a steel soil bin (1 m deep × 2 m long × 0·5 m wide), and a Plexiglas chamber (2·5 m tall × 2 m long × 1·5 m wide) to accommodate aerial plant parts, a heating and cooling system, and an environment monitoring and control system. The Plexiglas chamber is opaque to solar UV radiation of below 385 nm but transmits 96·6 ± 0·5 % of incoming PAR (wavelength 400–700 nm) (Zhao et al., 2003). During the experiment, the ambient total solar radiation (285–2800 nm) measured with a pyranometer (Model 4-48, Eppley Laboratory Inc., Newport, RI, USA) was 21·2 ± 0·5 MJ m−2 d−1. The air temperature and [CO2] in each SPAR unit were monitored and adjusted every 10 s throughout the day and night.
Plant culture
Six soybean genotypes were selected from 45 genotypes, varying in maturity groups that included glyphosate-tolerant and conventional varieties. The selection of genotypes was based on the amount of phenolics (ranging from 51·7 to 131·2 µg cm−2) accumulated under normal growing conditions as they are screening compounds for UV-B radiation tolerance (Cockell and Knowland, 1999). Genotypes D 88-5320 (matu D 88-5320 (maturity group VI, non-glyphosate-tolerant, phenolic content 96·7 μg cm−2), D 90-9216 (maturity group VII, non-glyphosate-tolerant, phenolic content 120·8 μg cm−2), Stalwart III (maturity group III, non-glyphosate-tolerant, phenolic content 131·2 μg cm−2), Plant Introduction (PI) 471938 (maturity group V, non-glyphosate-tolerant, phenolic content 91·1 μg cm−2), Delta Grow (DG) 5630RR (maturity group V, glyphosate-tolerant, phenolic content 72·5 μg cm−2), and Delta Pine (DP) 4933RR (maturity group IV, glyphosate-tolerant, phenolic content 57·8 μg cm −2) were sown on 5 August 2003 in 7·5-cm-diameter pots filled with fine sand.Thirty pots (five pots of each genotype) were arranged randomly in each SPAR chamber. Emergence was 5 d after sowing (DAS). The temperatures were maintained at 30/22 °C (day/night) in all the units, and the measured temperatures were 29·7 ± 0·24/20·7 ± 0·25 °C (control), 29·4 ± 0·28/21·3 ± 0·23 °C (5 kJ UV-B treatment), 29·4 ± 0·21/21·2 ± 0·22 °C (10 kJ UV-B treatment) and 28·7 ± 0·28/21·3 ± 0·23 °C (15 kJ UV-B treatment). The differences were not significant between the units. Plants were watered three times a day with half-strength Hoagland's nutrient solution delivered at 0800, 1200 and 1700 h to ensure favourable nutrient and water conditions for plant growth through an automated and computer-controlled drip system. Variable-density black shade cloths around the edges of plants were adjusted regularly to match plant height in order to simulate natural shading in the presence of other plants. First flower (FF) (R1 stage) was recorded on all the genotypes in all the treatments.
Treatments
Four treatments of biologically effective UV-B (280–320 nm) radiation intensities of 0 (control), 5, 10 and 15 kJ m−2 d−1 were imposed from emergence. Square-wave UV-B supplementation systems were used to provide respective UV-B radiation under near-ambient PAR. The Plexiglas of the SPAR chamber was opaque to solar UV-B radiation. The UV-B radiation was delivered to plants for 8 h, each day, from 0800 to 1600 h by eight fluorescent UV-313 lamps (Q-Panel Company, Cleveland, OH, USA) driven by 40 W dimming ballasts. The lamps were wrapped with presolarized 0·07 mm cellulose diacetate film to filter UV-C (<280 nm) radiation. The cellulose diacetate film was changed at 3 to 4 d intervals. The biologically effective UV-B energy delivered at the top of the plant canopy was checked daily at 0900 h with a UVX digital radiometer (UVP Inc., San Gabriel, CA, USA) and calibrated against an Optronic Laboratory (Orlando, FL, USA) Model 754 Spectroradiometer, which was used initially to quantify lamp output. The lamp output was adjusted, as needed, to maintain the respective UV-B radiation levels. A distance of 0·5 m from lamps to the top of plants was maintained throughout the experiment. The actual biologically effective UV-B radiation was measured during the crop growth period at six different locations in each SPAR unit corresponding to the pots arranged in rows. The weighted total UV-B radiation levels received at the top of the plants beneath the lamps were 0, 4·8 ± 0·05, 9·8 ± 0·16 and 14·3 ± 0·11 kJ m−2 d−1 for 0, 5, 10 and 15 kJ m−2 d−1 treatment set points, respectively, using the generalized plant response action spectrum (Caldwell, 1971) normalized at 300 nm. Although square wave supplementation system in controlled environments provide disproportionate spectral conditions on cloudy days, they are useful for quantifying the growth and developmental responses of plants to UV-B.
Measurements
Floral morphology
Length of the flower, standard petal, and staminal column were measured on 20 fresh flowers randomly picked from five plants of each genotype. Soybean has a typical papilionaceous flower with a tubular calyx of five unequal sepal lobes and a five-parted corolla consisting of posterior standard petal, two lateral wing petals and two anterior keel petals in contact with each other but not fused (Carlson and Larsten, 1987). Flower length was measured from the tip of the standard petal to the base of the calyx. The standard petal was stretched before measuring the length, and the length was measured from the point of insertion to the distal end. Staminal column was separated from flower and its length was measured.
Pollen number
Mature anthers were collected from five different inflorescences from five plants a day before anthesis to determine the number of pollen grains produced per anther. Pollen was counted by placing a single anther in a water drop on a glass slide and squashed with a needle, and the pollen grains dispersed in the drop of water were counted (Bennett, 1999).
Pollen germination and pollen tube lengths
Flowers were randomly selected from all the five plants in each genotype in the morning between 0900 and 1000 h from five plants, for pollen germination. Flowers were air-dried for 2 h and fresh pollen was then dusted onto modified in vitro germination medium (Gwata et al., 2003). The growth medium was prepared with 15 g sucrose (C12H22O11), 0·03 g calcium nitrate [Ca(NO3)2·4H2O], 0·01 g boric acid (H3BO3), and 0·6 g agar in 100 mL of distilled water and boiled for 10 min, and then 10 mL of the medium was poured into five 5-cm-diameter Petri dishes of for each genotype at each UV-B level (Salem et al., 2004). The pollen was dusted onto the solidified medium to allow a uniform distribution of grains on the surface of the medium. The plates were then covered and incubated at 30 °C (Precision Instruments, New York, USA) for 8–12 h. Pollen grain was considered germinated when its tube length at least equalled the grain diameter (Luza et al., 1987) using a Nikon SMZ 800 microscope (Nikon Instruments, Kanagawa, Japan) with a magnification of ×6·3 (five fields in each Petri dish). Pollen germination was determined as a percentage of total pollen. Pollen tube length was obtained by measuring 20 randomly selected pollen from each Petri dish using a microscope. The lengths were measured with an ocular micrometer fitted to the eye-piece of the microscope. A total of 100 pollen tubes were measured for each genotype at each UV-B level.
Combined response index (CRI) and UV-B sensitivity index (USI)
A combined response index (CRI), based on the concept of Dai et al. (1994), was calculated to evaluate the overall reproductive response of soybean to enhanced UV-B radiation using the following equation:
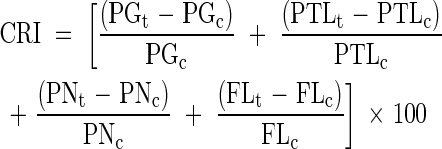 |
where CRI = combined response index, PG = pollen germination percentage, PTL = pollen tube length, PN = pollen number anther−1, and FL = flower length under t (treatment) and c (control) levels of UV-B radiation. A UV-B sensitivity index (USI) was calculated as the slope of the curve when CRI was regressed against UV-B radiation treatment. Based on the USI values, the genotypes were classified as tolerant and sensitive.
Pollen morphology
After the classification of genotypes based on USI, two genotypes, one tolerant (D 90-9216) and one sensitive (PI 471938), were selected for pollen morphological studies. Fresh flowers were collected between 1900 and 2100 h, a day before anthesis, and stored in FAA (formaldehyde–glacial acetic acid–ethyl alcohol) solution for scanning electron microscopy (SEM). Flower buds were removed from FAA and were fixed overnight in 3 % glutaraldehyde in 0·1 M phosphate buffer at pH 7·2 and 4 °C for SEM. After fixation, specimens were rinsed in buffer, post-fixed in 2 % osmium tetroxide (OsO4) in 0·1 M phosphate buffer for 2 h, rinsed in distilled water, dehydrated in an ethanolic series, and critical-point dried in a Polaron E 3000 Critical Point Dryer (Quorum Technologies, Newhaven, UK). Specimens were mounted on aluminium stubs, sputter-coated with gold in a Polaron E 5100 sputter coater (Quorum Technologies), and viewed in a LEO Stereoscan 360 SEM (LEO Electron Microscopy, Thornwood, NY, USA) at an accelerating voltage of 15 kV. Images were recorded on Polaroid Type 55 film (Polaroid, Cambridge, MA, USA).
Statistical analysis
Four treatments were randomly arranged in four identical SPAR units. Except for the treatment factors of UV-B radiation, the other growth conditions were the same in all units. Data were statistically analysed using a two-way analysis of variance (ANOVA) to test the significance of UV-B and genotype effect on flower morphological features and pollen germination percentage and pollen tube lengths by Genstat 6 for Windows (Genstat 6 Committee, 1997). The least significant difference (LSD) tests at P = 0·05 were employed to distinguish treatment differences. Data of pollen germination percentages were transformed using the arcsin transformation before statistical analysis. Differences between genotypes were determined by testing the heterogenity of slopes and comparison of intercepts of the linear models of CRI against UV-B dosage using Genstat 6 for Windows (Genstat 6 Committee, 1997). Genotypes were classified as tolerant [(>minimum USI + 1 s.d.], and sensitive (<minimum USI + 1 s.d.).
RESULTS
Floral morphology
Genotypic variation was observed for soybean in reaching the R1 stage, whereas the UV-B treatments did not modify this phenostage. Averaged over the UV-B treatments, first flower (FF) was produced on 33, 43, 44, 46, 49 and 52 d after emergence (DAE) in Stalwart III, DP 4933 RR, PI 471938, DG 5630 RR, D90-9216 and D88-5320, respectively. The effects of UV-B radiation on flower morphological characteristics are shown in Fig. 1. With increasing UV-B radiation, there were significant (P < 0·001) reductions in flower lengths (Fig. 1A) in all genotypes and genotypes varied significantly for flower lengths. A reduction of about 78 % was observed in Stalwart III at 15 kJ m−2 d−1 UV-B treatment, but the reduction was only 20 % in DG 5630RR (Table 1). The longest flowers with longer standard petals and staminal column lengths were observed in DG 5630RR, while the shortest flowers with shorter standard petal and staminal columns were observed in Stalwart III and PI 471938 genotypes under control conditions (Figs 1B and 2A).
Fig. 1.

Influence of UV-B radiation (UV-B) on (A) flower length, and (B) standard petal length of six soybean genotypes (G) (D 90-9216, Stalwart III, PI 471938, DP 4933RR, D 88-5320, and DG 5630RR). Bars represent standard errors (n = 25 for flower and standard petal lengths). Significance levels: *** P < 0·001.
Table 1.
Genotypic sensitivity to UV-B radiation based on percentage relative responses in flower length, pollen production, germination, and pollen tube length of six genotypes of soybean, in comparison to control
| UV-B (kJ m−2 d−1) |
Flower length |
Pollen production |
Pollen germination |
Pollen tube length |
Combined response index |
Rank |
|---|---|---|---|---|---|---|
| 5 | DG 5630RR (−10) | DG 5630RR (−8) | D 90-9216 (−30) | DP 4933RR (0) | D 90-9216 (−68) | 1 |
| D 90-9216 (−13) | PI 471938 (−11) | D 88-5320 (−32) | D 88-5320 (−5) | DG 5630RR (−72) | 2 | |
| PI 471938 (−20) | Stalwart III (−15) | DG 5630RR (−35) | D 90-9216 (−7) | D 88-5320 (−80) | 3 | |
| D 88-5320 (−22) | D 90-9216 (−18) | PI 471938 (−40) | DG 5630RR (−19) | PI 471938 (−117) | 4 | |
| DP 4933RR (−40) | D 88-5320 (−21) | Stalwart III (−45) | Stalwart III (−24) | Stalwart III (−128) | 5 | |
| Stalwart III (−44) | DP 4933RR (−60) | DP 4933RR (−52) | PI 471938 (−46) | DP 4933RR (−152) | 6 | |
| 10 | DG 5630RR (0) | DG 5630RR (−13) | D 90-9216 (−42) | D 90-9216 (−14) | DG 5630RR (−90) | 1 |
| D 90-9216 (−13) | PI 471938 (−23) | PI 471938 (−45) | D 88-5320 (−15) | D 90-9216 (−93) | 2 | |
| D 88-5320 (−22) | D 90-9216 (−24) | D 88-5320 (−51) | DG 5630RR (−19) | D 88-5320 (−117) | 3 | |
| PI 471938 (−30) | D 88-5320 (−29) | Stalwart III (−54) | DP 4933RR (−26) | PI 471938 (−156) | 4 | |
| DP 4933RR (−50) | Stalwart III (−34) | DG 5630RR (−58) | Stalwart III (−45) | Stalwart III (−189) | 5 | |
| Stalwart III (−56) | DP 4933RR (−71) | DP 4933RR (−65) | PI 471938 (−58) | DP 4933RR (−212) | 6 | |
| 15 | DG 5630RR (−20) | DG 5630RR (−21) | D 90-9216 (−48) | D 90-9216 (−21) | D 90-9216 (−121) | 1 |
| D 90-9216 (−25) | D 90-9216 (−27) | D 88-5320 (−53) | DG 5630RR (−24) | DG 5630RR (−132) | 2 | |
| D 88-5320 (−33) | D 88-5320 (−32) | DG 5630RR (−67) | D 88-5320 (−35) | D 88-5320 (−153) | 3 | |
| PI 471938 (−50) | PI 471938 (−36) | PI 471938 (−72) | DP 4933RR (−37) | PI 471938 (−221) | 4 | |
| DP 4933RR (−60) | Stalwart III (−38) | Stalwart III (−73) | Stalwart III (−48) | Stalwart III (−237) | 5 | |
| Stalwart III (−78) | DP 4933RR (−75) | DP 4933RR (−76) | PI 471938 (−63) | DP 4933 RR (−248) | 6 |
Ranking 1–6 is in the order of increasing sensitivity to UV-B radiation based on combined response index (the sum of relative responses).
Values in parentheses represent percentage relative responses.
Fig. 2.

Influence of UV-B radiation (UV-B) on (A) staminal column length (mm), and (B) pollen production (pollen anther−1) of six soybean genotypes (G) (D 90-9216, Stalwart III, PI 471938, DP 4933RR, D 88-5320 and DG 5630RR). Bars represent standard errors (n = 9 for pollen production and n = 25 for staminal column lengths). Significance levels: *** P < 0·001.
Pollen number per anther
Anthers borne on plants exposed to high levels of UV-B radiation had significantly (P < 0·001) less pollen, compared with those on control plants (Fig. 2B). Of all the genotypes, Stalwart III produced 543, 461, 358 and 338 pollen anther−1at 0, 5, 10 and 15 kJ m−2 d−1 UV-B, respectively, significantly more than any other genotype at those respective treatments. There were no significant differences in the number of anthers produced per flower between UV-B treatments (data not shown) in all the genotypes. The reduction in pollen production due to UV-B radiation was greater in DP 4933RR, where the relative response ranged from −60 to −75 across the UV-B radiation treatments, whereas the response was −8 to −21 in DG 5630RR (Table 1).
Pollen germination and tube lengths
All the genotypes exhibited considerable sensitivity to UV-B in terms of pollen germination and pollen tube length (Fig. 3). There were significant reductions (P < 0·001) in pollen germination and tube lengths of the genotypes under high levels of UV-B radiation, but genotypes differed significantly (P < 0·001) in the degree of their response to UV-B radiation (Fig. 3). The percentage of pollen germination ranged from 72 (D 88-5320) to 92 % (PI 471938 and DP 4933RR), and tube lengths ranged from 187 (DP 4933RR) to 329 μm (PI 471938) among treatments. Relative responses of pollen tube lengths were more in PI 471938 (−46 to −63) and less in D 90-9216 (−7 to −21) with high UV-B radiation levels when compared with the control (Table 1).
Fig. 3.

Influence of UV-B radiation (UV-B) on (A) pollen germination percentage, and (B) pollen tube lengths (μm) of six soybean genotypes (G) (D 90-9216, Stalwart III, PI 471938, DP 4933RR, D 88-5320 and DG 5630RR). Bars represent standard errors (n = 25 for pollen germination percentage, and n = 100 for pollen tube lengths). Significance levels: *** P < 0·001.
Combined response index (CRI)
The CRI, an integration of UV-B effects on pollen production, pollen germination, pollen tube lengths, and flower lengths, showed that soybean is sensitive to enhanced UV-B radiation. In this study, all the genotypes had a negative CRI (Table 1) indicating deleterious effects of UV-B. The CRI ranged from −67 (D 90-9216) to −152 (DP 4933RR) at 5 kJ, −90 (DG 5630RR) to −212 (DP 4933RR) at 10 kJ, and −118 (D 90-9216) to −248 (DP 4933RR) at 15 kJ m−2 d−1 of UV-B (Table 1). The genotypes were classified based on USI, the slope when CRI is plotted against UV-B (Fig. 4). The genotypes with USI > −7·43 were classified as tolerant (D 90-9216, DG 5630RR, and D 88-5320), and those with USI < −7·43 as sensitive (DP 4933R, Stalwart III and PI 471938). The genotypes differed significantly (P < 0·001) between slopes and intercepts of the linear models.
Fig. 4.
Combined response index, a sum of the relative responses in pollen germination, tube growth, pollen production, and flower lengths due to UV-B radiation regressed over respective levels of UV-B radiation in six soybean genotypes (D 90-9216, Stalwart III, PI 471938, DP 4933RR, D 88-5320 and DG 5630RR).
Pollen morphology
Although no gross morphological differences in the pollen grown under control conditions were visible between the sensitive (PI 471938) and tolerant (D 90-9216) genotypes, pollen abnormalities were observed in both genotypes with increasing UV-B radiation levels. The abnormalities were more evident in PI 471938 compared with D 90-9216. At the highest UV-B treatment of 15 kJ m−2 d−1, large numbers of the pollen grains were shrivelled compared with the pollen from plants grown in control conditions (Fig. 5D, H). There were no apertures on the pollen grains from 15 kJ m−2 d−1 UV-B irradiated plants, while the pollen produced in plants grown under control conditions were triporate, i.e. they had three protruding apertures (Fig. 5A and E). Differences in pollen exine structure were also observed (Fig. 6A–H). The columellae heads of the exine were less at high UV-B levels in both sensitive and tolerant genotypes, and the differences in the columellae heads were more pronounced in the sensitive genotype, resulting in an altered appearance of the exine.
Fig. 5.

Scanning electron microscopy images of pollen grains of two soybean genotypes, PI 471938 (UV-B sensitive) (A–D) and D 90-9216 (UV-B tolerant) (E–H), grown under four levels of UV-B radiation of 0 (control) (A and E), 5 (B and F), 10 (C and G) and 15 kJ m−2 d−1 (D and H). Arrows indicate shrivelled pollen grains without apertures at 15 kJ m−2 d−1 UV-B treatment. Scale bars = 20 μm.
Fig. 6.

Scanning electron microscopy images of pollen grain surface of soybean genotypes PI 471938, (UV-B stress sensitive) (A–D) and D 90-9216 (UV-B stress tolerant) (E–H), grown at four levels of UV-B radiation, 0 (control) (A and E), 5 (B and F), 10 (C and G) and 15 kJ m−2 d−1 (D and H). Arrows indicate disturbed exine ornamentation of pollen from 15 kJ m−2 d−1 UV-B treatment. Scale bars = 2 μm.
DISCUSSION
This is the first report to show that soybean plants exposed to higher levels of UV-B radiation produce smaller flowers with fewer pollen grains with altered pollen morphology and germination percentage, suggesting that UV-B damages reproductive efficiency. The smaller flowers in all the genotypes at higher levels of UV-B radiation were the result of flower-component part reductions such as standard petal and staminal column lengths. Similar reductions in flower lengths at elevated UV-B radiation levels were observed in cotton (Gossypium hirsutum) (Kakani et al., 2003b).
Flowers produced fewer pollen grains under enhanced UV-B radiation. The considerable genotypic differences in pollen produced under different levels of UV-B radiation in our study might be due to different adaptive mechanisms of the genotypes to UV-B radiation. In natural environments, such large reductions in pollen production could have far-reaching consequences on the reproductive success of plants. Reductions observed in germination and inhibition of pollen tube growth caused by high levels of UV-B radiation might decrease the effectiveness of pollination and fertilization, and consequently change the quantity and quality of seed (Demchik and Day, 1996; Van de Staaij et al., 1997). Similar reductions in the amount of pollen produced were observed in Brassica rapa with enhanced UV-B radiation simulating 16 and 32 % ozone depletions (Demchik and Day, 1996). An alteration in plant carbon allocation, increased allocation of resources towards repair mechanisms such as photoreactivation, excision repair (Taylor et al., 1996), and biosynthesis of UV-B flavonoid and related phenolic compounds (Caldwell et al., 1983) at the expense of reproductive structures, may cause a reduction in flower production (Sampson and Cane, 1999) and flower size (Kakani et al., 2003b).
Generally, it is thought that the reproductive system with developing pollen grains is well protected from UV-B (Martin, 1970; Flint and Caldwell, 1983) because perianths of most plants provide complete screening of reproductive organs from UV-B radiation since the corolla exhibits low UV-B transmittance (Flint and Caldwell, 1983). This may not be true in the natural conditions because UV-B can cause an indirect effect on pollen when the plant itself is exposed to high UV-B radiations. In our study, UV-B irradiation reduced pollen quality. Reductions in in vitro pollen germination were shown when pollen from normal plants was directly irradiated with UV-B radiation (Torabinejad et al., 1998; Musil et al., 1999; Feng et al., 2000). Pollen grain walls appear to transmit as much as 20 % of UV-B (Stadler and Uber, 1942). This relatively high transmittance may explain why several researchers have found reductions in in vitro germination of pollen when exposed to UV-B.
The CRI calculated is considered as a good indicator to assess plant sensitivity to UV-B radiation (Dai et al., 1994; Li et al., 2000; Yuan et al., 2002). Although parameters used in calculating CRI in this study were different from those used in previous studies, we found that the ranking of the genotypes for UV-B sensitivity was generally similar, regardless of whether ranking was assessed using individual parameters or an index. We classified all the six genotypes into tolerant and sensitive types based on the value of USI. Out of all the genotypes tested, D 90-9216, DG 5630RR and D88-5320 were found to be tolerant and DP 4933R, Stalwart III and PI 471938 were found to be sensitive. Similar genotype classifications based on CRI were performed for 20 wheat (Triticum aestivum) and soybean cultivars by Li et al. (2000) and Yuan et al. (2002), respectively, while studying enhanced UV-B radiation effects on vegetative and yield characteristics.
The present study is the first to report pollen abnormalities at high levels of UV-B radiation. The SEM results show that both sensitive (PI 471938) and tolerant (D 90-9216) genotypes grown under 15 kJ m−2 d−1 UV-B produced flattened and shrivelled pollen with abnormal exine surface ornamentation, but the differences were more pronounced in the sensitive genotype. In the control treatment, pollen was equipped with three apertures, which act as exits for the germinating pollen tube and also allow recognition of protein signals that initiate development of a pollen tube (Heslop-Harrison, 1971). The missing apertures could be the main reason for the reduction in pollen germination and tube growths observed under high UV-B radiation treatments. The inhibition of exine formation could be due to limitations in sporopollenin production, transport, or deposition. The exine sporopollenin originates from the tapetum (Dickinson and Potter, 1976) and normal pollen (gametophyte) development depends on a close interaction with the tapetal (sporophytic) tissue that composes of the innermost layer of the anther, which plays an important layer in microsporogenesis. The tapetum supplies nutrients necessary for pollen development (Dickinson, 1992) and provides the precursors of exine formation (Shivanna and Johri, 1985). Abnormalities observed in this study such as altered exine ornamentation with reduced columellae heads are indicative of the abnormal tapetum function. Although premature degeneration of the tapetal layer (Ahmed et al., 1992) could explain the abnormalities observed, the role of the tapetum was not described definitively. All these and other unknown factors may have contributed to the poor pollen germination observed in plants grown under UV-B radiation. The UV-B stress-induced morphological changes are intriguing and necessitate further investigation.
The experiment was conducted in sunlit-SPAR chambers under controlled environments using the square-wave UV-B delivery system. On cloudy days, the square-wave UV-B system usually overestimates UV-B dosages (Musil et al., 2002). Although evidence from published reports indicates no systematic differences among the chambers (Kakani et al., 2003b; Zhao et al., 2003), one chamber for each UV-B treatment and square-wave UV-B system might limit our strong conclusions in this study.
In summary, enhanced UV-B radiation had a significant effect on the reproductive biology of soybean. Genotypic variability was evident in response to UV-B radiation although all the genotypes were significantly affected. The altered floral morphology and decreased pollen production, along with altered pollen morphological characters and pollen germination capacity, will have a direct impact on the fertilization process and fruit set in sensitive genotypes. The differences in UV-B sensitivity identified among genotypes imply the possibility of selecting soybean genotypes with tolerance to elevated UV-B radiation. The UV-B tolerant genotypes identified in our study could be used as possible donors in breeding programmes. However, their genetic bases for these differences must be further examined. Further studies to relate pollen germination to fruit set are needed.
Supplementary Material
Acknowledgments
The project was funded in part by the USDA UV-B Monitoring Program and the RSTC-NASA. We thank D. Brand, K. Gourley and W. Ladner for their technical support and Bill Monroe, Electron Microscopy Center for his help during SEM studies. We thank Drs D. C. Gitz, L. G. Heatherly, M. M. Peet and C. S. Tara for reviewing the manuscript and for their helpful comments. We acknowledge Drs R. Smith and T. Carter for providing the seed of genotypes used in this study. Contribution from the Department of Plant and Soil Sciences, Mississippi State University, Mississippi Agricultural and Forestry Experiment Station paper no. J10477.
LITERATURE CITED
- Ahmed FE, Hall AE, DeMason. 1992. Heat injury during floral development in cowpea (Vigna unguiculata, Fabaceae). American Journal of Botany 79: 784–791. [Google Scholar]
- Ambasht M, Agarwal M, Agarwal M. 2003. Interactive effects of ozone and ultraviolet-B radiation on physiological and biochemical characteristics of soybean plants. Journal of Plant Biology 30: 37–45. [Google Scholar]
- Bennett SJ. 1999. Pollen-ovule ratios as a method of estimating breeding systems in Trifolium pasture species. Australian Journal of Agricultural Research 50: 1443–1450. [Google Scholar]
- Caldwell MM. 1971. Solar UV irradiation and the growth and development of higher plants. In: Giese AC, ed. Photophysiology. New York: Academic Press, 131–137. [Google Scholar]
- Caldwell MM, Robberecht R, Stephan DF. 1983. Internal filters: Prospects for UV-acclimation in higher plants. Physiologia Plantarum 58: 445–450. [Google Scholar]
- Caldwell MM, Flint SD, Searles PS. 1994. Spectral balance and UV-B sensitivity of soybean: a field experiment. Plant, Cell and Environment 17: 267–276. [Google Scholar]
- Carlson JB, Larsten NR. 1987. Reproductive morphology. In: Wilcox JR, ed. Soybeans: Improvement, Production and Uses. Wisconsin: ASA-CSSA-SSSA Inc., Publishers, 97–102. [Google Scholar]
- Cockell CS, Knowland J. 1999. Ultraviolet radiation screening compounds. Biological Reviews 74: 311–345. [DOI] [PubMed] [Google Scholar]
- Dai QJ, Peng SB, Chavez AQ, Vergara BS. 1994. Intraspecific responses of 188 rice cultivars to enhanced UV-B radiation. Environmental and Experimental Botany 34: 422–433. [Google Scholar]
- Demchik SM, Day TA. 1996. Effect of enhanced UV-B radiation on pollen quantity, quality, and seed yield in Brassica rapa (Brassicaceae). American Journal of Botany 83: 573–579. [Google Scholar]
- Dickinson HG, Potter U. 1976. The development of patterning in the alveolar sexine of Cosmos bipinnatus. New Phytologist 76: 543–550. [Google Scholar]
- Dickinson HG. 1992. Microspore derived emobryogenesis. In: Cresti M, Tiezzi A, eds. Sexual Plant Reproduction. Berlin: Springler Berlag, 1–5. [Google Scholar]
- Feng H, An L, Tan L, Hou Z, Wang X. 2000. Effect of enhanced ultraviolet-B radiation on pollen germination and tube growth of 19 taxa in vitro Environmental and Experimental Botany 43: 45–53. [Google Scholar]
- Flint SD, Caldwell MM. 1983. Influence of floral optical properties in the ultraviolet radiation environment of pollen. American Journal of Botany 70: 1416–1419. [Google Scholar]
- Genstat 6 Committee. 1997.Genstat 6 Release 3 Reference Manual. Oxford: Clarendon Press. [Google Scholar]
- Gwata ET, Wofford DS, Pfahler PL, Boote KJ. 2003. Pollen morphology and in vitro germination characteristics of nodulating and nonnodulating soybean (Glycine max L.) genotypes. Theoretical and Applied Genetics 106: 837–839. [DOI] [PubMed] [Google Scholar]
- Herman JR, McKenzie RL, Diaz S, Kerr J, Madronich S, Seckmeyer G. 1999. Ultraviolet radiation at the earth's surface. In: Albritton DP, Aucamp P, Megies G, Watson R, eds. Scientific assessment of ozone depletion. Geneva: World Meteorological Organization, chapter 9: 1–46. [Google Scholar]
- Heslop-Harrison J.S. 1971. The pollen wall: structure and development. In: Heslop-Harrison J, S. ed. Pollen: Development and Physiology. London: Butterworths, 75–98. [Google Scholar]
- Kakani VG, Reddy KR, Zhao D, Koti S. 2003. Field crop responses to ultraviolet-B radiation: A review. Agricultural and Forest Meteorology 120: 191–218. [Google Scholar]
- Kakani VG, Reddy KR, Zhao D, Mohammed AR. 2003. Effects of ultraviolet-B radiation on cotton (Gossypium hirsutum L.) morphology and anatomy. Annals of Botany 91: 817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JB, McElroy CT. 1993. Evidence for large upward trends of ultraviolet-B radiation linked to ozone depletion. Science 262: 1032–1034. [DOI] [PubMed] [Google Scholar]
- Krupa SV. 2003. Joint effects of elevated levels of ultraviolet-B radiation, carbon dioxide and ozone on plants. Photochemistry and Photobiology 78: 535–542. [DOI] [PubMed] [Google Scholar]
- Li Y, Zu YQ, Chen HY, Chen JJ, Yang JL, Hu ZD. 2000. Intraspecific responses in crop growth and yield of 20 wheat cultivars to enhanced UV-B radiation under field conditions. Field Crops Research 67: 25–33. [Google Scholar]
- Luza JG, Polito VS, Weinbaum SA. 1987. Staminate bloom and temperature responses of pollen germination and the growth of two walnut (Juglans nigra) species. American Journal of Botany 74: 1308–1403. [Google Scholar]
- Madronich S, Velders G, Daniel J, Lal M, McCulloch A, Slaper H. 1999. Halocarbon scenarios for the future ozone layer and related consequences. In: Albritton D, Aucamp P, Megie G, Watson R, eds. Scientific assessment of ozone depletion. Geneva: World Meteorological Organization, chapter 11, 1–38. [Google Scholar]
- Martin FW. 1970. The ultraviolet absorption profile of the stigmatic extracts. New Phytologist 69: 425–430. [Google Scholar]
- Mercado JA, Mar Trego M, Reid MS, Valpuesta V, Quesada MA. 1997. Effects of low temperature on pepper pollen morphology and fertility: evidence of cold induced exine alterations. Journal of Horticultural Science 72: 217–226. [Google Scholar]
- Monterroso VA, Wien HC. 1990. Flower and pod abscission due to heat stress in beans. Journal of the American Society of Horticultural Science 115: 631–634. [Google Scholar]
- Musil CF, Midgley GF, Wand SJE. 1999. Carry-over of enhanced ultraviolet-B exposure effects to successive generations of a desert annual: Interaction with atmospheric CO2 and nutrient supply. Global Change Biology 5: 311–329. [Google Scholar]
- Musil CF, Bjorn LO, Scourfield MWJ, Bodeker GE. 2002. How substantial are ultraviolet-B supplementation inaccuracies in experimental square-wave delivery systems? Environmental and Experimental Botany 47: 25–38. [Google Scholar]
- Peet MM, Sato S, Gardner RG. 1998. Comparing heat stress effects on male-fertile and male-sterile tomatoes. Plant, Cell and Environment 21: 225–231. [Google Scholar]
- Prasad PVV, Craufurd PQ, Summerfield RJ. 1999. Fruit number in relation to pollen production and viability in groundnut exposed to short episodes of heat stress. Annals of Botany 84: 381–386. [Google Scholar]
- Reddy KR, Hodges HF, Read JJ, McKinion JM, Baker JT, Tarpley L, Reddy VR. 2001. Soil-plant-atmosphere-research (SPAR) facility: A tool for plant research and modeling. Biotronics 30: 27–50. [Google Scholar]
- Salem MA, Kakani VG, Reddy KR. 2004. Temperature effects on in vitro pollen germination and pollen tube growth of soybean genotypes. Annual meetings of the Southern Branch of the American Society of Agronomy, 27–29 June 2004, Biloxi, Mississippi, MS, USA. [Google Scholar]
- Sampson BJ, Cane JH. 1999. Impact of enhanced ultraviolet-B radiation on flower, pollen and nectar production. American Journal of Botany 86: 108–114. [PubMed] [Google Scholar]
- Schindell RT, Rind D, Lonergan P. 1998. Increased polar stratospheric ozone losses and delayed eventual recovery owing to increasing greenhouse-gas concentrations. Nature 392: 589–592. [Google Scholar]
- Searles PS, Flint SD, Caldwell MM. 2001. A meta-analysis of plant field studies simulating stratospheric ozone depletion. Oecologia 127: 1–10. [DOI] [PubMed] [Google Scholar]
- Shen XY, Webster BD. 1986. Effects of water stress on pollen of Phaseolus vulgaris L. Journal of American Society for Horticultural Science 111: 807–810. [Google Scholar]
- Shivanna KR, Johri BM. 1985.The Angiosperm Pollen: Structure and Function. New Delhi: Wiley Eastern Limited, 5–83. [Google Scholar]
- Stadler LJ, Uber FM. 1942. Genetic effects of ultraviolet radiation in maize. IV. Comparison of monochromatic radiation. Genetics 27: 84–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RM, Nikaido O, Jordan BR, Rosamond J, Bray CM, Tobin AK. 1996. Ultraviolet B-induced DNA lesions and their removal in wheat (Triticum aestivum L.) leaves. Plant, Cell and Environment 19: 171–181. [Google Scholar]
- Torabinejad J, Caldwell MM, Flint SD, Durham S. 1998. Susceptibility of pollen to UV-B radiation: an assay of 34 taxa. American Journal of Botany 85: 360–369. [PubMed] [Google Scholar]
- UNEP. 2002. Executive Summary. Final of UNEP/WMO Scientific Assessment Panel of the Montreal Protocol on Substances that Deplete the Ozone Layer. UNEP, Nairobi. [Google Scholar]
- Van De Staaij JWM, Bolink E, Rozema J, Ernst WHO. 1997. The impact of elevated UV-B (180–320 nm) radiation levels on the reproductive biology of a highland and a lowland population of Silene vulgaris Plant Ecology 128: 173–179. [Google Scholar]
- Yuan L, Yanqun Z, Jianjun C, Haiyan C. 2002. Intraspecific responses in crop growth and yield of 20 soybean cultivars to enhanced ultraviolet-B radiation under field conditions. Field Crops Research 78: 1–8. [DOI] [PubMed] [Google Scholar]
- Zhao D, Reddy KR, Kakani VG, Read JJ, Sullivan JH. 2003. Growth and physiological responses of cotton (Gossypium hirsutum L.) to elevated carbon dioxide and ultraviolet-B radiation under controlled environmental conditions. Plant, Cell and Environment 26: 771–782. [Google Scholar]
- Zu Y, Yuan L, Haiyan C, Jianjun C. 2003. Intraspecific differences in physiological response of 20 soybean cultivars to enhanced ultraviolet-B radiation under field conditions. Environmental and Experimental Botany 50: 87–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



