Abstract
• Background and Aims The aim of this paper was to verify the variation in the loss of seed dormancy during after-ripening and the interspecific and interpopulation variability in the degree of dormancy of seven wild and two cultivated rice species comprising 21 populations and two cultivars.
• Methods Four wild rice species from South America, Oryza glumaepatula, O. latifolia, O. grandiglumis and O. alta, and two O. sativa cultivars were tested in one experiment. In a second experiment, five wild species, O. punctata, O. eichingeri, O.rufipogon, O. latifolia and O. glumaepatula, and one cultivated species (O. glaberrima) were evaluated. Initial germination tests were performed soon after the seeds were harvested and subsequently at 2-month intervals, for a total of six storage periods in the first experiment and three in the second. All tests were conducted in the dark at a temperature of 27 °C.
• Key Results Different patterns of after-ripening among populations within and between species were observed.
• Conclusions The cultivated species (O. sativa and O. glaberrima) and, amongst the wild species, the tetraploids O. latifolia, O. grandiglumis and the diploids O. eichingeri and O. punctata, had weak dormancy, losing it completely 2 months after harvest, while O. rufipogon and O. glumaepatula exhibited pronounced dormancy. The latter showed different patterns of after-ripening between populations indigenous to the Amazon region and those originating in the Paraguay River system. Seeds of Solimões (Amazon) and Japura origin showed weak dormancy whereas those of Paraguay origin showed deep dormancy. Ecological differences among natural habitats may be involved in such differentiation.
Key words: Brazil, Oryza sativa, O. glaberrima, O. glumaepatula, O. grandiglumis, O. rufipogon, O. latifolia, O. alta, O. punctata, O. eichingeri, rice germination, seed dormancy
INTRODUCTION
Distributed throughout the tropics and subtropics, the Oryza genus comprises two cultivated species (Oryza sativa L. and O. glaberrima Steud) and 21 wild species, grouped under four Sections and four Complexes. The two largest groups belong to the Oryza Section. The O. officinalis Complex with ten species [O. officinalis Wall ex Watt, O. eichingeri Peter, O. rhizomatis Vaughan, O. malapuzhaesis Krishnaswamy and Chandrasakaran, O. punctata Kotschy ex Steud., O. minuta JS Presl. ex CB Presl., O. alta Swallen, O. latifolia Desv., O. grandiglumis (Doell) Prod. and O. australiensis Domin.], and the O. sativa Complex with seven diploid species including the two cultivated species (O. sativa L., O. rufipogon Griff., O. meridionalis Ng; O. glaberrima Steud, O. barthii A. Chev., O. longistaminata Chev. et Roehr. and O. glumaepatula Steud). Two smaller groups are the O. ridleyi Complex (O. longiglumis Jansen and O. ridleyi Hook.) in the Ridleyanae Tateoka Section and the O. granulata Complex [O. granulata Nees et Arn ex Watt and O. meyeriana (Zoll. et Mor. ex Steud.) Baill.], in the Granulata Roschev. Section. Also included in the Ridleyanae Tateoka Section is the species O. schlechteri Pilger, a provisional classification (genome group unknown). Another species, O. brachyantha Chev. et Roehr., is grouped under the Brachyantha B. R. Lu Section (Vaughan et al., 2003).
Four wild rice species occur in South and Central America, three tetraploid (O. alta, O. latifolia and O. grandiglumis; CCDD genome) and a diploid species (O. glumaepatula; AgpAgp genome) (Morishima, 1994a). This last species was initially classified as O. rufipogon (Tateoka, 1964), and later was confirmed by Vaughan (1994) as an American form of the Asian species. Based on morphological and molecular differences in relation to the Asian form, O. glumaepatula was given its former species status (Juliano et al., 1998; Ge et al., 1999).
The presence of seed dormancy is generally considered a primitive trait of the wild species when compared with the cultivated species (Takahashi, 1984), which totally or partially lost this characteristic during domestication. Seed dormancy is an adaptation of the plant, which prevents premature germination and helps to preserve a supply of seed in the soil, an obvious selective advantage in wild plants, especially in environments that undergo favourable/unfavourable season cycles (Harlan, 1992).
According to Vaughan (1994), the majority of wild species possess a strong dormancy, although the author recognizes that for most species there is not sufficient information to support this. There are few data in the literature related to seed dormancy in the wild species of the Oryza genus (Oka and Morishima, 1967; Takahashi, 1984; Oliveira, 1992; Shimamoto et al., 1994), whereas the mean dormancy periods have been studied for cultivated species, showing a wide variation among cultivars of O. sativa and O. glaberrima (Roberts, 1961; Misra and Misro, 1970; Kalita et al., 1994). Varying degrees of dormancy have also been reported for the seeds of weedy rice (Perreto et al., 1993). Franco et al. (1997) verified that, in cultivated rice, depending on the cultivar, the seeds present post-harvest dormancy that may persist for 90–120 d.
The degree of dormancy may also vary considerably if seeds of O. sativa are harvested at various stages of ripeness (Roberts, 1961) and if stored at different temperatures, losing dormancy more rapidly at warmer temperatures than cooler temperatures (Roberts, 1965; Cohn and Hughes, 1981). The rate of dry after-ripening in rice is also a function of seed moisture content. The after-ripening process occurred readily at 11 % moisture content during a 4–6-week storage period at 20–30 °C (Cohn and Hughes, 1981). Leopold et al. (1988) observed rapid after-ripening in seeds of red rice (O. sativa) at 6–14 % moisture content, whereas after-ripening did not occur at >18 % moisture content and was severely inhibited at <5 % moisture content.
Seed dormancy has also been shown to have a hereditary component in a number of species, many of which are crops or serious weeds (Baskin and Baskin, 1998). Several studies have now shown that seed dormancy is a complex character influenced by many genetic and environmental factors (Li and Foley, 1997). In rice, previous studies have concluded that seed dormancy is a quantitative trait governed by polygenes with cumulative but unequal effects, and is strongly affected by environmental conditions during seed development (Chang and Tagumpay, 1973). Using molecular markers, researchers have identified several putative quantitative trait loci for seed dormancy (Wan et al., 1997; Lin et al., 1998; Cai and Morishima, 2000). Inheritance studies of seed dormancy in weedy rice (O. sativa), conducted by Gu et al. (2003), concluded that seed dormancy at 0 d after harvest was dominant, and that dominance for duration of seed dormancy was incomplete when judged by days to 50 % germination.
A high intrapopulation variability for seed dormancy among families of the American wild rice O. latifolia, ranging from 2·3 % to 90·0 %, was observed by Santos et al. (2001). The two O. glumaepatula populations evaluated in that study also showed significant differences (P < 0·01) among families, whereas O. grandiglumis and O. alta populations did not show intrapopulation variability. Viable seeds were observed among the non-germinated seeds, confirming the presence of seed dormancy.
The objective of this study was to evaluate both the inter- and intraspecific variation in the loss of seed dormancy during after-ripening of several wild rice taxa, including the American wild species and the two cultivated species, O. sativa and O. glaberrima.
MATERIALS AND METHODS
A two-stage experiment was conducted to study the seed dormancy breakdown starting with newly harvested seeds, and also to verify the interspecific and interpopulation variability of seed dormancy for seven wild and the two cultivated rice species. Nine populations of the American wild tetraploid species (O. grandiglumis, O. latifolia and O. alta) and the diploid O. glumaepatula, plus two cultivars of O. sativa (Table 1) were studied in the first stage. Seven germination tests were performed from April 2001 to April 2002, the first soon after the seeds were harvested and six at 2-month intervals. Seeds were collected from plants cultivated in the greenhouse in 5-kg plastic bags with a mixture of soil and sand. These bags were placed within water tanks to simulate their natural habitat along river banks. Mature seeds from each individual plant were collected by shaking the inflorescences into paper bags. Seeds were collected at different times, according to the seed maturity period of each population. As soon as enough seed for the whole test period was collected within a population, i.e. a bulked seed sample of 350 seeds per population from up to 16 plants, the test began for that population. This meant that the different populations had different test dates, with all of them starting between April and May 2001, to ensure that the seeds were newly harvested for each population. A period of no more than 20 d elapsed between the collection of the first and the last seeds for each of the bulked samples. The germination test then started right away for that particular population. The bulked seed samples that were not used in the first test were stored in paper bags at room temperature, at mean temperatures varying from 18·5 °C in June 2001 to 25·8 °C in January 2002 and relative humidity varying from 64·7 % in August to 89·6 % in January 2002 for the first experiment throughout the test periods.
Table 1.
Oryza spp. populations evaluated in both experiments, with species identification, population code number and origin
| Origin (germplasm source) |
||||||
|---|---|---|---|---|---|---|
| Species |
Code |
Hydrographic basin |
River (lake) |
|||
| Experiment 1 | ||||||
| O. latifolia | ARG5 | Paraguay/Paraná | Paraguay | |||
| O. latifolia | ARG8 | Paraguay/Paraná | Paraguay | |||
| O. latifolia | RPG1 | Paraguay | – | |||
| O. glumaepatula | RPG1 | Paraguay | – | |||
| O. glumaepatula | RJA4 | Japurá | Japurá | |||
| O. grandiglumis | RPU2 | Purus | Purus | |||
| O. grandiglumis | RSJN3 | Solimões | (Janauacá) | |||
| O. grandiglumis | RS3 | Solimões | (Paru) | |||
| O. alta | RI1 | Ribeira | Ribeira | |||
| O. sativa | ‘Maravilha’ | – | – | |||
| O. sativa | ‘Rio Verde’ | – | – | |||
| Experiment 2 | ||||||
| O. rufipogon | W0106 | Phulankara, India | – | |||
| O. rufipogon | W0107 | Pahala, Orissa, India | – | |||
| O. glaberrima | W0012 | From CRRI, Cuttack, Orissa, India | – | |||
| O. glaberrima | W0025 | Sierra Leone, Africa | – | |||
| O. eichingeri | W1525 | Bwamba, Toro, Uganda | – | |||
| O. punctata | W1564 | Ibadan, Nigeria | – | |||
| O. latifolia | ARG9 | Argentina, between Corrientes and Antiqueira | – | |||
| O. latifolia | MS1 | Paraguay | Paraguay | |||
| O. glumaepatula | RPG2 | Paraguay | Paraguay | |||
| O. glumaepatula | RPG3 | Paraguay | Paraguay | |||
| O. glumaepatula | RS17 | Solimões | (Coari) | |||
| O. glumaepatula | RS6 | Solimões | (Manacapuru) | |||
A second experiment including four germination tests, the first with newly harvested seed and three at 2-month intervals, was conducted from June 2002 to January 2003. This experiment evaluated the depth of dormancy and duration of the dry after-ripening period of 12 populations from the wild rice species O. glumaepatula, O. rufipogon, O. eichingeri, O. punctata and O. latifolia, originating from Asia and America, and the cultivated species O. glaberrima from Africa (Table 1). Seed samples were obtained from greenhouse-grown plants in a manner similar to that described in the first experiment. In the second experiment the bulked seed samples were collected from up to 12 plants for each population and stored in paper bags at room temperature. Mean temperatures varied from 17·7 °C in July 2002 to 25·4 °C in December 2003 and relative humidity from 54·2 % in October to 92·2 % in January 2003 during the test periods.
The germination test procedures were the same for both experiments. Two replicates of 25 intact seeds each, a total of 50 seeds for each population, were evaluated in a completely randomized design. The tests were conducted in the dark in a germination chamber at a constant temperature of 27 °C. Square plastic Gerboxes (11 × 11 cm) were used and the seeds placed on one sheet of Whatman no. 3 filter paper moistened with 10 mL distilled water. At daily evaluations, seeds were considered germinated when radicles attained a minimum of 5 mm. Each test lasted 28 d, which was longer than the 2 weeks considered ideal for germination tests (Baskin and Baskin, 1998), because some of the wild species were still germinating after 15 d, especially O. grandiglumis, which presented the slowest germination (data not shown). At the conclusion of each test, ungerminated seeds were checked for viability using the tetrazolium test (Delouche et al., 1976).
One-way ANOVAs were used to detect differences among populations and among species in seed germination rates at each test (storage period) for both series. To evaluate interpopulation variability, combined analyses of variance were used for both test series, considering all species and populations together and also each species separately, represented by two or more populations. Thus the effect of the storage period in the rates of seed dormancy loss was evaluated, as well as the interaction between populations and storage periods. The mean dormancy period, i.e. the mean time (days) taken from harvest for the individual seeds of a population to attain 50 % germination (Roberts, 1961; Misra and Misro, 1970) was estimated by plotting the results of the germination tests using a probability scale for percentage germination, with the normal distribution resulting in a straight line. From this figure, the mean dormancy periods were calculated for each population.
RESULTS
Experiment 1
Significant differences (P < 0·01) were observed among the 11 populations evaluated with the American wild species and O. sativa cultivars. These were not unexpected considering that different species were evaluated. Also, significant differences (P < 0·01) were observed among populations of each species, among storage periods, as well as for the interaction between storage periods and populations, indicating that the different populations presented different seed after-ripening dormancy loss.
At the first test, soon after the seeds were collected, all the wild species were dormant (Fig. 1) and viable (data not shown), which was confirmed by the tetrazolium tests. The Rio Verde cultivar of O. sativa exhibited low germination, while 66 % of the Maravilha cultivar germinated. Sixty days after harvest, seeds of the two O. sativa cultivars had lost their dormancy completely. The tetraploid species O. latifolia and O. grandiglumis presented high germination, above 50 %, compared with 26 % for O. alta, with a higher dormancy level. Both populations of the diploid O. glumaepatula were still highly dormant (Fig. 1).
Fig. 1.
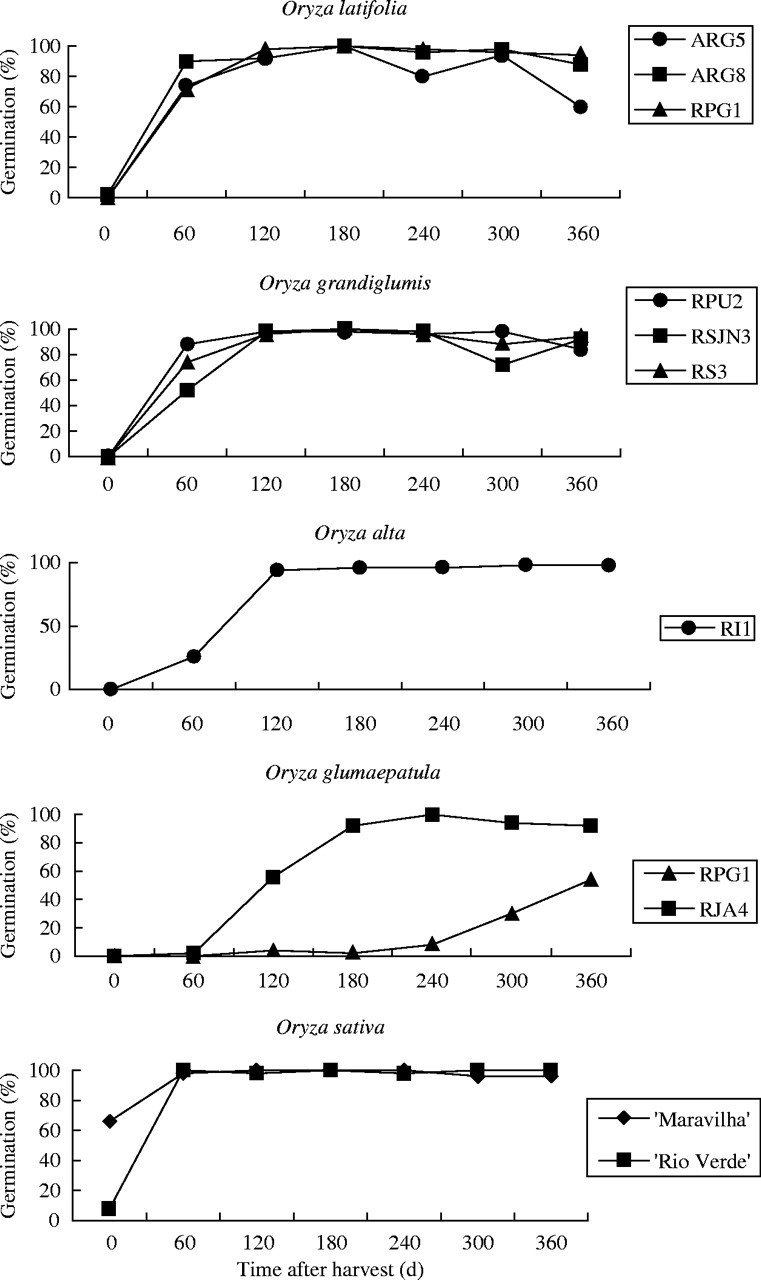
Germination percentage of Oryza latifolia, O. grandiglumis, O. alta, O. glumaepatula populations and O. sativa cultivars, plotted against time after harvest (expt 1).
Four months or 120 d after harvest, the seeds of all seven populations of the tetraploid American wild species were no longer dormant, with germination above 92 %. The O. glumaepatula population originating from the Paraguay River system (RPG1), collected at an area representative of the Pantanal ecosystem, continued highly dormant, while the Amazon population (RJA4) collected at the Rio Japura Basin showed less dormancy (44 % germination). This population lost its dormancy completely 180 d after harvest or after 6 months storage at room temperature, whereas the Pantanal population continued to present a high level of dormancy up to 360 d (Fig. 1); viability was confirmed in the tetrazolium test.
Experiment 2
Significant differences (P < 0·01) for after-ripening rates were observed among populations both in the joint analysis when considering the total of 12 populations and in the separate analysis of O. glumaepatula populations. No significant differences were observed between populations of O. latifolia, O. rufipogon and O. glaberrima. The interaction between storage periods and populations was only significant (P < 0·01) when the 12 populations were compared together and for the O. glumaepatula populations, showing that the latter species present differences in dormancy loss among populations, as already observed in the first experiment.
In the first germination test of this series, soon after the seeds were collected, most populations were shown to be completely dormant, although O. eichingeri, O. punctata and O. latifolia had, on average, 10–38 % germination (Fig. 2). In the second test, 60 d after seed harvest, only O. rufipogon and O. glumaepatula revealed high depths of dormancy. The two O. rufipogon populations maintained high levels of dormancy up to 180 d of seed harvest, when they had 54 % and 70 % germination. Oryza glumaepatula populations showed varying levels of germination 120 d after seed harvest, from 14 % to 98 %. At 180 d after harvest, germination still varied from 48 % to 100 %, revealing a high polymorphism for seed dormancy.
Fig. 2.
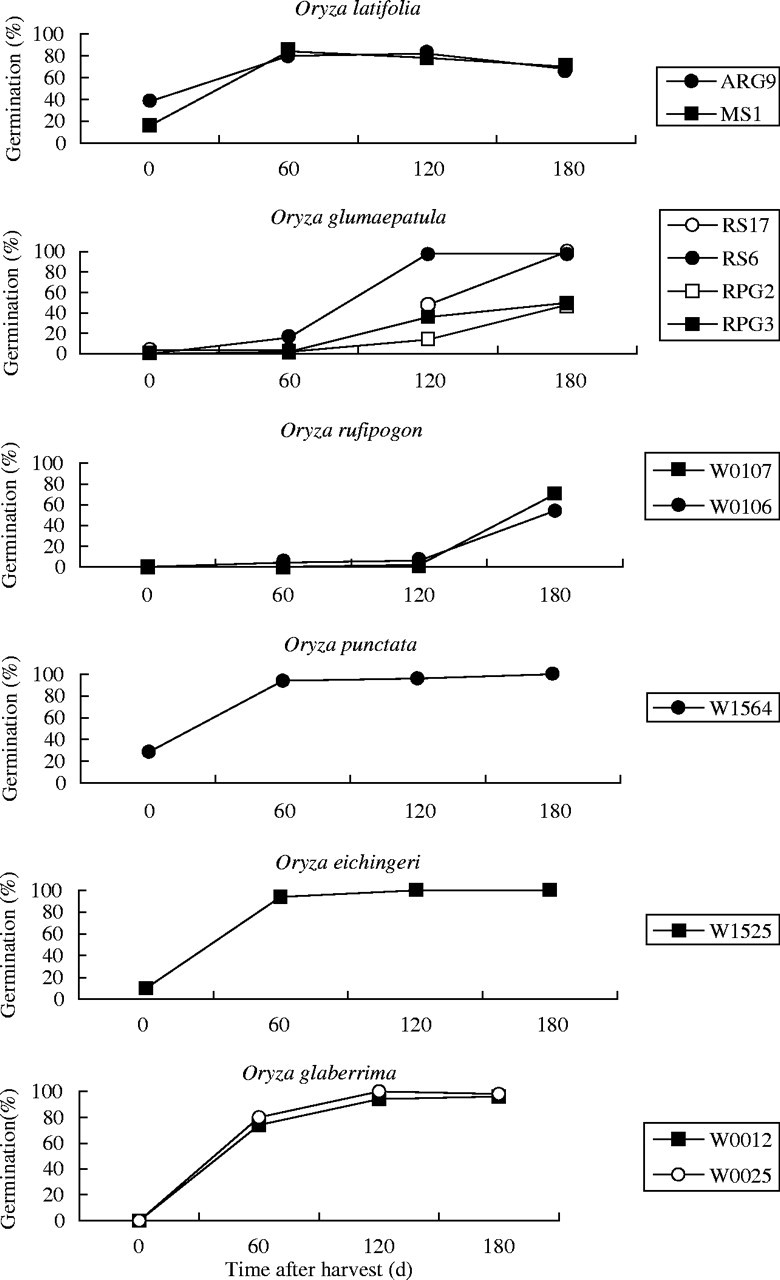
Germination percentage of Oryza latifolia, O. glumaepatula, O. rufipogon, O. punctata, O. eichingeri and O. glaberrima populations, plotted against time after harvest (expt 2).
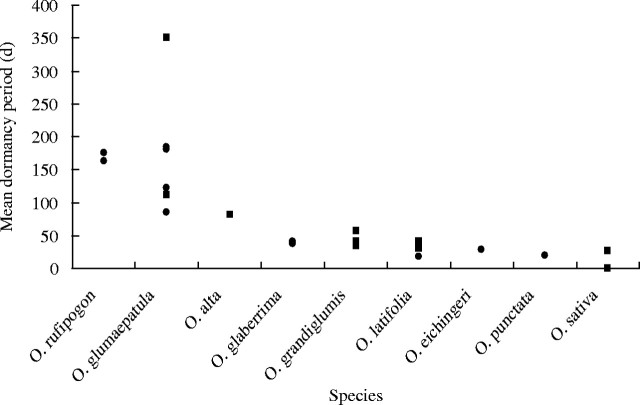
In a further comparison of the 23 populations within nine species of Oryza, including data from both sets of experiments (Fig. 3), the mean dormancy period for both cultivated species, O. glaberrima and O. sativa, and the wild species O. punctata, O. eichingeri, O. latifolia and O. grandiglumis ranged from 0 to 57 d. The only population available for these studies of the tetraploid O. alta had a longer dormancy period, up to 82 d, and the most dormant species were O. rufipogon and O. glumaepatula, the latter showing a wide range for the dormancy period, varying from 85 d up to 350 d to achieve 50 % germination. In O. glumaepatula populations, the order for the mean dormancy period from the most pronounced dormancy was RPG1 (350 d; Paraguay), RPG2 (183 d; Paraguay), RPG3 (180 d), RS17 (122 d; Solimões), RJA4 (112 d; Japura) and RS6 (85 d; Solimões).
Fig. 3.
Distribution of the mean dormancy periods (after-ripening time to 50 % germination) of 11 seed lots of expt 1 (squares) and 12 seed lots of expt 2 (circles) within nine species of Oryza.
DISCUSSION
There is little information in the literature related to germination and length of the dry after-ripening period for the American tetraploid wild rice species (Oliveira, 1992; Santos et al., 2001). The present results show that O. grandiglumis, O. latifolia and O. alta have high dormancy immediately after seed harvest, which is eliminated 60 d after harvest for the first two species and 120 d after harvest for O. alta when stored at room temperature in our climatic conditions.
Annual plants of wild rice produce seeds that can survive under various unfavourable conditions, whereas perennials produce diverse vegetative propagules in addition to seeds (Takahashi, 1984). This situation favours the presence of a higher length of the dry after-ripening period for the annuals in comparison with the perennials, as observed by Oka and Morishima (1967) for O. rufipogon, when comparing annual and perennial types. According to the present observations under the environmental conditions in Piracicaba, São Paulo, where they were identified as perennials, the tetraploid American species possess a longer life cycle than the diploid O. glumaepatula. However, Rubim (1994) observed a life cycle typical of annual plants for both O. glumaepatula and O. grandiglumis in their natural habitat in the Amazon. According to the author, the life cycle of these wild rice species is strongly influenced by the water level of the rivers, which has a variation range as large as 10 m between the dry and rainy seasons. The difference between these two species is that the new shoots which emerged in the terrestrial phase for O. grandiglumis were mostly ratoons from vegetative old stems, whereas in O. glumaepatula a varying percentage of good seeds were observed, depending on the time of year.
These different strategies may reflect the higher intensity of dormancy observed for O. glumaepatula, superior to the tetraploid species, as it depends on its seeds for survival in future generations. According to Takahashi (1984), if all seeds produced germinate simultaneously the standing density would be too high and the seedlings could be destroyed if exposed to unfavourable conditions, whereas gradual germination in a proper season ensures establishment of progeny plants, which is an apparent adaptive behaviour favoured by selection. On the other hand, tetraploid species that do not depend solely on seeds for survival, may have a shorter dry after-ripening period, or, as observed, lose their dormancy faster with time.
Seed of the cultivated species O. sativa and O. glaberrima had lower intensity of dormancy compared with the wild species, losing dormancy soon after harvesting, although the loss in dormancy was slower in O. glaberrima compared with O. sativa, similar to the observations of Ellis et al. (1983). However, a wide variation of mean dormancy periods has been observed among 137 varieties of O. sativa, from 0 to 110 d (Roberts, 1961). Also, a wide variation in the mean dormancy period, from 85 to 155 d, was observed for 35 O. glaberrima cultivars (Misra and Misro, 1970), showing that seed dormancy is not exclusive to the wild species.
The wild tetraploid species O. latifolia, O. grandiglumis and the diploids O. eichingeri and O. punctata, all grouped in the Officinalis Complex (Khush, 1997; Vaughan et al., 2003), showed a similar mean dormancy period in this study. The two diploids of the Sativa Complex, O. glumaepatula and O. rufipogon, showed the strongest dormancy. An interesting observation was the wide range of degree of dormancy among O. glumaepatula populations originating in the Amazon region and the Pantanal region in the Paraguay Hydrographic System. Populations originating in the Amazon lost their seed dormancy completely 180 d after seed harvest, whereas the Pantanal populations retained >90 % of its seeds in a dormant condition in the first experiment, and around 50 % in the second experiment.
These results may reflect an adaptation to the Pantanal ecosystem, that requires a longer dormancy period. In the Pantanal there are two seasons, wet and dry. Generally, the seasons are well defined: the dry season, from April to September, with a mean temperature around 21 °C, and the wet season from October to March, with mean temperatures around 32 °C. The mean annual temperature is about 24 °C and annual precipitation is between 1000 and 1400 mm, concentrated in the wet season, mostly during the summer months of December and January. In contrast, in the Amazon environment, there is little climate alteration during the year. Due to the high-energy values that occur on the surface, the air temperature shows little variation during the year, with the mean values between 24 °C and 26 °C. The mean annual precipitation in the Amazon region is about 2300 mm (Fisch et al., 2003). Therefore, it may not be necessary for the Amazonian O. glumaepatula seeds to maintain high dormancy rates for more than 4 or 6 months, whereas in the Pantanal region, with a well-defined dry period, the seeds of this species probably developed longer periods of dormancy awaiting the wet period when environmental conditions would again be favourable for germination and seedling survival.
Morphological, biochemical and molecular analyses suggest that O. glumaepatula consists of at least two main groups: one centred on the Amazon basin and the other in the Pantanal of Brazil and Caribbean regions (Vaughan et al., 2003). Morishima (1994b) also observed a weak dormancy for populations of O. glumaepatula collected in the Amazon when compared with Asian O. rufipogon populations, which presented strong dormancy for annual types and medium dormancy for perennial types.
Depending on the species, germination responses vary with latitude, elevation, soil moisture, soil nutrients, temperature, nature and density of plant cover and degree of habitat disturbance of the sites where the seeds matured, and one of the most common responses is the degree of dormancy (Baskin and Baskin, 1998). According to Turesson (1922), natural selection occurs in the various sites where a species grows, which results in local adaptive genotypic differentiation. The result of such ecotype differentiation is that variation within a species is correlated with habitat differences, which seems to have been the cause of the different degrees of dormancy in rice seeds observed in this work.
Acknowledgments
We thank the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for financial support and scholarships, the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for scholarships awarded, and Dr Tran Dang Hong, for reviewing the manuscript and providing valuable suggestions.
LITERATURE CITED
- Baskin CC, Baskin JM. 1998.Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego: Academic Press. [Google Scholar]
- Cai HW, Morishima H. 2000. Genomic regions affecting seed shattering and seed dormancy in rice. Theoretical Applied Genetics 100: 840–846. [Google Scholar]
- Chang TT, Tagumpay O. 1973. Inheritance of grain dormancy in relation to growth duration in 10 rice crosses. SABRAO Newsletter 5: 87–94. [Google Scholar]
- Cohn MA, Hughes JA. 1981. Seed dormancy in red rice (Oryza sativa). I. Effect of temperature on dry-afterripening. Weed Science 29: 402–404. [Google Scholar]
- Delouche JC, Still TW, Raspet M, Lienhard M. 1976.O teste de tetrazólio para viabilidade da semente. Brasília: Ministério da Agricultura, AGIPLAN. [Google Scholar]
- Ellis RH, Hong TD, Roberts EH. 1983. Procedures for the safe removal of dormancy from rice seed. Seed Science and Technology 11: 77–112. [Google Scholar]
- Fisch G, Marengo JA, Nobre CA. 2003. Clima da Amazônia. www2.cptec.inpe.br/products/climanalise/cliesp10a/fish.html (accessed 1 Aug. 2003). [Google Scholar]
- Franco DF, Petrini JA, Rodo A, Oliveira A, Tavares W. 1997. Métodos para superação da dormência em sementes de arroz. Lavoura Arrozeira 50: 11–15. [Google Scholar]
- Ge S, Oliveira GCX, Schaal BA, Gao LZ, Hong DY. 1999. RAPD variation within and between natural populations of the wild rice Oryza rufipogon from China and Brazil. Heredity 82: 638–644. [DOI] [PubMed] [Google Scholar]
- Gu XY, Chen ZX, Foley ME. 2003. Inheritance of seed dormancy in weedy rice. Crop Science 43: 835–843. [Google Scholar]
- Harlan JR. 1992.Crops & Man, 2nd edn. Madison, WI: American Society of Agronomy, Crop Science Society of America. [Google Scholar]
- Juliano AB, Naredo MEB, Jackson MT. 1998. Taxonomic status of Oryza glumaepatula Steud. I. Comparative morphological studies of New World diploids and Asian AA genome species. Genetic Resources and Crop Evolution 45: 197–203. [Google Scholar]
- Kalita UC, Baruah DK, Upadhaya LP. 1994. Seed dormancy on germplasm collection of rice (Oryza sativa L.) insensitive to photoperiod. Indian Journal of Agricultural Science 63: 160–164. [Google Scholar]
- Khush GS. 1997. Origin, dispersal, cultivation and variation of rice. Plant Molecular Biology 35: 25–34. [PubMed] [Google Scholar]
- Leopold AC, Glenister R, Cohn MA. 1988. Relationship between water content and afterripening in red rice. Physiologia Plantarum 74: 659–662. [Google Scholar]
- Li B, Foley ME. 1997. Genetic and molecular control of seed dormancy. Trends in Plant Science 2: 384–389. [Google Scholar]
- Lin SY, Sasaki T, Yano M. 1998. Mapping quantitative trait loci controlling seed dormancy and heading date in rice, Oryza sativa L., using backcross inbred lines. Theoretical and Applied Genetics 96: 997–1003. [Google Scholar]
- Misra PK, Misro B. 1970. Seed dormancy in the African cultivated rice (Oryza glaberrima Steud.). Indian Journal of Agricultural Science 40: 13–16. [Google Scholar]
- Morishima H. 1994. Background information about Oryza species in tropical America. In: Morishima H, Martins PS, eds. Investigations of plant genetic resources in the Amazon basin with the emphasis on the genus Oryza: Report of 1992/93 Amazon Project. Mishima: The Monbusho International Scientific Research Program, 4–5. [Google Scholar]
- Morishima H. 1994. Discussion: American wild rice as compared with Asian relatives. In: Morishima H, Martins PS, eds. Investigations of plant genetic resources in the Amazon basin with the emphasis on the genus Oryza: Report of 1992/93 Amazon Project. Mishima: The Monbusho International Scientific Research Program, 52–57. [Google Scholar]
- Oka HI, Morishima H. 1967. Variations in the breeding systems of a wild rice, Oryza perennis Evolution 21: 249–258. [DOI] [PubMed] [Google Scholar]
- Oliveira GCX. 1992.Padrões de variação fenotípica e ecologia de Oryzae (Poaceae) selvagens da Amazônia. Dissertation — Escola Superior de Agricultura “Luiz de Queiroz”, Universidade de São Paulo, Piracicaba, Brazil. [Google Scholar]
- Perreto EL, Peske ST, Galli J. 1993. Avaliação de sementes e plantas de arroz daninho. Revista Brasileira de Sementes 15: 49–54. [Google Scholar]
- Roberts EH. 1961. Dormancy of rice seed. I. The distribution of dormancy periods. Journal of Experimental Botany 12: 319–329. [Google Scholar]
- Roberts EH. 1965. Dormancy in rice seed. IV. Varietal responses to storage and germination temperatures. Journal of Experimental Botany 16: 341–349. [Google Scholar]
- Rubim MAL. 1994. A case study on life-history of wild rice — from germination to emergence of inflorescence. In: Morishima H, Martins PS, eds. Investigations of plant genetic resources in the Amazon basin with the emphasis on the genus Oryza: Report of 1992/93 Amazon Project. Mishima: The Monbusho International Scientific Research Program, 38–42. [Google Scholar]
- Santos PP, Mamani EM, Oliveira GCX, Veasey EA. 2001. Variabilidade inter e intrapopulacional para germinação de sementes de arroz selvagem. In: Proceedings of the Simpósio Internacional de Iniciação Científica da Universidade de São Paulo, 9 Nov. 2001. Piracicaba, Brazil: Universidade de São Paulo (CD-ROM). [Google Scholar]
- Shimamoto Y, Ohara M, Akimoto M. 1994. Habitat conditions and plant characters of wild rice in the Central Amazon. In: Morishima H, Martins PS, eds. Investigations of plant genetic resources in the Amazon basin with the emphasis on the genus Oryza: Report of 1992/93 Amazon Project. Mishima: The Monbusho International Scientific Research Program, 16–37. [Google Scholar]
- Takahashi N. 1984. Seed germination and seedling growth. In: Tsunoda T, Takahashi N, eds. Biology of rice. Amsterdam: Elsevier, 71–88. [Google Scholar]
- Tateoka T. 1964. Taxonomic studies of the genus Oryza In: International Rice Research Institute, ed. Rice genetics and cytogenetics. Amsterdam: Elsevier, 15–21. [Google Scholar]
- Turesson G. 1922. The genotypical response of the plant species to the habitat. Hereditas 3: 211–350. [Google Scholar]
- Vaughan DA. 1994.The wild relatives of rice: a genetic resources handbook. Los Baños, Philippines: International Rice Research Institute. [Google Scholar]
- Vaughan DA, Morishima H, Kadowaki K. 2003. Diversity in the Oryza genus. Current Opinion in Plant Biology 6: 139–146. [DOI] [PubMed] [Google Scholar]
- Wan J, Nakazaki T, Kawaura K, Ikehashi H. 1997. Identification of marker loci for seed dormancy in rice (Oryza sativa L.). Crop Science 37: 1759–1763. [Google Scholar]