Abstract
• Background and Aims Although some taxonomic studies in the genus Trigonella have been conducted, there has been no concerted effort to study the breeding system. This paper examines the floral structure and pollination system in a population of T. balansae, an annual pasture legume.
• Methods Floral morphology, hand and vector pollination, stigma receptivity, pollen tube growth, using scanning electron and fluorescence microscopy, were conducted.
• Key Results Measurements of floral structure from before to after anthesis indicates an inability for T. balansae to self-pollinate and a requirement for an external vector to effectively transfer pollen from the anthers onto the stigmas of this species. Seed set can be obtained by hand or honeybee manipulation of T. balansae flowers.
• Conclusions Trigonella balansae is a self-compatible species, but which requires vectors such as honeybees to bring about pollination.
Key words: Trigonella balansae, pollination, pollen tube growth, stigma receptivity, inbreeding depression
INTRODUCTION
An understanding of the breeding systems and pollination of agriculturally important crops, as well as native plants, is a major consideration in domestication (Bullita et al., 1993) and is also critical for plant improvement research. The amount and distribution of genetic variation within populations is greatly influenced by breeding characteristics, such as flower phenology, self-compatibility and the mating system (Loveless and Hamrick, 1984; Hamrick and Godt, 1989). Legumes offer an interesting opportunity to evaluate the interaction between self-compatibility, out-crossing rate and inbreeding depression, as many annual members of the group reproduce through a combination of selfing and out-crossing (Kittelson and Maron, 2000; Real et al., 2004). In the case of self-compatible species the temporal separation of male and female reproductive phases increases the probability of out-crossing (Richards, 1986). The proximity of pollen to the stigma when it is receptive helps in autonomous selfing (Lloyd and Schoen, 1992), while facilitated selfing may be high in plants with many inflorescences and where both the male and female phases are mature, as pollinators may forage longer among flowers of the same plant (Harder and Barrett, 1995). Inbreeding depression is a dynamic feature of populations that evolves with mating systems and is a powerful selective force for maintaining out-crossing in natural populations (Jarne and Charlesworth, 1993).
The genera Trigonella, Medicago, Trifolium and Melilotus belong to the subtribe Trigonellinae, tribe Trifolieae in the Leguminosae family. Several studies on the taxonomical relationship among these genera have been conducted (Small et al., 1987). The corolla of Trigonellinae is typically papilionaceous, comprising a standard petal, two wing petals and a keel made up of two marginally fused petals. The ten stamens are arranged in diadelphous fashion, with the staminal column (nine basally fused) surrounding the pistil. Considerable research on the tripping mechanism has been undertaken in Medicago, particularly in alfalfa, M. sativa (Small et al., 1981). Both Medicago and Trifolium are composed of species(s), which are either outbreeders or inbreeders. However, there has been no concerted effort to study the breeding system in the genus Trigonella.
Trigonella balansae is an annual legume of Eurasian origin, vegetatively productive and able to regenerate on alkaline soils receiving <400 mm of annual rainfall such as those in temperate Australia. Plants of this species have an upright growth habit and the proliferation of pods at the top of the canopy makes harvesting of seeds easier, compared with the traditional annual medics (Loi et al., 2000). Moreover, it is compatible with the native populations of Rhizobium meliloti associated with southern Australian medic pastures. Trigonella balansae thus has the potential to complement the role of annual medics in alkaline soil farming systems (Howie et al., 2001). Preliminary agronomic experiments have indicated the need to modify traits such as days to flowering in the introduced germplasm of T. balansae tested in Australia.
This paper examines the floral morphology, seed set following different pollination methods, stigma receptivity and pollen tube growth in a population of T. balansae. It also estimates the rate of selfing, amount of inbreeding depression and the self-compatibility index.
MATERIALS AND METHODS
Floral morphology
Trigonella balansae Boiss. and Reuter (Huber-Morath, 1970) accession, SA 5045 (CPI 19633, origin—University of Uppsala, Sweden) plants were grown in 200-mm pots containing modified University of California soil mix (Matkin and Chandler, 1957) in a glasshouse with temperature maintained at 20–25 °C. Fifteen plants of the accession were used in this study. Measurements of floral parts namely, length of the flower, corolla length, length of the stamen filaments and pistils were recorded at three stages: approx. 1 d before anthesis, at anthesis (flower opening) and approx. 1 d after anthesis.
Hand-pollination study
An additional 15 plants of the accession were used in this study. Responses to different methods of pollination were tested, namely:
Seed set without external vectors. Seed yield was determined on three flowers per plant that were covered with paper bags.
Seed set after hand manipulation of flowers. Within-flower transfer of pollen from anthers to stigma was achieved by depressing the keel petal of newly opened flowers using fine forceps. Three flowers per plant were thus manipulated (tripped), protected with a paper bag and the seed yield recorded.
Seed set after application of foreign pollen. Three flowers per plant were emasculated 1 d before anther dehiscence and protected with a paper bag. At anthesis, stigmas of these flowers were then pollinated using anthers from another plant of the same accession and seed yield determined.
Pollination-vector study
Seventy-five plants of the accession SA 5045 of T. balansae were grown in pots and used in these experiments:
Honeybee pollinator culture. Twenty-five plants were placed in a cloth-mesh crossing chamber in a glasshouse, with temperature maintained at 25 °C. A colony of honeybees (Apis mellifera) was introduced into the chamber for 3 weeks.
Field pollinator culture (natural pollination). Twenty-five plants were maintained in the field at Waite campus, Adelaide (34°58′S, 138°38′E) to attract free-ranging insect pollinators.
Vector-free culture. The 25 remaining plants were maintained in an insect-proof glasshouse.
Fifteen randomly selected plants from each treatment were used for recording pod and seed yield data. A total of three inflorescences per plant were selected. Pod set per inflorescence and number of seeds per pod were determined.
Measures of selfing rate and inbreeding depression
The selfing rate was calculated for T. balansae following Charlesworth (1988):
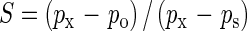 |
where px is percentage seed set following cross-pollination, ps is percentage seed set following self-pollination by hand tripping and po is percentage seed set following natural pollination.
Levels of inbreeding depression (δ) in T. balansae were determined on the basis of the relationship between the seed set of self-pollinated (ws) and of cross-pollinated flowers (wc) (Charlesworth and Charlesworth, 1987; Kephart et al., 1999).
The inbreeding depression level was calculated as:
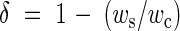 |
Self-compatibility index (SCI)
The SCI for T. balansae was calculated according to Lloyd and Schoen (1992). SCI is the mean seed set for manually self-pollinated flowers divided by the mean seed set for cross-pollinated flowers.
Stigma receptivity
Receptivity of the stigmas (n = 25) was tested by following the method suggested by Dafni and Maues (1998). The method involved soaking a Peroxtesmo esterase indicator paper (15 × 15 mm) in 1 mL of distilled water and applying a droplet of the solution directly onto the stigma. The receptivity of the stigma was indicated by the appearance of a blue colour.
Nectar production
The flowers of T. balansae (SA 5045; n = 46) were examined for the absence or presence of nectar at the base of the corolla tube.
Scanning electron microscopy
Samples (n = 15) of T. balansae stigmas (tripped and untripped) were fixed in Carnoy's fluid, followed by dehydration in a graded ethanol series, critical-point dried, coated with gold, and examined using a scanning electron microscope (Philips XL 30).
Pollen tube growth using fluorescence microscopy
Stigmas (n = 15) from flowers of T. balansae that were tripped and untripped were excised and fixed in Carnoy's fluid (3 : 1 acetic acid : ethanol) (Sharma and Sharma, 1980) for 2 h, followed by hydration with ethanol (70 % for 30 min, 30 % for 30 min) and then rinsed in reverse osmosis water twice for 30 min. Softening of the stigmas was done by treating them with 0·8 n NaOH at 60 °C for 1 h, followed by rinsing in water. Stigmas were then left in Gurr's Aniline Blue stain (BDH #34003) solution (7·67 g K3PO4 + 1·0 g Aniline Blue in 1 L distilled water, pH 11·5) overnight at 4 °C. The stigmas were mounted in 80 % glycerol, gently pressed under a coverslip and observed under a fluorescence microscope.
Statistical analyses
The length of the floral parts and the pod dimensions in the pollination vector study were analysed using an analysis of variance (F-test). Diagnostic plots of the residuals versus fitted values for each analysis displayed no departure from the homogeneity of variance. The number of seeds per pod response in the pollination vector study is counted data, therefore it is inappropriate to assume the data is normally distributed. Instead, the data set was analysed using a generalized linear model assuming a Poisson distribution and a log-link. This technique, also know as log-linear modelling, involves transforming the mean response with the log-link function, given by log (μ). The significance of the treatment term was tested by using a log-likelihood test statistic, otherwise known as scaled deviance (Dobson, 1990).
RESULTS
Flower morphology
Flowers of the species showed an increase (anova, F-test, P < 0·001) in size of corolla parts, stamen filaments and pistils from before to after anthesis (Table 1). The length of the corolla keel significantly increased from before to after anthesis but the styles did not increase in length after anthesis. The length of the stamen filament was consistently shorter than the length of the pistil before anthesis (ANOVA, F-test, P < 0·001).
Table 1.
Length of floral parts of Trigonella balansae compared across times (superscript letters) and within each time (subscript letters)
| Floral measurements (length in mm) |
Before anthesis |
At anthesis |
After anthesis |
|---|---|---|---|
| Flower | 6·320cA | 9·387bA | 12·480aA |
| Calyx | 4·000bD | 4·267abH | 4·640aF |
| Standard | 5·467cB | 8·907bB | 11·040aB |
| Wing | 4·160cD | 6·507bF | 8·160aE |
| Keel | 4·880cC | 7·360bE | 9·520aC |
| Filament | 3·867cD | 7·787bD | 9·120aD |
| Pistil | 5·147cBC | 8·373bC | 10·800aB |
| Style | 2·304bE | 5·013aG | 4·640aF |
Means with the same superscript or subscript letter are not significantly different (P < 0·001, n = 15).
After anthesis, the length of the pistil was significantly greater than the keel, which encloses it (ANOVA, F-test, P < 0·001). The pistil length was significantly (ANOVA, F-test, P < 0·001) greater than that of the filaments and this feature would indicate that T. balansae has the tendency to either extrude the stigma beyond the length of the stamens and keel or to retain the style and stigma within the keel but at increasing tensile pressure.
Examination of the anthers of this species indicated a difference in the deposition of pollen on the stigmas during anthesis. Anther dehiscence occurred before flower opening. However, in unopened flowers, no pollen was deposited onto the stigmas because of the difference in length of the stamens and pistil.
Pollination studies
There was no seed set in the flowers of T. balansae that were not tripped and protected from external vectors (Table 2). Hand-tripping of flowers, hand-cross pollinating, culture of plants in the glasshouse in the presence of honeybees and in the field resulted in high seed set (Table 2). A higher number of seeds per pod was obtained from insect-pollinated treatments in the glasshouse and field compared with hand-tripped flowers (χ2 test, P < 0·001) and there was no significant difference between the two insect pollinator treatments (χ2 test, P = 0·671). This would indicate that insect pollinators are more efficient in transferring viable pollen onto stigmas of flowers than human operators.
Table 2.
Mean pod yield, pod size and seed set of Trigonella balansae following different pollination treatments
| Pollination treatment |
No. of flowers/inflorescence |
No. of fertile pods/inflorescence |
Length of pod (mm) |
Width of pod (mm) |
No. of seeds/pod |
|---|---|---|---|---|---|
| Control (bagged in the glasshouse) | NA | 0 | NA | NA | NA |
| Bagged + hand tripped in the glasshouse | NA | NA | 13·33a | 3·08a | 1·5b |
| Hand cross-pollinated in the glasshouse | NA | NA | 12·50a | 3·08a | 1·83b |
| Honey bee pollinator culture in the glasshouse | 18·16 | 14·47a | 14·04a | 2·60a | 2·90a |
| Field pollinator culture | 17·13 | 14·38a | 13·00a | 2·73a | 3·08a |
| Vector-free culture in the glasshouse | 17·62 | 0 | NA | NA | NA |
NA, not applicable.
Means with the same superscript letter are not significantly different for each measurement (P < 0·001, n = 15).
Hand cross-pollination resulted in a non-significant difference in seeds per pod compared with hand self-pollinated flowers (χ2 test, P = 0·470). This indicates that there is no detectable level of self-incompatibility in this species. There was no significant difference between the different pollination treatments for pod length and for pod width (anova, F-test, P < 0·001).
Selfing rate, inbreeding depression and SCI
The selfing rate in T. balansae was estimated to be 0·13. The level of inbreeding depression recorded was 0·48 and the SCI was 0·51.
Stigma receptivity
Stigmas of accessions of both species tested with Peroxtesmo esterase indicator paper showed a positive blue reaction indicating that stigmas were receptive prior to anther dehiscence.
Nectar production
Forty-three out of 46 flowers from four inflorescences showed the presence of nectar.
Electron and fluorescence microscopy study
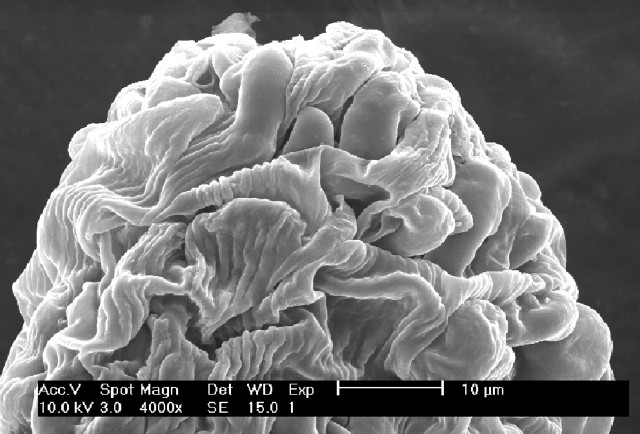
Microscopic examination of bagged and untripped florets showed no germinating pollen grains on the stigmas of T. balansae (Fig. 1). Hand-tripped and insect-pollinated florets of T. balansae showed the presence of germinating pollen grains (Fig. 2).
Fig. 1.
Scanning electron microscope view of an untripped stigmatic surface of Trigonella balansae.
Fig. 2.
Scanning electron microscope view of a tripped stigmatic surface of Trigonella balansae.
DISCUSSION
The results in this study indicate that Trigonella balansae is a self-compatible species, which requires vectors such as honeybees to bring about pollination and exhibits a mixed mating system. The lack of fertilization of florets of this species that have been protected from external pollination vectors (insect or human) is attributable to the spatial separation of stigma and pollen at the time of anthesis. The presence of nectar secretions in the corolla tube of T. balansae flowers would act as an attractant to pollinating insects. Manual tripping or tripping by insects resulted in the rupture of the stigmatic surface and facilitated pollen germination. Baum (1968) reported that the tripping mechanism was absent in Trigonella, but Lesins and Lesins (1979) suggested that the mechanism was present in some species. In comparison to the species belonging to Medicago and Melilotus, Small et al. (1987) placed T. balansae into the non-explosively tripping group. Barnes et al. (1972) reported that tripping ruptured the stigmatic membrane and was required for effective pollination in Medicago sativa. Lord and Heslop-Harrison (1984) found that, in the legume Vicia faba, pollen did not germinate until the stigmatic cuticle was disrupted and the lipid-rich secretions hydrated the pollen. Britten and Dundas (1985) found that some lines within the Psoralea patens complex (a semi-arid pasture legume) required outside intervention for the rupture of the stigmatic membrane, which resulted in the emergence of a sticky fluid engulfing the pollen grains so that germination occurred. As insects are commonly involved in pollination, there is the likelihood of cross-fertilization in this species. The structure of the florets of T. balansae during and after anthesis also supports the apparent tendency towards out-crossing. The observed lengthening of the pistils and extrusion of the stigmas is possibly an adaptive strategy to increase the chances of fertilization by foreign pollen. Real et al. (2004) in their floral development studies on Lotononis bainesii revealed that the upper stigma position related to the anther rings before anther dehiscence facilitated cross pollination. Anther dehiscence occurred at an advanced stage of the flower development when the stigma is already on top of the anthers and pollinating agents were required to set seed. Small et al. (1981) opined that inbreeding species tend to have wing petals shorter than the keel. They suggested that the prominence of the wings is an adaptive feature of the out-breeding species. In the present study the wing petals were significantly shorter than the keel in T. balanase before and after anthesis
The mating system largely influences the evolutionary dynamics of plant populations (Kittelson and Maron, 2000). The moderate value for the SCI indicated that T. balansae does not have any apparent self-incompatibility mechanism. Selfing is favoured when the values of inbreeding depression are <0·5 (Charlesworth and Charlesworth, 1987). Inbreeding appears universally to reduce fitness, but its magnitude and specific effects are highly variable because they depend on the genetic constitution of the species or populations and on how these genotypes interact with the environment (Hedrick and Kalinowski, 2000). However, the low selfing rate estimate in T. balansae suggests that out-crossing is more favoured. A low rate of selfing and a moderate amount of inbreeding depression was also reported in Lupinus arborreus, which has a mixed mating system (Kittelson and Maron, 2000). Pollinators can be opportunistic visitors (Waser et al., 1996) and habitat-mediated shifts in pollinator availability influence both out-crossing and inbreeding depression (Holsinger, 1996). A mixed mating system has been reported in the members of the related family, Fabaceae, in Dillwynia teuifolia (Rymer et al., 2002) and in Dinizia excelsa (Dick et al., 2003). Some theoretical models have suggested that the mixed mating exhibited by T. balansae should be transitory and evolve into a predominantly out-crossing system (Schemske and Lande, 1985; Lloyd, 1992). However, a mixed mating system may be stable from an evolutionary viewpoint (Holsinger, 1986; Holsinger, 1991; Lloyd, 1992; Lande et al., 1994).
The limitation of the present study is that only a single population of T. balansae was examined and hence the results cannot be used to generalize about the whole species.
Supplementary Material
Acknowledgments
The authors acknowledge Steve Hughes, Curator of the Australian Medicago Genetic Resources Centre, Adelaide for the supply of the germplasm used in this study. The technical assistance rendered by Steve Robinson and Jeff Hill is gratefully acknowledged. The Grains Research & Development Corporation (GRDC) Australia and Australian Wool Innovation Limited (AWI) are acknowledged for supporting this work as part of the National Annual Pasture Legume Improvement Program (NAPLIP), Australia.
LITERATURE CITED
- Barnes DK, Bingham ET, Axtell JD, Davis WH. 1972. The flower, sterility mechanism and pollination control. In: Hanson CH, ed. Alfalfa Science and Technology, Agronomy No. 15. Madison, WI: American Society of Agronomy, 123–141. [Google Scholar]
- Baum BR. 1968. A clarification of the generic limits of Trigonella and Medicago Canadian Journal of Botany 46: 741–749. [Google Scholar]
- Britten EJ, Dundas, IS. 1985. A dimorphic pollination system in a potentially valuable semi-arid pasture legume the Psoralea patens complex. In: Proceedings of the 15th International Grassland Congress. Kyoto, Japan. Kyoto: Science Council of Japan and Japan Society of Grassland Science, 209–210. [Google Scholar]
- Bullita S, Floris R, Hayward MD, Veronesi F. 1993. The reproductive system of a Lolium rigidum Gaud. population from Sardinia and its implication for breeding. Plant Breeding 111: 312–317. [Google Scholar]
- Charlesworth D. 1988. A method for estimating outcrossing rates in natural populations of plants. Heredity 61: 469–471. [Google Scholar]
- Charlesworth D, Charlesworth B. 1987. Inbreeding depression and its evolutionary consequences. Annual Review of Ecology and Systematics 18: 237–268. [Google Scholar]
- Dafni A, Maues MM. 1998. A rapid and simple procedure to determine stigma receptivity. Sexual Plant Reproduction 11: 177–180. [Google Scholar]
- Dick CW, Etchelecu G, Austerlitz F. 2003. Pollen dispersal of tropical trees (Dinizia excelsa: Fabaceae) by native insects and African honey bees in pristine and fragmented Amazonian rainforest. Molecular Ecology 12: 753–764. [DOI] [PubMed] [Google Scholar]
- Dobson AJ. 1990.An introduction to generalised linear models. Chapman & Hall. [Google Scholar]
- Hamrick JL, Godt MJW. 1989. Allozyme diversity in plant species. In: Brown ADH, Clegg MT, Kahler AL, Weir BS, eds. Population genetic resources in crop improvement. Sunderland, MA: Sinauer, 44–46. [Google Scholar]
- Harder LD, Barrett SCH. 1995. Mating cost of large floral displays in hermaphrodite plants. Nature 373: 512–515. [Google Scholar]
- Hedrick PW, Kalinowski ST. 2000. Inbreeding depression in conservation biology. Annual Review of Ecology and Systematics 31: 19–162. [Google Scholar]
- Holsinger KE. 1986. Dispersal and plant mating systems: the evolution of self-fertilisation in sub-divided populations. Evolution 40: 405–413. [DOI] [PubMed] [Google Scholar]
- Holsinger KE. 1991. Inbreeding depression and the evolution of plant mating systems. Trends in Ecology and Evolution 6: 307–308. [DOI] [PubMed] [Google Scholar]
- Holsinger KE. 1996. Pollination biology and the evolution of mating systems in flowering plants. Evolutionary Biology 29: 107–149. [Google Scholar]
- Howie J, Ballard RA, de Koning C, Sandral G, Charman N. 2001.Trigonella balansae – a new pasture legume for the alkaline soils of southern Australia? In: Proceedings of the 10th Australian Agronomy Conference, Hobart. Australian Society of Agronomy. Published online (http://www.regional.org.au/au/asa/2001/3/d/howie.htm). [Google Scholar]
- Huber-Morath A. 1970. Trigonella. In: Davis PH, ed. Flora of Turkey and the East Aegean Islands Vol. 3. Edinburgh: Edinburgh University Press, 452–482. [Google Scholar]
- Jarne P, Charlesworth D. 1993. The evolution of the selfing rate in functionally hermaphrodite plants and animals. Annual Review of Ecology and Systematics 24: 441–466. [Google Scholar]
- Kephart SR, Brown E, Hall J. 1999. Inbreeding depression and partial selfing: evolutionary implications of mixed-mating in a coastal endemic, Silene douglassi var. oraria (Caryophyllaceae). Heredity 82: 543–554. [DOI] [PubMed] [Google Scholar]
- Kittelson PM, Maron JL. 2000. Outcrossing rate and inbreeding depression in the perennial yellow bush lupine, Lupinus arboreus (Fabaceae). American Journal of Botany 87: 652–660. [PubMed] [Google Scholar]
- Lande R, Schemske DW, Schultz, DW. 1994. High inbreeding depression, selective interference among loci and the threshold selfing rate for purging recessive lethal mutations. Evolution 48: 965–978. [DOI] [PubMed] [Google Scholar]
- Lesins KA, Lesins I. 1979.Genus Medicago (Leguminosae). A taxogenetic study. The Hague: Dr. W Junk Publishers. [Google Scholar]
- Lloyd DG. 1992. Self- and cross-fertilization in plants. II. The selection of self-fertilisation. International Journal of Plant Sciences 153: 370–380. [Google Scholar]
- Lloyd DG, Schoen DJ. 1992. Self- and cross-fertilization in plants. I. Functional dimensions. International Journal of Plant Sciences 153: 358–369. [Google Scholar]
- Loi A, Nutt BJ, McRobb R, Ewing MA. 2000. Potential new alternative annual pasture legumes for Australian Mediterranean farming systems. Option Mediterraneennes 45: 51–54. [Google Scholar]
- Lord EM, Heslop-Harrison Y. 1984. Pollen-stigma interaction in the Leguminosae: stigma organisation and the breeding system in Vicia faba L. Annals of Botany 54: 827–836. [Google Scholar]
- Loveless MD, Hamrick JL. 1984. Ecological determinants of genetic structure in plant populations. Annual Review of Ecology and Systematics 15: 65–95. [Google Scholar]
- Matkin OA, Chandler PA. 1957. The U.C. type soil mixes. Section 5 in California Agricultural Experiment Station Manual 23. [Google Scholar]
- Real D, Rizza MD, Quesenberry KH, Echenique M. 2004. Reproductive and molecular evidence for allogamy in Lotononis bainesii Baker. Crop Science 44: 394–400. [Google Scholar]
- Richards AJ. 1986.Plant breeding systems. London: George Allen & Unwin. [Google Scholar]
- Rymer PD, Morris EC, Richardson BJ. 2002. Breeding system and population genetics of the vulnerable plant Dillwynia tenuifolia (Fabaceae). Austral Ecology 27: 241–248. [Google Scholar]
- Schemske DW, Lande R. 1985. The evolution of self-fertilisation and inbreeding depression in plants. II. Empirical observations. Evolution 39: 41–52. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Sharma A. 1980.Chromosome techniques, theory and practice, 3rd edn. London: Butterworths. [Google Scholar]
- Small E, Crompton CW, Brookes BS. 1981. The taxonomic value of floral characters in tribe Trigonelleae (Leguminosae), with special reference to Medicago Canadian Journal of Botany 59: 1578–1598. [Google Scholar]
- Small E, Lassen P, Brookes BS. 1987. An expanded circumscription of Medicago (Leguminsae, Trifolieae) based on explosive flower tripping. Willdenowia 16: 415–437. [Google Scholar]
- Waser NM, Chittka L, Price MV, Williams N, Ollerton J. 1996. Generalisation in pollination systems and why it matters. Ecology 77: 1043–1060. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.