Abstract
Molecular chaperones encompass a group of unrelated proteins that facilitate the correct assembly and disassembly of other macromolecular structures, which they themselves do not remain a part of. They associate with a large and diverse set of coregulators termed cochaperones that regulate their function and specificity. Amongst others, chaperones and cochaperones regulate the activity of several signaling molecules including steroid receptors, which upon ligand binding interact with discrete nucleotide sequences within the nucleus to control the expression of diverse physiological and developmental genes. Molecular chaperones and cochaperones are typically known to provide the correct conformation for ligand binding by the steroid receptors. While this contribution is widely accepted, recent studies have reported that they further modulate steroid receptor action outside ligand binding. They are thought to contribute to receptor turnover, transport of the receptor to different subcellular localizations, recycling of the receptor on chromatin and even stabilization of the DNA-binding properties of the receptor. In addition to these combined effects with molecular chaperones, cochaperones are reported to have additional functions that are independent of molecular chaperones. Some of these functions also impact on steroid receptor action. Two well-studied examples are the cochaperones p23 and Bag-1L, which have been identified as modulators of steroid receptor activity in nuclei. Understanding details of their regulatory action will provide new therapeutic opportunities of controlling steroid receptor action independent of the widespread effects of molecular chaperones.
Keywords: steroid receptors, nuclear receptors, chaperones, cellular transport
Introduction
In the early 1990’s, studies with the glucocorticoid receptor (GR; a member of the steroid/nuclear receptor superfamily) showed that immunoprecipitated GR, when incubated with reticulocyte lysate that contains molecular chaperones and a source of adenosine triphosphate (ATP), could be made to bind hormone in vitro [1]. These reconstitutions could also be achieved with purified GR, molecular chaperones (Hsp90, Hsp70) and cochaperones (Hsp60 or Hip, Hsp40 or its yeast equivalent YDJ-1 and p23), which displayed an orderly and dynamic assembly of the receptor. The first step in this assembly was the formation of a molecular complex (Hsp90)2.Hop.Hsp70.Hsp40, termed the foldosome [2,3] (Figure 1, A and B). Two of the mayor players of the foldosome, Hsp90 and Hsp70, contain nucleotide-binding domains that act as ATP/ADP-binding switches that allow them to assume different properties depending on which form of energy is bound. In its ATP-bound form, Hsp90 interacts with the cochaperone p23 and activates client protein activity through folding, whereas in its ADP-bound form it shows high affinity to hydrophobic proteins [4,5]. In the case of Hsp70, its ATPase activity is enhanced by the cochaperone, Hsp40, as well as a variety of other cochaperones [6,7]. In general, Hsp-interacting cochaperones can be grouped according to the presence or absence of tetratricopeptide repeats (TPR) in their sequence (Figure 2). The TPR domains are typically composed of three tandem repeats of a loosely conserved 34 amino acid sequence motif [9]. Each motif favors formation of two anti-parallel α-helices, and the core TPR domain consists of six total α-helices that form a saddle-like structure. The surface of the domain provides an interaction site that can accommodate specific peptide binding [10]. For example, TPR-containing cochaperones are able to interact with Hsp90 through its EEVD motif at the extreme C-terminus, as well as with Hsp70 via a EEVD-like sequence at its C-terminus [10,11]. The repertoire of the TPR-containing cochaperones known to regulate steroid receptor signaling pathways includes the Hsc70-interacting protein (Hip; p48), the Hsp70/Hsp90-organizing protein (Hop; p60), FK506 binding protein of 51 kDa (FKBP51), FK506 binding protein of 52 kDa (FKBP52), cyclophilin 40 (Cyp40), protein phosphatase 5 (PP5), FK506-binding protein like protein (FKBPL), and general cell UNC-45 (GCUNC-45) [12-15] (Figure 2). More recently, a small glutamine-rich TPR-containing protein alpha (SGTA) has been added to the growing diversity of TPR-containing cochaperones involved in the modulation of steroid receptor action [16,17] (Figure 2). It should be noted that although several cochaperones use TPR motifs to bind to the molecular chaperone [10,18], other cochaperones such as Prostaglandin E synthase 3 (p23), Activator of Hsp90 ATPase homolog 1 (Aha1) and Bcl-2-associated athanogene 1 (Bag-1) lack TPRs (Figure 2) and use their own, unique sequences to associate with Hsp90 and Hsp70. These cochaperones may have other activities of their own that are independent of their interaction with the molecular chaperones. Such actions were referred to in a recent quantitative analysis of the chaperone-cochaperone-client interaction networks in human cells, where the physical interaction landscape of all known Hsp70- and Hsp90-bound cochaperones was analyzed [19].
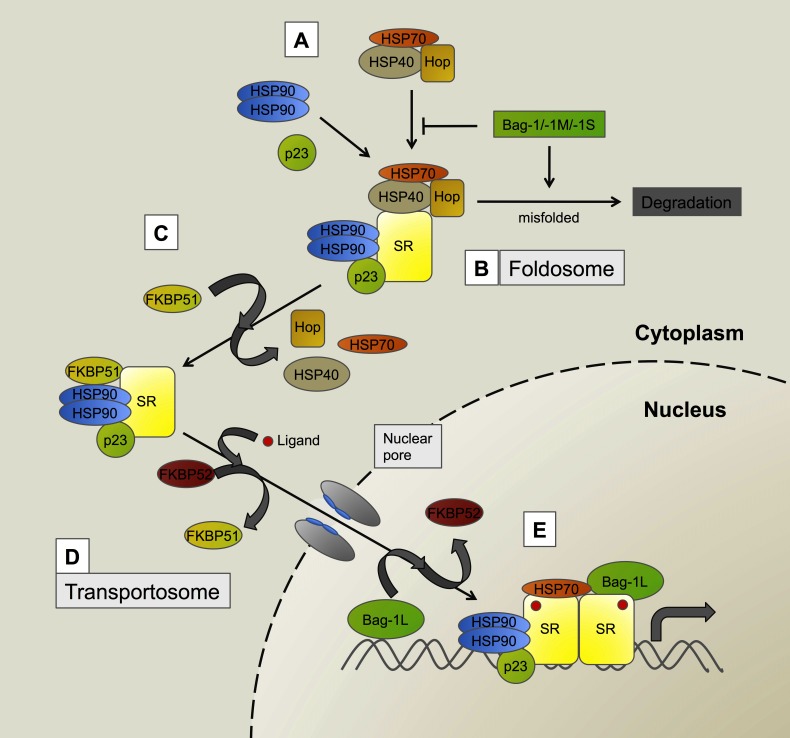
Figure 1. A model depicting some of the key steps of the maturation pathway of steroid receptors.
(A) Binding of the Hsp90, p23 and a preassembled complex of Hop, Hsp70 and Hsp40 assists a mature folding of the steroid receptor (SR). Cytoplasmic Bag-1 isoforms (Bag-1, -1M, -1S) control this process and mediate proteasomal degradation of misfolded SRs. Addition of Hsp90-dimers and p23 complete the assembled complex, termed the “foldosome” (B). Release of Hop, Hsp70 and Hsp40 and addition of any one of the TPR-containing cochaperones, for example FKBP51 (as shown here), further stabilize the SR in a high affinity form (C). After ligand binding FKBP51 is replaced by FKBP52, which mediates translocation to the nucleus via the microtubuli system (via dynein and dynamitin) in a molecular complex termed the “transportosome” (D). Within the nucleus FKBP52 is released and the receptor binds the response elements as an active dimer. Cochaperones, such as p23 and Bag-1L (that has been described to bind to chromatin prior to the nuclear entry of the receptor), enhance the activity of the SR most likely by stabilizing the active state of the receptor. The molecular chaperones Hsp90 and Hsp70 possibly also play a role in this process (E).
Figure 2. The structure of TPR-containing and TPR-lacking cochaperones.
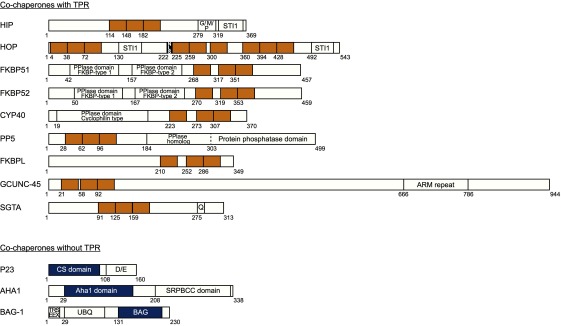
Top: Domain structure of cochaperones containing tetratricopeptide repeats (TPR) motifs. All TPR motifs are shown in yellow. Other important protein domains are indicated. Bottom: The domain structures of three cochaperones (p23, AHA1 and BAG-1) lacking the classical TPR motifs, with their Hsp90/Hsp70-binding domains highlighted in blue. All domain information (including residue numbers) were obtained from the RefSeq database (NCBI) [8]). STI1: Stress inducible protein 1 (Heat shock protein binding motif); NLS: Nuclear localization signal; PPlase: Peptidyl-prolyl cis-trans isomerase. ARM: Armadillo; CS: CHORD-containing protein SGT1; Aha1: Activator of Hsp90 ATPase; SRPBCC: START/RHOs_alpha_C/PITP/Bet v 1/CoxG/CalC: UBQ: Ubiquitin-like domain; BAG: Bcl-2-associated athanogene (Heat shock protein binding motif). Single alphabet letters (with or without separation by a slash) correspond to particular amino acids (or amino acid sequences) that are over-represented in a certain region.
Our views on the role of Hsp70 and Hsp90 in steroid receptor action are changing in recent times. Cryoelectron microscopy studies have demonstrated that Hsp70, known to facilitate GR delivery to Hsp90, actually inactivates GR through partial unfolding of the receptor [20]. Conversely, Hsp90 is able to reverse this function and promote GR activation. Although this unfolding/inactivation by Hsp70 and refolding/reactivation by Hsp90 might seem contradictory, this combination could in fact be complementary; constant rounds of Hsp70-mediated unfolding/ligand release and Hsp90-mediated refolding/ligand binding could allow for the non-liganded GR to remain in a non-aggregating, high-affinity state poised for a rapid response to changing hormone levels. In another cryoelectron microscopy study, Hop, once thought of as a mere adaptor protein for Hsp90 and Hsp70 binding which coordinates their actions on folding protein substrates [21], was shown to have additional functions in a reconstruction of the Hsp90/Hop complex. Here Hop formed extensive interactions with Hsp90, preorganizing its N-terminal domain for ATP hydrolysis and client protein binding [22]. In the classical model, Hop is eventually released from the complex and is replaced by one of the other TPR cochaperones such as FKBP51, FKBP52 or Cyp40 [18] (Figure 1C). This dynamic exchange occurs on Hsp90 dimers of the foldosome. Although any one of the TPR proteins can in principle replace Hop, only one TPR protein is found bound to the Hsp90 dimers at any one time [23]; it is as if Hsp90 surveys the local environment for available TPR proteins to bind to. There is some evidence suggesting that the type of ligand that ultimately associates with the receptor complex influences which TPR protein is recruited. For example, aldosterone binding to the mineralocorticoid receptor (MR) favors the exchange of FKBP51 for FKPB52, while binding of 11,19-oxidoprogesterone favors the association with the immunophilin-like PP5 [24].
In previous studies it was though that steroid receptors remain in the cytoplasm in complex with molecular chaperones and cochaperones in the absence of hormone [25]. Upon ligand binding, it was thought that the complex dissociates and the receptors are transported into the nucleus, where they bind chromatin and regulate the expression of multiple target genes. However, this classic model of molecular chaperones action is perhaps a bit too simple. Steroid receptors are known to shuttle between the cytoplasm and the nucleus [26] and some, for example, estrogen and progesterone receptors (ER and PR) prefer to remain in the nucleus bound to molecular chaperones in the absence of hormone [27,28]. Several questions therefore arise concerning how the molecular chaperones and cochaperones get transported into the nucleus. Are they transported on their own or in complex with the steroid receptors and what role do they play in the nucleus? More importantly, in view of the recent data on chaperone-independent functions of cochaperones, one might ask whether cochaperones exert specific effects on steroid hormone action. In this review, we will focus on two cochaperones, p23 and Bag-1L that are present in the nucleus, where they reportedly influence steroid receptor action through receptor recycling or via modulation of receptor binding to chromatin. We will describe how these cochaperones exert their action and suggest how cochaperone/steroid receptor action could be targeted for therapeutic purposes.
From foldosomes to transportosomes
The two step hypothesis of Jensen of a cytoplasmic/nuclear transportation of steroid receptors [29], together with the finding that molecular chaperones and cochaperones bind to nonliganded receptors, generated the concept that molecular chaperones confine steroid receptors in an inactive, cytoplasmic state. However nonliganded receptor/molecular chaperone complexes have been found to constantly shuttle between the cytoplasm and the nucleus [30,31]. Nevertheless, shuttling by the PR and ER, which are largely nuclear, may be mechanistically different from shuttling by the mineralocorticoid and androgen receptors (MR and AR respectively). This is also different from the shuttling of the GR, which is mainly localized in the cytoplasm, but can also be found at the nuclear periphery as part of a (non-liganded) GR/molecular chaperone complex in association with the integral nuclear pore glycoprotein Nup62 and importin β [32]. The Hsp90 cochaperone Aha 1 may also be involved in this complex as it was shown to contribute to the nucleocytoplasmic transport of the GR; cells lacking Aha 1 showed a reduced and impartial translocation of the receptor into the nucleus [33]. Whether Aha 1 is transported into the nucleus together with the GR is however not known. Several studies now show that molecular chaperones and cochaperones are transported along with the liganded steroid receptors into the nucleus [34,35]. In fact more recent studies have shown a constitutive requirement of Hsp90 throughout the functional lifetime of the GR and not just during the initial folding phase [20].
The ligand-dependent GR translocation to the nucleus was found to be considerably reduced using the Hsp90 inhibitor geldanamycin (GA) [36,37]. Furthermore, microinjection of an antibody against the cochaperone FKBP52, but not an isotype control also inhibited ligand-induced nuclear transport of the GR [38]. This suggests that FKBP52 contributes to the nuclear translocation of the GR. Additional evidence that FKBP52 plays a role in the nuclear transport of the GR is the finding that it directly binds to the motor protein dynein, via dynamitin [39,40]. Through this interaction, the receptor/chaperone complex is thought to move along the cytoskeleton to the nucleus on what has been described as the transportosome [41] (Figure 1D). The affinity of the FKBP52-receptor complex for dynein possibly determines the rate of transportation of the steroid receptors into the nucleus [40]. While GR has a high affinity for FKBP52, MR has a preferred affinity for FKBP51. Experiments carried out with cross-linked MR-Hsp90 or GR-Hsp90 heterocomplexes showed that these large heterocomplexes can be found in the nucleus in the presence of hormone, demonstrating that they can pass undissociated through the nuclear pore to the nucleus [32,42]. Once in the nucleus, the steroid receptor/molecular chaperone complex dissociates and the steroid receptor is converted into a DNA-binding form as has been shown by Davies et al. (2002) [43].
Mouse knockout models of FKBP51 and FKBP52 have been generated to find out how these cochaperones affect steroid hormone action in vivo [44]. Although FKBP51 and FKBP52 are implicated in steroid hormone action, knockout mice of the former cochaperone do not show any disruption in endocrine activity. In FKBP52 KO mice, no overt defects of GR-regulated physiology were observed. Mouse embryonic fibroblasts (MEFs) were therefore generated from wild-type (WT) and FKBP52-deficient animals and analyzed [45]. Contrary to expectation, loss of FKBP52 had no effect on the composition of hormone-free GR heterocomplexes nor did it show any effect on hormone-binding function or the ability of the GR to move to sites of chromatin action within the nucleus. Instead, FKBP52 was found to play an unexpected role as a gene-specific modulator of GR transcriptional activity. However, GR activity at endogenous glucocorticoid-target genes was not globally affected in FKBP52 KO cells. Reduced activity were observed at glucocorticoid-inducible leucine zipper (GILZ) and FKBP51, but not at serum- and glucocorticoid-kinase (SGK) and p21 genes [45]. The FKBP52 knockout mice however demonstrated the importance of this cochaperone for reproductive tissue development. Female knockout mice showed defects in uterine receptivity for embryo implantation and male knockouts displayed ambiguous external genitalia and dysgenic prostates [46,47]. Furthermore, in the female knockout mice FKBP52 was shown to be an essential regulator of PR action in the uterus, while being a non-essential but contributory regulator of steroid receptors in the mammary gland and ovaries [48]. These data may now provide the basis for selective targeting steroid-regulated physiology through co-chaperones.
Role of molecular chaperones in the nucleus
Studies using in vitro receptor/DNA interaction techniques and in vitro transcription experiments have provided hints that, in the nucleus, molecular chaperones function as modulators of the DNA-binding and transcriptional activities of steroid receptors [49,50]. One example is the work of Etienne Baulieu and colleagues in 1996 which showed that the binding of ERα to the estrogen response element from the vitellogenin A2 gene is inversely dependent on the relative concentration of Hsp90 [49]. In another assay, recombinant (ligand-free) PR was only able to bind to and induce transcription on (hormone-containing) chromatin templates in the presence of rabbit reticulocyte lysate rich in molecular chaperones [50]. The use of the Hsp90-specific inhibitor GA blocked the transcriptional activity of this receptor on chromatin [50], demonstrating a crucial role of Hsp90 in the nuclear function of the PR.
Additional experiments have provided more proof for a regulatory role of molecular chaperones on steroid receptor action at the chromatin level. Using live cell imaging, the groups of Gordon Hager, David Toft and Don DeFranco demonstrated that molecular chaperones contribute to the rapid mobility and dynamic exchange of steroid receptors at transcriptionally active chromatin sites [37]. They showed an impairment of nuclear mobility of GR and PR using transcriptionally active nuclei depleted in soluble factors. Receptor mobility was regained upon incubation of the nuclei with an ATP-dependent regenerating system and combinations of purified chaperones and cochaperones. A mixture of seven components (Hsp90, Hsp70, p23, Hop, Ydj-1, FKBP51) and CHIP was the most effective, but a mixture of five proteins (Hsp90, Hsp70, p23, Hop, and Ydj-1) or even three (Hsp90, Hsp70, and Hop) restored steroid receptor movement, albeit to a lesser extent [37]. Together these findings led to the conclusion that the molecular chaperones and cochaperones are involved in recycling of the receptors on chromatin.
Nuclear action of p23
Although the experiments described above argue that molecular chaperones and cochaperones enter the nucleus bound to the steroid receptors, some of the cochaperones appear to have a nuclear function of their own, independent of their regulation of receptor action. Two examples are the cochaperones p23 and Bag-1L. Mammalian p23 (or Sba1 in yeast) is composed of a simple molecular structure consisting of a compact eight β-strand antiparallel sandwich (the CS domain in Figure 2) followed by an acidic C-terminal tail [51,52]. This structure is conserved from humans to yeast with orthologs in both plants and protozoa. The main role of p23/Sba1 is to bind the ATP-engaged N-terminal domain of Hsp90, thereby stabilizing a high-affinity client binding conformation. At the same time this slows down the hydrolysis of ATP and increases the dwell time of client proteins in the Hsp90 chaperone complex [53]. However, it appears that p23 also has a chaperone activity of its own, independent of Hsp90. A large portion (approximately 69%–75%) of the p23/Sba1 interacting proteins (as determined by genetic and proteomic high throughput approaches in yeast) is not shared with Hsp90 [54]. Further analysis of the effect of Sba/p23 on chromatin events showed that deletion of the yeast p23 (Sba1Δ) reduced the number of DNase I hypersensitive sites in chromatin. The number of sites was decreased from 3260 in wild-type to 2439 in Sba1Δ cells. However, this reduction in the total number of DNase I hypersensitive sites in Sba1Δ cells (approximately a 25% loss), was not a mere reduction but was associated with the appearance of novel sites within chromatin. p23 does not harbor any obvious DNA-binding domains and it appears that its effect on transcription factors and chromatin is therefore mediated through protein-protein interactions with chromatin remodelers, such as the histone acetyltransferase GCN5 [55].
p23-mediated regulation of steroid receptor action on chromatin
Brian Freeman and Keith Yamamoto have previously suggested a genomic action of the cochaperone p23 in the dissociation of receptors from coactivators and response elements on chromatin [56]. Using chromatin immunoprecipitation (ChIP), they observed that molecular chaperones together with cochaperones are recruited to the response elements of the liver-specific, glucocorticoid-inducible tyrosine aminotransferase (TAT) and tryptophan oxygenase (TO) genes in rat hepatoma HTC cells. They could also show that p23 and Hsp90, but not Hsp70, were localized to the same response elements, in a hormone-dependent manner [57] (Figure 1E). In an unrelated immunofluorescence experiment, Hsp90, Hsp70 and p23 were visualized together with GR on an integrated array of glucocorticoid response elements (GRE) from the mouse mammary tumor virus (MMTV) DNA in the genome of a mouse adenocarcinoma cell line [58]. In this assay, the molecular chaperones and particularly Hsp90 and p23, were shown to stabilize GR binding. Inhibition of Hsp90 activity simultaneously reduced the binding of the molecular chaperones, p23 and GR to the response elements [58]. Accordingly contrasting explanations to the action of molecular chaperones and cochaperones have been presented. On the one hand their involvement in the dissociation of the receptor complex (as described by Brian Freeman and Keith Yamamoto [56]) and on the other hand their ability to stabilize the receptor complex (as outlined above). This discrepancy could be due to a key difference in the experimental setup of the two studies. In one study, Hsp90 function was disrupted by GA treatment and since this drug prevents p23 binding to Hsp90 [59], it was thought it would also affect p23 action [58]. In the other study, altered p23 activity was achieved by the addition of purified p23 to in vitro transcription assays [57]. Although p23 and Hsp90 normally act in concert within the chaperone complex, they may not act together on DNA. Therefore inhibiting the interaction of Hsp90 with p23 may not have the same effect as the addition of p23. However, both studies agree that Hsp90 and p23 modulate the action of the GR at the chromatin level.
The physiological function of GR requires the ligand to be presented to target cells in discrete pulses to agree with the pulsatile production of glucocorticoid from the adrenal gland [60]. Using ChIP, a connection between the function and cyclical action of GR and intranuclear molecular chaperones and cochaperones at glucocorticoid target genes was demonstrated [61]. When the chaperone activity was disrupted by GA treatment, pulsatile GR transcriptional activity was abrogated [61]. Furthermore it was shown that the ligand-bound GR complexes exchange rapidly and continuously with response elements in chromatin (in the time scale of seconds). During each exchange, the receptor may lose its ligand and require entry into the “chaperone cycle” (possibly through the foldosome activity) to re-acquire its ligand. Alternatively, the receptor may retain its ligand, but may need to return to the chromatin template with the help of the chaperone/cochaperone complexes [60]. These cycling reactions of the GR are different from those of the other steroid receptors, such as the AR or ER. Although cycling of AR and ER on chromatin have previously been described [62,63], these cycling events are intrinsic properties of these receptors and they depend on proteasome function and the degradation of the receptors. Furthermore, these processes occur in the presence of constant hormone levels, which is different from the oscillations of the GR.
Increased expression of p23 achieved by transfection in the estrogen-dependent breast cancer cell line MCF-7 increased ERα recruitment and activity at select regulatory elements of ER target genes [64]. Utilizing ChIP-sequencing (ChIP-seq), a 230% increase in the number of estrogen-induced ER-binding sites, compared with control cells, could be demonstrated in response to the increased expression of p23. Interestingly, motif analysis revealed that ERα bound to the same DNA sequences, regardless of p23 status. Thus, the increase in ER binding sites was not due to enhanced ER binding but most likely due to p23-mediate changes in histone modification; a consequence of this would be an increased chromatin accessibility and binding by the ERα [64]. More recently the action of the cochaperones p23 has been analyzed on AR activity. It was shown that p23 can enhance AR transactivation function through steps in both the cytoplasm (by increasing the ligand-binding capacity, possibly via direct interaction with AR) and the nucleus (by enhancing AR occupancy at target promoters) [65]. An interaction between AR and p23 was demonstrated even after treatment with the geldanamycin analog, 17-N-Allylamino-17-demethoxygeldanamycin (17-AAG), a Hsp90 inhibitor that displaces p23 from the complex with Hsp90 [65]. The effects of p23 on AR activity was, at least in part, Hsp90-independent, since a mutant form of p23 that was unable to bind Hsp90 increased AR activity nevertheless [65]. Collectively, these studies suggest that p23 has other functions in addition to its action as an Hsp90 cochaperone.
Knockout mouse model of p23
A knockout mouse model has been generated for studies on the role of p23 in steroid hormone action. p23 was found absolutely necessary for perinatal survival, but it was inessential for overall prenatal development and morphogenesis [66]. The skin barriers at the final fetal stages of development were incompletely formed and the lungs of p23 null embryos displayed underdeveloped airspaces and substantially reduced expression of surfactant genes [66]. The defects in skin and surfactant gene expression correlate with defects in glucocorticoid function in promoting lung maturation, and the development of epithelial barriers [67-69]. Accordingly, embryonic fibroblasts from p23 null mice displayed a defective glucocorticoid response [66]. The perinatal phenotype of the p23 null mice has prevented analysis of the contributions of this cochaperone to other endocrine functions in adult mice.
Nuclear action of Bag-1
The Bag-1 isoforms
Another cochaperone that has been shown to function in the nucleus in addition to p23 is Bag-1L, a member of the Bag-1 family of proteins. In humans, the Bag-1 family is made up of polypeptides translated from one mRNA by a leaky scanning mechanism [70,71]. This generates four differentially-sized isoforms; Bag-1L, Bag-1M, Bag-1 and Bag-1S (Figure 3A). These proteins differ at their N-terminal sequences, but have a conserved C-terminal Hsp70-binding domain (otherwise known as BAG domain) [74,75]. Additionally, Bag-1 proteins contain a ubiquitin-like domain (UBQ) through which they can be connected to the proteasome [76]. The UBQ was shown to be important in CHIP (carboxyl terminus of Hsp70-interacting protein)/E3 ligase-dependent degradation of the GR [77]. Bag-1 is therefore a coupling factor, which can link the chaperones and the proteolytic complex together and thereby plays a role in steroid receptor turnover (Figure 1A) [78].
Figure 3. The Bag-1 protein family and their structural domains.
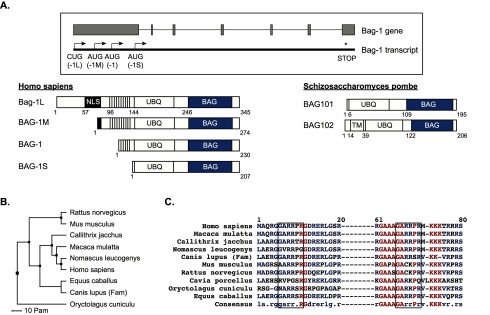
A. Top: Intron-exon structure of the human Bag-1 gene and corresponding transcript. The start codons for the different Bag-1 transcripts are indicated by arrows. Note, Bag-1L, the longest family member, is the only one with a CUG start codon. Bottom: The domain structures of the four human Bag-1 isoforms (left) and the two isoforms of Schizosaccharomyces pombe (right), with their Hsp70/Hsc70-binding domains (BAG) highlighted in blue. The TR/QSEEX repeat region is shown as vertical lines and other functional domains are indicated. The domain information (including residue numbers) for the human Bag-1 isoforms were obtained from the RefSeq database (NCBI), while the domain information for the yeast homologues were taken from Kriegenburg et al. 2014 [72]. NLS: Nuclear localization signal; UBQ: Ubiquitin-like domain; TM: Transmembrane domain. B, C. Phylogenetic tree (B) and sequence alignment (C) of the first 80 N-terminal amino acids of the human Bag-1L protein compared with Bag-1 isoforms in other organisms. Both graphs were generated using the MultAlin website [73].
The Bag-1 proteins do not contain a TPR motif but instead use their BAG domain to bind the Hsp70 ATPase. The BAG domain is approximately 100 amino acids in length and is made up of three antiparallel alpha helices, which serve as a protein-protein interaction surface for a number of cellular proteins [79]. In a 1.9 Å crystal structure in complex with the ATPase of Hsc70 (a homologous protein of Hsp70), the BAG domain was shown to induce a conformational switch in the ATPase that is incompatible with further nucleotide binding. A similar switch was observed in the bacterial Hsp70 homolog DnaK, when bound by the structurally unrelated nucleotide exchange factor GrpE. The Bag-1 proteins and in particular Bag-1 are therefore often described as mammalian nucleotide exchange factors of Hsp70 [75]. Furthermore, the interaction of Bag-1 with Hsp70 and with the unliganded GR has made it a member of the foldosome complex [80]. In fact, early studies showed that Bag-1 is involved in the release of Hop from the foldosome complex [80] (Figure 1A and B) but later analysis demonstrated that it actually competes with Hip for binding to the Hsp70 ATPase domain [6].
Bag-1L, the largest member of the family, possesses a N-terminal nuclear localization sequence (NLS) and is therefore exclusively localized to the nucleus. The other Bag-1 isoforms are mainly cytoplasmic [71], although under stress conditions these members and notably Bag-1M, are reported to also localize to the nucleus [81]. Intriguingly, two orthologs of Bag-1, Bag101 and Bag102 (Figure 3A), have been identified in the fission yeast Schizosaccharomyces pombe that show about 20% sequence identity and 45% sequence homology with the human Bag-1 isoforms [82]. Bag101 and 102 also contain BAG and UBQ domains and have the ability to bind to Hsp70 and the 26S proteasome [82]. Like the human Bag-1 proteins, the fission yeast proteins have different cellular localizations. While Bag101 is localize to the cytosol, Bag102 is found exclusively in the nuclear envelope. In accordance, genetic and biochemical assays show that only Bag102 has a nuclear function. It is required for a nuclear chaperone-assisted degradation mechanism and is involved in protein nuclear quality control and kinetochore integrity [82].
Nuclear action of human Bag-1L
The human Bag-1 proteins were shown to either positively or negatively regulate the action of many steroid/nuclear receptors ranging from GR, PR, MR, AR, ER and vitamin D receptor (VDR) to retinoid X receptor (RXR) [79,83]. In particular ERα, ERβ, AR and VDR were reported to be positively regulated by Bag-1L, and to a lesser extend by Bag-1M [84-86]. This suggests that the (NLS-containing) N-terminus of Bag-1L, which is absent in Bag-1M, contributes to the positive effect of Bag-1L on steroid receptor action. To confirm this experimentally, the cytoplasmic Bag-1 proteins (Bag-1M and Bag-1S) were tagged with a SV40 NLS and their effect on AR regulation was monitored. Although NLS-Bag-1M and NLS-Bag-1S translocated to the nucleus and were able to exert positive regulation on AR transactivation, they did not achieve the regulatory activity of Bag-1L [87,88]. This suggests that the N-terminus of Bag-1L has a function distinct from the cochaperone activity of its C-terminal BAG domain.
In addition to the presence of a NLS at the N-terminus, Bag-1L binds the AR, ERα and VDR [85,88,89] and harbors two additional, unique functions. First, the region between amino acids 72 to 79 is reported to bind non-specifically to DNA. This region contains positively charged sequences of three consecutive lysine and three arginine residues, separated by a centrally located neutral residue. Mutational analysis has identified both trimeric blocks as essential for DNA binding [90,91]. Second, sequences between amino acids 17-50 have been described as important for the nuclear retention of Bag-1L. It is thought that this region holds the cochaperone anchored to structures in the nucleus, possibly histone proteins [87]. Together, these regulatory elements keep Bag-1L in the nucleus and contribute to its effect as a modulator of steroid receptor action. In ChIP experiments, Bag-1L was bound to chromatin along with the AR at androgen-regulated target genes [88,92].
The GARRPR motif of Bag-1L
More recently a duplicated sequence “GARRPR” at positions 6-11 and 66-71 at the N-terminus of Bag-1L was shown to interact with the AR and the ERα [92]. Mutation of these motifs destroyed binding of Bag-1L to the AR but did not impair the chromatin binding potential of the mutant Bag-1L [92]. This indicates that chromatin and receptor binding are not linked for this protein. While the C-terminal BAG domain shows a high degree of sequence homology among the Bag-1 proteins found throughout evolution (in yeast, invertebrates, amphibians, mammals and plants), the first 128 N-terminal amino acids containing the duplicated GARRPR motif are less well conserved [93]. Nevertheless, a high sequence homology exists among the GARRPR motifs of human and monkey (M. mulatta, N. leucogenys and C. jacchus) (Figures 3B and C). Protein binding studies using peptides encompassing the GARRPR motifs showed that they bind to the ligand-binding domain (LBD) of the AR to a region termed binding function-3, which allosterically modulates the activity of the receptor [92]. Structure-based sequence alignments of the LBD of multiple steroid receptors show that the BF-3 pocket is highly conserved among steroid receptors as well as being present in other major nuclear receptors [94]. It is therefore expected that the GARRPR motif would also bind to other steroid/nuclear receptors. However, in vitro experiments showed that the GARRPR motif bound only to the AR and ERα and not the PR or GR [92]. This suggests that other receptor sequences contribute to the binding to the GARRPR motif. So far the only other protein that has been found to bind the BF-3 region is the cochaperone FKBP52 that has a GARRPR-like sequence [92,95].
It is interesting to note that for both Bag-1L and FKBP52 the sequences that interact with the BF-3 domain are different from those reported to bind Hsp70 and Hsp90. Another interesting observation is that mutation of the GARRPR motifs in both proteins results in a gain-of-function phenotype [92,96]. In Bag-1L the mutations do not completely inhibit AR-mediated gene expression but rather increase the expression of a subset of androgen-regulated genes involved in metabolic processes [92].
The BAG domain of Bag-1L
In addition to the regulation of steroid receptor action through the GARRPR motif, several lines of evidence suggest that Bag-1L may indirectly regulate the activity of the steroid receptors through its BAG domain. First, deletion of the BAG domain destroys the ability of Bag-1L to enhance the activity of the AR [88,97]. Second, mutation of amino acids in the BAG domain involved in the interaction with the ATPase domain of Hsc70, not only destroyed the interaction between Bag-1L and Hsc70, but simultaneously obliterate the ability of Bag-1L to enhance the transactivation function of the AR [97]. Combined, these results show that the BAG domain of Bag-1L, which also acts as a nucleotide exchange factor for Hsp70, contributes to the regulation of steroid receptor action, at least for AR. Intriguingly, protein-protein interaction studies showed that the BAG domain of Bag-1L does not interact with the AR-LBD, but rather with its N-terminal transactivation domain [88]. This region of the AR is intrinsically disordered, which means that it exists without a stable tertiary structure. However, the lack of structure has several advantages. For example, it provides a large interaction surface compared with other globular proteins of the same size. Secondly, intrinsically disordered proteins have short linear motifs (SLiMs) that allow them to recognize binding partners by undergoing coupled folding and binding processes. SLiMs have extremely compact protein interaction interfaces that are generally encoded by less than four major (affinity- and specificity-determining) residues within a stretch of 2–10 amino acids [98]. The occurrence of SLiMs in intrinsically disordered regions gives way to specific, yet transient, interactions that enable them to play central roles in signaling pathways and allow them to act as hubs for protein interaction networks [99]. It is likely that the ability to be involved in such protein-protein interactions allows the BAG domain of Bag-1L to exert a great impact on AR function. Since several steroid receptors have SLiMs-containing regions at their intrinsically disordered N-termini [100], it seems plausible that they could also be targeted by the BAG domain of Bag-1L. It is likely that they already account for the reported effects of Bag-1L on steroid receptor action [84-86].
Knockout mouse model of Bag-1
Bag-1 knockout mice are available for studies of how the deletion of this gene affects steroid hormone action. Regrettably, the Bag-1 null mice are lethal between embryonic days 12.5 and 13.5 (E12.5 and E13.5). Bag-1 is reported to have an essential role in the survival of differentiating neurons and hematopoietic cells [101]. Consequently, Bag-1 knockout mice display massive apoptosis in cells of the fetal liver and developing nervous system [101]. However, Bag-1 heterozygous mice are viable and show no difference in development, growth and body size compared to their wild-type counterparts. They also show no obvious defects in the endocrine system (our unpublished observations). The Bag-1 gene is separated by only 414 bp from Chmp 5, a gene that codes for charged multivesicular body protein 5. These two genes are encoded by different DNA strands and their 5’ ends are positioned head-to-head [102]. Targeted disruption of Bag-1 resulted in a double knockout, ablating the expression of both Bag-1 and of Chmp 5 (our unpublished data). The Chmp 5 knockout, like the Bag-1 knockout, is embryonic [102]. This double knockout has hampered analysis of the contribution of Bag-1 to steroid receptor action in the mouse. A new strategy for Bag-1 knockout mice (Bag1tm1a(EUCOMM)Hmgu/Ics) has been generated by the European conditional mouse mutagenesis (EUCOMM) program. Future characterization of these mice will determine the contribution of Bag-1 to steroid receptor action.
Conclusion
Although the molecular chaperones Hsp70 and Hsp90 are present in both the cytoplasm and the nucleus, a clear role of these proteins in the regulation of steroid receptor function at the chromatin level has not been identified. Instead, unambiguous evidence exists that cochaperones that regulate the activity of these molecular chaperones, and are themselves recruited into the nucleus, where they modulate the transcriptional activities of the steroid receptors on chromatin. To date, two cochaperones have been characterized in this respect: p23 and Bag-1L. Both proteins belong to the family of cochaperones that do not interact with Hsp70 and Hsp90 through the classical TPR domains. Instead these proteins employ domains that are multifunctional and are also involved in chaperone-independent activities. This is particularly the case for Bag-1L, where in fact two domains have been identified for regulating cellular processes; the BAG domain that serves as a nucleotide exchange factor for Hsp70 and steroid receptor binding, and the GARRPR motif at its N-terminus that is essential for AR and ERα binding. It is currently unclear whether the two domains functionally cooperate in regulating steroid receptor action or if they act independent from one another. Similarly, p23 mutational and inhibitor studies have shown that the sequences it uses to bind Hsp90 are different from those for interaction with the AR. Combined, these findings therefore offer great opportunities for the discovery of small molecular-weight chemicals that can specifically target these interaction surfaces and thereby inhibit the steroid receptor action in a novel way without affecting the overall chaperone activity. Some interesting candidate compounds have already emerged. For example, 2-((2-(2,6-dimethylphenoxy)ethyl)thio)-1H-benzo[d]imidazole competes with the binding of the Bag-1L GARRPR motif to the AR [92]. Thio-2 binds to the BAG domain of Bag-1 [103] and Gedunin binds to the N-terminus of p23 [104]. In the latter two cases, the inhibitors are only “partially dissociated” compounds and therefore still inhibit Hsp70 and Hsp90 action, albeit weakly. Future detailed studies on how these cochaperones regulate steroid receptor activity independent of their activity through the molecular chaperones will open new avenues to the identification of more specific compounds that can produce a new generation of steroid receptor therapeutics.
Acknowledgements
This work was supported, in part, by grants from the Deutsche Krebshilfe (110237) to A. C. B. C. and from the National Cancer Institute (1P01CA163227) and (P50CA090381) to M. B.
Abbreviations:
- 17-AAG
17-Allylamino-17-demethoxygeldanamycin
- ADP
Adenosine diphosphate
- AR
Androgen receptor
- BF-3
Binding function-3
- ChIP
Chromatin immunoprecipitation
- ChIP-seq
Chromatin immunoprecipitation-sequencing
- ER
Estrogen receptor
- GA
Geldanamycin
- GILZ
Glucocorticoid-inducible leucine zipper
- GR
Glucocorticoid receptor
- GRE
Glucocorticoid response element
- HTC
Hepatoma cells
- KO
Knock out
- LBD
Ligand-binding domain
- MMTV
Mouse mammary tumor virus
- MR
Mineralocorticoid receptor
- NLS
Nuclear localization sequence
- PR
Progesterone receptor
- RXR
Retinoid X receptor
- SLiMs
short linear motifs
- SR
Steroid receptor
- TAT
Tyrosine aminotransferase
- TO
Tryptophan oxygenase
- TPR
Tetratricopeptide repeats
- UBQ
Ubiquitin-like domain
- VDR
Vitamin D receptor
- WT
Wild-type
Footnotes
The authors declare no conflicts of interest.
References
- 1.Hutchison KA, Dittmar KD, Pratt WB: All of the factors required for assembly of the glucocorticoid receptor into a functional heterocomplex with heat shock protein 90 are preassociated in a self-sufficient protein folding structure, a "foldosome". J Biol Chem 1994, 269:27894-27899. [PubMed] [Google Scholar]
- 2.Dittmar KD, Banach M, Galigniana MD, Pratt WB: The role of DnaJ-like proteins in glucocorticoid receptor.hsp90 heterocomplex assembly by the reconstituted hsp90.p60.hsp70 foldosome complex. J Biol Chem 1998, 273:7358-7366. 10.1074/jbc.273.13.7358 [DOI] [PubMed] [Google Scholar]
- 3.Pratt WB, Dittmar KD: Studies with Purified Chaperones Advance the Understanding of the Mechanism of Glucocorticoid Receptor-hsp90 Heterocomplex Assembly. Trends Endocrinol Metab 1998, 9:244-252. 10.1016/S1043-2760(98)00059-9 [DOI] [PubMed] [Google Scholar]
- 4.Wandinger S, Richter K, Buchner J: The Hsp90 chaperone machinery. J Biol Chem 2008, 283:18473-18477. 10.1074/jbc.R800007200 [DOI] [PubMed] [Google Scholar]
- 5.Toft DO: Recent Advances in the Study of hsp90 Structure and Mechanism of Action. Trends Endocrinol Metab 1998, 9:238-243. 10.1016/S1043-2760(98)00060-5 [DOI] [PubMed] [Google Scholar]
- 6.Kanelakis KC, Murphy PJ, Galigniana MD, Morishima Y, Takayama S, Reed JC, Toft DO, Pratt WB: Hsp70 interacting protein Hip does not affect glucocorticoid receptor folding by the hsp90-based chaperone machinery except to oppose the effect of BAG-1. Biochemistry 2000, 39:14314-14321. 10.1021/bi001671c [DOI] [PubMed] [Google Scholar]
- 7.Prapapanich V, Chen S, Toran EJ, Rimerman RA, Smith DF: Mutational analysis of the hsp70-interacting protein Hip. Mol Cell Biol 1996, 16:6200-6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pruitt KD, Brown GR, Hiatt SM, Thibaud-Nissen F, Astashyn A, Ermolaeva O, Farrell CM, Hart J, Landrum MJ, McGarvey KM, Murphy MR, O’Leary NA, Pujar S, Rajput B, Rangwala SH, Riddick LD, Shkeda A, Sun H, Tamez P, Tully RE, Wallin C, Webb D, Weber J, Wu W, DiCuccio M, Kitts P, Maglott DR, Murphy TD, Ostell JM: RefSeq: an update on mammalian reference sequences. Nucleic Acids Res 2014, 42:D756-763. 10.1093/nar/gkt1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith DF: Tetratricopeptide repeat cochaperones in steroid receptor complexes. Cell Stress Chaperones 2004, 9:109-121. 10.1379/CSC-31.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheufler C, Brinker A, Bourenkov G, Pegoraro S, Moroder L, Bartunik H, Hartl FU, Moarefi I: Structure of TPR domain-peptide complexes: critical elements in the assembly of the Hsp70-Hsp90 multichaperone machine. Cell 2000, 101:199-210. 10.1016/S0092-8674(00)80830-2 [DOI] [PubMed] [Google Scholar]
- 11.Liu FH, Wu SJ, Hu SM, Hsiao CD, Wang C: Specific interaction of the 70-kDa heat shock cognate protein with the tetratricopeptide repeats. J Biol Chem 1999, 274:34425-34432. 10.1074/jbc.274.48.34425 [DOI] [PubMed] [Google Scholar]
- 12.Echeverria PC, Picard D: Molecular chaperones, essential partners of steroid hormone receptors for activity and mobility. Biochim Biophys Acta 2010, 1803:641-649. [DOI] [PubMed]
- 13.Smith DF, Toft DO: Minireview: the intersection of steroid receptors with molecular chaperones: observations and questions. Mol Endocrinol 2008, 22:2229-2240. 10.1210/me.2008-0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKeen HD, McAlpine K, Valentine A, Quinn DJ, McClelland K, Byrne C, O’Rourke M, Young S, Scott CJ, McCarthy HO, Hirst DG, Robson T: A novel FK506-like binding protein interacts with the glucocorticoid receptor and regulates steroid receptor signaling. Endocrinology 2008, 149:5724-5734. 10.1210/en.2008-0168 [DOI] [PubMed] [Google Scholar]
- 15.Chadli A, Graham JD, Abel MG, Twila A, Gordon DF, Wood WM, Felts SJ, Horwitz KB, Toft D: GCUNC-45 is a novel regulator for the progesterone receptor/hsp90 chaperoning pathway. Mol Cell Biol 2006, 26:1722-1730. 10.1128/MCB.26.5.1722-1730.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchanan G, Ricciardelli C, Harris JM, Prescott J, Yu ZCL, Jia L, Butler LM, Marshall VR, Scher HI, Gerald WL, Coetzee GA, Tilley WD: Control of androgen receptor signaling in prostate cancer by the cochaperone small glutamine rich tetratricopeptide repeat containing protein alpha. Cancer Res 2007, 67:10087-10096. 10.1158/0008-5472.CAN-07-1646 [DOI] [PubMed] [Google Scholar]
- 17.Paul A, Garcia YA, Zierer B, Patwardhan C, Gutierrez O, Hildenbrand Z, Harris DC, Balsiger HA, Sivils JC, Johnson JL, Buchner J, Chadli A, Cox MB: The cochaperone SGTA (small glutamine-rich tetratricopeptide repeat-containing protein alpha) demonstrates regulatory specificity for the androgen, glucocorticoid and progesterone receptors. J Biol Chem 2014, 289:15297-15308. 10.1074/jbc.M113.535229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young JC, Obermann WM, Hartl FU: Specific binding of tetratricopeptide repeat proteins to the C-terminal 12-kDa domain of hsp90. J Biol Chem 1998, 273:18007-18010. 10.1074/jbc.273.29.18007 [DOI] [PubMed] [Google Scholar]
- 19.Taipale M, Tucker G, Peng J, Krykbaeva I, Lin Z-Y, Larsen B, Choi H, Berger B, Gingras A-C, Lindquist SA: Quantitative Chaperone Interaction Network Reveals the Architecture of Cellular Protein Homeostasis Pathways. Cell 2014, 158: 434–448. 10.1016/j.cell.2014.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirschke E, Goswami D, Southworth D, Griffin PR, Agard DA: Glucocorticoid Receptor Function Regulated by Coordinated Action of the Hsp90 and Hsp70 Chaperone Cycles. Cell 2014, 157: 1685–1697. 10.1016/j.cell.2014.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carrigan PE, Sikkink LA, Smith DF, Ramirez-Alvarado M: Domain:domain interactions within Hop, the Hsp70/Hsp90 organizing protein, are required for protein stability and structure. Protein Sci 2006, 15:522-532. 10.1110/ps.051810106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Southworth DR, Agard DA: Client-loading conformation of the Hsp90 molecular chaperone revealed in the cryo-EM structure of the human Hsp90:Hop complex. Mol Cell 2011, 42: 771–781. 10.1016/j.molcel.2011.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silverstein AM, Galigniana MD, Kanelakis KC, Radanyi C, Renoir JM, Pratt WB: Different regions of the immunophilin FKBP52 determine its association with the glucocorticoid receptor, hsp90, and cytoplasmic dynein. J Biol Chem 1999, 274:36980-36986. 10.1074/jbc.274.52.36980 [DOI] [PubMed] [Google Scholar]
- 24.Gallo LI, Ghini AA, Piwien PG, Galigniana MD: Differential recruitment of tetratricorpeptide repeat domain immunophilins to the mineralocorticoid receptor influences both heat-shock protein 90-dependent retrotransport and hormone-dependent transcriptional activity. Biochemistry 2007, 46:14044-14057. 10.1021/bi701372c [DOI] [PubMed] [Google Scholar]
- 25.Picard D, Khursheed B, Garabedian MJ, Fortin MG, Lindquist S, Yamamoto KR: Reduced levels of hsp90 compromise steroid receptor action in vivo. Nature 1990, 348: 166–168. 10.1038/348166a0 [DOI] [PubMed] [Google Scholar]
- 26.Hache RJG, Tse R, Reich T, Savory JGA, Lefebvre YA: Nucleocytoplasmic Trafficking of Steroid-free Glucocorticoid Receptor. J Biol Chem 1999, 274: 1432–1439. 10.1074/jbc.274.3.1432 [DOI] [PubMed] [Google Scholar]
- 27.King WJ, Greene GL: Monoclonal antibodies localize oestrogen receptor in the nuclei of target cells. Nature 1984, 307:745-747. 10.1038/307745a0 [DOI] [PubMed] [Google Scholar]
- 28.Perrot-Applanat M, Logeat F, Groyer-Picard MT, Milgrom E: Immunocytochemical study of mammalian progesterone receptor using monoclonal antibodies. Endocrinology 1985, 116:1473-1484. 10.1210/endo-116-4-1473 [DOI] [PubMed] [Google Scholar]
- 29.Jensen EV, Suzuki T, Kawashima T, Stumpf WE, Jungblut PW, DeSombre ER: A two-step mechanism for the interaction of estradiol with rat uterus. Proc Natl Acad Sci U S A 1968, 59:632-638. 10.1073/pnas.59.2.632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guiochon-Mantel A, Delabre K, Lescop P, Milgrom E: The Ernst Schering Poster Award. Intracellular traffic of steroid hormone receptors. J Steroid Biochem Mol Biol 1996, 56:3-9. 10.1016/0960-0760(95)00268-5 [DOI] [PubMed] [Google Scholar]
- 31.Pratt WB: The role of heat shock proteins in regulating the function, folding, and trafficking of the glucocorticoid receptor. J Biol Chem 1993, 268:21455-21458. [PubMed] [Google Scholar]
- 32.Echeverría PC, Mazaira G, Erlejman A, Gomez-Sanchez C, Piwien Pilipuk G, Galigniana MD: Nuclear import of the glucocorticoid receptor-hsp90 complex through the nuclear pore complex is mediated by its interaction with Nup62 and importin beta. Mol Cell Biol 2009, 29:4788-4797. 10.1128/MCB.00649-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Echeverría PC, Bernthaler A, Dupuis P, Mayer B, Picard D: An interaction network predicted from public data as a discovery tool: application to the Hsp90 molecular chaperone machine. PLoS One 2011, 6:e26044. 10.1371/journal.pone.0026044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grossmann C, Ruhs S, Langenbruch L, Mildenberger S, Strätz N, Schumann K, Gekle M: Nuclear shuttling precedes dimerization in mineralocorticoid receptor signaling. Chem Biol 2012, 19:742-751. 10.1016/j.chembiol.2012.04.014 [DOI] [PubMed] [Google Scholar]
- 35.Galigniana MD: Steroid receptor coupling becomes nuclear. Chem Biol 2012, 19:662-663. 10.1016/j.chembiol.2012.06.001 [DOI] [PubMed] [Google Scholar]
- 36.Galigniana MD, Scruggs JL, Herrington J, Welsh MJ, Carter-Su C, Housley PR, Pratt WB: Heat shock protein 90-dependent (geldanamycin-inhibited) movement of the glucocorticoid receptor through the cytoplasm to the nucleus requires intact cytoskeleton. Mol Endocrinol 1998, 12:1903-1913. 10.1210/mend.12.12.0204 [DOI] [PubMed] [Google Scholar]
- 37.Elbi C, Walker DA, Romero G, Sullivan WP, Toft DO, Hager GL, DeFranco DB: Molecular chaperones function as steroid receptor nuclear mobility factors. Proc Natl Acad Sci U S A 2004, 101:2876-2881. 10.1073/pnas.0400116101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Czar MJ, Lyons RH, Welsh MJ, Renoir JM, Pratt WB: Evidence that FK506-binding immunophilin heat shock protein 56 is required for the trafficking of the glucocorticoid receptor from the cytoplasm to the nucleus. Mol Endocrinol 1995, 9:1549-1560. [DOI] [PubMed] [Google Scholar]
- 39.Galigniana MD, Radanyi C, Renoir JM, Housley PR, Pratt WB: Evidence that the peptidylprolyl isomerase domain of the hsp90-binding immunophilin FKBP52 is involved in both dynein interaction and glucocorticoid receptor movement to the nucleus. J Biol Chem 2001, 276:14884-14889. 10.1074/jbc.M010809200 [DOI] [PubMed] [Google Scholar]
- 40.Wochnik GM, Rüegg J, Abel GA, Schmidt U, Holsboer F, Rein T: FK506-binding proteins 51 and 52 differentially regulate dynein interaction and nuclear translocation of the glucocorticoid receptor in mammalian cells. J Biol Chem 2005, 280:4609-4616. 10.1074/jbc.M407498200 [DOI] [PubMed] [Google Scholar]
- 41.Pratt WB, Silverstein AM, Galigniana MD: A model for the cytoplasmic trafficking of signalling proteins involving the hsp90-binding immunophilins and p50 cdc37. Cell Signal 1999, 11:839-851. 10.1016/S0898-6568(99)00064-9 [DOI] [PubMed] [Google Scholar]
- 42.Galigniana MD, Erlejman AG, Monte M, Gomez-Sanchez C, Piwien-Pilipuk G: The hsp90-FKBP52 complex links the mineralocorticoid receptor to motor proteins and persists bound to the receptor in early nuclear events. Mol Cell Biol 2010, 30:1285-1298. 10.1128/MCB.01190-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davies TH, Ning YM, Sánchez ER: A new first step in activation of steroid receptors: hormone-induced switching of FKBP51 and FKBP52 immunophilins. J Biol Chem 2002, 277:4597-4600. 10.1074/jbc.C100531200 [DOI] [PubMed] [Google Scholar]
- 44.Yong W, Yang Z, Periyasamy S, Chen H, Yucel S, Li W, Lin LY, Wolf IM, Cohn MJ, Baskin LS, Sánchez ER, Shou W: Essential role for Co-chaperone Fkbp52 but not Fkbp51 in androgen receptor-mediated signaling and physiology. J Biol Chem. 2007, 282:5026-5036. 10.1074/jbc.M609360200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolf IM, Periyasamy S, Hinds T, Jr, Yong W, Shou W, Sanchez ER: Targeted ablation reveals a novel role of FKBP52 in gene-specific regulation of glucocorticoid receptor transcriptional activity. J Steroid Biochem Mol Biol 2009, 113:36-45. 10.1016/j.jsbmb.2008.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tranguch S, Cheung-Flynn J, Daikoku T, Prapapanich V, Cox MB, Xie H, Wang H, Das SK, Smith DF, Dey SK: Cochaperone immunophilin FKBP52 is critical to uterine receptivity for embryo implantation. Proc Natl Acad Sci U S A 2005, 102:14326-14331. 10.1073/pnas.0505775102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheung-Flynn J, Prapapanich V, Cox MB, Riggs DL, Suarez-Quian C, Smith DF: Physiological role for the cochaperone FKBP52 in androgen receptor signaling. Mol Endocrinol 2005, 19:1654-1666. 10.1210/me.2005-0071 [DOI] [PubMed] [Google Scholar]
- 48.Yang Z, Wolf IM, Chen H, Periyasamy S, Chen Z, Yong W, Shi S, Zhao W, Xu J, Srivastava A, Sánchez ER, Shou W: FK506-binding protein 52 is essential to uterine reproductive physiology controlled by the progesterone receptor A isoform. Mol Endocrinol 2006, 20:2682-2694. 10.1210/me.2006-0024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sabbah M, Radanyi C, Redeuilh G, Baulieu EE: The 90 kDa heat-shock protein (hsp90) modulates the binding of the oestrogen receptor to its cognate DNA. Biochem J 1996, 314:205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thackray VG, Toft DO, Nordeen SK: Novel activation step required for transcriptional competence of progesterone receptor on chromatin templates. Mol Endocrinol 2003, 17:2543-2553. 10.1210/me.2003-0200 [DOI] [PubMed] [Google Scholar]
- 51.Felts SJ, Toft DO: P23, a simple protein with complex activities. Cell Stress Chaperones 2003, 8:108-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ali MM, Roe SM, Vaughan CK, Meyer P, Panaretou B, Piper PW, Prodromou C, Pearl LH: Crystal structure of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature 2006, 440:1013-1017. 10.1038/nature04716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bose S, Weikl T, Bügl H, Buchner J: Chaperone function of Hsp90-associated proteins. Science 1996, 274:1715-1717. 10.1126/science.274.5293.1715 [DOI] [PubMed] [Google Scholar]
- 54.Echtenkamp FJ, Zelin E, Oxelmark E, Woo JI, Andrews BJ, Garabedian M, Freeman BC: Global functional map of the p23 molecular chaperone reveals an extensive cellular network. Mol Cell 2011, 43:229-241. 10.1016/j.molcel.2011.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zelin E, Zhang Y, Toogun OA, Zhong S, Freeman BC: The p23 molecular chaperone and GCN5 acetylase jointly modulate protein-DNA dynamics and open chromatin status. Mol Cell 2012, 48:459-470. 10.1016/j.molcel.2012.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Freeman BC, Yamamoto KR: Continuous recycling: a mechanism for modulatory signal transduction. Trends Biochem Sci 2001, 26:285-290. 10.1016/S0968-0004(01)01834-5 [DOI] [PubMed] [Google Scholar]
- 57.Freeman BC, Yamamoto KR: Disassembly of transcriptional regulatory complexes by molecular chaperones. Science 2002, 296:2232-2235. 10.1126/science.1073051 [DOI] [PubMed] [Google Scholar]
- 58.Stavreva DA, Müller WG, Hager GL, Smith CL, McNally JG: Rapid glucocorticoid receptor exchange at a promoter is coupled to transcription and regulated by chaperones and proteasomes. Mol Cell Biol 2004, 24:2682-2697. 10.1128/MCB.24.7.2682-2697.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith DF, Whitesell L, Nair SC, Chen S, Prapapanich V, Rimerman RA: Progesterone receptor structure and function altered by geldanamycin, an hsp90-binding agent. Mol Cell Biol 1995, 15:6804-6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stavreva D, Wiench M, John S, Conway-Campbell B, McKenna M, Pooley J, Johnson T, Voss T, Lightman S, Hager G: Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nat Cell Biol 2009, 11:1093-1102. 10.1038/ncb1922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Conway-Campbell BL, George CL, Pooley JR, Knight DM, Norman MR, Hager GL, Lightman SL: The HSP90 molecular chaperone cycle regulates cyclical transcriptional dynamics of the glucocorticoid receptor and its coregulatory molecules CBP/p300 during ultradian ligand treatment. Mol Endocrinol 2011, 25:944-954. 10.1210/me.2010-0073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Métivier R, Penot G, Hübner MR, Reid G, Brand H, Kos M, Gannon F: Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 2003, 115:751-763. 10.1016/S0092-8674(03)00934-6 [DOI] [PubMed] [Google Scholar]
- 63.Kang Z, Pirskanen A, Jänne OA, Palvimo JJ: Involvement of proteasome in the dynamic assembly of the androgen receptor transcription complex. J Biol Chem 2002, 277:48366-48371. 10.1074/jbc.M209074200 [DOI] [PubMed] [Google Scholar]
- 64.Simpson NE, Gertz J, Imberg K, Myers RM, Garabedian MJ: Research resource: enhanced genome-wide occupancy of estrogen receptor α by the cochaperone p23 in breast cancer cells. Mol Endocrinol 2012, 26:194-202. 10.1210/me.2011-1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reebye V, Cano L, Lavery D, Brooke G, Powell S, Chotai D, Walker M, Whitaker H, Wait R, Hurst H, Bevan C: Role of the HSP90-associated cochaperone p23 in enhancing activity of the androgen receptor and significance for prostate cancer. Mol Endocrinol 2012, 26:1694-1706. 10.1210/me.2012-1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grad I, McKee TA, Ludwig SM, Hoyle GW, Ruiz P, Wurst W, Floss T, Miller CA, Picard D: The Hsp90 cochaperone p23 is essential for perinatal survival. Mol Cell Biol 2006, 26:8976-8983. 10.1128/MCB.00734-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Grier DG, Halliday HL: Effects of glucocorticoids on fetal and neonatal lung development. Treat Respir Med 2004, 3:295-306. 10.2165/00151829-200403050-00004 [DOI] [PubMed] [Google Scholar]
- 68.Aszterbaum M, Feingold KR, Menon GK, Williams ML: Glucocorticoids accelerate fetal maturation of the epidermal permeability barrier in the rat. J Clin Invest 1993, 91:2703-2708. 10.1172/JCI116509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hanley K, Feingold KR, Kömüves LG, Elias PM, Muglia LJ, Majzoub JA, Williams ML: Glucocorticoid deficiency delays stratum corneum maturation in the fetal mouse. J Inves Dermatol 1998, 111:440-444. 10.1046/j.1523-1747.1998.00303.x [DOI] [PubMed] [Google Scholar]
- 70.Yang X, Chernenko G, Hao Y, Ding Z, Pater MM, Pater A, Tang SC: Human BAG-1/RAP46 protein is generated as four isoforms by alternative translation initiation and overexpressed in cancer cells. Oncogene 1998, 17:981-989. 10.1038/sj.onc.1202032 [DOI] [PubMed] [Google Scholar]
- 71.Takayama S, Krajewski S, Krajewska M, Kitada S, Zapata JM, Kochel K, Knee D, Scudiero D, Tudor G, Miller GJ, Miyashita T, Yamada M, Reed JC: Expression and location of Hsp70/Hsc-binding anti-apoptotic protein BAG-1 and its variants in normal tissues and tumor cell lines. Cancer Res 1998, 58:3116-3131. [PubMed] [Google Scholar]
- 72.Kriegenburg F, Jakopec V, Poulsen EG, Nielsen SV, Roguev A, Krogan N, Gordon C, Fleig U, Hartmann-Petersen R: A chaperone-assisted degradation pathway targets kinetochore proteins to ensure genome stability. PLoS Genet 2014, 10:e1004140. 10.1371/journal.pgen.1004140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Corpet F: Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 1988, 16:10881-10890. 10.1093/nar/16.22.10881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brehmer D, Rudiger S, Gassler CS, Klostermeier D, Packschies L, Reinstein J, Mayer MP, Bukau B: Tuning of chaperone activity of Hsp70 proteins by modulation of nucleotide exchange. Nat Struct Biol 2001, 8:427-432. 10.1038/87588 [DOI] [PubMed] [Google Scholar]
- 75.Sondermann H, Scheufler C, Schneider C, Hohfeld J, Hartl FU, Moarefi I: Structure of a Bag/Hsc70 complex: convergent functional evolution of Hsp70 nucleotide exchange factors. Science 2001, 291:1553-1557. 10.1126/science.1057268 [DOI] [PubMed] [Google Scholar]
- 76.Alberti S, Esser C, Hohfeld J: BAG-1 - a nucleaotide exchange factor of Hsc70 with multiple cellular functions. Cell Stress Chaperones 2003, 8: 225-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lüders J, Demand J, Höhfeld J: The ubiquitin-related BAG-1 provides a link between the molecular chaperones Hsc70/Hsp70 and the proteasome. J Biol Chem 2000, 275:4613-4617. 10.1074/jbc.275.7.4613 [DOI] [PubMed] [Google Scholar]
- 78.Demand J, Alberti S, Patterson C, Höhfeld J: Cooperation of a ubiquitin domain protein and an E3 ubiquitin ligase during chaperone/proteasome coupling. Curr Biol 2001, 11:1569-1577. 10.1016/S0960-9822(01)00487-0 [DOI] [PubMed] [Google Scholar]
- 79.Cato AC, Mink S: BAG-1 family of cochaperones in the modulation of nuclear receptor action. J Steroid Biochem Mol Biol 2001, 78:379-388. 10.1016/S0960-0760(01)00114-5 [DOI] [PubMed] [Google Scholar]
- 80.Kanelakis KC, Morishima Y, Dittmar KD, Galigniana MD, Takayama S, Reed JC, Pratt WB: Differential effects of the hsp70-binding protein BAG-1 on glucocorticoid receptor folding by the hsp90-based chaperone machinery. J Biol Chem 1999, 274:34134-34140. 10.1074/jbc.274.48.34134 [DOI] [PubMed] [Google Scholar]
- 81.Niyaz Y, Zeiner M, Gehring U: Transcriptional activation by the human Hsp70-associating protein Hap50. J Cell Sci 2001, 114:1839-1845. [DOI] [PubMed] [Google Scholar]
- 82.Kriegenburg F, Jakopec V, Poulsen EG, Nielsen SV, Roguev A, Krogan N, Gordon C, Fleig U, Hartmann-Petersen R: A chaperone-assisted degradation pathway targets kinetochore proteins to ensure genome stability. PLoS Genet 2014, 10:e1004140. 10.1371/journal.pgen.1004140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Knapp RT, Steiner A, Schmidt U, Hafner K, Holsboer F, Rein T: BAG-1 diversely affects steroid receptor activity. Biochem J 2012, 441:297-303. 10.1042/BJ20111456 [DOI] [PubMed] [Google Scholar]
- 84.Froesch BA, Takayama S, Reed JC: BAG-1L protein enhances androgen receptor function. J Biol Chem 1998, 273:11660-11666. 10.1074/jbc.273.19.11660 [DOI] [PubMed] [Google Scholar]
- 85.Cutress RI, Townsend PA, Sharp A, Maison A, Wood L, Lee R, Brimmell M, Mullee MA, Johnson PWM, Royle GT, Bateman AC, Packham G: The nuclear BAG-1 isoform, BAG-1L, enhances oestrogen-dependent transcription. Oncogene 2003, 22:4973-4982. 10.1038/sj.onc.1206688 [DOI] [PubMed] [Google Scholar]
- 86.Lee SS, Crabb SJ, Janghra N, Carlberg C, Williams AC, Cutress RI, Packham G, Hague A: Subcellular localisation of BAG-1 and its regulation of vitamin D receptor-mediated transactivation and involucrin expression in oral keratinocytes: implications for oral carcinogenesis. Exp Cell Res 2007, 313:3222-3238. 10.1016/j.yexcr.2007.06.010 [DOI] [PubMed] [Google Scholar]
- 87.Knee DA, Froesch BA, Nuber U, Takayama S, Reed JC: Structure-function analysis of Bag1 proteins. Effects on androgen receptor transcriptional activity. J Biol Chem 2001, 276:12718-12724. 10.1074/jbc.M010841200 [DOI] [PubMed] [Google Scholar]
- 88.Shatkina L, Mink S, Rogatsch H, Klocker H, Langer G, Nestl A, Cato AC: The cochaperone Bag-1L enhances androgen receptor action via interaction with the NH2-terminal region of the receptor. Mol Cell Biol 2003, 23:7189-7197. 10.1128/MCB.23.20.7189-7197.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Witcher M, Yang X, Pater A, Tang SC: BAG-1 p50 isoform interacts with the vitamin D receptor and its cellular overexpression inhibits the vitamin D pathway. Exp Cell Res 2001, 265:167-173. 10.1006/excr.2001.5176 [DOI] [PubMed] [Google Scholar]
- 90.Zeiner M, Niyaz Y, Gehring U: The hsp70-associating protein Hap46 binds to DNA and stimulates transcription. Proc Natl Acad Sci U S A 1999, 96:10194-10199. 10.1073/pnas.96.18.10194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schmidt U, Wochnik GM, Rosenhagen MC, Young JC, Hartl FU, Holsboer F, Rein T: Essential role of the unusual DNA-binding motif of BAG-1 for inhibition of the glucocorticoid receptor. J Biol Chem 2003, 278:4926-4931. 10.1074/jbc.M212000200 [DOI] [PubMed] [Google Scholar]
- 92.Jehle K, Cato L, Neeb A, Muhle-Goll C, Jung N, Smith E, Buzon V, Carbo L, Estebanez-Perpina E, Schmitz K, Fruk L, Luy B, Chen Y, Cox M, Brase S, Brown M, Cato A: Coregulator control of androgen receptor action by a novel nuclear receptor-binding motif. J Biol Chem 2014, 289:8839-8851. 10.1074/jbc.M113.534859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Takayama S, Reed JC: Molecular chaperone targeting and regulation by BAG family proteins. Nat Cell Biol 2001, 3:E237-241. 10.1038/ncb1001-e237 [DOI] [PubMed] [Google Scholar]
- 94.Buzon V, Carbo LR, Estruch SB, Fletterick RJ, Estebanez-Perpina E: A conserved surface on the ligand binding domain of nuclear receptors for allosteric control. Mol Cell Endocrinol 2012, 348:394-402. 10.1016/j.mce.2011.08.012 [DOI] [PubMed] [Google Scholar]
- 95.De Leon JT, Iwai A, Feau C, Garcia Y, Balsiger HA, Storer CL, Suro RM, Garza KM, Lee S, Kim YS, Chen Y, Ning YM, Riggs DL, Fletterick RJ, Guy RK, Trepel JB, Neckers LM, Cox MB: Targeting the regulation of androgen receptor signaling by the heat shock protein 90 cochaperone FKBP52 in prostate cancer cells. Proc Natl Acad Sci U S A 2011, 108:11878-11883. 10.1073/pnas.1105160108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Riggs DL, Cox MB, Tardif HL, Hessling M, Buchner J, Smith DF: Noncatalytic role of the FKBP52 peptidyl-prolyl isomerase domain in the regulation of steroid hormone signaling. Mol Cell Biol 2007, 27:8658-8669. 10.1128/MCB.00985-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Briknarova K, Takayama S, Brive L, Havert ML, Knee DA, Velasco J, Homma S, Cabezas E, Stuart J, Hoyt DW, Satterthwait AC, Llinas M, Reed JC, Ely KR: Structural analysis of BAG1 cochaperone and its interactions with Hsc70 heat shock protein. Nat Struct Biol 2001, 8:349-352. 10.1038/86236 [DOI] [PubMed] [Google Scholar]
- 98.Davey NE, Van Roey K, Weatheritt RJ, Toedt G, Uyar B, Altenberg B, Budd A, Diella F, Dinkel H, Gibson TJ: Attributes of short linear motifs. Mol Biosyst 2012, 8:268-281. 10.1039/c1mb05231d [DOI] [PubMed] [Google Scholar]
- 99.Wright PE, Dyson HJ: Linking folding and binding. Curr Opin Struct Biol 2009, 19:31-38. 10.1016/j.sbi.2008.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xue R, Zakharov MN, Xia Y, Bhasin S, Costello JC, Jasuja R: Research resource: EPSLiM: ensemble predictor for short linear motifs in nuclear hormone receptors. Mol Endocrinol 2014, 28:768-777. 10.1210/me.2014-1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Götz R, Wiese S, Takayama S, Camarero GC, Rossoll W, Schweizer U, Troppmair J, Jablonka S, Holtmann B, Reed JC, Rapp UR, Sendtner M: Bag1 is essential for differentiation and survival of hematopoietic and neuronal cells. Nat Neurosci 2005, 8:1169-1178. 10.1038/nn1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shim JH, Xiao C, Hayden MS, Lee KY, Trombetta ES, Pypaert M, Nara A, Yoshimori T, Wilm B, Erdjument-Bromage H, Tempst P, Hogan BLM, Mellman I, Ghosh S: CHMP5 is essential for late endosome function and down-regulation of receptor signaling during mouse embryogenesis. J Cell Biol 2006, 172:1045-1056. 10.1083/jcb.200509041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Enthammer M, Papadakis ES, Gachet MS, Deutsch M, Schwaiger S, Koziel K, Ashraf MI, Khalid S, Wolber G, Packham G, Cutress RI, Stuppner H, Troppmair J: Isolation of a novel thioflavin S-derived compound that inhibits BAG-1-mediated protein interactions and targets BRAF inhibitor-resistant cell lines. Mol Cancer Ther 2013, 12:2400-2414. 10.1158/1535-7163.MCT-13-0142 [DOI] [PubMed] [Google Scholar]
- 104.Patwardhan CA, Fauq A, Peterson LB, Miller C, Blagg BSJ, Chadli A: Gedunin inactivates the co-chaperone p23 protein causing cancer cell death by apoptosis. J Biol Chem 2013, 288:7313-7325. 10.1074/jbc.M112.427328 [DOI] [PMC free article] [PubMed] [Google Scholar]