Abstract
• Background and Aims Salvia divinorum produces several closely related neoclerodane diterpenes. The most abundant of these, salvinorin A, is responsible for the psychoactive properties of the plant. To determine where these compounds occur in the plant, various organs, tissues and glandular secretions were chemically analysed. A microscopic survey of the S. divinorum plant was performed to examine the various types of trichomes present and to determine their distribution.
• Methods Chemical analyses were performed using thin layer chromatographic and histochemical techniques. Trichomes were examined using conventional light microscopy and scanning electron microscopy.
• Key Results It was found that neoclerodane diterpenes are secreted as components of a resin that accumulates in peltate glandular trichomes, specifically in the subcuticular space that exists between the trichome head cells and the cuticle that encloses them. Four main types of trichomes were observed: peltate glandular trichomes, short‐stalked capitate glandular trichomes, long‐stalked capitate glandular trichomes and non‐glandular trichomes. Their morphology and distribution is described. Peltate glandular trichomes were only found on the abaxial surfaces of the leaves, stems, rachises, bracts, pedicles and calyces. This was consistent with chemical analyses, which showed the presence of neoclerodane diterpenes in these organs, but not in parts of the plant where peltate glandular trichomes are absent.
• Conclusions Salvinorin A and related compounds are secreted as components of a complex resin that accumulates in the subcuticular space of peltate glandular trichomes.
Key words: Salvia divinorum, Labiatae, diviner’s sage, salvinorin A, salvinorins, neoclerodane diterpenes, trichomes, thin layer chromatography, histochemistry, morphology
INTRODUCTION
Salvia divinorum (Labiatae) is a powerful psychoactive herb (Fig. 1). It is endemic to a relatively small region in the mountainous north‐eastern corner of the Mexican state of Oaxaca. The Mazatec Indians that inhabit the area cultivate the plant and use its leaves in medico‐religious ceremonies. When ingested in the traditional manner, it produces an altered state of awareness that is deeply introspective in nature. Depending on a person’s sensitivity and the amount consumed, the effects range from subtle to profound. When the effects are intense, people often experience extraordinary dream‐like visions. Mazatec shamans reportedly utilize these visionary states to venture into supernatural realms and to contact divine entities, whose aid they often petition to remedy the complaints of their clients. They also use the herb remedially at sub‐visionary doses to treat a variety of conditions. These include arthritis, headache and eliminatory complaints (Wasson, 1962; Valdés et al., 1983).

Fig. 1.Salvia divinorum plant in bloom. This specimen was photographed at Cerro Camarón, in the Mazatec region of Oaxaca, Mexico.
The species has several distinctive morphological characteristics that, taken together, make it possible to identify flowering specimens with relative ease. A detailed description of the morphology and distribution of its trichomes is presented here. Other aspects of the plant’s anatomy have previously been described elsewhere (Epling and Játiva‐M., 1962; Valdés, 1983; Reisfield, 1987, 1993).
The psychoactive principle of the plant has been identified as the neoclerodane diterpene salvinorin A (Siebert, 1994). Its structure (Fig. 2) was characterized by two independent research groups working concurrently (Ortega et al., 1982; Valdés, 1983; Valdés et al., 1984; Koreeda et al., 1990). This compound has been identified as an extremely potent and highly selective kappa‐opioid receptor agonist. Consequently, it has attracted much attention from pharmacologists. It is the first naturally occurring, non‐nitrogenous, opioid‐receptor‐subtype‐selective agonist discovered (Roth et al., 2002; Sheffler and Roth, 2003; Chavkin et al., 2004). The potential of salvinorin A as an antidepressant medication is being explored (Hanes, 2001, 2003).
Fig. 2. Structural diagrams of salvinorins A–F, divinatorins A–C and (–)‐hardwickiic acid.
The concentration of salvinorin A in leaves at various stages of development on an individual plant is often remarkably consistent. However, leaves collected from separate plants can vary considerably, even when they are genetically identical. The specific growing conditions and environmental factors that influence salvinorin A content have not been identified. In a previous study, quantitative analysis of 20 dry leaf samples, each collected from separate plants, showed salvinorin A contents that ranged from 0·089 to 0·37 %, with most samples being close to the total average of 0·245 %. Whole stem tissue was also analysed, but the amount of salvinorin A detected was too small to accurately quantify. The concentration in the stems was roughly estimated to be approx. 4 % of the level typically found in leaves (Gruber et al., 1999). This compound is quite stable under ordinary storage conditions. In an analysis performed by the author, the salvinorin A content of a leaf obtained from a 40‐year‐old herbarium specimen was found to be substantially similar to that typically found in freshly harvested leaves.
The closely related compounds, salvinorin B, salvinorin C, salvinorin D, salvinorin E, salvinorin F, divinatorin A, divinatorin B, divinatorin C and (–)‐hardwickiic acid (Fig. 2) also occur in the plant, but at lower concentrations than salvinorin A (Valdés et al., 1984; Valdés et al., 2001; Bigham et al., 2003; Munro and Rizzacasa, 2003). Salvinorin B, salvinorin C and salvinorin D are the most abundant of these other compounds. The concentrations of salvinorin E, salvinorin F, divinatorin A, divinatorin B, divinatorin C and (–)‐hardwickiic acid are considerably lower. The salvinorins and divinatorins are thus far only known to occur in S. divinorum. No other natural source has been identified. (–)‐Hardwickiic acid, which is structurally similar to the divinatorins, has been identified in many other species. Several other terpenoid compounds have been detected in the plant; some of these have been isolated and structurally characterized (Stewart et al., 2001; Bigham et al., 2003). Receptor assays have demonstrated that salvinorin B, salvinorin D and salvinorin E are inactive at kappa‐opioid receptors (Chavkin et al., 2004; B. L. Roth and T. A. Munro, pers. comm.). Bioassays of salvinorin B in mice indicate that it is pharmacologically inactive (Valdés et al., 1984). Self‐experiments performed by the author have also shown this compound to be inactive. Receptor assays of salvinorin C are in progress. Preliminary results suggests that it might be weakly active at kappa‐opioid receptors, but certainly much less active than salvinorin A, and possibly inactive (B. L. Roth and T. A. Munro, pers. comm.). Self‐experiments performed by the author indicate that salvinorin Cis not psychoactive.
Most labiates have glandular trichomes that emerge from the epidermal surface of their leaves, stems and reproductive structures. These glands, which are often microscopic, secrete various types of compounds. Terpenes usually constitute the major lipophilic components of these secretions. A growing body of experimental evidence shows that terpene biosynthesis also takes place within these trichomes (Croteau and Johnson, 1984; Hay and Svoboda, 1993; Duke et al., 2000; Hallahan, 2000). In the Labiatae, glandular trichomes are generally classified as either capitate (clavate) or peltate (subsessile), based on morphological characteristics (Fahn, 2000). The compounds secreted by capitate glandular trichomes are mostly excreted to the surrounding environment, apparently through pores in the cuticle of the head cell(s). Whereas in peltate glandular trichomes, the secretions accumulate in a capacious subcuticular space that is formed by the separation of the head cell walls from the cuticular dome that encloses them, and they remain there until the cuticle is physically ruptured. Thus, peltate glandular trichomes function as repositories for the specialized phytochemicals that they secrete (Hay and Svoboda, 1993). The epidermal localization of peltate glandular trichomes allows for three approaches to selective extraction of the compounds they secrete: selective solvent extraction, isolation of the trichomes from the plant, and mechanical removal of material from the subcuticular space in situ. All three approaches were utilized in the experiments reported here.
The investigations described herein are motivated by interest in the pharmacological properties of the species. The identification of structures responsible for salvinorin production and accumulation is useful from an economic standpoint because it can be used to develop strategies for maximizing yields of useful compounds. This information is also meaningful from an ecological perspective because it can help us better understand what role these compounds may have in the plant’s ecology, and may lead to a better understanding of natural plant protection.
MATERIALS AND METHODS
Plant material
Most of the plant material used in these experiments was asexually propagated (as stem cuttings) from a specimen of S. divinorum Epling and Játiva‐M. collected in 1962 by Sterling Bunnell in Huautla de Jiménez, Oaxaca, Mexico. A herbarium specimen of the originally collected material is deposited in the University Herbarium of the University of California, Berkeley. Cotyledons were harvested from seedlings that were germinated from seeds obtained from plants grown on the north side of the Hawaiian island of Oahu. The parent plants had been asexually propagated from a plant collected near Llano de Arnica, Oaxaca, in 1991, by anthropologist Bret Blosser. Additional specimens were obtained from other introductions, and these were examined separately. In most respects, the specimens were found to be chemically and morphologically indistinguishable from each other.
Microscopy
The morphology and distribution of the various types of trichomes on the plant was determined by carefully examining each part using a conventional light microscope. To improve the visibility of peltate glandular trichomes, many of the live tissue samples were dipped briefly (approx. 2 s) in chloroform—these trichomes turn milky‐white as the solvent evaporates. Interestingly, when this process was used on extremely young leaves the peltate glandular trichomes became iridescent. Scanning electron microscopy (SEM) was used to examine the trichomes of selected regions in greater detail. The SEM images were produced by Michael Dunlap at the Chemical Engineering and Materials Sciences Central Facilities Electromicroscopy Laboratary of the University of California, Davis.
Sequential extraction of fresh leaves
Four beakers, each containing 200 mL of room temperature reagent grade chloroform, were arranged in a row. Two freshly harvested, fully developed S. divinorum leaves were immersed for 30 s in the first beaker. The leaves were held by the petioles, which were not immersed, and very gently agitated throughout the immersion period. The leaves were removed and then immediately immersed for 30 s in the second beaker. Once again, the leaves were removed and then immersed for another 30 s in the third beaker. The procedure was repeated with additional pairs of leaves until a total of ten medium‐sized leaves had been dipped three times (16·5 g total f. wt). Following the three dips, the leaves were dried and powdered so that they could be extracted more thoroughly. The leaf powder was then added to the chloroform in the fourth beaker, and this was stirred occasionally over the course of 2 h. This final extraction was performed to determine the remaining salvinorin content of the leaves, and to thus assess the effectiveness of the three preceding 30‐s extractions. The relative concentration of salvinorins in each of the four beakers was determined by spotting a TLC plate with volumetrically equal samples from each beaker.
Collection of glandular secretions from the epidermal surfaces of leaves and stems
Glandular secretions were collected from both the abaxial and adaxial surfaces of fresh leaves and from the abaxial stem surface using chloroform‐soaked cotton swabs. The swabs were gently brushed over the epidermis, with care being taken not to damage the epidermal surface. A glass capillary tube was used to collect fluid from the swabs, and this was then spotted onto a TLC plate.
Extraction of various parts of the plant
Individual extracts were made from the roots, external stem tissue, internal stem tissue, internal petiole tissue, leaves, cotyledons, rachises, bracts, pedicles, calyces and corollas. These were prepared by placing fresh specimens of each plant part into a separate vial of room temperature chloroform. Each specimen weighed 100 mg and each vial contained 1 mL of chloroform. The vials were capped and then shaken several times over a 30‐min period. Samples of each extract were spotted onto TLC plates.
Isolation of peltate glandular trichomes from the abaxial leaf surface
Clear adhesive carton sealing tape was used to remove leaf trichomes. The tape used was manufactured by Manco® Inc. (Avon, OH, USA) and is marketed under the brand name, ‘High Performance Crystal Clear’. The adhesive was first tested to determine that it was not alcohol soluble and did not contain chemicals that would react with the vanillin reagent used to visualize terpenes in the chromatograms of the isolated trichome samples.
A fully developed leaf, 16 cm in length, was divided into two equal halves by cutting away the central rib. One of the halves was laid on a flat surface with the abaxial surface facing up. Adhesive tape was placed over the abaxial surface, gently rubbed and then removed. Although short‐stalked capitate glandular trichomes are also present on this surface, the adhesive mostly adhered to the peltate glandular trichomes because they are larger and extend out farther from the leaf surface. The adhesive also removed non‐glandular trichomes from the veins, where they are confined. Care was taken not to press the tape too hard, both to minimize removal of capitate glandular trichomes and to avoid removing any of the epidermis. Viewing the tape under a light microscope revealed that the adhesive had effectively removed about 50 % of the peltate glandular trichomes. Areas of the trichome‐containing adhesive that corresponded to the intervenous areas of the leaf were then swabbed with ethanol using a fine‐point polyester foam swab. The samples were taken from the intervenous areas because here peltate glandular trichomes were the main type removed. A TLC plate was spotted using the tip of the swab. The reserved leaf half and the half that had the trichomes partially removed were dried separately and powdered. A 70 mg portion of the powder from each half was extracted into 0·3 mL of room temperature chloroform for 30 min, with occasional agitation. A TLC plate was spotted with volumetrically equal samples (0·5 µL) of both extracts.
Removal of secretory products from the subcuticular reservoirs of individual peltate glandular trichomes
A 1 × 3 cm section was cut from a fresh leaf. This was quickly dipped in chloroform (approx. 2 s) and then secured to a microscope slide with the abaxial surface facing up. The specimen was placed on the stage of a light microscope with the magnification set to ×100. A no. 10 beading needle was used to pierce the cuticles of 50 peltate glandular trichomes. When pierced, the fragile cuticle bursts open and some of the subcuticular contents adheres to the needle. Following each five piercings, the needle was dipped into a small vial containing 0·1 mL chloroform. In this way, the collected material was transferred into the solvent. This was then spotted onto a TLC plate.
Extraction of leaves at six different stages of development
Two sets of leaves, each consisting of six pairs of primary leaves, were harvested from two separate stalks, each from a separate S. divinorum plant. These were labelled ‘set A’ and ‘set B’. The leaves were harvested from the six uppermost nodes of the two stalks. They were harvested in pairs because the plants produce a pair of primary, opposite leaves at each node. The six pairs of leaves in each set represented six stages of leaf development. The nascent leaves at the top of the stalk were of course the smallest and least developed. Those progressively lower down were progressively larger and more developed.
The leaves from the uppermost node ranged in length from 3·5 to 4 cm. Those removed from the next node down ranged in length from 7 to 8 cm. Leaves removed from the third node down ranged in length from 9·75 to 12 cm. Leaves removed from the fourth node down ranged in length from 10·5 to 15 cm. Leaves removed from the fifth node down ranged in length from 12·5 to 16 cm. Leaves removed from the sixth node down ranged in length from 15·5 to 18·5 cm.
The leaves from each node were dried and powdered separately. A 20 mg portion of the powder from each leaf pair was extracted into 0·1 mL of room temperature chloroform for 30 min, with occasional agitation. A TLC plate was spotted with volumetrically equal samples (0·5 µL) of the six extracts from set A. A second TLC plate was spotted in the same manner with the six extracts from set B.
Thin layer chromatography
Thin layer chromatography (TLC) was performed using Whatman® silica gel plates (catalogue no. 4410222). The plates were developed in ethyl acetate/hexanes (1 : 1). Terpenes were visualized by spraying the plates with vanillin reagent, and then heating at 110 °C. The vanillin reagent was prepared by mixing 50 mL ethanol, 0·3 mL sulfuric acid, and 1 g vanillin. The salvinorins react with this chromogenic reagent to produce pinkish‐purple spots on the plates. The chromatograms were digitally scanned into a computer. To improve the visibility of faint spots in the images, the colour contrast and saturation were increased using Adobe Photoshop 7.0.
This proved to be a very sensitive method for detecting salvinorins. Through preliminary experimentation it was determined that salvinorin A can readily be detected in solution at concentrations as low as one part per 50 000 (0·002 %), and by overspotting sample applications, it can be detected at far lower concentrations.
Salvinorin A, salvinorin B, salvinorin C and salvinorin D were identified in the plant extracts by cochromatography with authenticated reference compounds. To confirm correct identification, several of the extracts were also chromatographed using two other solvent systems: acetone/dichloromethane (1 : 9), chloroform/methanol/water (100 : 10 : 1). These alternative mobile phases proved especially useful for resolving salvinorin B and salvinorin D (the two overlapped to produce a single spot when using the ethyl acetate/hexanes solvent system). The ethyl acetate/hexanes solvent system was more effective at resolving salvinorin A and salvinorin C (the two compounds produced overlapping spots when using the other mobile phases).
Salvinorin A was isolated by the author. Salvinorin B was obtained from D. J. Stewart (Department of Pharma cognosy, University of Mississippi). Salvinorin C and salvinorin D were obtained from T. A. Munro (School of Chemistry, University of Melbourne). Each of these had been authenticated using NMR spectroscopy.
Histochemistry
The vanillin reagent that was used to visualize salvinorins on the TLC plates was also used to visually locate the occurrence of these compounds directly in plant tissue. Small dried sections of stems, leaves, rachises, bracts, pedicles and calyces were dipped briefly (approx. 1 s) in the reagent and then heated in an oven at 110 °C for 3 min. The sections were then examined using light microscopy. Trichomes were removed from fully developed fresh leaves using adhesive tape as described previously. The tape was then dipped in the reagent, heated and examined in the same manner as the previous material.
RESULTS
The variety of trichome types in S. divinorum and their distribution on the plant
Several kinds of trichomes are present in S. divinorum. These can be divided into two general types: non‐glandular trichomes and glandular (secretory) trichomes. The glandular trichomes are divided into two types: peltate and capitate. The capitate glandular trichomes are further divided into short‐stalked and long‐stalked varieties.
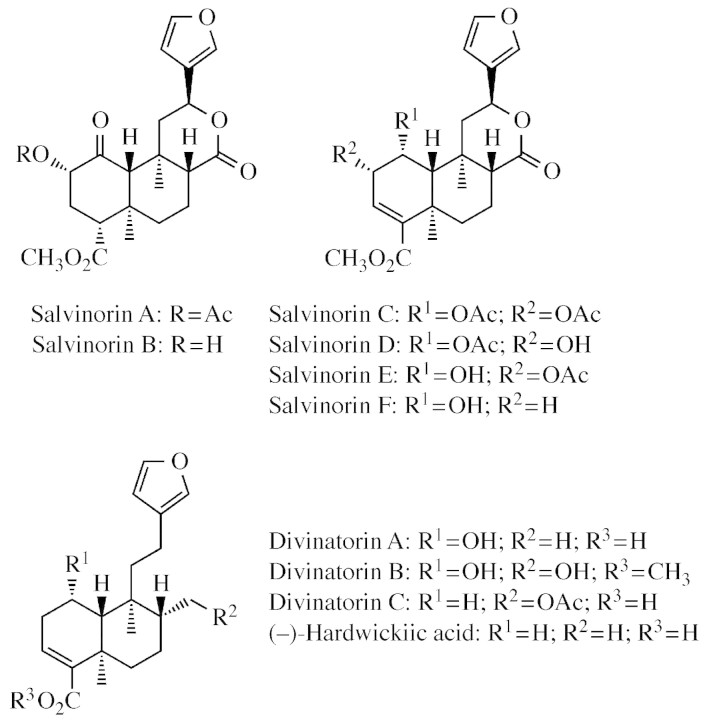
The non‐glandular trichomes are composed of a basal epidermal cell and one or more additional cells arranged linearly to form simple hair‐like structures that are unbranched, uniseriate, verrucose and tapered towards the apex. The most abundant of these are 100–200 µm long hairs, usually consisting of six to eight cells. These cover the stems, rachises, petioles, the abaxial veins of the leaves and bracts and the primary adaxial leaf veins; in these locations they are antrorse and usually arcuate (Fig. 3C). Similar trichomes are abundant on the abaxial surfaces of the pedicels and calyces, but here they are usually straight or only slightly curved (Fig. 3F). Hair‐like trichomes are also present on the abaxial surface of the corolla, but these are usually considerably longer (0·5–2 mm), relatively straight, and often comprise eight to ten cells. Inside the calyx there are many small antrorse hairs that are typically 50–90 µm long. Short, stout, upright, conical trichomes are scattered sparsely on the adaxial leaf surface. These are usually comprised of four to six cells and are typically 120–220 µm in length. They are often supported by a slightly raised pedestal formed by the immediately adjacent epidermal cells (Fig. 3D). In seedling plants, the first few pairs of true leaves produce exceptionally long (8–12 mm), rigid, hair‐like trichomes that stand straight up from the adaxial leaf surface; these usually consist of five to seven cells (Fig. 4). The seedling stem and adaxial surface of the cotyledons are covered with short (50–90 µm) upright hairs. These usually consist of three or four cells.
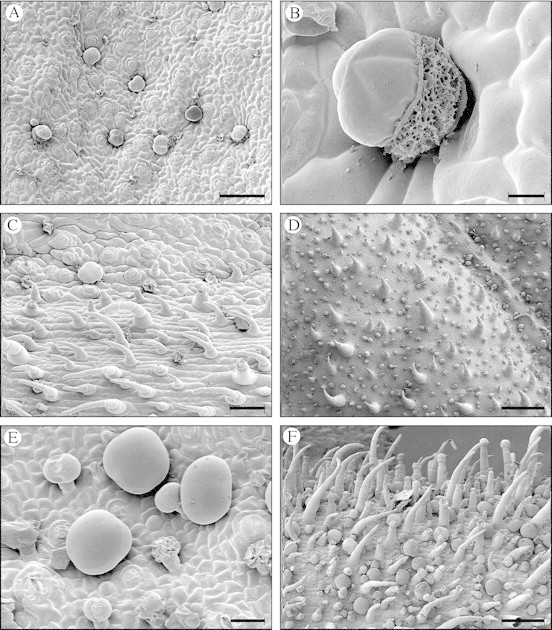
Fig. 3. Scanning electron micrographs showing S. divinorum trichomes. (A) An intervenous area of the abaxial leaf surface with several peltate glandular trichomes clearly visible. Many of these are slightly sunken in the epidermis. Although difficult to see because of their much smaller size, several short‐stalked capitate glandular trichomes are also present in this frame; many of these have withered. Scale bar = 100 µm. (B) A peltate glandular trichome on the abaxial leaf surface. The cuticle is broken, revealing the salvinorin‐rich resinous secretion that accumulates in the subcuticular cavity. The spongy appearance of the secretion suggests that it accumulates as a heterogeneous emulsion. Scale bar = 10 µm. (C) Non‐glandular trichomes on a vein of the abaxial leaf surface. A peltate glandular trichome is visible in the upper left quadrant of the frame. Scale bar = 50 µm. (D) Adaxial surface of a young leaf with non‐glandular trichomes and short‐stalked capitate glandular trichomes. A large percentage of the capitate glandular trichomes here have two head cells. The non‐glandular trichomes are particularly stout on this surface, except for those on the veins, where they are slender. The trichomes would be scattered much further apart on a fully developed leaf. Scale bar = 200 µm. (E) Peltate glandular trichomes and short‐stalked capitate glandular trichomes on the abaxial calyx surface. The capitate glandular trichomes shown in this frame all have two head cells, some of which have collapsed and shrivelled. Scale bar = 20 µm. (F) The abaxial surface of a young calyx showing a dense covering of non‐glandular trichomes, peltate glandular trichomes, and both short‐stalked and long‐stalked capitate glandular trichomes. Scale bar = 100 µm.
Fig. 4. Long, rigid, upright non‐glandular trichomes on the adaxial leaf surface of a S. divinorum seedling. These are only present on the first few sets of true leaves.
The peltate glandular trichomes consist of six cells: a basal epidermal cell, a short stalk cell and four head cells that are enclosed in a smooth cuticle. The head cells are grouped together above the stalk cell, surrounding its central axis. They secrete a terpene‐rich resin that accumulates in a relatively large subcuticular space (Fig. 3B). The secretions cause the cuticle to lift and expand, giving it a tumescent, somewhat globular appearance. The fully developed head is quadrilobate and 35–45 µm in diameter (Fig. 3A–C, E and F). Peltate glandular trichomes are abundant on the abaxial surfaces of the leaves, stems, rachises, bracts, pedicles and calyces. They are entirely absent from the adaxial surfaces. When young, these trichomes appear colourless or milky‐white. With age they often become honey‐coloured. On the abaxial leaf surface, these trichomes are sometimes slightly sunken in the epidermis (Fig. 3A). The density of peltate glandular trichomes on the abaxial surface of a fully developed leaf is typically about 1600 per cm2. The density is consistent over the entire abaxial leaf surface. A typical, fully developed leaf measuring 16 cm long has an approximate surface area of 80 cm2 and would therefore contain about 128 000 peltate glandular trichomes.
The capitate glandular trichomes consist of a basal epidermal cell, one or more stalk cells and one or two head cells. When there are multiple stalk cells, they are uniseriate. When there are two head cells, they are both attached to the stalk, adjacent to each other and enclosed by a single cuticle. Most of these trichomes are short‐stalked, having one or two short stalk cells and one or two head cells. The short‐stalked capitate glandular trichomes are typically 15–20 µm tall, with a head that is 10–20 µm in diameter. They are present on the stems, leaves, cotyledons, rachises, pedicles, bracts and calyces. These trichomes are apparently relatively short‐lived, since they frequently appear shrivelled and collapsed on the fully developed organs of the plant. A large percentage of the short‐stalked capitate glandular trichomes have two head cells; these are particularly abundant on the adaxial surface of the young leaves (Fig. 3D). Another type of short‐stalked capitate glandular trichome is sparsely present on the abaxial surface of the cotyledons. These have a milky‐white head measuring 25–30 µm in diameter. Long‐stalked capitate glandular trichomes consist of one or more (usually one to three) elongated stalk cells and a single head cell. Usually there is also a neck cell between the head and stalk, this differing morphologically from the two. Long‐stalked capitate glandular trichomes are typically 20–100 µm long, with a head that is 20–30 µm in diameter. These long‐stalked trichomes occur primarily on the pedicles, bracts and calyces. They are particularly abundant on the calyces (Fig. 3F).
Sequential leaf extracts
The leaves had been exposed to chloroform for 30 s in the first beaker, and during this time the solvent did not visibly penetrate the epidermis. By the time they were removed from the second beaker they had been exposed to chloroform for a total of 60 s. When they were removed from the third beaker they had been in contact with the solvent for a total of 90 s. Although the leaves spent only 30 s in each of these three beakers, the progressively longer total time in contact with the solvent caused progressively larger amounts of solvent penetration and visible cellular damage and, consequently, some extraction of chlorophylls and other compounds from the cellular tissues.
Examination of the developed TLC plate revealed that the first 30‐s extract contained the highest concentration of salvinorins. This extract was remarkably clean, with no chlorophylls or other pigments evident. The concentration of these terpenes progressively diminished in the second, third and fourth extracts. It was clear that the salvinorins had been mostly extracted in the first three extractions, since very little remained in the powdered leaves extracted in the fourth (Fig. 5A).
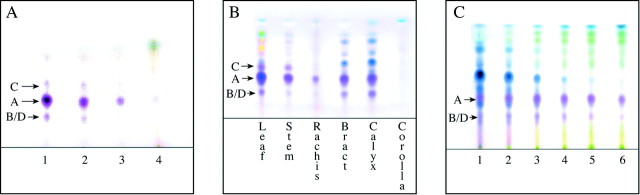
Fig. 5. Thin layer chromatograms. Arrows: A = salvinorin A, B/D = salvinorin B and salvinorin D together as an unresolved single spot, C = salvinorin C. (A) Chromatogram of sequential chloroform extracts of fresh leaves. Lane 1, first 30‐s extract; lane 2, second 30‐s extract; lane 3, third 30‐s extract; lane 4, 2‐h chloroform extract prepared from the previously extracted leaves after they had been dried and powdered. (B) Chromatogram of chloroform extracts prepared from six organs of S. divinorum. The stems and rachises both contain a high percentage of pith and vascular tissue. The low concentration of salvinorins in the rachis extract reflects this. The stem extract shown here was prepared from the external stem tissue only, and therefore the concentration of salvinorins is much higher than it would be if whole stems had been used. Of the other plant parts analysed, but not pictured here, only the pedicles contained salvinorins. (C) Comparative analysis of leaves at six progressive stages of development. Lane 1, leaves from the uppermost node of the stalk (the youngest leaves); lane 2, leaves from the second node down; lane 3, leaves from the third node down; lane 4, leaves from the fourth node down; lane 5, leaves from the fifth node down; lane 6, leaves from the sixth node down. The concentration of salvinorin C in these leaves is too low to detect in this chromatogram. This compound is more concentrated in leaves that are older than those analysed here.
Glandular secretions collected from the epidermal surfaces of leaves and stems
The chromatogram of the chloroform‐swabbed surfaces revealed that salvinorins are highly concentrated in the glandular secretions on the abaxial surfaces of the leaves and stems. Salvinorins were not detected on the adaxial leaf surface.
Extracts from various parts of the plant
TLC of the various plant part extracts revealed the presence of salvinorins in the external stem tissue, leaves, rachises, bracts, pedicles and calyces. Salvinorins were not detected in the roots, internal stem tissue, internal petiole tissue, cotyledons or corollas (Fig. 5B).
Isolated peltate glandular trichomes and the leaf from which they had been removed
TLC of the alcohol swab sample revealed the presence of high concentrations of salvinorins in the peltate glandular trichomes that had been removed with adhesive tape. Comparative TLC of the extracts from the trichome‐reduced and normal leaf halves showed that the salvinorin content was reduced about 50 % in the half that had its trichomes partially removed. This corresponded closely with the percentage of trichomes that had been removed.
Subcuticular contents of peltate glandular trichomes
TLC of the resinous material collected from the subcuticular cavities of peltate glandular trichomes showed that salvinorins are highly concentrated in these secretions.
Leaves at six stages of development
The concentration of salvinorin A appeared remarkably similar in all six samples of both sets of leaves analysed. Salvinorin C was not detected in these chromatograms. This compound is apparently more concentrated in leaves that are older than those analysed in this experiment (Fig. 5C). Several unidentified compounds reacted with the vanillin reagent to produce dark blue spots on the TLC plates. Some of these are interesting in that they progressively diminish in concentration as the leaves develop. One of these compounds is especially concentrated in the very young leaves. In these young leaves it occurs at substantially higher concentrations than does salvinorin A (Fig. 5C). It appears that several of these blue‐spot‐producing compounds are identical to compounds that are also prominent in the calyces, pedicles and bracts (Fig. 5B).
Histochemical analysis
Glandular trichomes on the stems, leaves, rachises, bracts, pedicles and calyces turned violet‐black after being treated with the vanillin reagent. Only the glandular trichomes reacted in this way. The rest of the tissue appeared unchanged. The glandular trichomes that were isolated from the fresh leaves with adhesive tape also turned deep violet after being treated with this reagent. This reaction is not specific for salvinorins. TLC shows that the bracts, calyces and young leaves contain significant quantities of other compounds that react with the reagent to produce dark‐coloured products, so, in these organs at least, the reaction does not necessarily indicate that the glandular trichomes contain salvinorins. More telling is the lack of a reaction in the non‐glandular tissues. The vanillin reagent is quite sensitive to salvinorins, so a lack of a reaction is a clear indication that salvinorins are absent in these tissues.
DISCUSSION
A single 30‐s chloroform dip proved to be a remarkably simple, fast and efficient method for selectively extracting terpenoid glandular secretions from fresh S. divinorum leaves without significant contamination from other leaf components. Increasing the duration of the chloroform immersion resulted in a more thorough extraction of the glandular secretions, but this also increased the amount of material extracted from the cellular tissues. The fact that most of the salvinorin content of fresh leaves can be extracted into chloroform without the solvent penetrating the epidermis indicates that these compounds are secreted externally to the epidermis. This is also demonstrated by the presence of these compounds in glandular secretions removed by swabbing the abaxial surfaces of leaves and stems with chloroform.
Chemical analysis of peltate glandular trichomes isolated from the abaxial leaf surface reveals that these structures are rich in salvinorins. More specifically, it was found that these compounds accumulate in the extracellular subcuticular space of these trichomes. This was determined though collection and analysis of the resinous material contained therein. The various chemical analyses performed show that salvinorins are only present in parts of the plant that contain peltate glandular trichomes. Analysis of leaves that had their peltate glandular trichomes partially removed shows that the salvinorin content is reduced in accordance with the percentage of peltate glandular trichomes removed. These observations suggest that production of salvinorins is specific to these trichomes. Histochemical analyses of various parts of the plant show that salvinorins are absent from the non‐glandular tissues. The absence of salvinorins on the adaxial leaf surface shows that these compounds are not secreted by the capitate glandular trichomes there. If capitate glandular trichomes elsewhere on the plant secrete salvinorins, their contribution to the total accumulation in the plant would be minor because their storage capacity, relative to that of peltate glandular trichomes, is insignificant.
The concentrations of the salvinorins, both individually and relative to one another, vary in different organs, and also in individual organs at different stages of growth. The chemical profiles of the mature leaves, stems and rachises are visibly similar to each other (Fig. 5B). This is also the case with the young leaves, bracts, pedicles and calyces (Fig. 5B, C). The ratio of salvinorin C to salvinorin A is greater in the mature leaves, stems and rachises, than in the young leaves, bracts, pedicles and calyces.
The extracts of the young leaves, bracts, pedicles and calyces produced several prominent dark blue spots on the chromatograms, thus revealing the presence of compounds that are largely absent from, or only weakly present in, the other plant parts analysed. The identity of these compounds has not been determined. Their reaction to the vanillin reagent suggests that they are probably not neoclerodane diterpenes. They clearly do not correspond with any of the salvinorins thus far reported. One of these compounds is especially concentrated in the very young leaves (Fig. 5C). An apparently identical compound also occurs at high concentrations in the bracts, pedicles and calyces (Fig. 5B). Given that the calyces enclose the reproductive organs, and that the leaves are particularly vulnerable during early development, the presence of such high concentrations of this compound could indicate that it has a protective function.
The inflorescence emits a pleasant fragrance that is not present in other parts of the plant. This suggests the presence of volatile terpenes. This fragrance appears in the early stages of inflorescence development, weeks before the corollas emerge. It may be that these odoriferous compounds are secreted by the particularly large long‐stalked capitate glandular trichomes that occur primarily on the bracts, pedicles and calyces.
The dramatically lower yields of salvinorin A previously reported for whole stems compared with whole leaves is primarily due to the fact that the stems are largely composed of woody vascular tissue and pith, which do not contain salvinorins. Stems typically weigh about ten times as much as leaves when dry sections of both organs with equal abaxial surface areas are compared. The density of peltate glandular trichomes on the abaxial stem surface is about the same as that on the abaxial leaf surface. A comparison of equivalent chloroform swab samples taken from both of these surfaces indicates that the surface concentrations of salvinorins are substantially similar. As expected, the concentration of these compounds in whole stems and whole leaves differs in a manner that roughly corresponds to their different ratios of weight to abaxial surface area.
Peltate glandular trichomes are crowded closely together on the very young leaves. Although additional trichomes continue to initiate and develop during leaf development, their overall density decreases as the leaves enlarge. The fact that the concentration of salvinorin A in the leaves remains relatively even throughout much of their development suggests that it progressively accumulates within the trichomes as the leaves enlarge. Although peltate glandular trichomes appear fully developed very early during leaf development, it is apparent that their chemical composition continues to change as the leaves mature.
The fact that salvinorins are localized in trichomes that are distributed over much of the plant’s exterior suggests that they serve a protective function. The compartmentalization of these compounds in extracellular spaces implies that they are not involved in the normal physiological processes of the plant. Considering the biological activity of salvinorin A, it is reasonable to suppose that the selection pressures that favoured the evolution of salvinorin‐secreting glandular trichomes were those of interspecies interactions. Most likely these secretions function as feeding deterrents. Although many types of insects seem to relish S. divinorum (Díaz, 1975; Valdés, 1983), there may be others that are negatively affected by the salvinorins it contains. This possibility is supported by reports of other neoclerodane diterpenes having antifeedant activity in insects (Cole, 1992; Simmonds and Blaney, 1992; Sosa et al., 1994; Rodríquez‐Hahn et al., 1995). It seems likely that herbivorous mammals such as deer would be susceptible to the psychoactive effects of salvinorin A. If so, it would likely discourage further consumption of the plant. Although now rare due to over‐hunting, deer were once common in the Mazatec region. This is reflected in the Náhuatl word Mazatec, which is derived from the place name Mazatlán, which means ‘land of deer’.
In conclusion, the experimental evidence presented here shows that salvinorins are secreted as components of a complex resin that accumulates in the subcuticular space of peltate glandular trichomes. Although it is yet to be experimentally demonstrated, given what is known about the biochemistry of related species, it seems highly probable that peltate glandular trichomes are not only sites of salvinorin accumulation, but also sites of salvinorin biosynthesis in S. divinorum. The chemical similarity of the various neoclerodane diterpenes in S. divinorum strongly suggests that they are all products of the same biosynthetic system, so, although only the four most abundant of these were clearly identified in the chromatographic analyses reported here, it is probable that the others are also localized in peltate glandular trichomes. Other types of terpenes have previously been localized in glandular trichomes of Labiatae species; however, the localization of diterpenes in trichomes of this family is apparently novel.
ACKNOWLEDGEMENTS
Special thanks go to my wife, Pernille Thisted Siebert, and to David Jeremy Stewart for their invaluable assistance. I am also grateful to Aaron Shai Reisfield for kindly allowing me to use his photograph of a blooming S. divinorum plant.
Supplementary Material
Received: 9 June 2003; Returned for revision: 9 December 2003; Accepted: 2 February 2004; Published electronically: 15 April 2004
References
- BighamAK, Munro TA, Rizzacasa MA, Robins‐Browne RM.2003. Divinatorins A–C, new neoclerodane diterpenoids from the controlled sage Salvia divinorum Journal of Natural Products 66: 1242–1244. [DOI] [PubMed] [Google Scholar]
- ChavkinC, Sud S, Jin W, Stewart J, Zjawiony JK, Siebert DJ, Toth BA, Hufeisen SJ, Roth BL.2004. Salvinorin A, an active component of the hallucinogenic sage Salvia divinorum, is a highly efficacious κ‐opioid receptor agonist: structural and functional considerations. Journal of Pharmacology and Experimental Therapeutics 308: 1197–1203. [DOI] [PubMed] [Google Scholar]
- ColeMD.1992. The significance of the terpenoids in the Labiatae. In: Harley RM, Reynolds T, eds. Advances in labiate science Kew: The Royal Botanic Gardens, 315–324. [Google Scholar]
- CroteauR, Johnson MA.1984. Biosynthesis of terpenoids in glandular trichomes. In: Rodriguez E, Healey PL, Mehta I, eds. Biology and chemistry of plant trichomes. New York: Plenum Press, 133–185. [Google Scholar]
- DíazJL.1975. Etnofarmacología de algunos psicotrópicos vegetales de México. In: Díaz JL, ed. Etnofarmacología de plantas alucinógenas latinoamericanas, Vol. 4 México, DF: Centro Mexicano de Estudios en Farmacodependencia (CEMEF) Cuadernos Científicos, 135–201. [Google Scholar]
- DukeSO, Canel C, Rimando AM, Tellez MR, Duke MV, Paul RN.2000. Current and potential exploitation of plant glandular trichome productivity. In: Hallahan DL, Gray JC, eds. Advances in botanical research. Incorporating advances in plant pathology. Vol. 31. Plant trichomes London: Academic Press, 121–151. [Google Scholar]
- EplingC, Játiva‐M. CD.1962. A new species of Salvia from Mexico. Botanical Museum Leaflets, Harvard University 20: 75–76. [Google Scholar]
- FahnA.2000. Structure and function of secretory cells. In: Hallahan DL, Gray JC, eds. Advances in botanical research. Incorporating advances in plant pathology. Vol. 31. Plant trichomes London: Academic Press, 37–75. [Google Scholar]
- GruberJW, Siebert DJ, Der Marderosian AH, Hock RS.1999. High performance liquid chromatographic quantification of salvinorin A from tissues of Salvia divinorum Epling and Játiva‐M. Phyto chemical Analysis 10: 22–25. [Google Scholar]
- HallahanDL.2000. Monoterpenoid biosynthesis in glandular trichomes of labiate plants. In: Hallahan DL, Gray JC, eds. Advances in botanical research. Incorporating advances in plant pathology. Vol. 31. Plant trichomes London: Academic Press, 77–120. [Google Scholar]
- HanesKR.2001. Antidepressant effects of the herb Salvia divinorum: a case report. Journal of Clinical Psychopharmacology 21: 634–635. [DOI] [PubMed] [Google Scholar]
- HanesKR.2003.Salvia divinorum: clinical and research potential. MAPS Bulletin 13: 18–20. [Google Scholar]
- HayRKM, Svoboda KP.1993. Botany. In: Hay RKM, Waterman PG, eds. Volatile oil crops: their biology, biochemistry, and production Harlow, Essex: Longman Scientific and Technical, 5–22. [Google Scholar]
- KoreedaM, Brown L, Valdés III LJ.1990. The absolute stereochemistry of salvinorins. Chemistry Letters 19: 2015–2018. [Google Scholar]
- MunroTA, Rizzacasa MA.2003. Salvinorins D–F, new neoclerodane diterpenoids from Salvia divinorum, and an improved method for the isolation of salvinorin A. Journal of Natural Products 66: 703–705. [DOI] [PubMed] [Google Scholar]
- OrtegaA, Blount JF, Manchand PS.1982. Salvinorin, a new trans‐neoclerodane diterpene from Salvia divinorum (Labiatae). Journal of the Chemical Society, Perkin Transactions 1: 2505–2508. [Google Scholar]
- ReisfieldAS.1987.Systematic studies in Salvia L. (Lamiaceae) with special emphasis on subgenus Calosphace Benth. section Dusenostachys Epl. MSc Thesis, University of Wisconsin, USA. [Google Scholar]
- ReisfieldAS.1993. The botany of Salvia divinorum (Labiatae). Sida 15: 349–366. [Google Scholar]
- Rodríquez‐HahnL, Esquivel B, Cárdenas J.1995. Neo‐clerodane diterpenoids from American Salvia species. In: Arnason JT, Mata R, Romeo JT, eds. Phytochemistry of medicinal plants. Recent advances in phytochemistry. Vol. 29 New York: Plenum Press, 311–332. [Google Scholar]
- RothBL, Baner K, Westkaemper R, Siebert DJ, Rice KC, Steinberg S, Ernsberger P, Rothman R.2002. Salvinorin A: a potent, naturally occurring, non‐nitrogenous κ‐opioid selective agonist. Proceedings of the National Academy of Sciences of the USA 99: 11934–11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ShefflerDJ, Roth BL.2003. Salvinorin A: the ‘magic mint’ hallucinogen finds a molecular target in the κ‐opioid receptor. Trends in Pharmacological Sciences 24: 107–109. [DOI] [PubMed] [Google Scholar]
- SiebertDJ.1994.Salvia divinorum and salvinorin A: new pharmacologic findings. Journal of Ethnopharmacology 43: 53–56. [DOI] [PubMed] [Google Scholar]
- SimmondsMSJ, Blaney WM.1992. Labiate‐insect interactions: effects of labiate‐derived compounds on insect behavior. In: Harley RM, Reynolds T, eds. Advances in labiate science. Kew: Royal Botanic Gardens, 375–392. [Google Scholar]
- SosaME, Tonn CE, Giordano OS.1994. Insect antifeedant activity of clerodane diterpenoids. Journal of Natural Products 57: 1262–1265. [DOI] [PubMed] [Google Scholar]
- StewartDJ, Schühly W, Zjawiony J.2001. Phytochemical studies of the Mexican hallucinogenic plant Salvia divinorum. Poster presentation at the 42nd annual meeting of the American Society of Pharmacognosy, Oaxaca, Mexico. 4–18 July 2001. Abstract published in: 42nd annual meeting of the American Society of Pharmacognosy, program and abstracts (poster 70), 147. [Google Scholar]
- ValdésIII LJ.1983.The pharmacognosy of Salvia divinorum (Epling and Játiva‐M.): an investigation of ska María Pastora. PhD Thesis, University of Michigan, USA. [DOI] [PubMed] [Google Scholar]
- ValdésIII LJ, Butler WM, Hatfield GM, Paul AG, Koreeda M.1984. Divinorin A, a psychotropic terpenoid, and divinorin B from the hallucinogenic Mexican mint Salvia divinorum Journal of Organic Chemistry 49: 4716–4720. [Google Scholar]
- ValdésIII LJ,Chang HM, Visger DC, Koreeda M.2001. Salvinorin C, a new neoclerodane diterpene from a bioactive fraction of the hallucinogenic Mexican mint Salvia divinorum Organic Letters 3: 3935–3937. [DOI] [PubMed] [Google Scholar]
- WassonRG.1962. A new Mexican psychotropic drug from the mint family. Botanical Museum Leaflets, Harvard University 20: 77–84. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.