Abstract
• Background and Aims Pollination through sexual mimicry, also known as pseudocopulation, has been suggested to occur in some genera of the Neotropical orchid subtribe Maxillariinae. However, it has been demonstrated so far only for Trigonidium obtusum. This study reports and illustrates pollination through sexual mimicry in Mormolyca ringens.
• Methods A total of 70 h were dedicated to the observation of flowers and pollinator behaviour, which was photographically recorded. Flower features involved in pollinator attraction were studied using a stereomicroscope and by SEM analyses. Preliminary observations on the plant breeding system were made by manually self‐pollinating flowers. The chemical composition of the fragrance volatiles was determined by GC/MS analysis.
• Key Results The flower features of M. ringens parallel those of other pseudocopulatory flowers. The labellum shape and indument are reminiscent of an insect. Sexually excited drones of Nannotrigona testaceicornis and Scaptotrigona sp. (both in the Apidae: Meliponini) attempt copulation with the labellum and pollinate the flower in the process. In both bee species, the pollinarium is attached to the scutellum. Pollinator behaviour may promote some degree of self‐pollination, but preliminary observations indicate that M. ringens flowers are self‐incompatible. Flowers are produced all the year round, which ties in with the production of bee males several times a year. The phylogenetic relationships of M. ringens are discussed and a number of morphological and phenological features supporting them are reported.
• Conclusions It is expected that further research could bring to light whether other Maxillariinae species are also pollinated through sexual mimicry. When a definitive and robust phylogeny of this subtribe is available, it should be possible to determine how many times pseudocopulation evolved and its possible evolutionary history.
Key words: Maxillariinae, Mormolyca, Maxillaria, Orchidaceae, pollination, pseudocopulation, sexual mimicry, Meliponini
INTRODUCTION
A significant number of orchid species offer no flower reward to their pollinators (van der Pijl and Dodson, 1966; Dressler, 1993). These flowers are pollinated through different types of deceit. Many orchids are ‘food‐frauds’ meaning that they bear some flower features, such as flower shape and fragrances, that attract food‐seeking animal pollinators (van der Pijl and Dodson, 1966). A much more complex pollination strategy is so‐called ‘pseudocopulation’. Flowers belonging to this category have sets of flower features that attract sexually excited male insects, mostly Hymenoptera (Kullenberg, 1961). Most orchid flowers with this ‘pseudocopulatory’ syndrome display hairy, insect‐like labella (the modified median petal of orchids), and also these flowers emit fragrances mimicking the sexual pheromones of female insects (Borg‐Karlson, 1990; van der Cingel, 1995, 2001; Ayasse et al., 2000). Such fragrances are responsible for long‐distance insect attraction. Attracted male insects attempt copulation with the insect‐like labellum and, during this behaviour, promote pollination (Kullenberg, 1961; van der Pijl and Dodson, 1966; van der Cingel, 1995, 2001).
A remarkable exception to this role is the pollination of Trigonidium obtusum Lindl. The upright, funnel‐like flowers of this orchid lack any insect‐like flower part. Sexually attractive fragrances are secreted either by the lateral sepals or lateral petals. The attracted drones of Plebeia droryana (Apidae: Meliponini) attempt copulation with the lateral sepals or petals, slip and get briefly trapped in the flower cavity. When trying to escape, one of the bees dislodges the pollinarium and eventually leaves the flower. A drone laden with a pollinarium that subsequently falls inside another flower will deposit the pollinia in the concave stigmatic cavity, thus leading to pollination (Singer, 2002).
The phenomenon of flowers pollinated by pseudocopulation has been very well documented for Afro‐European and Australian terrestrial orchids (van der Pijl and Dodson, 1966, and references therein; van der Cingel, 1995, 2001). Although pseudocopulation has also been suggested to occur in a number of Neotropical orchid genera (van der Pijl and Dodson, 1966), most of these reports are sketchy and lack the wealth of information of the Old World ones (Borg‐Karlson and Tengo, 1986; van der Cingel, 1995, 2001; Ayasse et al., 2000). For this reason, the phenomenon of pseudocopulation in Neotropical orchids deserves additional study, in order to allow a complete understanding of this process in the family Orchidaceae.
Among orchids of subtribe Maxillariinae, pseudocopulation has been suggested to occur in the genera Cyrtidiorchis, Chrysocycnis, Mormolyca and Trigonidium (van der Pijl and Dodson, 1966). As mentioned above, pseudocopulation in combination with trap‐flowers has recently been confirmed for the Brazilian Trigonidium obtusum (Singer, 2002). All the other genera display hairy insect‐like labella, as do the Old World genera, but to date the occurrence of pseudocopulatory pollination in these plants remains speculative.
In observing cultivated plants of Mormolyca ringens, we noticed that their flowers were often pollinated. These preliminary records prompted more detailed observations that confirmed the pseudocopulatory nature of these orchids. The aim of the present contribution is to report and illustrate the pollination process in M. ringens. In addition, flower features and fragrances involved in attracting pollinators are described and discussed. This information is considered in a phylogenetic context.
MATERIALS AND METHODS
The species M. ringens (Lindl.) Schltr. is widespread, and probably common, from Mexico to northern Costa Rica (Atwood and Retana, 1999) and to date it has been considered non‐native to Brazil. The only Mormolyca species that has been reported for the Brazilian Flora is M. galeata (Scheidw.) Garay & M. Wirth, discussed by Hoehne as Maxillaria galeata Scheidw (Hoehne, 1953; Pabst and Dungs, 1977). Hoehne (1953), however, cast doubt on the reported distribution of this species. According to him, the few existing vouchers came from Mexico and were mistaken as Brazilian plants (Hoehne, 1953). A putative living specimen of Mormolyca galeata is currently in cultivation at the Instituto de Botânica de São Paulo (São Paulo, Brazil, voucher no. 16981). The plant is registered as from the Atlantic Rain Forest of North‐eastern São Paulo State (Municipality of Ubatuba) but no population of this species is known for this region. The plant has been identified as M. cf. galeata, but we considered its flowers, as well as its vegetative morphology very similar to the earlier described M. ringens.
This study was performed at the orchidarium of the ‘Escola Superior de Agronomia Luiz de Queiroz, Universidade de São Paulo’, Piracicaba, São Paulo State, south‐eastern Brazil. It was based on ten individuals cultivated in semi‐open conditions that allowed pollinators to access plants. Plant vouchers (ESA 7247, ESA 16802 and ESA 1648) are deposited at ESA and UEC herbaria. The observations were carried out from 1 August to 15 October 2003 between 0700–1700 h. A total of 70 h were dedicated to the observation of the flowers. Pollinator behaviour was recorded with the help of a 35 mm camera. A chronometer was used to record the time that pollinators spent in the flowers.
Flower features were studied from fresh flowers in a Nikon SMZ‐U binocular stereomicroscope with a Nikon FD‐X 35 mm camera attached. The labella of three fresh flowers were also observed with a scanning electron microscope (SEM JEOL 5800LV) at 10 kV. In order to avoid alteration of the fine hairs that cover the flower (indument), the labella were observed fresh through low‐vacuum scanning (Davies et al., 2000). The morphological concepts of Dressler (1993) are followed.
Preliminary observations of the breeding system of M. ringens were performed by manually self‐pollinating 30 flowers. This approach was used since M. ringens produce flowers throughout the year but in small numbers. Thus, priority was given to determinate whether M. ringens is able to set fruits after self‐pollination.
Flower odour sampling
In order to ascertain the composition of flower volatiles involved in pollinator attraction, the fragrance of M. ringens flowers was analyzed. The flower volatiles of fresh, unpollinated M. ringens flowers (one flower at a time) were placed in a glass vessel (7 cm long, 4 cm internal diameter) attached to an absorption trap containing Porapak Q 80–100 mesh (50 mg). Volatiles were drawn through the trap with the help of a battery‐operated pump (500 mL min–1) over 4 h. Trapped volatiles were extracted with bidistilled dichloromethane (1 mL), concentrated under a N2 flux to a final volume of 0·2 mL, and analysed by GC/MS.
Gas chromatography/mass spectrometry
Analyses were carried out using a HP 6890/5973 system equipped with HP‐5 fused silica capillary column (30 m × 0·25 mm × 0·25 µm); column temperatures were programmed from 50 °C to 310 °C at 4 °C min–1 for integrating purposes. Injector temperatures were 240 °C. Helium was used as carrier gas, flow rate 1 mL min–1, splitless mode. The injection volume was 0·5 µL of the dicloromethane solution obtained as described above. The retention indices (van den Dool and Kratz, 1963) were obtained by co‐injecting the fragrance sample with a C10–C24 normal hydrocarbon mixture. The mass spectrometry (MS) was taken at 70 eV. Scanning speed was 0·84 scans s–1 from m/z 40 to 550.
The identification of the volatile components (Table 1, Fig. 4) was made on the basis of retention index comparisons as well as by computerized matching of the obtained mass spectra with those stored in the Wiley/NBS mass spectral library of the GC/MS data system and other published mass spectra (Adams, 1995).
Table 1.
Volatile components of Mormolyca ringens, percentage and retention indices (RI) determined by GC/MS
| Peak numbers | Compound | RI, calculated | RI, literature | Relative concentration (%) |
| 1 | 6‐methyl‐5‐hepten‐2‐one | 989 | 985 | 2·7 |
| 2 | n‐octanal | 999 | 1001 | 2·9 |
| 3 | Benzyl alcohol | 1034 | 1032 | 0·8 |
| 4 | cis‐arbusculone | 1051 | 1052 | 1·1 |
| 5 | n‐octanol | 1071 | 1070 | 2·3 |
| 6 | n‐undecane | 1100 | 1100 | 2·5 |
| 7 | n‐nonanal | 1104 | 1102 | 10·0 |
| 8 | n‐hexyl butirate | 1188 | 1192 | 1·1 |
| 9 | n‐dodecane | 1199 | 1199 | 1·4 |
| 10 | n‐decanal | 1205 | 1204 | 7·4 |
| 11 | Phenyl butyl alcohol isomer 1 | 1244 | – | 6·5 |
| 12 | Phenyl butyl alcohol isomer 2 | 1249 | – | 0·1 |
| 13 | Phenyl butyl alcohol isomer 3 | 1255 | – | 5·6 |
| 14 | 3‐ethylacetophenone | 1262 | – | 6·2 |
| 15 | 4‐ethylacetophenone | 1281 | – | 3·0 |
| 16 | α‐copaene | 1374 | 1376 | 3·1 |
| 17 | n‐hexyl n‐hexanoate | 1386 | 1383 | 18·0 |
| 18 | Unknown | 1399 | – | 1·5 |
| 19/ | n‐dodecanal | 1409 | 1407 | 1·4 |
| 20 | Alloaromadendrene | 1458 | 1461 | 0·5 |
| 21 | γ‐muurolene | 1476 | 1477 | 1·7 |
| 22 | ar‐curcumene | 1484 | 1483 | 1·2 |
| 23 | α‐muurolene | 1501 | 1499 | 0·7 |
| 24 | γ‐cadinene | 1513 | 1513 | 1·1 |
| 25 | cis‐calamenene | 1522 | 1521 | 3·0 |
| 26 | Unknown | 1530 | – | 1·3 |
| 27 | Unknown | 1612 | – | 2·0 |
| 28 | Unknown | 1663 | – | 1·2 |
| 29 | Unknown | 1788 | – | 7·0 |
| 30 | isopropyl myristate | 1817 | – | 1·0 |
| 31 | Tetradecanol | 1820 | – | 1·7 |
| Total | 100·0 |
Peak numbers refer to Fig. 4.
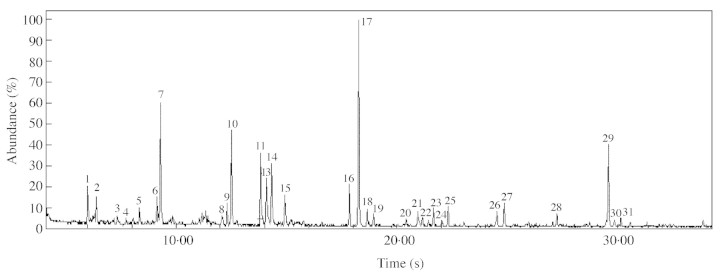
Fig. 4. Total ion current chromatogram of the volatiles of Mormolyca ringens. Peak numbers refer to Table 1.
RESULTS AND DISCUSSION
Plant features
Only diagnostic and gross morphological features pertinent to pollination will be discussed. Readers interested in details of vegetative parts, as well as variation of perianth shape are referred to Atwood and Mora de Retana (1999).
Mormolyca ringens is an epiphytic orchid, bearing clustered, smooth, unifoliate pseudobulbs. The solitary flowers are borne on long (approx. 20 cm), wiry pedicels. Flowers are produced throughout the year. One to four flowers are gradually produced laterally to each new pseudobulb. The flower shape is distinctive when compared to other Maxillariinae orchids, with patent sepals and lateral petals (Fig. 1A). This renders Mormolyca flowers remarkably ‘open’ in comparison with most orchids of subtribe Maxillariinae, where campanulate to tubulose flower presentation prevails.

Fig. 1. Flower features of Mormolyca ringens. (A) Flower in lateral view. (B) Labellum in dorsal view. (C–F) Column. (C) Intact. (D) With anther cap removed, exposing the pollinarium. (E) With pollinarium removed, exposing the scar left after the removal of the tegula. (F) Column of a pollinated flower, showing the closing of the stigmatic surface. AC, anther cap; Sc, scar after tegula removal; Stg, stigmatic cavity; Tg, tegula or tegular stipes; Vs, viscidium.
Flower features of M. ringens are otherwise widespread in the subtribe Maxillariinae (Dressler, 1993). Flower coloration is mostly tan, with some external dark brown longitudinal stripes on the sepals and lateral petals (Fig. 1A). Sepals are 14–16 mm long and 7–8 mm wide. The labellum is 7–8 mm long and 4–6 mm wide (Atwood and Mora de Retana, 1999). Flowers may keep their fresh appearance for about 10 days. The labellum, which is articulated at the base of the column, is trilobed, reddish‐brown spotted and papillose with acute lateral lobes (Fig. 1B). The indument and spotting of the labellum resemble those of several Old World Ophrys species (subfamily Orchidoideae). The column is stout and erect, with a reduced column foot (Atwood and Mora de Retana, 1999). The anther is incumbent (Fig. 1A and C) and holds a pollinarium made up of four unequal pollinia, a broad tegular stipe (Rasmussen, 1986) and an arcuate, slender viscidium (Fig. 1D). Unpollinated flowers show a broad and concave stigmatic surface (Fig. 1C–E). The fruit is a capsule.
SEM observations
Low‐vacuum SEM analyses revealed that many areas of the lip surface of Mormolyca ringens are densely trichomatic (Fig. 2A, B). The labellum indument resembles, although superficially, that of the hairy areas of an insect tegument. It was also noticed that many areas of the labellum surface, especially the median region, are glabrous and smooth (Fig. 2C). The hairy parts are particularly concentrated at the basal lateral margins of the labellum and on a triangular purple‐coloured spot just below the column. This set of flower features parallels those of the Old World Ophrys (Orchidoideae) species. The labellum of Ophrys species is also densely trichomatic and insect‐like in shape (Kullenberg, 1961; van der Pijl and Dodson, 1966). It has been proposed that the labellum indument in Ophrys may visually and mechanically guide the pollinators (Kullenberg, 1961; van der Pijl and Dodson, 1966). We believe that the labellum indument and colouration of M. ringens may also have a similar function.
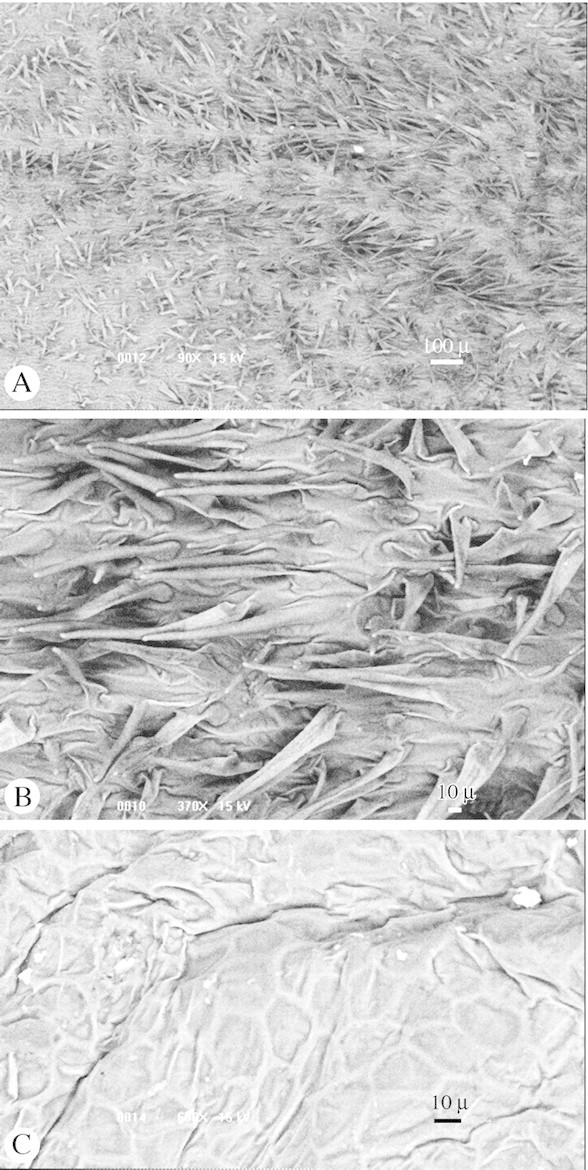
Fig. 2. SEM (low‐vaccuum) of the labellum surface. (A) View of a densely trichomatic portion. (B) Detail of the trichomes. (C) Detail of a smooth region located in the median region of the labellum.
Pollination process and pollinator behaviour
The recorded flower visitors and pollinators were drones of Nannotrigona testaceicornis (Fig. 3A–D) and Scaptotrigona sp. (Fig. 3E) (Apidae: Meliponini). Nannotrigona males were far much more common, whereas Scaptotrigona drones were sighted only a few times. The two bee species were able to dislodge and deposit pollinaria, thus acting as pollinators. Pollinator activity started around 1000 h and lasted until approx. 1700 h. During this period, the flowers were intensively visited. In general, consecutive visits to an attractive flower were separated by intervals of less than 1 min.

Fig. 3. The pollination process in Mormolyca ringens. (A–D) Pollination by drones of Nannotrigona testaceicornis (Apidae: Meliponini). (A) Two males attempting copulation with the labellum. (B) Drone with a pollinarium adhered on its scutelum. (C) Drone laden with pollinarium leaving the flower. (D) Close‐up of a drone laden with a pollinarium. (E) Male of Scaptotrigona sp. (Apidae: Meliponini) attempting copulation with the labellum surface. Notice that a pollinarium has already been deposited at the stigmatic cavity.
Pollinator behaviour at the flowers was basically the same for the two bee species. The bees hovered for a few seconds in the front of the flower and then landed on the labellum (Fig. 3A–C, E). The bees then attempted copulation with the dorsal surface of the labellum, as was indicated by spasmodic abdominal movements as well as by the clear extrusion of their genitalia. Bees of Nannotrigona spent from 1·5–92 s on the labellum (n = 109; mean = 15·95), while individuals of Scaptotrigona were observed to spend between 15–40 s (n = 3; mean = 28·33).
Some behavioural differences were perceived among males of Nannotrigona and Scaptotrigona. Males of Nannotrigona were frequently seen to vibrate their wings, often with an audible buzzing, while attempting copulation with the labellum. This behaviour did not occur in the few recorded visits by males of Scaptotrigona. One to three bees may visit a flower at the same time. As was previously also recorded for Trigonidium obtusum (Singer, 2002), some drones were observed attempting copulation with already landed bees, thus supporting the pseudocopulatory nature of the process.
Pollinarium removal occurs when drones back out of the flowers and press their scutellum against the rostellum (Fig. 3B–D). Then, the arcuate viscidium firmly embraces and adheres on the bee scutellum and the pollinarium is removed (Fig. 3B–D). The anther cap that covers the pollinarium immediately falls off. Pollination takes place when a pollinarium‐laden drone backs out of a flower. The globose pollinia are arrested at the concave stigmatic surface. According to our observations, the bees can deposit either the whole pollinarium or a pair of pollinia. It was observed that, if more than one flower is available, a drone that has just removed a pollinarium can return to the same flower or the same plant a few times. Such behaviour can promote some degree of self‐pollination and, at least on one occasion, we observed a drone to perform geitonogamy. Remarkably, there are no morphological flower features hindering self‐pollination. In Trigonidium obtusum, recently removed pollinaria are too large to enter the stigmatic cavity and dehydration is needed for pollination to be accomplished (Singer, 2002). In contrast, the stigmatic cavity of M. ringens is broad enough to arrest fresh pollinaria. In other words, unlike T. obtusum, cross‐pollination in M. ringens completely relies on pollinator behaviour.
The stigmatic surface of pollinated flowers readily closes after the deposition of the pollinarium (Fig. 1F), the closing being perceived as soon as 3 h after pollination. Recently pollinated flowers are still attractive to pollinators. However, on the day following the pollination process, the flowers are completely ignored by the bees, even though they retain their fresh appearance for a few days after pollination. This suggests that volatile emissions may decrease or cease after pollination. Since Mormolyca ringens is apparently non‐native to Brazil, these observations were only possible because bees of the genera Nannotrigona and Scaptotrigona are widespread in the Neotropics (Michener, 2000).
The chemical composition of Mormolyca ringens floral volatiles
The GC/MS analysis revealed that the floral odour was composed of 31 major components (Table 1). This is in remarkable contrast to the fragrance composition of the previously studied (and also pseudocopulatory) Trigo nidium obtusum (Flach et al., 2004), where the fragrance was found to be mainly composed of pentadecane. However, preliminary bioassays with baits of pentadecane showed no attractivity for the bee pollinators of T. obtusum (drones of Plebeia droryana, Apidae: Meliponini). Many of the fragrance compounds of M. ringens derive from the polyketide pathway (fatty acid derivatives, aromatic compounds) and the mevalonic acid pathway (mono‐ and sesquiterpenes). The presence of phenyl‐butanol isomers (peaks 11, 12 and 13, Fig. 4) was suggested by the fragmentation pattern obtained. The main fragments of the three isomers were similar, depicting the molecular ion at m/z 150 and the base peak was at m/z 79. The latter was associated with ions at m/z 77 and m/z 78 that are characteristic of monosubstituted benzene derivatives possessing a carbinol group linked to the aromatic ring (Davis and Frearson, 1987; McLafferty and Turecek, 1993). In this species the series of n‐aldehydes begins with n‐octanal (2 %), n‐nonanal (10 %), n‐decanal (7·5 %) and n‐dodecanal (1·7%). A survey of Maxillaria floral volatiles revealed that the presence of normal aldehyde is a common feature but the predominance of the nonanal remains unique (Kaiser, 1993). The predominance of the polyketide route is again present in the n‐hexyl‐hexanoate, 18 %.
Preliminary GC–EAG (gas chromatography–electroantenography) experiments have also demonstrated that the volatiles of Mormolyca ringens elicit a very strong response in Nannotrigona males. This matter is currently being studied by our group.
Preliminary observations on the breeding system
All of the 30 manually self‐pollinated flowers aborted. Although sampling in more individuals is desirable, the evidence obtained strongly suggests that M. ringens is self‐incompatible. There are no previous reports on the breeding system of this species and we believe self‐incompatibility may be frequent in subtribe Maxillariinae (R. B. Singer and S. Koehler, pers. obs.). All self‐pollinated flowers show a considerable degree of column and ovary swelling. However, they start becoming yellowish about a week after pollination and flower abscision takes place approx. 10 d after pollination. Most flowers at the ESALQ‐USP orchid nursery are visited and many of them are pollinated. Remarkably, only a fraction (less than 10 %) of these flowers develop into fruits. This may, at least in part, be explained by the occurrence of self‐incompatibility. Another factor that may explain low fruit set is the low genetic diversity of the individuals available for study. Drones of both recorded bee species were often seen depositing pollinaria, which (in our opinion) excludes the possibility of inefficient pollination.
Ecological considerations
As previously described for Trigonidium obtusum (Singer, 2002), a number of flower features of M. ringens differ from those of other orchid species also pollinated by pseudocopulation (van der Pijl and Dodson, 1966; van der Cingel, 1995, 2001). Most orchids of this type produce multi‐flowered inflorescences, which start blooming just when seasonal male insects are born (van der Pijl and Dodson, 1966). In these orchid species, pollination takes place during a brief period of time when insect females are absent or scarce in the environment. Consequently, flowers are mistaken for females and the pollination process takes place. Once adult female insects emerge, the males are able to distinguish the females from flowers, avoiding the latter. As a result of the learning ability of the pollinators, these orchids usually display low fruit set (Neiland and Wilcock, 1998). Nonetheless, Ayasse et al. (2003) demonstrated that Ophrys speculum flowers are still attractive to males even after females have emerged. In fact, sometimes males prefer the orchid rather than the available female.
Nannotrigona and Scaptotrigona, like other eusocial bees, live in perennial hives where large quantities of males are produced several times a year. Thus, the extended, sequential flowering of M. ringens turns out to be very advantageous, since large quantities of males are available for most parts of the year. This correlation between flowering period and production of males was also suggested for Trigonidium obtusum, which also produces flowers throughout the year (Singer, 2002). When considering the ability of the pollinators to distinguish the real pheromone from the flower fragrance, our observations indicated that there is no decrease in the interest of the bees in relation to the flowers. This phenomenon persisted over 2 months, during which the bee pollinators were constantly recorded. It is possible that the amounts of attractive compounds in the plant fragrance vary among individuals or even among different periods in the same plant, but experimental work is necessary to prove this. This has already been demonstrated for some European Ophrys species (Ayasse et al., 2000). Such variations may promote cross‐pollination, since it becomes more difficult for the bees to learn the composition of the false pheromone.
Taxonomic affinities
A preliminary phylogeny of Maxillariinae orchids based on sequence data from ITS nrDNA is already available at www.flmnh.ufl.edu/natsci/herbarium/max. Ongoing molecular research strongly suggests that Mormolyca is nested within a clade of Maxillaria species, such as M. rufescens, M. hedwigiae and M. acutifolia (N. Williams and M. Whitten, pers. comm.). This view is also supported by both vegetative and flower features. Most species in this complex, including the genus Mormolyca, present aggregate, smooth, unifoliate pseudobulbs. Shared flower features include slender pedicels with reduced, inconspicuous bracts, stout columns with reduced column‐foot, trilobed labella with acute lateral lobes and pollinaria with broad tegulae and arcuate viscidia. Also all species within this clade have extended flowering periods; that is, plants produce flowers almost throughout the year, although flowering is more intense in the spring and summer.
The taxonomic affinities of Mormolyca ringens pose an interesting question. It has been suggested that sexual mimicry in Orchidaceae could have evolved from rewardless (possibly food‐fraud) ancestors (Dafni and Bernhardt, 1990; Singer, 2002), since flower features in rewardless orchids, such as long‐lived, showy, fragrant flowers, could be pre‐adapted for the evolution of pseudocopulation (Singer, 2002). Contrary to this hypothesis, however, flowers of some Maxillaria species related to Mormolyca ringens offer floral rewards to their pollinators. The flowers of Maxillaria rufescens offer dense cushions of trichomes in the median region of the lip. These trichomes are probably filled with starch and oily secretions (Porsch, 1905). At the campus of the ‘Universidade Estadual de Campinas’, Trigona workers (Meliponini) were observed collecting these trichomes (R. B. Singer, pers. obs.). Yet, these bees were too small to dislodge pollinaria and thereby pollinate the plants. We have observed similar tufts of trichomes in M. acutifolia. On the other hand, a yellowish, viscous secretion was observed on the lip surface of M. hedwigiae.
Conclusions
The pseudocopulatory nature of the flowers of Mormolyca ringens has been demonstrated. This constitutes the second well‐supported case of sexual mimicry in the subtribe Maxillariinae. Contrary to Trigonidium, which displays trap‐flowers and lacks any insect‐like flower parts, the flowers and the pollination process in M. ringens parallel those of the Old World genus Ophrys (subfamily Orchidoideae). There is considerable support for the monophyly of the orchid subtribe Maxillariinae (Whitten et al., 2000). However, generic delimitations are still ambiguous and unconvincing (Dressler, 1993; Whitten et al., 2000). Preliminary molecular research suggests that the genus Mormolyca is nested within the large and artificial genus Maxillaria (N. Williams and M. Whitten, pers. comm.). As previously discussed, many vegetative and floral features support a close relationship among a group of Maxillaria species and Mormolyca ringens. We hope that this contribution inspires colleagues in other Neotropical countries to do analogous research with other genera of Maxillariinae in which pseudocopulation has been suggested to occur, such as Chrysocycnis, Cyrtidium and Cyrtidiorchis. Research with other species of Mormolyca and Trigonidium would also be of great value. We hope that once a robust phylogeny of Maxillariinae orchids is available, the relation of flower reward to the evolution of pseudocopulation, as well as to other pollination processes, can be better understood. Thus, reliable, well‐supported scenarios could be proposed. For the time being an important question remains to be answered: how many times did sexual mimicry evolve within the Maxillariinae orchids?
Acknowledgements
We are grateful for grants from the ‘Fundação de Amparo a Pesquisa do Estado de São Paulo’ (FAPESP) (A.F. 02/07029‐0, R.B.S. 01/08958‐1, S.K. 02/02161‐7), which made possible the present study; and also thank the ‘Conselho Nacional de Pesquisa e Tecnologia’ (CNPq) for scholarships and support. The authors are greatly indebted to Elizabeth Ann Veasey and Josué Pontes, of the Escola Superior de Agricultura Luiz de Queiroz (ESALQ‐USP, Piracicaba), for allowing the study of cultivated plants. We are very grateful to Adriane Sprogis (Laboratório de Microscopia Eletrônica, Unicamp) for all the help with the SEM analysis, to N. Williams and M. Whitten for kindly sharing preliminary molecular data, and to J. Alcock and M. Ayasse for the valuable comments on the manuscript. G. A. R. Melo (UFPR) confirmed the generic identity of the Scaptotrigona drones. Favízia Freitas de Oliveira (Laboratório de Abelhas‐USP) confirmed the identity of the Nannotrigona.
Received: 15 December 2003; Returned for revision: 21 January 2004; Accepted: 11 February 2004; Published electronically: 29 March 2004
References
- AdamsRP.1995.Identification of essential oil by gas chromatography/mass spectroscopy. Illinois: Allured Publishing Co. [Google Scholar]
- AtwoodJT, Mora de Retana DE.1999. Orchidaceae: Tribe Maxillarieae: Subtribes Maxillariinae and Oncidiinae. Fieldiana 40: 1–182. [Google Scholar]
- AyasseM, Schiestl FP, Paulus Lofstedt C, Hanson B, Ibarra F, Francke W.2000. Evolution of reproductive strategies in the sexually deceptive orchid Ophrys sphegodes: how does flower‐specific variation of odor signals influence reproductive success? Evolution 54: 1995–2006. [DOI] [PubMed] [Google Scholar]
- AyaseeM, Schiestl FP, Paulus HF, Ibarra F, Francke W.2003. Pollinator attraction in a sexually deceptive orchid by means of unconventional chemicals. Proceedings of the Royal Society of London. Series B: Biological Sciences 270: 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg‐KarlsonAK.1990. Chemical and ethological studies of pollination in the genus Ophrys (Orchidaceae). Phytochemistry 29: 1359–1387. [Google Scholar]
- Borg‐KarlsonAK, Tengo J.1986. Odor mimetism? Journal of Chemical Ecology 12: 1927–1941. [DOI] [PubMed] [Google Scholar]
- DaviesKL, Winters C, Turner MP.2000. Pseudopollen: its structure and development in Maxillaria (Orchidaceae). Annals of Botany 85: 887–895. [Google Scholar]
- DavisR, Frearson M.1987.Mass spectrometry John Wiley & Sons, 1–430. [Google Scholar]
- DresslerRL.1993.Phylogeny and classification of the orchid family. Portland: Dioscorides Press. [Google Scholar]
- DafniA, Bernhardt P.1990. Pollination of terrestrial orchids of Southern Australia and the Mediterranean Region. Systematics, ecological and evolutionary implications. Evolutionary Biology 24: 193–253. [Google Scholar]
- FlachA, DondonRC, Singer RB, Koehler S, Amaral MEC and Marsaioli AJ.2004. The chemistry of pollination in selected Brazilian Maxillariinae orchids: floral rewards and fragrance. Journal of Chemical Ecology 30: 1039–1050. [DOI] [PubMed] [Google Scholar]
- HoehneFC.1953. Orchidaceas. In: Hoehne FC, ed. Flora Brasilica, fasc 10, vol. 12(7). São Paulo: Secretaria da Agricultura. [Google Scholar]
- KaiserR.1993.The scents of orchids, olfactory and chemical investigations. Basel: Elsevier, Editiones Roche. [Google Scholar]
- KullenbergB.1961. Studies in Ophrys pollination. Zoologiska Bidrag fran Uppsala 34: 1–340. [Google Scholar]
- McLaffertyFW, Turecek F.1993.Interpretation of mass spectra, 4th edition. California: University Science Books 1–359. [Google Scholar]
- MichenerCD.2000.The bees of the world. Baltimore, MD: Johns Hopkins University Press. [Google Scholar]
- NeilandMR, Wilcock CC.1998. Fruit set, nectar reward and rarity in the Orchidaceae. American Journal of Botany 85: 1657–1671. [PubMed] [Google Scholar]
- PabstGF, Dungs F.1977.Orchidaceae Brasilienses II. Hildesheim: Brucke‐Verlag. [Google Scholar]
- PorschO.1905. Beitrage zur histologischen Blütenbiologie I. Österreichische Botanische Zeitschrift 55: 165–173. [Google Scholar]
- RasmussenFN.1986. On the various contributions by which pollinia are attached to viscidia. Lindleyana 1: 21–32. [Google Scholar]
- SingerRB.2002. The pollination mechanism in Trigonidium obtusum Lindl. (Orchidaceae: Maxillariinae): Sexual mimicry and Trap‐flowers. Annals of Botany 89: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der CingelNA.1995.An Atlas of orchid pollination: European orchids. Rotherdam: Balkema Publishers. [Google Scholar]
- van der CingelNA.2001.An Atlas of orchid pollination. America, Africa, Asia and Australia. Rotherdam: Balkema Publishers. [Google Scholar]
- van den DoolH and Kratz DJ.1963. A generalization of the retention index system including liner temperature programmed gas‐liquid partition chromatography. Journal of Chromatography 11: 463–471. [DOI] [PubMed] [Google Scholar]
- van der PijlL, Dodson CH.1966.Orchid flowers – their pollination and evolution. Coral Gables: University of Miami Press. [Google Scholar]
- WhittenWM, Williams NH, Chase MW.2000. Subtribal and generic relationships of Maxillarieae (Orchidaceae) with emphasis in Stanhopeinae. American Journal of Botany: 87: 1842–1856. [PubMed] [Google Scholar]


