Abstract
• Background and aims Eryngium alpinum (Apiaceae) is an endangered perennial, characteristic of the Alpine flora. Because the breeding system influences both demographic (reproductive success) and genetic (inbreeding depression, evolutionary potential) parameters that are crucial for population maintenance, the reproductive ecology of E. alpinum was investigated. Specifically, the aims of the study were (1) to determine the factors (resources and/or pollen) limiting plant fitness; and (2) to assess the potential for gene flow within a plant, within a patch of plants, and across a whole valley where the species is abundant.
• Methods Field experiments were performed at two sites in the Fournel valley, France, over three consecutive years. Studies included a phenological survey, observations of pollinators (visitation rates and flight distances), dispersal of a fluorescent powder used as a pollen analogue, the use of seed traps, determination of the pollen/ovule ratio, and an experiment to test whether seed production is limited by pollen and/or by resources.
• Key results E. alpinum is pollinated by generalist pollinators, visitation rates are very high and seed set is resource‐ rather than pollen‐limited. The short flights of honeybees indicate a high potential for geitonogamy, and low pollen and seed dispersals suggest strong genetic structure over short distances. These results are interpreted in the light of previous molecular markers studies, which, in contrast, showed complete outcrossing and high genetic homogeneity.
• Conclusions. The study highlights the usefulness of adopting several complementary approaches to understanding the dynamic processes at work in natural populations, and the conservation implications for E. alpinum are emphasized. Although the studied populations do not seem threatened in the near future, long‐term monitoring appears necessary to assess the impact of habitat fragmentation. Moreover, this study provides useful baseline data for future investigations in smaller and more isolated populations.
Key words: Conservation biology, Eryngium alpinum, Apiaceae, breeding system, phenology, pollen/ovule ratio, pollination ecology, pollen limitation, pollen and seed dispersal, reproductive success, resource allocation, molecular markers
INTRODUCTION
Because of its crucial role in reproductive success and in the level and distribution of genetic variability, the breeding system lies at the very heart of population health and maintenance. Although once controversial (Lande, 1988), there is now a wide consensus among conservation biologists that, in addition to the loss and destruction of suitable habitat (very often the primarily causes of extinction), the maintenance of populations is strongly influenced by both demographic and genetic mechanisms (Frankham and Ralls, 1998; Saccheri et al., 1998). These two kinds of mechanisms do not act independently from each other but, rather, interact and the breeding system lies at the interface between them: the reproductive success partly determines the growth rate of a population whereas mating patterns (gene flow through pollen) and gene flow through seeds influence the level and distribution of genetic variability. These dispersal capacities have consequences both at the individual level, through the inbreeding coefficient and possible inbreeding depression, and at the population level through the evolutionary potential. Therefore, because the breeding system determines both quantitatively (demographic) and qualitatively (genetic) crucial parameters, the characterization of the reproductive biology of endangered species provides invaluable information to suggest appropriate conservation measures.
The breeding system of a species or a population is determined by a large number of pre‐ and post‐fertilization factors, either biotic or abiotic (Barrett, 1998). Among pre‐fertilization factors, nutrient and pollen availability are of paramount importance (Haig and Westoby, 1988; Parra‐Tabla et al., 1998). The level of abiotic resources acts upon both pollen and seed production, and 62 % of the species have been shown to be pollen‐limited (Burd, 1994; Byers, 1995), either because of low pollen production, or because of low pollinator activity. Some other determinants of the breeding system are floral biology (e.g. the relative positions of stigmas and stamens), spatial population structure (e.g. density, patchiness) and plant–pollinator interactions (identity, specificity and activity of pollinators).
Eryngium alpinum (Apiaceae) is an endangered sub‐alpine species (Gillot and Garraud, 1995) patchily distributed in France, Italy, Switzerland and Austria, and possibly in Rumania, ex‐Yugoslavia and Slovakia (Cherel and Lavagne, 1982). The plant is characteristic of the Alpine flora, growing in open habitats (avalanche corridors or hayfields) at altitudes between 1500 m and 2000 m. The species is threatened by human activities, mainly by picking for commercial use (flowering stems used to be extensively cut and sold as dried bouquets in towns) and by changes in land use leading to habitat destruction and fragmentation. Indeed, former hayfields are now either used as pastures, which limits flowering, or are abandoned, leading to habitat closure unfavourable to E. alpinum. Although locally abundant, some populations are known to have decreased in size while others have disappeared. Eryngium alpinum is now protected all over Europe (European Habitat Directive, see Wyse‐Jackson and Akeroyd, 1994), and is considered vulnerable by the International Union for the Conservation of Nature (IUCN; Gillot and Garraud, 1995). The species is especially abundant in the 12 km‐long Fournel valley, in the French Alps, where it is found either as restricted patches of plants or as large populations.
The present study was conducted with a conservation perspective. Various field experiments were performed at two sites in the valley and over 3 consecutive years. The aims were to determine the factors limiting the male (pollen production) and female (seed production) fitness of the species, and to quantify the potential for gene flow (important since it influences both the risk of inbreeding depression and the ability of the plants to adapt to a changing environment). More specifically, the main questions addressed were: (1) is the species dependent on insects for pollination, and what are the main pollinators? (2) How far are pollen and seeds dispersed, and may gene flow occur across the valley (dependant on pollen and seed dispersal, but also on the synchronization of the flowering periods among different sites)? (3) Is seed set limited by resources and/or pollen availability? (4) Is pollen production limited by resources? The results show that pollination is opportunistic and very efficient, but that seed production is resource‐limited and that pollen and seed dispersal are low. The observed patterns are interpreted in the light of previous studies of genetic structure (M. Gaudeul, unpubl. res.) and selfing rate (Gaudeul and Till‐Bottraud, 2003), showing the usefulness of using different approaches to elucidate the processes at work in natural populations. In addition, conservation implications of the results are discussed.
MATERIALS AND METHODS
Study species
Eryngium alpinum L. is a perennial Apiaceae with a longevity probably greater than 15–20 years. Plants reach sexual maturity after 2–3 years, and subsequently flower mostly every other year (Cherel and Lavagne, 1982). Each flowering individual generally produces one to five stems, each of them bearing one to five inflorescences (one terminal and zero to four axillary inflorescences). Each inflorescence produces 200–300 flowers that have a helicoidal repartition and open in sequence from bottom to top. The helices spanning from the bottom to the top of a given inflorescence are all composed of the same number of flowers (hereafter referred to as the ‘number of ranks of flowers of the inflorescence’). Each flower produces two ovules and five stamens, which are exerted a few hours before dehiscence. Fruits are schizocarpous diachenes.
Study sites
The experiments were performed at two sites in the Fournel valley (44°79′N, 6°53′E), located 10 km south of the city of Briançon, France. Eryngium alpinum is patchily distributed throughout the 12 km‐long, east–west orientated valley, included into the peripheral zone of the Ecrins National Park. The first site, called ‘Bernards’, is at the eastern entrance of the valley, 1550 m high, and is an abandoned 1 ha hayfield, which is currently undergoing extensive shrub development. The second one, ‘Deslioures’, is 8 km deeper into the valley, at the bottom of an avalanche corridor and 1600 m high. Deslioures is the densest and largest E. alpinum site in Europe (approx. 12 ha), containing several hundred thousand plants. The climate is continental, with great differences in temperature through the year (maximum in July) and low precipitation (minimum in June). The snow cover usually lasts for 6 months, from November to April.
Phenology
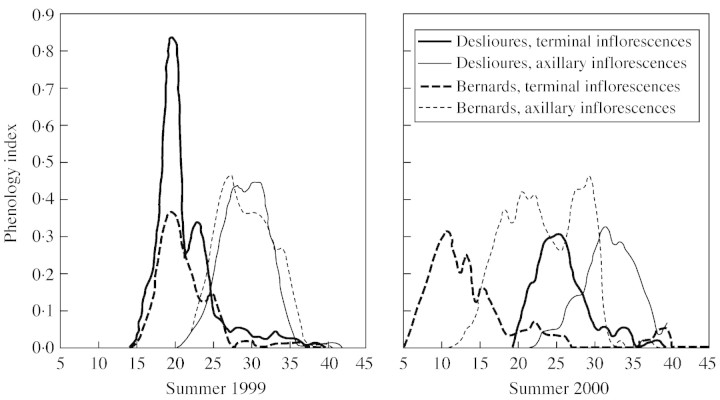
In 1999 and 2000, 20 randomly chosen individuals were marked at each site when stems and inflorescences were still green (10 and 14 July, respectively). For all inflorescences in 1999, and for all inflorescences of one stem per individual in 2000, the number of ranks of flowers with visible stamens was monitored daily until the end of flowering (10 and 15 August, respectively). For each inflorescence, a daily phenology index was calculated as the proportion of ranks with visible stamens, and the population index was calculated as the average of the inflorescence indices.
Floral visitor observations
Observations were conducted at both sites during summers of 1998, 1999 and 2000. A random (terminal or axillary) inflorescence was chosen and the number and duration of pollinators’ visits were monitored in 20‐min observation intervals. The taxonomic family of each visitor was noted. All observations were made on inflorescences of similar size and phenology (dehiscent stamens on at least half of the inflorescence) and on sunny days, between 1200 h and 1400 h (solar time). No pollinators were observed on the flowers early or late in the day and when the weather was cloudy (M. Gaudeul, pers. obs.). For a given observation interval, the total duration of visits was calculated by summing the visitation time across all visitors that arrived during a given observation interval. A one‐way analysis of variance (ANOVA) was performed to test the effect of the inflorescence type (terminal or axillary, fixed factor) on the number and total duration of visits among the 3 years. Identification of pollinators was also done once during the night.
In summer 2000, the flight distance of randomly chosen pollinators was measured, and it was noted whether the inflorescence visited next belonged to the same plant or not. In order to test if foraging insects were species‐constant, similar observations were made in 1998 on 35 bees and five bumblebees, but the insects were marked by a small, white paint spot, and followed for as long as possible.
Fluorescent powder dispersal
Fluorescent dye (Radiant Color™) was used as a pollen analogue to track visitors’ movements (Waser and Price, 1982; Waser, 1988). Because it was impossible to mark only the stamens, the powder was applied to the whole inflorescence with a paintbrush. We checked that pollinators showed no behavioural changes when encountering dye‐marked flowers compared with unmarked flowers (unpubl. res.). This was done at 0700 h (solar time) for the daytime experiments (seven in 1998, four in 2000) and at 1900 h for the nocturnal experiments (three in 2000). The marked inflorescences were always located in areas with a homogeneous and dense cover of plants (about 5 plants m–2). At 2000 h (0300 h for the nocturnal experiments), all the inflorescences found along two perpendicular transects centred on the marked inflorescence were observed with an ultraviolet lamp. For each of them, the distance to the marked inflorescence was noted together with the presence or absence of fluorescence. Experiments were performed on different days and in different areas so that they did not interfere with each other. In 1998, three different dyes were used on very close inflorescences to distinguish the transport of pollen by pollinators and by wind. Wind pollination was suspected because mature stamens are largely exerted from the inflorescences. One inflorescence was marked with red dye (insects + wind transport), another one was marked with blue dye and bagged with fine nylon mesh (negative control), and the third one was marked with yellow dye and bagged with wide nylon mesh (wind transport only). In 2000, only one inflorescence was marked with red dye to estimate insects + wind transport.
Seed dispersal
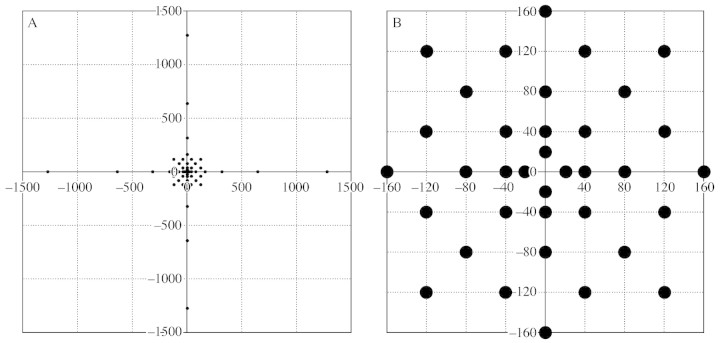
At the end of summer 2000 (12 September) in Deslioures, seed traps were placed around ten isolated stems with only one (terminal) inflorescence. Seed‐traps consisted of 140 mm diameter Petri dishes in which a fine layer of grease was smeared in order to fix the seeds after they fell. Traps were placed following a precise scheme at known distances between 20–1280 cm from the target plant (48 traps per plant, of which 36 were within a 160 cm radius; Fig 1). The inflorescences were bagged with fine nylon mesh, and mature seeds were allowed to fall and disperse only once the experimental procedure was set up. It was initially planned to monitor the number of seeds that fell in each trap daily for at least 7 d, but, because of rain, this was done for 3 d only. However, it was observed that most seeds had fallen by that time.
Fig. 1. Spatial distribution of seed traps for the study of seed dispersal. (A) Overall pattern around the target plant. (B) Enlarged pattern in a 160 cm radius around the target plant. The size of circles approximately corresponds to the size of seed traps.
Pollen/ovule ratio
During the summers of 1998 and 1999, stamens were collected before dehiscence and were stored individually in Eppendorf tubes. In 1998, stamens were sampled from 23 and 20 individuals from Deslioures and Bernards, respectively. For each individual, one stamen was collected from two flowers on each of two terminal and two axillary inflorescences. In 1999, stamens were collected on only one terminal inflorescence per plant, on 25 and 21 individuals in Deslioures and Bernards, respectively. In the laboratory, each stamen was crushed manually with a small pestle, sonicated in a small volume of 95 % alcohol to destroy pollen aggregates, and evaporated. Then, pollen grains were resuspended in 100 µL of a 20 % sucrose/20 % glycerol solution, and counted under microscope using a 2 × 1 µL hematocytometer (two counts per stamen). With each flower producing two ovules and five stamens, the pollen: ovule (P/O) ratio of a flower was calculated as follows: P/O = number of pollen grains in 1 µL × 50 × 5. ANOVAs were performed on the resulting P/O ratios: (1) for each year, to test the effects of population (fixed), individual (random, nested in population), inflorescence type (fixed), inflorescence (random, nested in individual) and flower (random, nested in inflorescence); and (2) on the overall data set, adding the year as a fixed factor. In the latter case, only data on terminal inflorescences were included in the 1998 data set. When needed, P/O ratios were square‐root transformed to meet the normality assumption of residuals.
Resource/pollen limitation
In 1998, before flowering began, 60 stems were randomly marked at the site Deslioures and assigned to one of three treatments. Twenty stems served as controls. On another 20 stems, the terminal inflorescence and all but one axillary inflorescence were removed to determine if this would result in a reallocation of nutrient resources to the remaining inflorescence. And on the 20 remaining stems, pollen was manually supplemented on one axillary inflorescence to test for possible pollen‐limitation of the seed set. Pollen was collected from randomly chosen, bagged inflorescences and spread onto stigmas with a paintbrush. At the end of flowering, the inflorescences were bagged in fine nylon mesh to prevent the seeds from dispersing, and seeds were collected when ripe. Some inflorescences did not fully develop (the flowers did not open) and produced no seeds at all or very few. The other inflorescences were fully developed and had a high seed set (0·6–0·7 seed/ovule ratio; Gaudeul and Till‐Bottraud, 2003) or only partly developed and had a reduced seed set (about 0·2–0·4 seed/ovule). Therefore, seed set was estimated according to three classes: high, medium or low. In 1999, the same protocol was followed at both sites and on 2 × 50 stems per site. The pollen supplementation treatment was not performed as a consequence of the results of the 1998 experiment (see below). χ2 tests were performed to test for a significant seed‐set difference between inflorescence types, treatments, or study sites.
RESULTS
Phenology
Within a plant, the periods of sexual maturity of flowers largely overlap, both within and among inflorescences (Table 1). At each site, the flowering season was 3–4 weeks long and, for a given type of inflorescence, flowering was highly synchronous within a site (Fig. 2). Moreover, although a delay was observed between Bernards and Deslioures in summer 2000, their flowering periods largely overlapped.
Table 1.
Summary statistics for phenology for the summers of 1999 and 2000. Data obtained at both sites (Bernards and Deslioures) were pooled and expressed as mean ± s.d. All durations and time‐lags are in days
| 1999 | 2000 | |
| Duration of the flowering period within an inflorescence | 7·1 ± 2·0 (n = 209) | 6·9 ± 2·5 (n = 118) |
| Average number of simultaneously flowering ranks | 9·0 ± 2·0 (n = 209) | 8·9 ± 3·6 (n = 118) |
| Average total number of ranks on an inflorescence (terminal and axillary) | 17·7 ± 3·2 (n = 209) | 16·6 ± 3·2 (n = 118) |
| Duration of the flowering period on a whole plant | 17·8 ± 3·9 (n = 40) | – |
| Time lag between terminal inflorescences | 4·0 ± 3·8 (n = 32) | – |
| Time lag between axillary inflorescences | 1·2 ± 1·3 (n = 52) | 1·4 ± 1·4 (n = 30) |
| Time lag between terminal and axillary inflorescences | 6·9 ± 1·0 (n = 63) | 7·0 ± 2·2 (n = 28) |
Fig. 2. Phenology of terminal and axillary inflorescences at the two sites investigated in 1999 and 2000. The phenology index was calculated as the proportion of flowers with dehiscent stamens. Along the x‐axis, days are numbered from 1 (1 July) to 45 (14 August).
Floral visitor observations
Species observed on E. alpinum inflorescences are listed in Appendix 1. Lepidoptera, Coleoptera and Hymenoptera were the most represented orders in terms of the number of species observed. Two distinct guilds of pollinators could be identified: one during the night (mainly composed of Lepidoptera), the other during the day (mainly composed of Hymenoptera). During the day, honeybees accounted for the highest number and duration of visits for all years of study. Across years, they averaged 84 % (± 1 %) and 85 % (± 0·4 %) of the total number of visits and total visit duration, respectively. They visited a high number of flowers within a single inflorescence by moving from one to another, most often from bottom to top rows (M. Gaudeul, pers. obs.). Pollinators showed constancy in flower‐ foraging: after visiting E. alpinum, no insect was seen on flowers of another species.
In a total of 188 20‐min observation periods, the total length of visits averaged 19·3 ± 25·3 min, and the mean number of visits was 8·9 ± 9·2. There was no significant difference among years or among sites, but terminal inflorescences were significantly more visited than axillary ones (ANOVA on the overall data set, P = 0·0067 and P = 0·0023 for number and total duration of visits, respectively; Fig. 3).
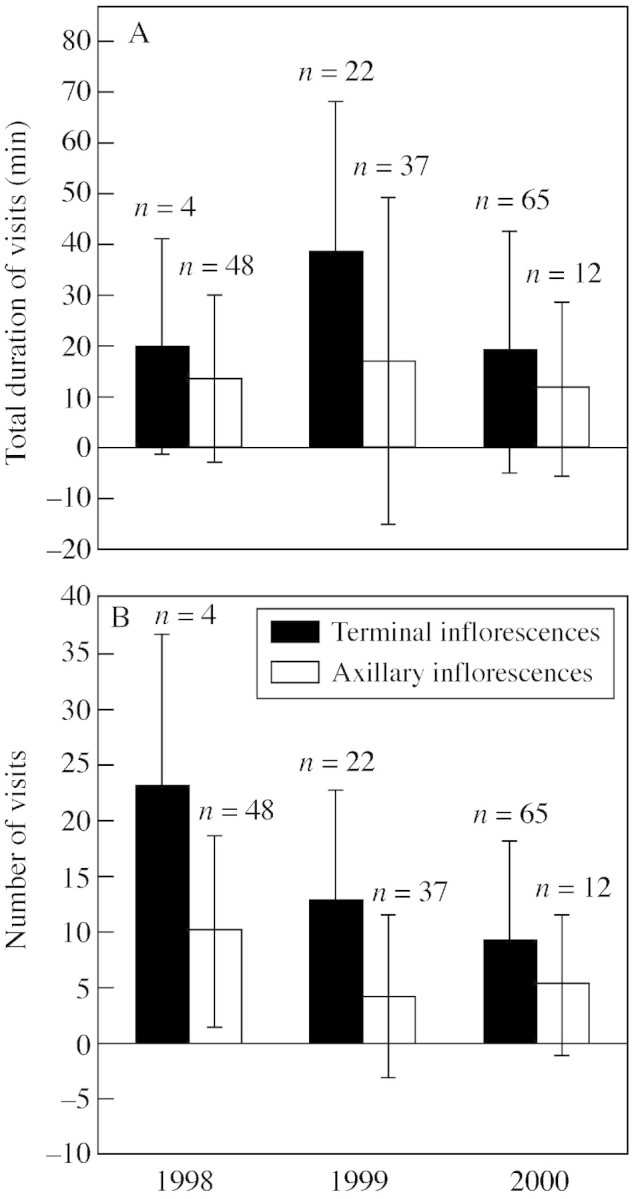
Fig. 3. (A) Total duration and (B) number of pollinator visits on terminal and axillary inflorescences in 1998, 1999 and 2000.
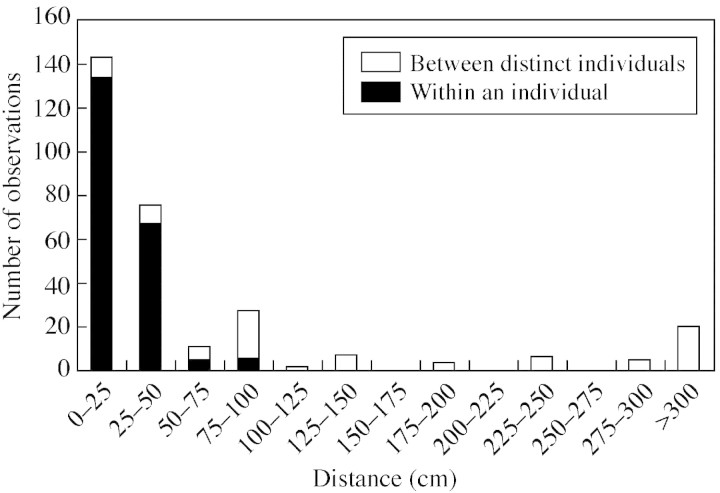
The distribution of flight distances was leptokurtic (Fig. 4): insects flew less than 50 cm away in 72 % of the cases, and more than 3 m away in 7 % of the cases. Moreover, 70 % of the flights occurred within a single plant.
Fig. 4. Travel distances for pollinators and proportion of within‐ vs. between‐plants flights in 2000.
Fluorescent powder dispersal
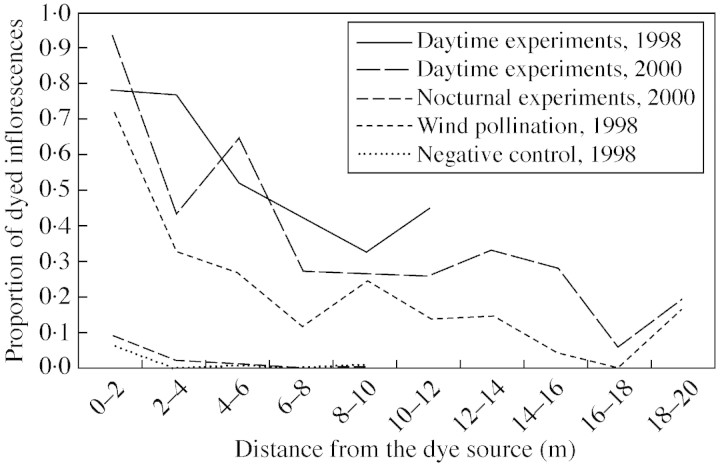
In 1998, the proportions of inflorescences observed with yellow (wind transport) or blue (control) dyes were very similar and very low (Fig. 5). The red dye (insects + wind transport) was very commonly detected within a 2 m radius (about 80 % of the observed inflorescences) and subsequently declined when the distance increased. In 1998, the same trend was observed on the number of fluorescent grains per stigma (data not shown). Fluorescent powder was found on about 30 % of the observed inflorescences located 10 m away from the source, and the maximal distance travelled by the powder varied from 5·6–22·3 m in the 1998 experiments, from 2·4–18·8 m in the daytime 2000 experiments, and from 4·3–15·2 m in the nocturnal 2000 experiments. Dispersal patterns did not show any directional trend.
Fig. 5. Dispersal patterns of the fluorescent powder used as a pollen analogue for different experimental treatments. The ‘proportion of dyed inflorescences’ represents the proportion of observed inflorescences with dye particles on them. Experiments of the same type (nocturnal, daytime, wind pollination and negative control) were pooled for each year (1998 and 2000).
Seed dispersal
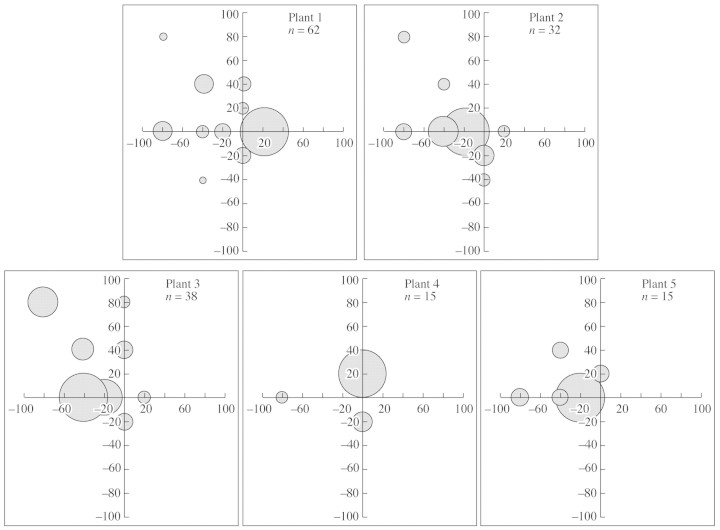
The number of seeds collected in the traps ranged from 3–62 per plant, averaging 19·2 ± 18·2. Given that each inflorescence produces up to 200–300 seeds, only a low proportion of the total seed production was thus trapped, indicating that the protocol is probably not very well suited to this species. A spatially continuous design of seed collection (rather than this discrete one) would probably be more efficient. Despite this experimental caveat, which is not likely to strongly modify the observed patterns of seed dispersal, it was found that all the seeds remained very close to their mother‐plant, and the highest dispersal distance was 110 cm (Fig. 6).
Fig. 6. Patterns of seed dispersal observed on the five mother‐plants for which the highest number of seeds were collected (n from 15–62 seeds per plant). Distances from the mother‐plant are indicated in cm along both axes and the size of the circles is proportional to the number of seeds in the corresponding seed‐trap.
Pollen/ovule ratio
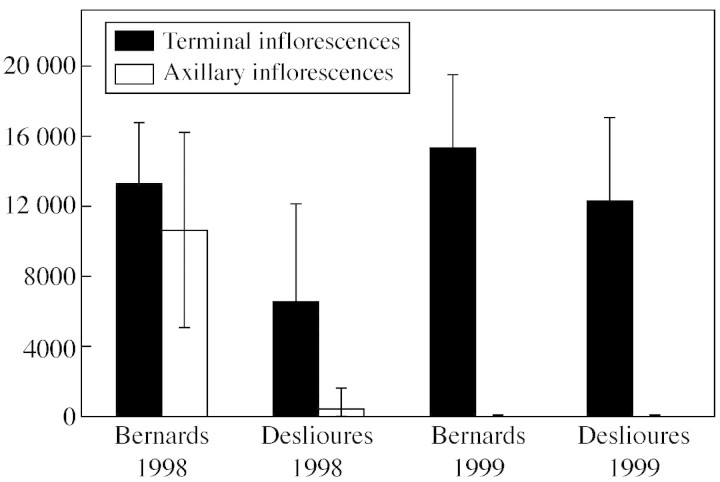
In 1998, terminal inflorescences had a significantly higher P/O ratio than axillary ones at both sites (P < 0,001; Fig. 7, no data in 1999), and this difference was much stronger at Deslioures than at Bernards (population × type interaction, P < 0·001). In 1998 and 1999, plants at Bernards had a significantly higher P/O than at Deslioures (P < 0·001). At both sites, pollen production of terminal inflorescences was significantly lower in 1998 than in 1999 (P < 0·0001) and significant effects of the individual plant and of the inflorescence were detected (P < 0·001), but not of the flower.
Fig. 7. Pollen/ovule ratios obtained for terminal and axillary inflorescences at both sites (1998 and 1999).
Resource/pollen limitation
On control stems, seed set was significantly lower in axillary compared with terminal inflorescences in both years and at both sites (χ2 tests, P = 0·002 in 1998 and P < 0·001 at both sites in 1999; Table 2). Pollen supplementation of one axillary inflorescence did not increase its seed‐set (P > 0·05). In contrast the early removal of the terminal and other axillary inflorescences lead to a significant increase in the seed set of the remaining axillary inflorescence in both years and at both sites (P = 0·003 in 1998 and P < 0·001 in 1999 at both sites). However, seed‐set of these axillary inflorescences was still lower than seed set of terminal ones in two experiments out of three (P > 0·05 in 1998 and P < 0·001 at both sites in 1999).
Table 2.
Resource/pollen limitation. Seed set estimates of terminal and axillary inflorescences assigned to different treatments in summer 1998 and 1999
| Control | Terminal inflorescence removed | Pollen supplementation | |||||
| Year | Site | Seed‐set | Terminal | Axillary | Axillary | Terminal | Axillary |
| 1998 | Deslioures | High | 13 | 8 | 9 | 14 | 2 |
| Medium | 3 | 4 | 7 | 4 | 7 | ||
| Low | 4 | 24 | 4 | 2 | 27 | ||
| 1999 | Deslioures | ||||||
| High | 41 | 5 | 14 | – | – | ||
| Medium | 4 | 15 | 30 | – | – | ||
| Low | 0 | 53 | 13 | – | – | ||
| 1999 | Bernards | ||||||
| High | 18 | 1 | 5 | – | – | ||
| Medium | 4 | 5 | 17 | – | – | ||
| Low | 1 | 36 | 10 | – | – | ||
DISCUSSION
In the two large populations that were studied, pollinators were diverse and abundant, and seed set did not seem to be pollen‐limited. About 50 species of both diurnal and nocturnal insects were observed on E. alpinum inflorescences and no effect of wind could be detected. Honeybees (Apis mellifera) and bumblebees were the most frequent visitors. Thus, E. alpinum is opportunistic for its pollination and relies on abundant insects. High pollen availability was confirmed by the experimental pollen supplementations of axillary inflorescences, which did not lead to increased seed set. The highly attractive nature of the species is very probably linked to the mass‐flowering phenomenon. In large populations, the density is quite high (1–10 plants m–2) and the phenological survey showed that flowering was limited to a 3–4‐week period. Mass‐flowering is often observed in harsh environments, such as alpine ones, where the short breeding season causes a large amount of blossoms to appear and open over a short period in order to complete seed maturation (Bliss, 1971). Colour (a very intense blue‐violet in E. alpinum) is often regarded as a global advertisement to pollinators over relatively long distances, whereas the ultimate (short‐distance) attractants are pollen and nectar (Waser, 1983).
In contrast to pollen, abiotic resources appeared to be limiting. A much lower pollen and seed production in axillary compared with terminal inflorescences was observed. For seed production, the higher visitation rate of terminal compared with axillary inflorescences may increase pollen availability for terminal inflorescences and lead to a higher seed set. However, the pollen supplementation treatment had no significant effect on seed set and allowed the rejection of this hypothesis. Testing whether manual pollination is efficient in E. alpinum is difficult. This would require inflorescences to be pollinated, then bagged to exclude herbivores, and finally to have seed‐set monitored; but earlier studies, involving controlled crosses, have shown that bagging inflorescences immediately after pollination can lead to a significant reduction in seed set (Gaudeul and Till‐Bottraud, 2003). Nevertheless, it was observed earlier that manual self‐pollination lead to either equal or higher seed set than natural self‐pollination, and inflorescences were bagged in both cases (Gaudeul and Till‐Bottraud, 2003). Moreover, in the present experiment, inflorescences were bagged long after pollination. Thus, we believe that pollen supplementation was efficient.
The resource allocation experiment showed enhanced seed set of axillary inflorescences when all other inflorescences were removed, suggesting that resources were limited, and that there was a differential allocation within the plant: terminal inflorescences were favoured over axillary ones for both seed maturation and pollen production (as shown by P/O ratios). An alternative interpretation of the increased seed set of axillary inflorescences when other inflorescences are removed is increased pollination. However, given the high density of plants, pollinators would more probably visit other terminal inflorescences rather than the axillary one. Moreover, if removing neighbouring inflorescences causes any change in the visitation rate of the axillary inflorescence, it may actually be to reduce visits, as the overall attractiveness of the individual plant as a whole would be lower. Finally, it was shown that pollen was not limiting seed set, leading us to exclude this alternative explanation of increased pollination. Interestingly, the difference between pollen production of terminal and axillary inflorescences was larger at the more resource‐limited site, Deslioures, which exhibited lower P/O ratios than the Bernards site. This lower level of abiotic resources was also suggested by the lower species’ richness in Deslioures (M. Gaudeul, pers. obs.) and by the different history of each site: Bernards used to be exploited for hay and probably fertilized, whereas Deslioures has never been. Differential resource allocation within a single inflorescence was also often observed, as flowers from bottom rows (the first to flower) set more seeds than flowers from top rows (M. Gaudeul, pers. obs.). This was congruent with the fact that most E. alpinum plants do not flower every year but, rather, every other year (Cherel and Lavagne, 1982). They probably store resources in their taproot during one year and make use of this energy to produce flowers and seeds the following year.
Given the energetic cost of floral production, the almost null reproductive success of axillary inflorescences raises the question of why they have been maintained through evolution. Possible (non‐exclusive) hypotheses are (1) maximization of the reproductive output in a variable environment where soil resources and/or pollinators may be transiently abundant (‘bet‐hedging’ strategy; Moody‐Weiss and Heywood, 2001); (2) reproductive assurance in case the terminal inflorescence is broken or eaten by animals; (3) increased attractiveness of the plant thanks to intense colour and nectar production; and (4) enhanced male fitness of the plant via pollen dispersal and fertilization of ovules (Queller, 1983). This latter mechanism could lead to gender‐specialization of flowers, which has been shown to occur in some species in response to dichogamy (e.g. in the protogynous Pseudocymopteyrus montanus; Schlessman and Graceffa, 2002) or to a negative correlation between male and female reproductive success (e.g. in the andromonoecious Solanum carolinense; Elle and Meagher, 2000). Additional studies are required to determine which of these mechanisms is the most important one in E. alpinum.
Both direct (insects) and indirect (fluorescent powder) observations suggested that pollen transfer was most likely to happen within a plant either between (70 % of the flights) or within inflorescence. Within an inflorescence, foraging insects very often transported pollen from stamens to stigmas of the same flower or of a neighbouring flower. However, the male and female phases of an inflorescence are temporally separated by a strong protandry, and controlled crosses showed partial self‐incompatibility (Gaudeul and Till‐Bottraud, 2003). Moreover, estimates of outcrossing rates from maternal seed progenies using molecular markers were close to 100 % (Gaudeul and Till‐Bottraud, 2003). Thus, although a high rate of geitonogamous selfing was expected from pollinators observations, this was not achieved. A high outcrossing rate is in agreement with the high pollen/ovule ratio: outcrossing causes pollen loss when pollen is transferred from one plant to another, and this is often compensated by increased pollen production (Cruden, 1977). This provides a clear example of the need to bring together different types of results in order to reach a reliable understanding of the mechanisms at work in populations.
The study of dispersal distances gave another example of the complementary nature of field observations and molecular markers. Even when pollen was transferred among plants, it was transported over relatively short distances: insect flights were less than 50 cm in 72 % of the cases and more than 3 m in only 7 % of the cases, the fluorescent powder was never found more than 22 m away from the source inflorescence and primary seed dispersal was very restricted, with seeds falling in the immediate vicinity of the mother‐plant. These results suggested strong within‐population structure or, at least, strong differentiation between patches of plants within the valley. Once again, contrary to this expectation based on field observations, a genetic survey carried throughout the Fournel valley showed a very high genetic homogeneity and could detect any population substructure within the valley. Pairwise differentiation tests performed among five a priori defined groups of plants only detected significant divergence in three cases out of ten, when the most distant groups, separated by 4–10 km, were considered (M. Gaudeul, unpubl. res.). This discrepancy might be explained by several factors, either due to intrinsic differences between direct (field) and indirect (molecular) methods in measuring dispersal, to more specific characteristics of our field experiments, or to the biology of the species.
First, both insects observations and fluorescent powder tracking experiments often underestimate pollen dispersal (Campbell and Dooley, 1992; Ouborg et al., 1999; Sork et al., 1998, 1999; Fenster et al., 2003). Occasional fluorescent grains (or pollinators) might travel far away and remain undetected because the search becomes more and more difficult as the distance from the source increases. In contrast, even occasional successful long‐distance dispersal events are detected with molecular markers. Second, field and genetics studies would probably infer different dispersal patterns if pollen carry‐over occurs. If several insects successively transport pollen from its mother‐plant to intermediate inflorescences, and then to its final recipient‐plant, this would potentially increase the dispersal distance and would not be taken into account by the type of field observations that we carried out (but it could be studied through other experimental designs). It is interesting to note that field and molecular data were much more congruent when restricted areas were studied. Spatial autocorrelation analyses, carried out in two 50 × 10 m quadrats where plants were sampled every 2 m, allowed us to estimate average gene dispersal distances of 1·10 and 1·30 m, respectively (M. Gaudeul, unpubl. res.), thus very close to the pollen and seed dispersal distances that we observed in the field. Third, whereas direct observations provide information on current and potential gene flow (without any insight into the outcome of the fertilization, germination and early survival processes), molecular markers are informative on past and effective gene flow (Sork et al., 1998, 1999; Austerlitz et al., 1999; Ouborg et al., 1999).
Other possible factors responsible for the underestimation of pollen dispersal distances are more strictly related to our own experimental designs and may at least partly explain the difference between field and molecular studies. First, given that the number of available plants increases when the distance from the source inflorescence increases, the proportion of marked inflorescences decreases but the absolute number of inflorescences reached by the powder (or pollen) can still be quite high. Second, we mainly studied daytime pollination and although our nocturnal observations do not seem to confirm this trend, it has been shown that nocturnal pollinators (mostly Lepidopteres) often transport pollen further than diurnal ones (mostly Hymenopteres; Handel, 1983; Herrera, 1987; Young, 2002). Third, our field experiments took place during peak flowering and it has been observed that pollen dispersal distances are higher earlier and later in the season because flowering plants are less numerous and, consequently, more distant from each other (Eguiarte et al., 1993). Thus, gene flow through pollen is certainly more extensive than our field results showed and, given that the flowering periods of the different sites largely overlap, some long‐range pollen flow could exist between sites within the valley. Secondary seed transport might also be important as mature fruits have barbs on the dried sepals: animals, humans or tractors can probably transport seeds, and farmers occasionally find some in sheep’s wool and in hay. The extent of this long‐range pollen and seed gene flow is, however, impossible to determine through direct observations. Finally, the biology of the species may also at least partly explain the absence of genetic structure over short distances. Eryngium alpinum is a long‐lived perennial with overlapping generations, two parameters known to be responsible for homogenizing gene frequency and increasing genetic inertia when disturbance events (such as fragmentation) occur (Austerlitz et al., 1999; Austerlitz et al., 2000).
From a conservation perspective, this study showed that the high attractiveness of synchronously flowering patches of plants makes pollination very efficient. Moreover, pollen production is high. Thus, the limitation of seed production of axillary inflorescences (very few of them set seeds, while seed‐set of terminal inflorescences averages 0·70 seeds per ovule; Gaudeul and Till‐Bottraud, 2003) is resource‐ rather than pollen‐driven. However, because environmental heterogeneity is known to be strong, several years of monitoring of pollen and resource limitation are needed (e.g. Baker et al., 2000; Wilcock and Neiland, 2002).
The present study was performed at two sites where visitation rates may be higher than in most E. alpinum populations. The first factor responsible for this is the presence of hives in the vicinity of both sites, and the second is that these sites are the largest and densest in the whole distribution area of the species. Small populations should therefore be studied because land closure and weaker floral attractiveness may lead to lower visitation rates, reduced fecundity and poor offspring performance (Allee effect; e.g. Roll et al., 1997; Groom, 1998; Kéry et al., 2000). In this respect, this study provides baseline data regarding the reproductive biology of the endangered plant E. alpinum, which should help for the design of further experimental assessments of population viability and, ultimately, allow effective management plans to be proposed.
Moreover, based on two examples (the outcrossing rate and the genetic substructure within the valley), this study shows the value of bringing together several kinds of data in conservation biology (field experiments, molecular markers surveys, population dynamics monitoring, etc.). Molecular methods allow investigations on broader spatial and temporal scales than field experiments, but both kinds of data are relevant in a conservation perspective. For instance, field experiments give more insight into the exact, mechanistic processes at work in natural populations and, because they measure current dispersal rather than a past average, they may detect the impact of fragmentation or other recent changes on gene dispersal much more rapidly than molecular markers.
ACKNOWLEDGEMENTS
We thank all the students who helped in collecting and analysing data: S. Bonin, O. Duchemin, H. N. Fournier, A. Gardès, N. Juillet, F. Noël, A. M. Nordström, V. Ravigné, A. Rivat, G. Rouhan and P. Saccone. We are grateful to the Parc National des Ecrins, to the Office National des Forêts (ONF) and to the farmers working in the Fournel Valley for their logistic support and frequent advice. We also thank three anonymous reviewers for helpful comments on a previous version of this manuscript. M.G. was supported by a grant from the French Ministère de l’Education Nationale, de la Recherche et de la Technologie. This project was partly financed by the French Ministère de l’Aménagement du Territoire et de l’Environnement and the Région Rhône‐Alpes.
APPENDIX 1.
Non‐exhaustive list of E. alpinum visitors
| Order | Family | Species |
| Coleoptera | Cerambycidae | Judolia sexmaculata |
| Corymbia rubra | ||
| Clytus arietis | ||
| Cartalum ebulinum | ||
| Tillius sp. | ||
| Cetonidae | Trichius fasciatus | |
| Cetonia aurata | ||
| Chrysomelidae | Clytra quadripuncatat | |
| Oreina geminata | ||
| Elateridea | sp. | |
| Cantharidae | sp. | |
| Cleridae | sp. | |
| Dermaptera | sp. | |
| Diptera | Tachimidae | Tachina fera |
| Asilidae | sp. | |
| Muscidae | sp. | |
| Hemiptera | Pentatomidae | Graphosoma italicum |
| Palonema parasina | ||
| Lygaeidae | Lygaeus equestris | |
| Cydinadea | sp. | |
| Miridae | sp. | |
| Anthochoridae | sp. | |
| Homoptera | Anthophoridae | Nomada sp. |
| Cicadoidea | sp. | |
| Hymenoptera | Apidae | Apis mellifera |
| Bombus sp. | ||
| sp. | ||
| Megachilidae | Heriades sp. | |
| Formicidae | Formica sp. | |
| Vespidae | sp. | |
| Sphecidae | sp. | |
| Tenthredinidae | sp. | |
| Lepidoptera | Geometridae | Scatopterix diniensis |
| Mninoa murinata | ||
| Noctuidae | Orthosia minosia | |
| Euxoa vitta | ||
| sp. | ||
| Arctiidae | Eilema complana | |
| Pyralidae | sp. | |
| Adelaidae | Adela sp. | |
| Nymphalidae | Cinclidia phoebe | |
| Zygaenidae | Zygaena sp. | |
| Lycaenidae | Lysandra coridon | |
| Psychidae | sp. | |
| Pterophoridae | sp. | |
| Tortricidae | sp. | |
| Mercoptera | Panorpa | sp. |
| Orthoptera | Acridinae | sp. |
| Tettigoniidae | sp. |
Received: 18 August 2003; Returned for revision: 13 January 2003; Accepted: 23 February 2004; Published electronically: 21 April 2004
References
- AusterlitzF,Brachet S, Couvet D, Frascaria‐Lacoste N, Jung‐Muller B, Kremer A, Streiff R.1999.Flux génétiques chez les arbres forestiers. Synthèse bibliographique Commission Ressources Génétiques Forestières, Bureau des Ressources Génétiques. http://www.brg.prd.fr/brg/textePdfs/Arbrequicache.pdf 18 Aug. 2003. [Google Scholar]
- AusterlitzF,Mariette S, Machon N, Gouyon P‐H, Godelle B.2000. Effects of colonization processes on genetic diversity: differences between annual plants and tree species. Genetics 154: 1309–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BakerAM,Barrett SCH, Thompson JD.2000. Variation of pollen limitation in the early flowering Mediterranean geophyte Narcissus assoanus (Amaryllidaceae). Oecologia 124: 529–535. [DOI] [PubMed] [Google Scholar]
- BarrettSCH.1998. The evolution of mating strategies in flowering plants. Trends in Plant Science 9: 335–341. [Google Scholar]
- BlissLC.1971. Arctic and alpine plant life cycles. Annual Review of Ecology and Systematics 2: 405–438. [Google Scholar]
- BurdM.1994. Bateman’s principle and plant reproduction: the role of pollen limitation in fruit and seed set. Botanical Review 60: 80–139. [Google Scholar]
- ByersDL.1995. Pollen quantity and quality as explanations for low seed set in small populations exemplified by Eupatorium (Asteraceae). American Journal of Botany 82: 1000–1006. [Google Scholar]
- CampbellDR,Dooley JL.1992. The spatial scale of genetic differentiation in a hummingbird‐pollinated plant: comparison with models of isolation by distance. American Naturalist 139: 735–748. [Google Scholar]
- CherelO,Lavagne A.1982. Aire de répartition, phénologie, biologie, reproduction d’Eryngium alpinum, ‘‘la Reine des Alpes’’, dans la vallée du Fournel. Propositions de mesures de protection de l’espèce. Travaux Scientifiques du Parc National des Ecrins 2: 53–92. [Google Scholar]
- CrudenRW.1977. Pollen–ovule ratios: a conservative indicator of breeding systems in flowering plants. Evolution 31: 32–46. [DOI] [PubMed] [Google Scholar]
- EguiarteLE, Búrquez A, Rodríguez J, Martínez‐Ramoz M, Sarukhán J, Piñero D.1993. Direct and indirect estimates of neighborhood and effective population size in a tropical palm, Astrocaryum mexicanum Evolution 47: 75–87. [DOI] [PubMed] [Google Scholar]
- ElleE,Meagher TR.2000. Sex allocation and reproductive success in the andromonoecious perennial Solanum carolinense (Solanaceae). II. Paternity and functional gender. American Naturalist 156: 622–636. [DOI] [PubMed] [Google Scholar]
- FensterCB,Vekemans X and Hardy OJ.2003. Quantifying gene flow from spatial genetic structure data in a metapopulation of Chamaecrista fasciculata (Leguminosae). Evolution 57: 995–1007. [DOI] [PubMed] [Google Scholar]
- FrankhamR,Ralls K.1998. Inbreeding leads to extinction. Nature 392, 441–442. [Google Scholar]
- GaudeulM,Till‐Bottraud I.2003. Low selfing rate in an endangered perennial plant, Eryngium alpinum L. American Journal of Botany 90: 716–723. [DOI] [PubMed] [Google Scholar]
- GillotP,Garraud L.1995.Eryngium alpinum (L.). In: Museum National d’Histoire Naturelle, Conservatoire Botanique National de Porquerolles, Ministère de l’Environnement, eds. Livre Rouge de la Flore Menacée, Vol. 1. Paris, France, 185. [Google Scholar]
- GroomMJ.1998. Allee effect limits population viability of an annual plant. American Naturalist 151: 487–496. [DOI] [PubMed] [Google Scholar]
- HaigD,Westoby M.1988. On limits to seed production. American Naturalist 131: 397–398. [Google Scholar]
- HandelSN.1983. Pollination ecology, plant population structure, and gene flow. In: Real L, ed. Pollination biology London: Academic Press, 163–211. [Google Scholar]
- HerreraCM.1987. Components of pollinator ‘‘quality’’: comparative analysis of a diverse insect assemblage. Oikos 50: 79–90. [Google Scholar]
- KéryM,Matthies D, Spillmann H‐H.2000. Reduced fecundity and offspring performance in small populations of the declining grassland plants Primula veris and Gentiana lutea Journal of Ecology 88: 17–30. [Google Scholar]
- LandeR.1988. Genetics and demography in biological conservation. Science 241: 215–244. [DOI] [PubMed] [Google Scholar]
- Moody‐WeissJ,Heywood JS.2001. Pollination limitation to reproductive success in the Missouri evening primrose Oenothera macrocarpa (Onagraceae). American Journal of Botany 88: 1615–1622. [PubMed] [Google Scholar]
- OuborgNJ,Piquot Y, van Groenendal JM.1999. Population genetics, molecular markers and the study of dispersal in plants. Journal of Ecology 87: 551–568. [Google Scholar]
- Parra‐TablaV,Vargas CF, Eguiarte LE.1998. Is Echeveria giggiflora (Crassulaceae) fecundity limited by pollen availability? An experimental study. Functional Ecology 12: 591–595. [Google Scholar]
- PritchardJK,Stephens M, Donnelly P.2000. Inference of population structure using multilocus genotype data. Genetics 155: 945–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- QuellerDC.1983. Sexual selection in a hermaphroditic plant. Nature 305: 706–707. [Google Scholar]
- RollJ,Mitchell RJ, Cabin RJ, Marshall DL.1997. Reproductive success increases with local density of conspecifics in a desert mustard (Lesquerella fendleri). Conservation Biology 11: 738–746. [Google Scholar]
- SaccheriI,Kuussaari M, Kankare M, Vikman P, Forteliust W, Hanski I.1998. Inbreeding and extinction in a butterfly metapopulation. Nature 392: 491–494. [Google Scholar]
- SchlessmanMA,Graceffa LM.2002. Protogyny, pollination, and sex expression of andromonoecious Pseudocymopterus montanus (Apiaceae, Apioideae). International Journal of Plant Sciences 163: 409–417. [Google Scholar]
- SorkVL,Campbell D, Dyer R, Fernandez J, Nason J, Petit R, Smouse R, Steinberg E.1998.Proceedings from a workshop on gene flow in fragmented, managed, and continuous populations Research Paper No. 3. National Center For Ecological Analysis and Synthesis, Santa Barbara, California. http://www.nceas.ucsb.edu/nceas‐web/projects/2057/nceas‐paper3/, accessed on 18 Aug. 2003. [Google Scholar]
- SorkVL,Nason J, Campbell DR, Fernandez, JF.1999. Landscape approaches to historical and contemporary gene flow in plants. Trends in Ecology and Evolution 14: 219–224. [DOI] [PubMed] [Google Scholar]
- WaserNM.1983. The adaptive nature of floral traits: ideas and evidence. In: Real L, ed. Pollination biology London: Academic Press, 241–285. [Google Scholar]
- WaserNM.1988. Comparative pollen and dye transfer by pollinators of Delphinium nelsonii Functional Ecology 2: 41–48. [Google Scholar]
- WaserNM,Price MV.1982. A comparison of pollen and fluorescent dye carry‐over by natural pollinators of Ipomopsis aggregata (Polemoniaceae). Ecology 63: 1168–1172. [Google Scholar]
- WilcockC,Neiland R.2002. Pollination failure: why it happens and when it matters. Trends in Plant Science 7: 270–277. [DOI] [PubMed] [Google Scholar]
- Wyse‐JacksonPS,Akeroyd JR.1994.Guidelines to be followed in the design of plant conservation or recovery plans. Strasbourg, France: Council of Europe. [Google Scholar]
- YoungHJ.2002. Diurnal and nocturnal pollination of Silene alba (Caryophyllaceae). American Journal of Botany 89: 433–440. [DOI] [PubMed] [Google Scholar]