Abstract
• Background and Aims The problem of increasing CO2 concentration [CO2] and associated climate change has generated much interest in modelling effects of [CO2] on plants. While variation in growth and productivity is closely related to the amount of intercepted radiation, largely determined by leaf area index (LAI), effects of elevated [CO2] on growth are primarily via stimulation of leaf photosynthesis. Variability in LAI depends on climatic and growing conditions including [CO2] concentration and can be high, as is known for agricultural crops which are specifically emphasized in this report. However, modelling photosynthesis has received much attention and photosynthesis is often represented inadequately detailed in plant productivity models. Less emphasis has been placed on the modelling of leaf area dynamics, and relationships between plant growth, elevated [CO2] and LAI are not well understood. This Botanical Briefing aims at clarifying the relative importance of LAI for canopy assimilation and growth in biomass under conditions of rising [CO2] and discusses related implications for process‐based modelling.
• Model A simulation exercise performed for a wheat crop demonstrates recent experimental findings about canopy assimilation as affected by LAI and elevation of [CO2]. While canopy assimilation largely increases with LAI below canopy light saturation, effects on canopy assimilation of [CO2] elevation are less pronounced and tend to decline as LAI increases. Results from selected model‐testing studies indicate that simulation of LAI is often critical and forms an important source of uncertainty in plant productivity models, particularly under conditions of limited resource supply.
• Conclusions Progress in estimating plant growth and productivity under rising [CO2] is unlikely to be achieved without improving the modelling of LAI. This will depend on better understanding of the processes of substrate allocation, leaf area development and senescence, and the role of LAI in controlling plant adaptation to environmental changes.
Key words: Elevated CO2, leaf area index, modelling, photosynthesis, plant growth, scaling
INTRODUCTION
It has been estimated that atmospheric CO2 concentration [CO2] will increase to between 540 and 970 µmol mol–1 [CO2] by 2100 (IPCC, 2001), which will affect plant and vegetation growth as demonstrated by numerous experiments and simulation studies. However, plant responses to rising [CO2] vary (e.g. Kimball et al., 2002) as a result of relationships that are not well understood and that involve complex responses of underlying growth and development to changes in [CO2] and other environmental conditions.
Photosynthesis is without doubt the process that has been studied and modelled the most, not least because of the direct effect of [CO2] on photosynthetic rate. However, at the more integrated plant and ecosystem level there is little evidence for a predictive relationship between leaf photosynthesis and growth. Instead, biomass production is closely related to light interception, which is mainly determined by leaf area index (LAI), as has been demonstrated for agricultural crops (e.g. Monteith, 1977) and other vegetation types (e.g. Hirose et al., 1997). LAI varies depending on a number of factors including seasonal climate, water and nitrogen availability, and to some extent [CO2] elevation (e.g. Drake et al., 1997; Ewert and Pleijel, 1999; Hartz‐Rubin and DeLucia, 2001; Kimball et al., 2002; Cowling and Field, 2003). Recent investigations suggest that canopy photosynthesis increases with LAI (Rochette et al., 1995, 1996; Campbell et al., 2001; Rodriguez et al., 2001) and that effects of elevated [CO2] on canopy photosynthesis decrease as LAI increases (Brooks et al., 2000). Understanding of the relative importance of such relationships for vegetation growth and productivity is limited but essential for modelling systems responses to [CO2].
Process‐based models are increasingly used to predict effects of [CO2] on crop and vegetation productivity (Amthor and Loomis, 1996; Tubiello and Ewert, 2002). These models integrate responses at the process level to the higher system level (van Oijen, 2002) and usually have some form of representation of LAI and photosynthesis depending on the underlying conceptual framework of the specific model. Results from comparison of models suggest that while model performance is often satisfactory at the system level, model behaviour at the process level is more critical (Ewert et al., 2002). It is unclear to what extent uncertainties in estimating productivity changes due to rising [CO2] are associated with inaccurate model assumptions about LAI.
The aim of this Botanical Briefing is to provide some clarification about the relative importance of LAI for plant responses under rising [CO2] and to discuss related implications for process‐based modelling. Relationships are analysed between canopy assimilation, elevated [CO2] and LAI for an example wheat crop with reference to experimental data from Rodriguez et al. (2001) and Manderscheid et al. (2003). For the simulations performed here, the WIMOVAC modelling system was used (Humphries and Long, 1995). This has been applied in earlier studies to assess responses of photosynthesis and its characteristics at leaf and canopy level in relation to environmental conditions such as temperature, radiation or tropospheric ozone and [CO2] (e.g. Burkart et al., 2000; Rogers and Humphries, 2000; Martin et al., 2001; Sage, 2002). Results from selected simulation studies are also reviewed (Ewert et al., 1999, 2002; van Oijen and Ewert, 1999; Jamieson et al., 2000) in order to discuss model uncertainties related to simulations of LAI.
APPROACHES OF PROCESS‐BASED MODELLING
Processes affected by [CO2]
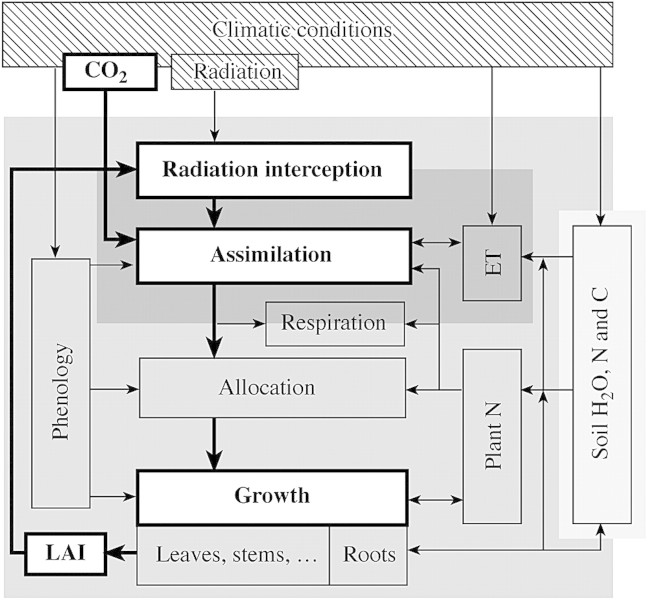
Process‐based models calculate biomass production from underlying growth and development processes (van Oijen, 2002) that are affected directly or indirectly by elevated [CO2] (Fig. 1). An enormous number of these models have been developed over the last few decades (see reviews by Reynolds et al., 1996; Tubiello and Ewert, 2002) with substantial differences between models in their structure and mechanistic detail. As estimating the effects of rising [CO2] was not an original modelling aim, most models were adapted for this application at a later stage with more or less extensive modifications of the original model version (Tubiello and Ewert, 2002). The emerging diversity of [CO2] modelling approaches has been reviewed elsewhere (Amthor and Loomis, 1996; Reynolds et al., 1996; Boote et al., 1997; Tubiello and Ewert, 2002) and needs no repetition here. Instead, a brief overview is given of approaches that are most commonly used in productivity models to simulate growth, light interception (including LAI) and photosynthesis.
Fig. 1. Growth and developmental processes that are affected by elevated [CO2] and are commonly used in process‐based productivity models. Processes and relationships that are shown in white boxes and bold arrows are the ones primarily emphasized in the text. Grey areas indicate the temporal resolution of different processes ranging from seconds‐to‐hours (dark grey), to days (grey) and to decades‐to‐months (light grey).
Growth
Growth in biomass is either calculated as proposed by Monteith (1977) for agricultural crops from intercepted radiation and the efficiency with which energy is converted into dry matter (Table 1, eqn 1), or from net assimilation, which is computed as the result of canopy gross assimilation and respiration, often distinguished into growth and maintenance respiration (Table 1, eqn 2). However, the approximate constancy of energy conversion (e.g. Monteith 1977; Sinclair and Muchow, 1999), henceforth ‘radiation use efficiency’ (RUE), has made the RUE approach rather attractive for modelling growth, avoiding more complicated calculation and parameterization of carbon accumulation from leaf photosynthesis and respiration. Increase in RUE with [CO2] (e.g. Mulholland et al., 1998, Ewert et al., 1999) has been modelled empirically using linear (e.g. Jamieson et al., 2000) or curvilinear (e.g. Stockle et al., 1992) multipliers (Table 1, eqn 1a). Recent studies report considerable variation in RUE depending on species, developmental stage and environmental conditions (e.g. Ruimy et al., 1994; Sinclair and Muchow, 1999). Some attempts have been made to explore further such variations in RUE (Haxeltine and Prentice, 1996; Dewar, 1996; Ewert and Porter, 2000; Choudhury, 2001) and improve the modelling of RUE (Wolf et al., 2002; Green et al., 2003; Sitch et al., 2003).
Table 1.
Summary of approaches with explanation of parameters for modelling responses to [CO2] of growth, radiation interception and photosynthesis commonly used in crop and vegetation productivity models (see text for further explanation and references)
| Approach | Equation no. | Parameters* |
| Growth rate | ||
| I dC/dt = Siϵ | 1 | C, growth; t, time; Si, intercepted solar radiation; ϵ, radiation use efficiency |
| ϵ = ϵ f(ca) f(nc) f(FIdiff) | 1a | ca, atmospheric CO2 concentration; nc, canopy nitrogen content; FI,diff, fraction of diffuse radiation |
| II dC/dt = Ag – (Rg + Rm) | 2 | Ag, gross photosynthesis; Rg, growth respiration; Rm, maintenance respiration |
| Radiation interception and LAI | ||
| I Si/S0 = e–kL | 3 | S0, solar radiation above canopy; k, light extinction coefficient; L, leaf area index |
| L = ClLsa | 3a | Cl, leaf growth; Lsa, specific leaf area |
| Leaf photosynthesis | ||
| I θAl2 – Al(αIl + Asat)Al + αIlAsat = 0 | 4 | Al, leaf gross assimilation rate; Asat, leaf maximum assimilation rate; Il, intercepted PAR at leaf; α, quantum efficiency; θ, curvator |
| Asat = Asat f(ca) f(T) f(nl) | 4a | T, temperature; nl, leaf nitrogen content |
| α = α f(ca) f(T) | 4b | |
| II A = min{Aq, Ar} – Rd | 5 | A, leaf net assimilation rate; Aq, light limited assimilation; Ar, Rubisco limited assimilation; Rd, day respiration |
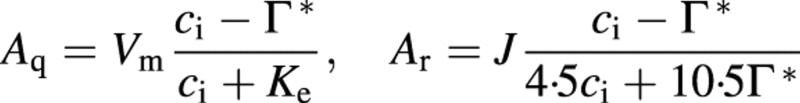
|
5a, 5b | Vm, maximum carboxylation velocity; ci, intercellular CO2 concentration; Γ*, CO2 compensation point in the absence of respiration; Ke, function of enzyme; J, electron transport rate |
| ci = ca – A/gsc | 5c | gsc, stomatal conductance |
| gsc = g0 + a1f(D)A/(ca – Γ) | 5d | f(D), function of humidity deficit; Γ, CO2 compensation point; g0, residual conductance; a1, empirical parameter |
| Canopy photosynthesis | ||
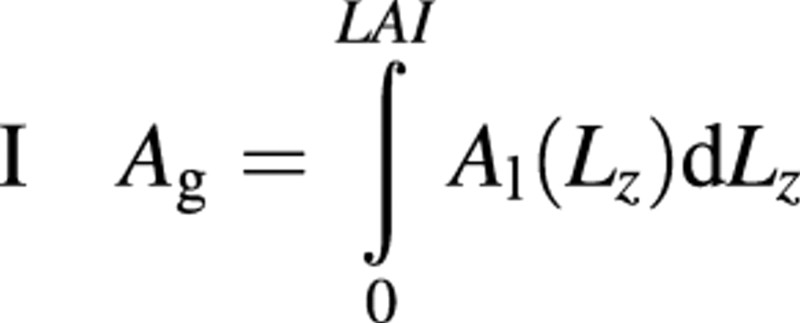
|
6 | LAI, leaf area index; Lz, LAI at level z of the canopy |
| II An = AFI/k | 7 | An, canopy net photosynthesis; fI, fraction of incident PAR absorbed by the canopy |
* Explanation of each parameter is given at its first use in an equation
Light interception and leaf area index
Computation of light interception usually follows Lambert–Beer’s function (Monsi and Saeki, 1953; Table 1, eqn 3). Interception of photosynthetically active radiation is usually calculated separately for direct and diffuse light (e.g. Spitters, 1986; Goudriaan and van Laar, 1994; Choudhury, 2001). Recent analysis of multi‐species and/or multi‐site data‐sets report substantial variation in LAI with ranges between 1–10 and more (Ewert and Pleijel, 1999; Choudhury, 2001; Cowling and Field, 2003). LAI depends on a number of environmental factors and modelling LAI remains difficult due to the complexity of relationships determining substrate allocation and growth and development of leaf area. Methodological differences and associated difficulties in measuring LAI cause additional errors (see Levy and Jarvis, 1999; Bréda, 2003), which are not further addressed in this report. Most productivity models simulate dry matter partitioning using descriptive allometry models based on empirically derived ratios between growth rates or relative growth rates (see review by Marcelis et al., 1998; Table 1, eqn 3a). Such ratios are usually assumed to change with phenological development, but effects of environmental conditions have only recently been incorporated using more mechanistic modelling approaches (e.g. Dewar et al., 1998; Marcelis et al., 1998; Thornley, 1998).
Photosynthesis
Physiology‐based models that calculate growth in biomass from leaf photosynthesis (see Table 1, eqn 2) have some formulation to account for the non‐linear relationship between assimilation rate and intercepted radiation. Simple empirical approaches employ exponential (Goudriaan and van Laar, 1994) or hyperbolic functions (Boote and Loomis, 1991; Table 1, eqn 4). Empirical relationships have been used to model [CO2] effects on quantum efficiency and light‐saturated photosynthesis rate (Table 1, eqn 4a, b). Despite its simplicity, this approach has been demonstrated satisfactorily to reproduce observed photosynthetic responses for a range of environmental conditions including elevated [CO2] (Cannell and Thornley, 1998; Thornley, 1998; Rodriguez et al., 2001). However, the most common approach to modelling photosynthesis is the one described first by Farquhar et al. (1980) (Table 1, eqn 5) where photosynthesis rate, limited by either light or Rubisco, is modelled in more detail from underlying biochemical relationships (Table 1, eqn 5a, b). This approach and its improved versions (e.g. Collatz, 1990), and parameter responses to temperature and nitrogen (e.g. Long 1991; Harley et al., 1992; Sage, 1994, 2002; Drake et al., 1997; Medlyn et al., 2002), have been described in detail many times and will not be repeated here. Effects of elevated [CO2] on leaf photosynthesis are calculated from intercellular [CO2] (Table 1, eqn 5a), which is often computed iteratively through coupling of a photosynthesis model with equations for stomatal conductance and intercellular [CO2] (e.g. Leuning, 1995; Table 1, eqn 5c, d).
Scaling photosynthesis from leaf to canopy and acclimation to [CO2]
Vegetation models that are based on photosynthesis need some approach to integrate responses from the leaf to the canopy level. The most conventional approach is the multi‐layer model where leaf photosynthesis is integrated down the canopy, following radiation interception (e.g. Thornley, 1998; Table 1, eqn 6). Much emphasis has been on the development of simpler models such as the ‘big‐leaf approach’ (e.g. Sellers et al., 1992; Kull and Jarvis, 1995; Friend et al., 1997; Woodward and Lomas, 2001; Table 1, eqn 7) where the whole canopy is treated as one big leaf, often separated into a sunlit and sun‐shaded part. Despite its wide acceptance, the physiological basis of the assumptions behind the big‐leaf approach have recently been questioned (Kull and Kruijt, 1998; Friend, 2001). Since leaf assimilation rate is closely related to leaf incident radiation and nitrogen content, a number of studies have aimed at improving the understanding and modelling of radiation and nitrogen distribution within the canopy (see Dewar, 1996; Medlyn, 1998; Choudhury, 2001; Kull 2002) including relationships to [CO2] elevation (Long and Drake, 1991; Hirose et al., 1997; Hartz‐Rubin and DeLucia, 2001). Optimization of nitrogen distribution within the canopy (Field 1983; Hirose and Weger, 1987; Badeck, 1995) is commonly used to explain photosynthetic acclimation to low radiation.
Acclimation to [CO2], i.e. the failure of plants to sustain the initial, maximal stimulation of photosynthesis (e.g. Gunderson and Wullschleger, 1994; Drake et al., 1997), can occur after long‐term exposure to elevated [CO2] and reduced N supply (Sage, 1994; Drake et al., 1997). A related decrease in maximum carboxylation velocity of Rubisco (Rogers and Humphries, 2000) is caused by limitation of sink development (Rogers et al., 1998) and a temporal shift of leaf ontogeny (Ludewig and Sonnewald, 2000). Mechanisms that explain acclimation and adaptation to [CO2] at the whole‐plant level are more complex and are not well understood (Wolfe et al., 1998), and modelling remains difficult.
EFFECTS ON CANOPY ASSIMILATION
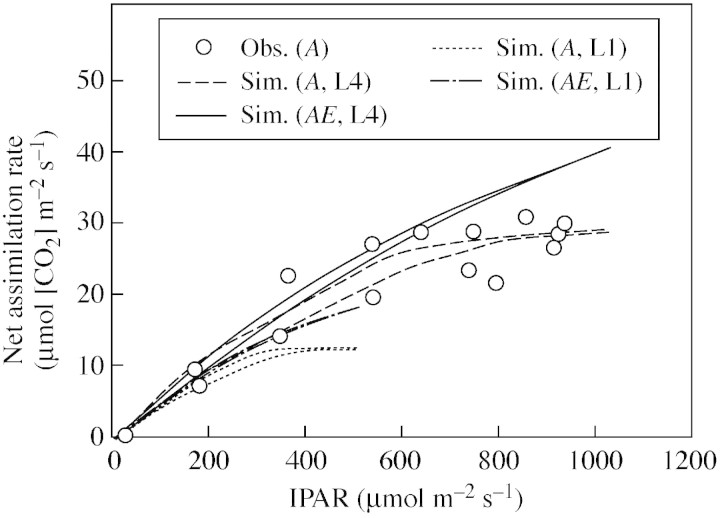
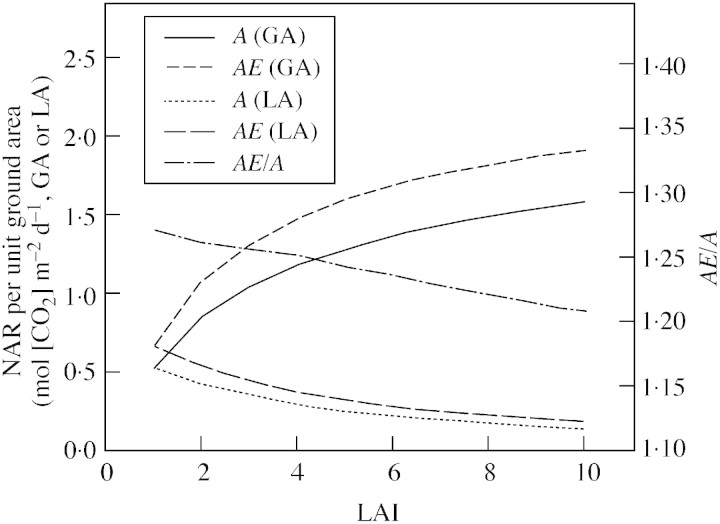
A well‐validated multi‐layer model of canopy photosynthesis was used to demonstrate characteristic responses of instantaneous and daily canopy net assimilation to LAI reported for annual, single‐species vegetation stands (Figs 2–4). Briefly, canopy photosynthesis increases with LAI (Baldocchi, 1994; Rochette et al., 1995, 1996; Campbell et al., 2001; Rodriguez et al., 2001; Sakai et al., 2001) but the effect of LAI depends on radiation level and is particularly high at noon when incident radiation is high (Fig. 2). LAI largely affects the canopy radiation saturation point (Fig. 3; see also Baldocchi, 1994; Rochette et al., 1995, 1996; Campbell et al., 2001), so that in dense canopies assimilation further increases with incident radiations above the radiation saturation point of individual leaves. The effect of LAI on the initial slope of the regression between canopy assimilation and intercepted radiation is comparably small (Fig. 3). This suggests that for canopy sub‐saturated radiation conditions effects on instantaneous RUE of LAI are small (Fig. 3; see also Medlyn, 1998) and are likely to be insignificant (Choudhury, 2001, Green et al., 2003; but see Campbell et al., 2001). The implication for daily integrated responses is shown in Fig. 4. Clearly, as LAI increases more radiation is intercepted per unit ground area resulting in higher assimilation rates, which tend to level out at high LAI. In contrast, intercepted radiation and assimilation rate decrease with increasing LAI on a per unit leaf area basis (Fig. 4).
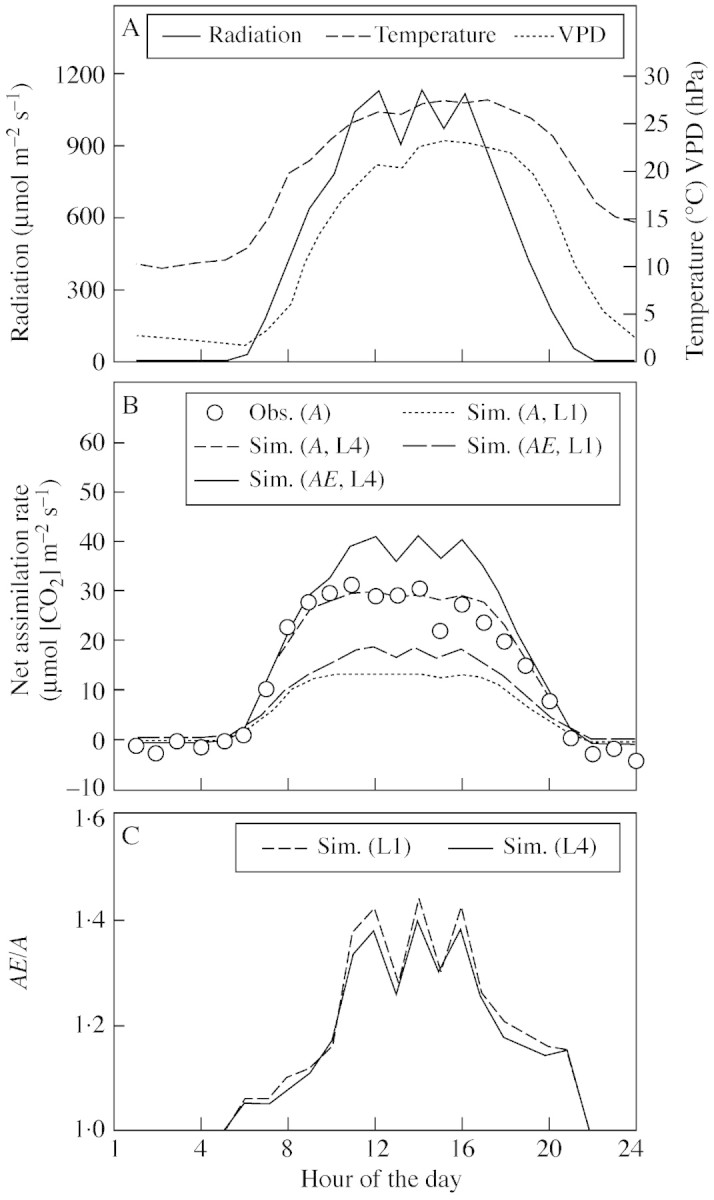
Fig. 2. Diurnal course of (A) measured air temperature, incident radiation and vapour pressure deficit (VPD) used as model input; (B) observed and simulated (WIMOVAC) instantaneous net assimilation rate of two wheat canopies with LAI = 1 (L1) and LAI = 4 (L4) at 360 µmol mol–1 [CO2] (A) and 720 µmol mol–1 [CO2] (AE); and (C) the simulated relative effects of [CO2] elevation on canopy assimilation (AE/A) for L1 and L4. Measured assimilation rates are shown in (B) for A and LAI = 4·2. Information about experimental conditions and measurements are available in Rodriguez et al. (2001) and Manderscheid et al. (2003).
Fig. 3. Relationships between simulated (WIMOVAC) and observed (only for A and LAI = 4·2) instantaneous canopy net assimilation rate and intercepted photosynthetically active radiation (IPAR) for LAI = 1 (L1) and LAI = 4 (L4) at ambient (A) and elevated (AE) [CO2]. Data refer to simulations and observations presented in Fig. 2.
Fig. 4. Simulated (WIMOVAC) relationships between daily canopy net assimilation rate per unit ground area (GA) and per unit leaf area (LA) and LAI for ambient (A) and elevated (AE) [CO2]. Relative [CO2] effects are calculated from AE/A. Climate input data and [CO2] concentrations were the same as in Figs 2 and 3.
The present simulations further demonstrate that elevated [CO2] increases canopy assimilation, particularly at noon (Fig. 2; see also Brooks et al., 2000; Rodriguez et al., 2001) and in sparse canopies (Figs 3, 4; Brooks et al., 2000). Thus, as LAI increases less radiation is intercepted per unit leaf area. This results in a smaller stimulation of photosynthetic assimilation at elevated [CO2], a mechanism apparently sufficient to explain interactions between LAI and [CO2] observed in the field (Brooks et al., 2000).
It can be seen from the simulations (Fig. 4) that an additional increase in LAI by 10–30 % due to [CO2] elevation (e.g. Ewert et al., 1999; Rodriguez et al., 2001; Kimball et al., 2002) would have a relatively small effect on radiation interception (not shown) and canopy assimilation, particularly when LAI is high and close to radiation saturation (see also Drake et al., 1997; Brooks et al., 2000; Manderscheid et al., 2003).
Acclimation and adaptation to [CO2] were not considered in the present example since simulations were performed for unstressed conditions. However, modification in canopy architecture (Brooks et al., 2000) may reduce the stimulatory effect of [CO2] elevation (Figs 2–4). Leaves of C3 plants tend to be more erectophile in ambient compared to elevated [CO2], with the implication that solar radiation is distributed more uniformly (i.e. increase in k, eqn. 3), which results in increased canopy assimilation (Brooks et al., 2000).
Importantly, for the range of conditions considered here, canopy assimilation was largely affected by LAI below canopy radiation saturation and to a lesser extent by [CO2] elevation. Interactive effects between [CO2] elevation and LAI were relatively small.
EFFECTS ON BIOMASS: EVIDENCE FROM MODEL TESTING
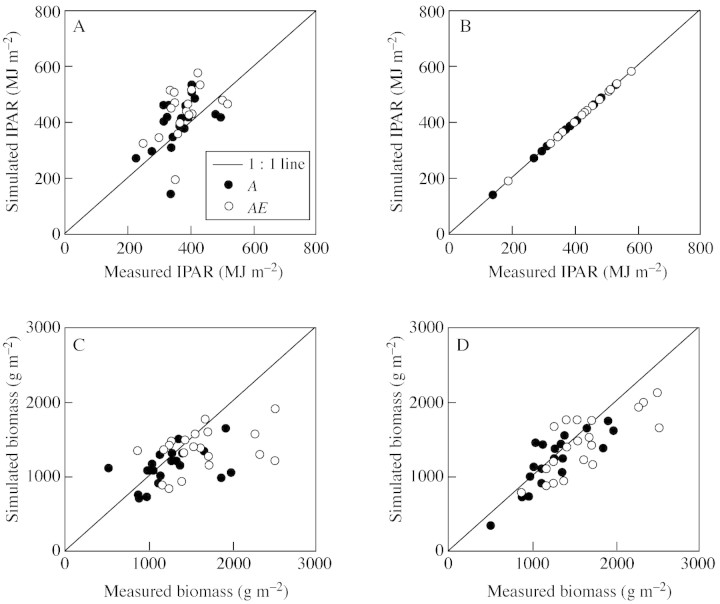
There is a remarkable imbalance between the large number of models available and applied for estimating [CO2] effects on field‐grown plants and the limited number of studies that have actually tested models against the few available data sets (Tubiello and Ewert, 2002). Most model‐testing exercises have demonstrated satisfactory model results even for conditions where resources such as H2O or N were limiting (see Tubiello and Ewert, 2002). However, a few studies have reported unsatisfactory simulations and differences among models, particularly for experiments that represented a larger range in climatic and growing conditions (Ewert et al., 1999, 2002; van Oijen and Ewert, 1999). Further analysis indicates that difficulties in estimating LAI form an important source of model uncertainty (Fig. 5; see also Wolf et al., 2002). Accurate prediction of LAI can improve model behaviour at the systems level significantly, both under ambient and elevated [CO2] (Fig. 5). This even applies to conditions of limited N or H2O supply and seems independent of the model’s approach for canopy assimilation (Jamieson et al., 2000; Ewert et al., 2002). However, there are large differences among models in simulating LAI, with important implications for simulations of biomass (e.g. Jamieson et al., 1998), which has also been reported for elevated [CO2] (Ewert et al., 2002).
Fig. 5. Simulated (AFRCWHEAT2‐O3) vs. measured IPAR (A, B) and biomass at harvest maturity (C, D) for spring wheat ‘Minaret’ grown at ambient (A) and elevated (2 × ambient, AE) [CO2] at eight location across Europe between 1994–1996. Simulations were performed using simulated LAI (A, C) or observed LAI (B, D) as model input. Original simulations of biomass (C) that used model estimates of LAI were unsatisfactory (see also Ewert et al., 1999) but improved substantially when observed LAI data were used as model input (D). The remaining unexplained variability was due to factors mainly associated to the use of open‐top chambers that were not considered in the model (see Ewert and Porter, 2000; Ewert et al., 2002).
THE PROBLEM OF SCALING‐UP
Numerous studies have aimed at scaling responses of leaf photosynthesis to changes in [CO2] and other environmental conditions from the leaf to the canopy, plant, ecosystem and even global level. However, aggregation of fine (leaf‐) scale photosynthetic variability to higher spatial and temporal scales remains difficult since systems’ functions and responses to environmental conditions generally change with scale. Hierarchy theory (Allen and Star, 1982; O’Neill, 1986) suggests that it is seldom necessary to look more than one level down in search of a mechanistic explanation of a system’s behaviour. Instead, representation of important processes and consistency in modelling detail within hierarchical layers are important criteria for process‐based modelling (Leffelaar, 1999). Photosynthesis models such as the one by Farquhar et al. (1980) were originally developed to explain [CO2] exchange by leaves to environmental conditions (Farquhar et al., 1980, 2001). Such detail in process modelling with a time‐step of seconds to hours is in contrast to other processes that are also important at the plant and ecosystem level with characteristic time‐steps of days and larger (Fig. 1). It has recently been shown that the temporal resolution (hours, days, months or years) of input data has a significant impact on RUE (Ruimy et al., 1994; Medlyn, 1998; van Wijk and Bouten, 2002). As the time scale increases, RUE becomes less variable (e.g. Medlyn, 1998). Consequently, other processes become more important for explaining systems’ behaviour.
In this brief report I have tried to demonstrate the importance of LAI for determining variation in plant productivity under ambient and elevated [CO2]. The examples considered here refer to unstressed conditions. However, recent evidence suggests that limited supply of H2O (Jamieson et al., 1998) and N (Jamieson et al., 2000; Poorter and Nagel, 2000) also affect plant growth significantly via changes in LAI. Interactions between these factors and [CO2] elevation for growth and productivity at field and larger scales are not well understood but are likely to be small. There is recent evidence that elevated [CO2] has little or no effect, respectively, on the role of LAI to control growth responses to H2O and N limitation (Fig. 6).
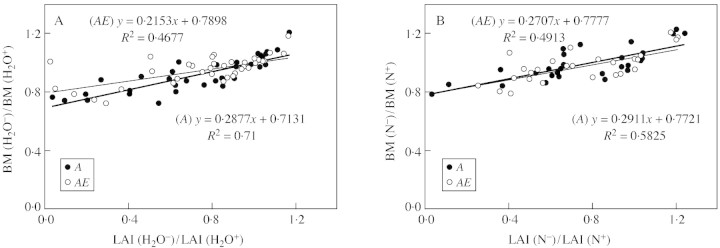
Fig. 6. Relationships between effects on biomass (BM) and effects on LAI of limited (A) water and (B) N supply for ambient (A) and elevated (1·5 × ambient, AE) [CO2] of wheat ‘Yecora Rojo’ grown at Maricopa, Arizona in 1992–93 and 1993–94 (H2O limitation) and 1995–96 and 1996–97 (N‐limitation). Ratios were calculated from measurements of biomass and LAI at different occasions throughout each growing season in water‐stressed (H2O–) and well‐watered (H2O+) and N‐limited (N–) and non‐limited (N+) treatments, respectively. References about data sources with information about experimental performances can be obtained from Jamieson et al. (2000) and Ewert et al. (2002).
Modelling LAI is still in its infancy, particularly for stressed conditions. Recent advances in modelling allocation (Dewar et al., 1998), leaf area development and senescence (Jamieson and Semenov, 2000; Yin et al., 2000; Franklin and Ågren, 2002) offer promising concepts but require further evaluation and, eventually, consideration in plant productivity models. Importantly, processes determining LAI should be viewed as properties of the canopy (Franklin and Ågren, 2002) or even of the ecosystem rather than of that of a single leaf. In this respect, investigations about the role of LAI in controlling plant adaptation to environmental changes (Hirose et al. 1997; Jamieson et al., 1998; Jamieson and Semenov, 2000), including optimization of canopy photosynthesis (Anten et al., 1995, Franklin and Ågren, 2002), are of particular interest.
SUMMARY
This brief report has demonstrated the relative importance of LAI for canopy assimilation and growth in biomass under conditions of rising [CO2] and the need for satisfactory representation of LAI in plant productivity models. Interactions between LAI and [CO2] effects on canopy assimilation are relatively small but require further investigation. Effects of LAI on RUE are also not well understood but are likely to be small. My conclusion is that progress in estimating future plant productivity under conditions of rising [CO2] is unlikely to be achieved without improving the modelling of LAI, particularly for vegetation types with a large variability in LAI, such as agricultural crops. Improved modelling of LAI will depend on better understanding of the processes of substrate allocation, leaf area development and senescence, and the role of LAI in controlling plant adaptation to environmental changes.
ACKNOWLEDGEMENTS
Funding by the European Commission via the projects ESPACE‐Wheat (EV5V‐CT93‐0301), MODEXCROP (ENV4‐CT95‐0142), IMPETUS (ENV4‐CT97‐0496) and ATEAM (EVK2‐2000‐00075) is acknowledged. The author thanks Mark van Wijk and Ed Rowe for helpful discussions and comments on an earlier draft of the manuscript.
Received: 14 November 2003; Returned for revision: 28 January 2004; Accepted: 25 February 2004; Published electronically: 21 April 2004
References
- AllenTFH, Starr TB.1982.Hierarchy: perspectives for ecological complexity. Chicago: University of Chicago Press. [Google Scholar]
- AmthorJS, Loomis RS.1996. Integrating knowledge of crop responses to elevated CO2 and temperature with mechanistic simulation models: model components and research needs. In: Koch GW, Mooney HA, eds. Carbon Dioxide and Terrestrial Ecosystems. San Diego: Academic Press, 317–345. [Google Scholar]
- AntenNPR, Schieving F, Medina E, Werger MJA, Schuffelen P.1995. Optimal leaf area indices in C3 and C4 mono‐ and dicotyledonous species at low and high nitrogen availability. Physiologia Plantarum 95: 541–550. [Google Scholar]
- BadeckFW.1995. Intra‐leaf gradient of assimilation rate and optimal allocation of canopy nitrogen: a model on the implications of the use of homogeneous assimilation functions. Australian Journal of Plant Physiology 22: 425–439. [Google Scholar]
- BaldocchiD.1994. A comparative study of mass and energy exchange rates over a closed C3 (wheat) and an open C4 (corn) crop: II. CO2 exchange and water use efficiency. Agricultural and Forest Meteorology 67: 291–321. [Google Scholar]
- BooteKJ, Loomis RS.1991. The prediction of canopy assimilation. In: Boote KJ, Loomis RS, eds. Modeling crop photosynthesis – from biochemistry to canopy. Madison: CSSA Special Publication 19, 109–137. [Google Scholar]
- BooteKJ, Pickering NB, Allen LH, Jr.1997. Plant modeling: advances and gaps in our capability to predict future crop growth and yield in response to global climate change. In: Allen LH, Jr, Kirkham MB, Olszyk DM, Whitman CE, eds. Advances in Carbon Dioxide Research. Madison: ASA Special Publication 61, 179–228. [Google Scholar]
- BrédaNJJ.2003. Ground‐based measurements of leaf area index: a review of methods, instruments and current controversies. Journal of Experimental Botany 54: 2403–2417. [DOI] [PubMed] [Google Scholar]
- BrooksTJ, Wall GW, Pinter PJ, Jr, Kimball BA, LaMorte RL, Leavitt SWet al.2000. Acclimation response of spring wheat in a free‐air CO2 enrichment (FACE) atmosphere with variable soil nitrogen regimes. 3. Canopy architecture and gas exchange. Photosynthesis Research 66: 97–108. [DOI] [PubMed] [Google Scholar]
- BurkartS, Manderscheid R, Weigel HJ.2000. Interacting effects of photosynthetic photon flux density and temperature on canopy CO2 exchange rate of spring wheat under different CO2‐concentrations. Journal of Plant Physiology 157: 31–39. [Google Scholar]
- CampbellCS, Heilman JL, McInnes KJ, Wilson LT, Medley JC, Wu G, Cobos DR.2001. Seasonal variation in radiation use efficiency of irrigated rice. Agricultural and Forest Meteorology 110: 45–54. [Google Scholar]
- CannellMGR, Thornley JHM.1998. Temperature and CO2 Responses of Leaf and Canopy Photosynthesis: a Clarification using the Non‐rectangular Hyperbola Model of Photosynthesis. Annals of Botany 82: 883–892. [Google Scholar]
- ChoudhuryBJ.2001. Estimating gross photosynthesis using satellite and ancillary data: approach and preliminary results. Remote Sensing of Environment 75: 1–21. [Google Scholar]
- CollatzGJ, Berry JA, Farquhar GD Pierce J.1990. The relationship between the Rubisco reaction mechanism and models of photosynthesis. Plant, Cell and Environment, 13: 219–225. [Google Scholar]
- CowlingSA, Field CB.2003. Environmental control of leaf area production: implications for vegetation and land‐surface modeling. Global Biogeochemical Cycles 17: 1–14. [Google Scholar]
- DewarRC.1996. The correlation between plant growth and intercepted radiation: an interpretation in terms of optimal plant nitrogen content. Annals of Botany 78: 125–136. [Google Scholar]
- DewarRCMedlyn BE, McMurtie RE.1998. A mechanistic analysis of light and carbon use efficiencies. Plant, Cell and Environment 21: 573–588. [Google Scholar]
- DrakeBG, Gonzalez‐Meler MA, Long SP.1997. More efficient plants: a consequence of rising atmospheric CO2? Annual Review of Plant Physiology and Plant Molecular Biology 48: 609–639. [DOI] [PubMed] [Google Scholar]
- EwertF, Pleijel H.1999. Phenological development, leaf emergence, tillering and leaf area index, and duration of spring wheat across Europe in response to CO2 and ozone. European Journal of Agronomy 10: 171–184. [Google Scholar]
- EwertF, van Oijen M, Porter JR.1999. Simulation of growth and development processes of spring wheat in response to CO2 and ozone for different sites and years in Europe using mechanistic crop simulation models. European Journal of Agronomy 10: 231–247. [Google Scholar]
- EwertF, Porter JR.2000. Ozone effects on wheat in relation to elevated CO2: modelling short‐term and long‐term responses of leaf photosynthesis and leaf duration. Global Change Biology 6: 735–750. [Google Scholar]
- EwertF, Rodriguez D, Jamieson P, Semenov MA, Mitchell RAC, Goudriaan Jet al.2002. Effects of elevated CO2 and drought on wheat: testing crop simulation models for different experimental and climatic conditions. Agriculture, Ecosystems and Environment 93: 249–266. [Google Scholar]
- FarquharGD, von Caemmerer S, Berry JA.1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149: 78–90. [DOI] [PubMed] [Google Scholar]
- FarquharGD, von Caemmerer S, Berry JA.2001. Models of photosynthesis. Plant Physiology 125: 42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FieldC.1983. Allocating leaf nitrogen for maximization of carbon gain: leaf age as a control on the allocation program. Oecologia 56: 341–347. [DOI] [PubMed] [Google Scholar]
- FranklinO, Agren GI.2002. Leaf senescence and resorption as mechanisms of maximizing photosynthetic production during canopy development at N limitation. Functional Ecology 16: 727–733. [Google Scholar]
- FriendAD.2001. Modelling canopy CO2 fluxes: are ‘big‐leaf’ simplifications justified? Global Ecology and Biogeography 10: 603–619. [Google Scholar]
- FriendAD, Stevens AK, Knox RG, Cannell MGR.1997. A process‐based, terrestrial biosphere model of ecosystem dynamics (Hybrid v3.0). Ecological Modelling 95: 249–287. [Google Scholar]
- GoudriaanJ, Van Laar HH.1994.Modelling potential crop growth processes. Dordrecht: Kluwer Academic Publishers. [Google Scholar]
- GreenDS, Erickson JE, Kruger EL.2003. Foliar morphology and canopy nitrogen as predictors of light‐use efficiency in terrestrial vegetation. Agricultural and Forest Meteorology 115: 163–171. [Google Scholar]
- GundersonCA, Wullschleger SD.1994. Photosynthetic acclimation in trees to rising atmospheric CO2: a broader perspective. Photo synthesis Research 39: 369–388. [DOI] [PubMed] [Google Scholar]
- HarleyPC, Thomas RB, Reynolds JF, Strain BR.1992. Modelling photosynthesis of cotton grown in elevated CO2 Plant, Cell and Environment 15: 271 282. [Google Scholar]
- Hartz‐RubinJS, DeLucia EH.2001. Canopy development of a model herbaceous community exposed to elevated atmospheric CO2 and soil nutrients. Physiologia Plantarum 113: 258–266. [DOI] [PubMed] [Google Scholar]
- HaxeltineA, Prentice IC.1996. A general model for the light‐use efficiency of primary production. Functional Ecology 10: 551–561. [Google Scholar]
- HiroseT, Werger MJA.1987. Maximizing daily canopy photosynthesis with respect to the leaf nitrogen allocation pattern in a canopy. Oecologia 72: 520–526. [DOI] [PubMed] [Google Scholar]
- HiroseT, Ackerly DD, Traw MB, Ramseier D, Bazzaz FA.1997. CO2 elevation, canopy photosynthesis, and optimal leaf area index. Ecology 78: 2339–2350. [Google Scholar]
- HumphriesSW, Long SP.1995. WIMOVAC: a software package for modelling the dynamics of plant leaf and canopy photosynthesis. Computer Applications in the Biosciences 11: 361–371. [DOI] [PubMed] [Google Scholar]
- IPCC.2001. The scientific basis. In: Houghton JT, Ding Y, Griggs DJ, Noguer M, van der Linden PJ, Dai X, Maskell K, Johnson CA, eds. Third assessment report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press. [Google Scholar]
- JamiesonPD, Semenov MA.2000. Modelling nitrogen uptake and redistribution in wheat. Field Crops Research 68: 21–29. [Google Scholar]
- JamiesonPD, Porter JR, Goudriaan J, Ritchie JT, van Keulen H, Stol W.1998. A comparison of the models AFRCWHEAT2, CERES‐Wheat, Sirius, SUCROS2 and SWHEAT with measurements from wheat grown under drought. Field Crop Research 55: 23–44. [Google Scholar]
- JamiesonPD, Bernsten J, Ewert F, Kimball BA, Olesen JE, Pinter Jr PJ, Porter JR, Semenov MA.2000. Modelling CO2 effects on wheat with varying nitrogen supplies. Agriculture, Ecosystems and Environment 82: 27–37. [Google Scholar]
- KimballBA, Kobayashi K, Bindi M.2002. Responses of agricultural crops to free‐air CO2 enrichment. Advances in Agronomy 77: 293–368. [PubMed] [Google Scholar]
- KullO.2002. Acclimation of photosynthesis in canopies: models and limitations. Oecologia 133: 267–279. [DOI] [PubMed] [Google Scholar]
- KullO, Jarvis PG.1995. The role of nitrogen in a simple scheme to scale up photosynthesis from leaf to canopy. Plant, Cell and Environment 18: 1174–1182. [Google Scholar]
- KullO, Kruijt B.1998. Leaf photosynthetic light response: a mechanistic model for scaling photosynthesis to leaves and canopies. Functional Ecology 12: 767–777. [Google Scholar]
- LeffelaarPA, ed.1999. On systems analysis and simulation of ecological processes; with examples in CSMP, FST and FORTRAN. In: Current Issues in Production Ecology, 4, 2nd edn. Dordrecht: Kluwer. [Google Scholar]
- LeuningR.1995. A critical appraisal of a combined stomata‐photosynthesis model for C3 plants. Plant, Cell and Environment 18: 339–355. [Google Scholar]
- LevyPE, Jarvis PG.1999. Direct and indirect measurements of LAI in millet and fallow vegetation in HAPEX‐Sahel. Agricultural and Forest Meteorology 97: 199–212. [Google Scholar]
- LongSP.1991. Modification of the response of photosynthesis productivity to rising temperature by atmospheric CO2 con centration: Has its importance been underestimated? Plant, Cell and Environment 14: 729–739. [Google Scholar]
- LongSP, Drake BG.1991. Effect of the long‐term elevation of CO2 concentration in the field on the quantum yield of photosynthesis of the C3 sedge, Scrirpus olnyei Plant Physiology 96: 221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LudewigF, Sonnewald U.2000. High CO2‐mediated down‐regulation of photosynthetic gene transcripts is caused by accelerated leaf senescence rather than sugar accumulation. FEBS Letters 479: 19–24. [DOI] [PubMed] [Google Scholar]
- ManderscheidR, Burkart S, Bramm A, Weigel H‐J.2003. Effect of CO2 enrichment on growth and daily radiation use efficiency of wheat in relation to temperature and growth stage. European Journal of Agronomy 19: 411–425. [Google Scholar]
- MarcelisLFM, Heuvelink E, Goudriaan J.1998. Modelling biomass production and yield of horticultural crops: a review. Scientia Horticulturaee 74: 83–111. [Google Scholar]
- MartinMJ, Host GE, Lenz KE, Isebrands JG.2001. Simulating the growth response of aspen to elevated ozone: a mechanistic approach to scaling a leaf‐level model to ozone effects on photosynthesis to complex canopy architecture. Environmental Pollution 115: 425–436. [DOI] [PubMed] [Google Scholar]
- MedlynBE.1998. Physiological basis of the light use efficiency model. Tree Physiology 18: 167–176. [DOI] [PubMed] [Google Scholar]
- MedlynBE,Dreyer E, Ellsworth D, Forstreuter M, Harley PC, Kirschbaum MUFet al.2002. Temperature response of parameters of a biochemically based model of Photosynthesis. II. A review of experimental data. Plant, Cell and Environment 25: 1167–1179. [Google Scholar]
- MonsiM, Saeki T.1953. Über den Lichtfaktor in Pflanzengesellschaften und seine Bedeutung für die Stoffproduktion. Japanese Journal of Botany 14: 22–52. [Google Scholar]
- MonteithJL.1977. Climate and the efficiency of crop production in Britain. Philosophical Transactions of the Royal Society of London, Series B 281: 277–294. [Google Scholar]
- MulhollandBJ, Craigon J, Black CR, Colls JJ, Atherton J, Landon G.1998. Growth, lighter interception and yield responses of spring wheat (Triticum aestivum L.) grown under elevated CO2 and O3 in open‐top chambers. Global Change Biology 4: 21–130. [Google Scholar]
- O’NeillRV, DeAngelis DL, Waide JB, Allen TFH.1986.A hierarchical concept of ecosystems. Princeton: Princeton University Press. [Google Scholar]
- PoorterH, Nagel O.2000. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Australian Journal of Plant Physiology 27: 595–607. [Google Scholar]
- ReynoldsJF, Kemp PR, Acock B, Chen J‐L, Moorhead DL.1996. Progress, limitations, and challenges in modeling the effects of elevated CO2 on plants and ecosystems. In: Koch G, Mooney HA, eds. Carbon Dioxide and Terrestrial Ecosystems San Diego: Academic Press, 347–380. [Google Scholar]
- RochetteP, Desjardins RL, Pattey E, Lessard R.1995. Crop net carbon dioxide exchange rate and radiation use efficiency in soybean. Agronomy Journal 87: 22–28. [Google Scholar]
- RochetteP, Desjardins RL, Pattey E, Lessard R.1996. Instantaneous measurement of radiation and water use efficiencies of a maize crop. Agronomy Journal 88: 627–635. [Google Scholar]
- RodriguezD, Ewert F, Goudriaan J, Manderscheid R, Burkart S, Weigel HJ.2001. Modelling the response of wheat canopy assimilation to atmospheric CO2 concentrations. New Phytologist 150: 337–346. [Google Scholar]
- RogersA, Humphries SW.2000. A mechanistic evaluation of photosynthetic acclimation at elevated CO2 Global Change Biology 6: 1005–1011. [Google Scholar]
- RogersA, Fischer BU, Bryant J, Frehner M, Blum H, Raines CA, Long SP.1998. Acclimation of photosynthesis to elevated CO2 under low‐nitrogen nutrition is affected by the capacity for assimilate utilization. Perennial ryegrass under free‐air CO2 enrichment. Plant Physiology 118: 683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RuimyA, Saugier B, Dedieu G.1994. Methodology for the estimation of terrestrial net primary production from remotely sensed data. Journal of Geophysical Research 99: 5263–5283. [Google Scholar]
- SageRF.1994. Acclimation of photosynthesis to increasing atmospheric CO2 The gas exchange perspective. Photosynthesis Research. 39: 351–368. [DOI] [PubMed] [Google Scholar]
- SageRF.2002. Variation in the k(cat) of Rubisco in C‐3 and C‐4 plants and some implications for photosynthetic performance at high and low temperature Journal of Experimental Botany 53: 609–620. [DOI] [PubMed] [Google Scholar]
- SakaiH, Yagi K, Kobayashi K, Kawashima S.2001. Rice carbon balance under elevated CO2 New Phytologist 150: 241–249. [Google Scholar]
- SellersPJ, Berry JA, Collatz GJ, Field CB, Hall FG.1992. Canopy reflectance, photo‐synthesis, and transpiration. III. A reanalysis using improved leaf models and a new canopy integration scheme. Remote Sensing of the Environment 42: 187–216. [Google Scholar]
- SinclairTR, Muchow RC.1999. Radiation use efficiency. Advances in Agronomy 65: 215–265. [Google Scholar]
- SitchS, Smith B, Prentice IC, Arneth A, Bondeau A, Cramer Wet al.2003. Evaluation of ecosystem dynamics, plant geography and terrestrial carbon cycling in the LPJ dynamic global vegetation model. Global Change Biology 9: 161–185. [Google Scholar]
- SpittersCJT.1986. Separating the diffuse and direct component of global radiation and its implications for modeling canopy photosynthesis. II. Calculation of canopy photosynthesis. Agricultural and Forest Meteorology 38: 231–242. [Google Scholar]
- StockleCO, Williams JR, Rosenberg NJ, Jones CA.1992. A method for estimating the direct and climatic effects of rising atmospheric carbon dioxide on growth and yield of crops: Part I. Modification of the EPIC model for climate change analysis. Agricultural Systems 38: 225–238. [Google Scholar]
- ThornleyJHM.1998. Dynamic model of leaf photosynthesis with acclimation to light and nitrogen. Annals of Botany 81: 421–430. [Google Scholar]
- TubielloF, Ewert F.2002. Simulating the effects of elevated CO2 on crops: approaches and applications for climate change. European Journal of Agronomy 18: 57–74. [Google Scholar]
- van OijenM.2002. On the use of specific publication criteria for papers on process‐based modelling in plant science. Field Crops Research 74: 197–205. [Google Scholar]
- van OijenM, Ewert F.1999. The effects of climatic variation in Europe on the yield response of spring wheat cv. Minaret to elevated CO2 and O3: an analysis of open‐top chamber experiments by means of two crop growth simulation models. European Journal of Agronomy 10: 249–264. [Google Scholar]
- van WijkMT, Bouten W.2002. Simulating daily and half‐hourly fluxes of forest carbon dioxide and water vapor exchange with a simple model of light and water use. Ecosystems 5: 567–610. [Google Scholar]
- WolfJ, van Oijen M, Kempenaar C.2002. Analysis of the experimental variability in wheat responses to elevated CO2 and temperature. Agriculture, Ecosystems and Environment 93: 227–247. [Google Scholar]
- WolfeDW, Gifford RM, Hilbert D, Luo Y, Luo YQ.1998. Integration of photosynthetic acclimation to CO2 at the whole‐plant level. Global Change Biology 4: 879–893. [Google Scholar]
- WoodwardFI, Lomas M.2001. Integrating fluxes from heterogeneous vegetation. Global Ecology and Biogeography 10: 595–601. [Google Scholar]
- YinX, Schapendonk AHCM, Kropff MJ, van Oijen M, Bindraban PS.2000. A generic equation for nitrogen‐limited leaf area index and its application in crop growth models for predicting leaf senescence. Annals of Botany 85: 579–585. [Google Scholar]