Abstract
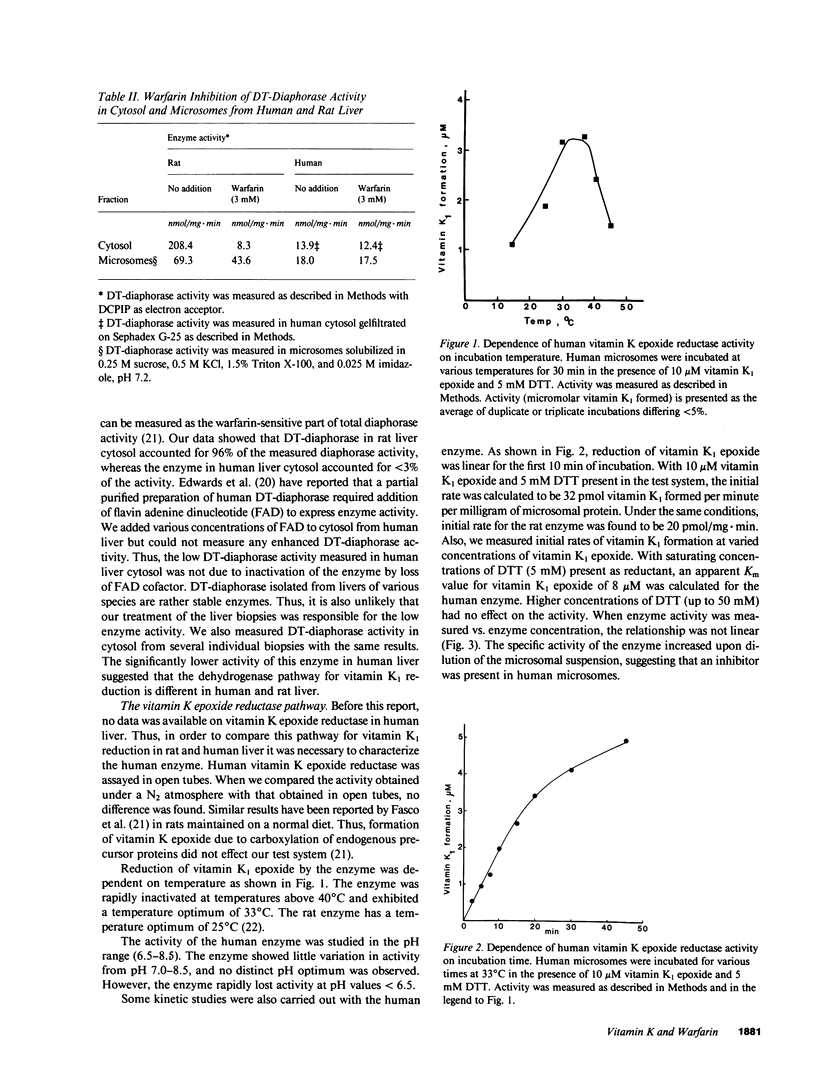
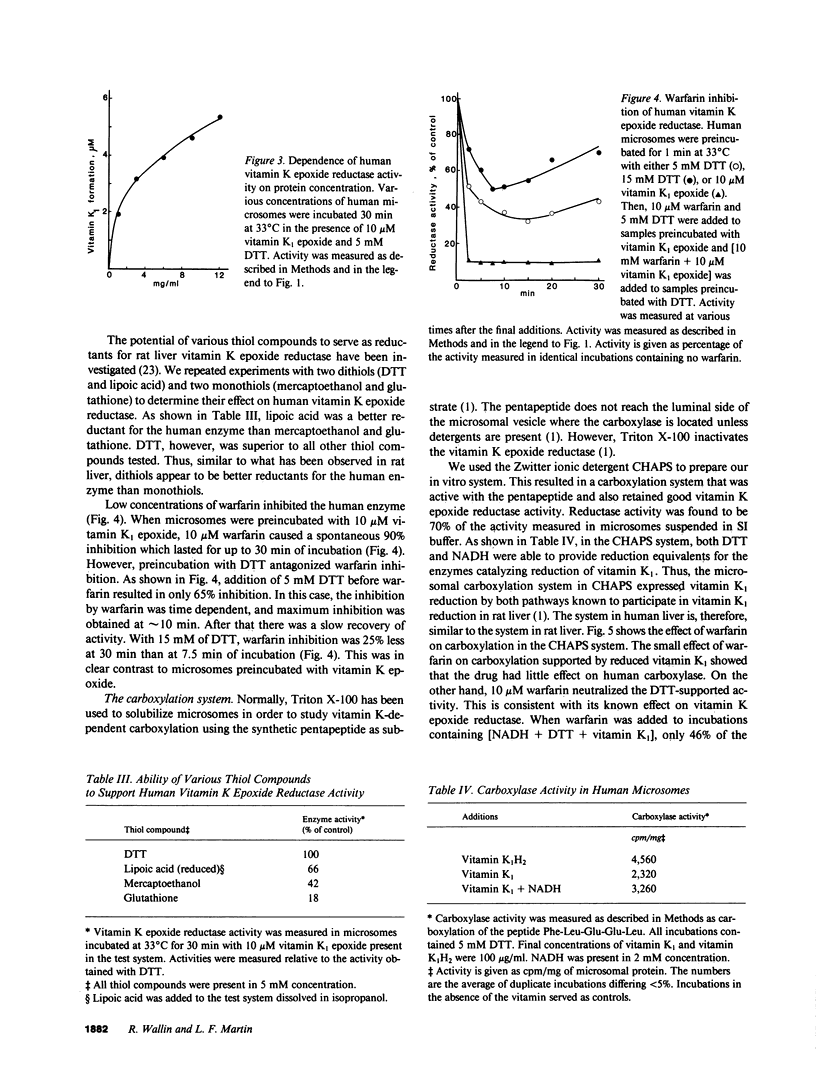
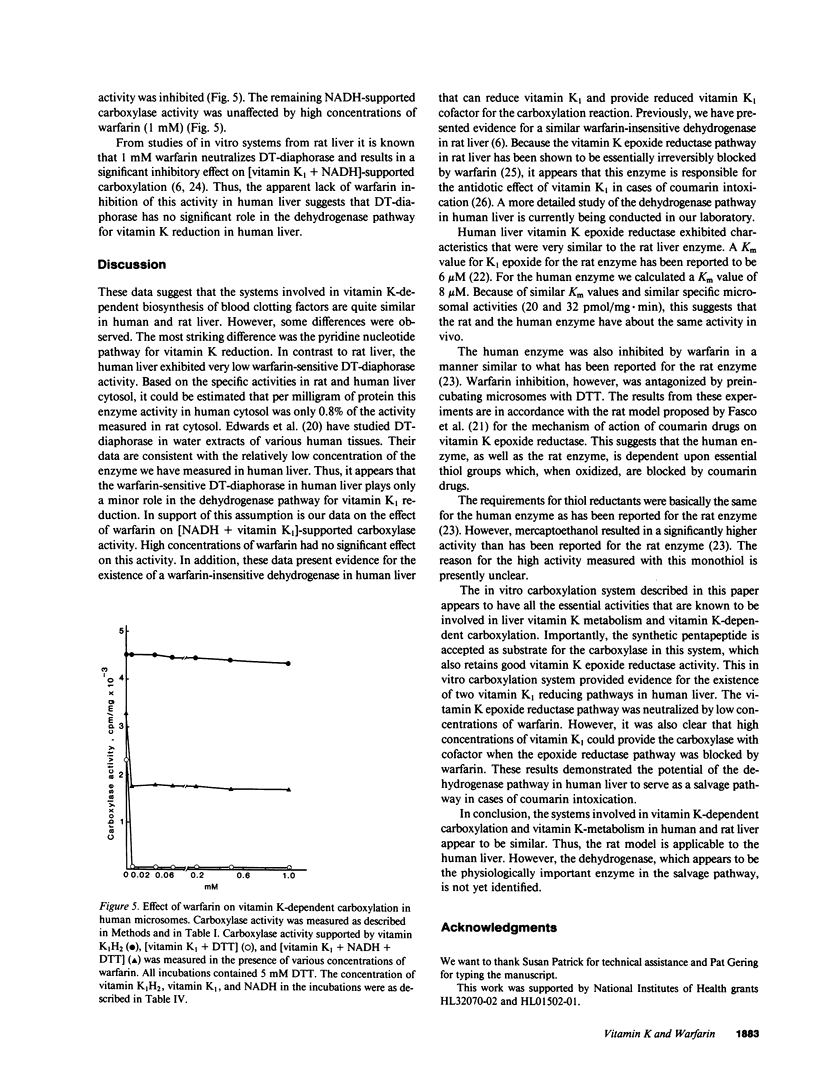
The systems involved in vitamin K-dependent carboxylation and vitamin K metabolism have been extensively studied in rat liver. To determine how clinically applicable this information is, similar in vitro studies were completed using human liver. One major difference exists in the pathways that provide reduced vitamin K1 cofactor for the carboxylation reaction. The coumarin-sensitive DT-diaphorase (EC.1.6.99.2) in human liver appears to play a relatively minor role in the dehydrogenase pathway. However, similar to rat liver, the human liver contains a warfarin-insensitive enzyme in this dehydrogenase pathway. The data suggest that this enzyme is responsible for the antidotic effect of vitamin K1 in cases of coumarin intoxication. Human vitamin K epoxide reductase, which constitutes the other pathway for vitamin K1 reduction, has kinetic and enzymological characteristics that are very similar to the rat enzyme. This enzyme exhibited similar activity in rat and human microsomes. Initial velocities for vitamin K1 epoxide reduction in rat and human microsomes were 20 and 32 pmol/mg X min, respectively. The human enzyme is highly sensitive to warfarin inhibition. The mechanism for this inhibition appears to be similar to what has been proposed for the rat enzyme. Also, a vitamin K-dependent carboxylation system is described that allows both pathways to support the carboxylation reaction with reduced vitamin K1 cofactor. The effect of warfarin on this in vitro system is consistent with the current model for the mechanism of action of coumarin anticoagulant drugs in the rat.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edwards Y. H., Potter J., Hopkinson D. A. Human FAD-dependent NAD(P)H diaphorase. Biochem J. 1980 May 1;187(2):429–436. doi: 10.1042/bj1870429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernster L., Lind C., Rase B. A study of the DT-diaphorase activity of warfarin-resistant rats. Eur J Biochem. 1972 Jan 31;25(1):198–206. doi: 10.1111/j.1432-1033.1972.tb01685.x. [DOI] [PubMed] [Google Scholar]
- Esmon C. T., Suttie J. W. Vitamin K-dependent carboxylase. Solubilization and properties. J Biol Chem. 1976 Oct 25;251(20):6238–6243. [PubMed] [Google Scholar]
- Fasco M. J., Hildebrandt E. F., Suttie J. W. Evidence that warfarin anticoagulant action involves two distinct reductase activities. J Biol Chem. 1982 Oct 10;257(19):11210–11212. [PubMed] [Google Scholar]
- Fasco M. J., Principe L. M. R- and S-Warfarin inhibition of vitamin K and vitamin K 2,3-epoxide reductase activities in the rat. J Biol Chem. 1982 May 10;257(9):4894–4901. [PubMed] [Google Scholar]
- Fasco M. J., Principe L. M., Walsh W. A., Friedman P. A. Warfarin inhibition of vitamin K 2,3-epoxide reductase in rat liver microsomes. Biochemistry. 1983 Nov 22;22(24):5655–5660. doi: 10.1021/bi00293a031. [DOI] [PubMed] [Google Scholar]
- Hildebrandt E. F., Preusch P. C., Patterson J. L., Suttie J. W. Solubilization and characterization of vitamin K epoxide reductase from normal and warfarin-resistant rat liver microsomes. Arch Biochem Biophys. 1984 Feb 1;228(2):480–492. doi: 10.1016/0003-9861(84)90014-6. [DOI] [PubMed] [Google Scholar]
- Hildebrandt E. F., Suttie J. W. Mechanism of coumarin action: sensitivity of vitamin K metabolizing enzymes of normal and warfarin-resistant rat liver. Biochemistry. 1982 May 11;21(10):2406–2411. doi: 10.1021/bi00539a020. [DOI] [PubMed] [Google Scholar]
- Hollander P. M., Ernster L. Studies on the reaction mechanism of DT diaphorase. Action of dead-end inhibitors and effects of phospholipids. Arch Biochem Biophys. 1975 Aug;169(2):560–567. doi: 10.1016/0003-9861(75)90200-3. [DOI] [PubMed] [Google Scholar]
- Lee J. J., Fasco M. J. Metabolism of vitamin K and vitamin K 2,3-epoxide via interaction with a common disulfide. Biochemistry. 1984 May 8;23(10):2246–2252. doi: 10.1021/bi00305a024. [DOI] [PubMed] [Google Scholar]
- Lind C., Höjeberg B. Biospecific adsorption of hepatic DT-diaphorase on immobilized dicoumarol. II. Purification of mitochondrial and microsomal DT-diaphorase from 3-methylcholanthrene-treated rats. Arch Biochem Biophys. 1981 Mar;207(1):217–224. doi: 10.1016/0003-9861(81)90027-8. [DOI] [PubMed] [Google Scholar]
- Preusch P. C., Suttie J. W. Relationship of dithiothreitol-dependent microsomal vitamin K quinone and vitamin K epoxide reductases inhibition of epoxide reduction by vitamin K quinone. Biochim Biophys Acta. 1984 Mar 22;798(1):141–143. doi: 10.1016/0304-4165(84)90022-9. [DOI] [PubMed] [Google Scholar]
- Sadowski J. A., Esmon C. T., Suttie J. W. Vitamin K-dependent carboxylase. Requirements of the rat liver microsomal enzyme system. J Biol Chem. 1976 May 10;251(9):2770–2776. [PubMed] [Google Scholar]
- Sherman P. A., Sander E. G. Vitamin K epoxide reductase: evidence that vitamin K dihydroquinone is a product of vitamin K epoxide reduction. Biochem Biophys Res Commun. 1981 Dec 15;103(3):997–1005. doi: 10.1016/0006-291x(81)90908-6. [DOI] [PubMed] [Google Scholar]
- Suttie J. W., Hageman J. M. Vitamin K-dependent carboxylase. Development of a peptide substrate. J Biol Chem. 1976 Sep 25;251(18):5827–5830. [PubMed] [Google Scholar]
- Suttie J. W. Mechanism of action of vitamin K: synthesis of gamma-carboxyglutamic acid. CRC Crit Rev Biochem. 1980;8(2):191–223. doi: 10.3109/10409238009105469. [DOI] [PubMed] [Google Scholar]
- Vermeer C., Soute B. A., Hemker H. C. In vitro prothrombin synthesis from a purified precursor protein. II. Partial purification of bovine carboxylase. Biochim Biophys Acta. 1978 Apr 12;523(2):494–505. doi: 10.1016/0005-2744(78)90052-9. [DOI] [PubMed] [Google Scholar]
- Wallin R., Gebhardt O., Prydz H. NAD(P)H dehydrogenase and its role in the vitamin K (2-methyl-3-phytyl-1,4-naphthaquinone)-dependent carboxylation reaction. Biochem J. 1978 Jan 1;169(1):95–101. doi: 10.1042/bj1690095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin R., Hutson S. Vitamin K-dependent carboxylation. Evidence that at least two microsomal dehydrogenases reduce vitamin K1 to support carboxylation. J Biol Chem. 1982 Feb 25;257(4):1583–1586. [PubMed] [Google Scholar]
- Wallin R., Suttie J. W. Vitamin K-dependent carboxylation and vitamin K epoxidation. Evidence that the warfarin-sensitive microsomal NAD(P)H dehydrogenase reduces vitamin K1 in these reactions. Biochem J. 1981 Mar 15;194(3):983–988. doi: 10.1042/bj1940983. [DOI] [PMC free article] [PubMed] [Google Scholar]



