Abstract
• Aims To highlight the importance of sphingolipids and their metabolites in plant biology.
• Scope The completion of the arabidopsis genome provides a platform for the identification and functional characterization of genes involved in sphingolipid biosynthesis. Using the yeast Saccharomyces cerevisiae as an experimental model, this review annotates arabidopsis open reading frames likely to be involved in sphingolipid metabolism. A number of these open reading frames have already been subject to functional characterization, though the majority still awaits investigation. Plant‐specific aspects of sphingolipid biology (such as enhanced long chain base heterogeneity) are considered in the context of the emerging roles for these lipids in plant form and function.
• Conclusions Arabidopsis provides an excellent genetic and post‐genomic model for the characterization of the roles of sphingolipids in higher plants.
Key words: Arabidopsis thaliana, ceramide, desaturase, lipid metabolism, long chain base, post‐genomics, Saccharomyces cerevisiae, signalling, sphingolipid
INTRODUCTION
Sphingolipids are ubiquitous and essential components of eukaryotic cells that were classically viewed as structural components of the membrane. However, it is now clear that sphingolipids and their metabolites are also dynamic regulators of many cellular processes. In particular, studies have shown that sphingolipids control crucial events in mammalian cells that determine the normal development and fate of living organisms, including proliferation, differentiation and apoptosis. Far less is known about sphingolipid functions in plants, but recent studies indicate that they have important signalling roles in plants as well. For example, sphingosine‐1‐phosphate plays a role in Ca2+‐mediated guard cell closure, a sphingosine transfer protein is involved in programmed cell death, and plant resistance to fungal toxins is mediated by a plant orthologue of a yeast gene involved in ceramide synthesis (for recent reviews, see Ng and Hetherington, 2001; Worrall et al., 2003). An important step toward delineating the function of sphingolipids has been the isolation of sphingolipid metabolism mutants in Saccharomyces cerevisiae and the identification of yeast genes encoding the enzymes responsible for sphingolipid biosynthesis and metabolism. These studies provide the groundwork for investigating the functions of sphingolipids in plants by a ‘reverse genetic’ approach because genome analysis indicates that many of the enzymes have been conserved throughout evolution. Arabidopsis thaliana has emerged as one of the best model organisms for studying the biology of higher plants, as well as the first plant genome to be fully sequenced. The aim of this review is to use this post‐genomic platform to examine the biosynthesis and metabolism of plant sphingolipids, in particular in the context of S. cerevisiae, which serves as the primary genetic and molecular model for sphingo‐biology. Moreover, this serves as a complement to previous reviews, which have compared sphingolipid metabolism in yeast and mammals (Dickson, 1998).
STRUCTURE AND OCCURRENCE OF PLANT SPHINGOLIPIDS
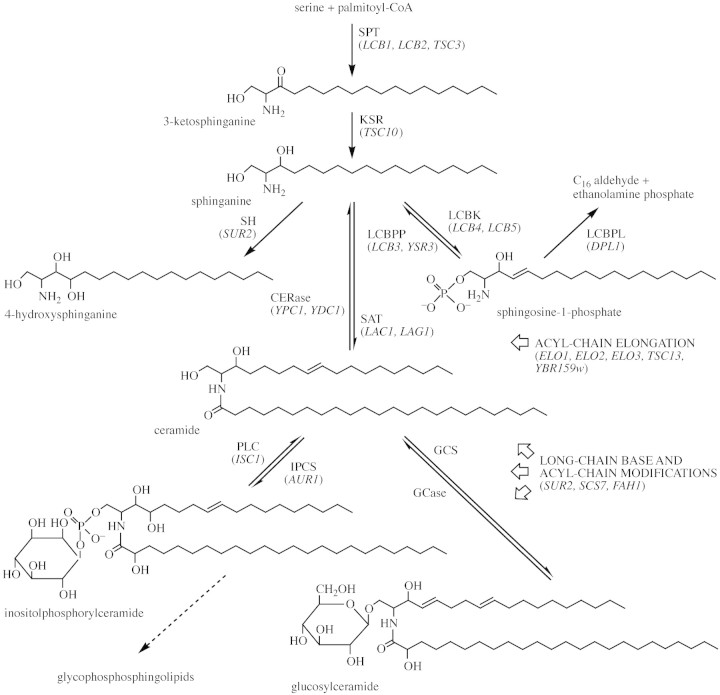
Complex sphingolipids are formed by the addition of various sugar residues or phosphate‐containing headgroups to a ceramide (Cer) backbone composed of a long‐chain base (LCB) that is amide‐linked to a fatty acid; thus, an LCB becomes acylated to generate a ceramide, which in turn is modified to yield a sphingolipid (see Fig. 1 for structural information as well as biosynthetic pathway). Sphingolipids are a diverse group of molecules with several hundred different molecular species known. This complexity arises from both the large array of possible polar headgroups, and from differences in the chain lengths, degrees of unsaturation, and hydroxylation states of both the LCB and fatty acid moieties of the ceramide. The predominant sphingolipid classes in mammals are sphingomyelin and the neutral and acidic glycolipids, while inositolphosphorylceramides (IPCs) are prevalent in yeast (Lester and Dickson, 1993). The predominant sphingolipids in plant tissues are generally considered to be glucosylceramides (GlcCers) (Lynch et al., 1993b), although complex glycophosphosphingolipids, including IPCs, are also found in plant tissues (Lester and Dickson, 1993; Lynch and Dunn, 2004). The absolute levels of sphingolipid classes in plant tissues have not been subject to unambiguous determination.
Fig. 1. A representation of the metabolic pathway displaying the enzymatic interconversions and structures of plant sphingolipids. Known yeast gene orthologues are listed in parentheses following abbreviated enzyme designations. Plant genes predicted to be involved in sphingolipid metabolism are not shown but are described in the text. Enzymatic steps modifying long‐chain bases and amide‐linked acyl chains via hydroxylation and/or desaturation are thought to utilize ceramide, glucosylceramide or inositolphosphorylceramide as substrate, but acyl‐chain elongation precedes ceramide formation, and the hydroxylation of free sphinganine has been demonstrated. Inositolphosphorylceramide is thought to be the precursor to complex glycophosphosphingolipids but the glycosyltransferases presumably required have not been identified. In addition to free sphinganine (d18:0) and 4‐hydroxysphinganine (t18:0, phytosphingosine), other long‐chain bases present in plants include cis and trans isomers of 8‐sphingenine (d18:1Δ8), 4,8‐sphingadienine (d18:2Δ4,8) and 4‐hydroxy‐8‐sphingenine (t18:1Δ8), shown here as constituents of ceramide, glucosylceramide and inositolphosphorylceramide, respectively. Sphingosine (d18:1Δ4trans, 4‐sphingenine) is shown as the phosphorylated derivative. The acyl chain amide‐linked in the ceramide shown is a nonhydroxy saturated C24 chain (lignocerate), whereas that of inositolphosphorylceramide and glucosylceramide is the α‐hydroxylated counterpart. Enzyme abbreviations: CERase, ceramidase; GCase, glucosylceramidase; GCS, glucosylceramide synthase; IPCS, inositolphophorylceramide synthase; KSR, 3‐ketosphinganine reductase; LCBK, long‐chain base kinase; LCBPL; long‐chain base‐phosphate lyase; LCBPP, long‐chain base‐phosphate phosphatase; PLC, phospholipase C; SAT, sphinganine acyltransferase; SH, sphinganine hydroxylase; SPT, serine palmitoyltransferase.
Considerable structural diversity exists among the complex sphingolipids (Cer, GlcCer and IPC) from various plant tissues with respect to LCB and fatty acid composition (Imai et al., 1995, 1997; Norberg et al., 1996). The most abundant LCBs are cis and trans isomers of 4,8‐sphingadienine (d18:2), 4‐hydroxy‐8‐sphingenine (t18:1), and 8‐sphingenine (d18:1). Note that the prevalent LCB of mammalian sphingolipids, sphingosine (trans‐4‐sphingenine), is a very minor component of most plant tissues. Sphinganine (d18:0, dihydrosphingosine; DHS) and 4‐hydroxysphinganine (t18:0, phytosphingosine; PHS) are typically minor constituents of the complex sphingolipids, but may be present as free LCBs in some plant tissues. Saturated and monenoic α‐hydroxy (h) fatty acids ranging in length from 16 to 26 carbons account for >90 % of the total fatty acids of GlcCer, Cer and IPC (with monenoic hydroxy fatty acids reported only in GlcCer from cold hardy cereals and Brassica species). In Arabidopsis, the predominant fatty acids, together constituting >55 % of the total, are 24:1h and 24:0h, with lesser amounts (<15 % each) of 16:0h, 22:0h, 26:1h and 26:0h being present. Cis and trans isomers of t18:1 are the predominant LCBs, comprising 57 and 32 %, respectively, while isomers of d18:1 comprise <10 % of the total LCBs present in arabidopsis. In routine lipid analyses, the presence of sphingolipids is often overshadowed by more abundant chloroplast galactolipids in leaves or triacylglycerols in seeds. This is attributable to the localization of complex sphingolipids (at least glucosylceramide) to the plasma membrane and tonoplast. However, cumulatively sphingolipids account for as much as 10 % of total plant lipids. It is clear, therefore, that they are both an overlooked and underestimated component of plant metabolism. Interestingly, the distribution of LCBs between GlcCer and IPC sphingolipids is not uniform, with 4,8‐sphingadienine (d18:2) LCBs accumulating predominantly in the GlcCer fraction, whereas 4‐hydroxy‐8‐sphingenine (t18:1) accumulates mainly in IPC. This may represent some aspect of (currently unknown) membrane functionality, though the accumulation of Δ8‐cis‐desaturated LCBs has been correlated with low temperature tolerance.
SPHINGOLIPID FUNCTION IN PLANTS
Sphingolipids are known to function in animal and fungal cells as membrane structural components, in cell–cell interactions, and as bioactive molecules involved in signal transduction and cell regulation. Following the discovery of complex glycophosphosphingolipids in seed tissues in the late 1950s, plant lipidologists seemingly overlooked sphingolipids until GlcCer was shown to be a quantitatively important component of plasma membrane (Lynch, 1993a) and tonoplast (Yoshida, 1986; Haschke et al., 1990; Tavernier et al., 1993). The heavily hydroxylated species of GlcCer present in many plant tissues may contribute to the overall integrity of the plasma membrane and tonoplast (Karlsson, 1982; Boggs, 1987). Plant GlcCer demonstrates unusual physical behaviour (Lynch et al., 1990; Lynch et al., 1992; Norberg et al., 1996) and has been implicated (but not mechanistically proven) to play a role in chilling and freezing tolerance in plants (Steponkus and Lynch, 1989; Uemura and Steponkus, 1994; Uemura et al., 1995). The location and function of glycophosphosphingolipids in plant cells have not been investigated. In plants glycosylphosphatidylinositol (GPI)‐anchored proteins on the cell surface have IPC as the lipid anchor (Morita et al., 1996; Sherrier et al., 1999; Svetek and Nothnagel, 1999; Thompson and Okuyama, 2000). A recent bioinformatics study catalogued the predicted number of GPI‐anchored proteins present in the arabidopsis genome (Borner et al., 2003), though the chemical composition and synthesis of such lipid anchors are currently undefined.
Evidence of bioactive sphingolipids in plants is limited but intriguing. Exogenously added sphingosine was shown to modulate plasma membrane ferricyanide reductase in response to blue light in oat mesophyll cells (Dharmawardhane et al., 1989) and stimulate tonoplast proton‐pumping pyrophosphatase activity in Chenopodium rubrum suspension cultures (Bille et al., 1992). Recent results suggesting that sphingosine‐1‐phosphate (S‐1‐P) is involved in calcium mobilization (Worrall et al., 2003), possibly via G‐protein linked pathways (Coursol et al., 2003) to an integral role for sphingolipids in guard cell signal transduction (Ng et al., 2001). Mycotoxins such as fumonisin and AAL toxin, which disrupt sphingolipid metabolism by inhibiting sphinganine N‐acyltransferase, promote apoptosis in tomato (Wang et al., 1996) and can induce a lethal accumulation of LCBs in a variety of plant tissues (Abbas et al., 1994). Studies of AAL toxin and sphingolipids in tomato genotypes (Asc/Asc and asc/asc) suggest that an interaction between or a sensing of the levels of ceramide and free LCBs may be important in triggering apoptosis (Spassieva et al., 2002). Consistent with a role for sphingolipids in apoptosis, the recently identified arabidopsis mutant Accelerated Cell Death 11 (ACD11) is deficient in a putative sphingolipid LCB transfer protein (Broderson et al., 2002) and, more recently, the ACD5 gene has been identified as a lipid kinase with specificity for (presumptively non‐structural) short‐chain fatty acyl ceramides (Liang et al., 2003). GlcCer species (and derived Cer) from rice blast fungus also act as elicitors in rice plants, inducing phytoalexins and pathogen‐related proteins, thereby protecting rice plants from subsequent infections (Umemura et al., 2000).
YEAST SPHINGOLIPID MUTANTS AS A TOOL FOR DEFINING PLANT SPHINGOLIPID GENES AND ENZYMES
The vast amount of information garnered from numerous studies of sphingolipids in mammals and fungi (especially S. cerevisiae) has provided useful paradigms in attempting to understand the structure, function and synthesis of plant sphingolipids. It is now appreciated that plant sphingolipids have many structural features distinct from those of either mammals or yeast. However, it appears that significant similarities/homologies exist between plants and yeast with respect to the enzymes involved in sphingolipid metabolism. With information derived from the genomes of yeast, arabidopsis and other plant species, a reverse genetic approach to characterizing the genes and gene products involved in plant sphingolipid metabolism is under way. This allows for a more systematic approach to defining the functions of specific sphingolipids in plants. Recent comparative reviews have focused on the similarity between mammalian and yeast sphingolipid biosynthetic genes (Dickson, 1998), but with the surge of interest in plant sphingolipid metabolism, it is essential to have a comprehensive catalogue of genes and enzyme activities which highlights the nuances of higher plants.
Genetic strategies using the yeast system have been especially useful for defining the sphingolipid biosynthetic pathway. Many of the proteins responsible for sphingolipid synthesis and turnover have been identified in yeast, and this has allowed their orthologues from higher eukaryotic cells to be identified by bioinformatic approaches. Furthermore, heterologous expression of candidate sphingolipid genes in yeast has proved to be a valuable strategy for assigning functions to the genes. The large collection of yeast mutants with defects in the endogenous sphingolipid genes enhances this approach because the heterologously expressed gene products can be assayed in a mutant background selected to optimize assignment of function to the heterologously expressed gene. For example, a mutant lacking the corresponding endogenous activity can be used; alternatively, a mutant that accumulates high levels of the suspected substrate of the heterologous gene product can be used. This gene discovery strategy has resulted in the assignment of function to a rapidly growing number of higher eukaryotic genes involved in sphingolipid metabolism. In particular, the yeast sphingolipid genes/mutants have facilitated the identification of candidate arabidopsis sphingolipid genes, and the current status of the functional characterization of the plant orthologues is detailed below (summarized in Table 1).
Table 1.
A list of yeast genes involved in sphingolipid metabolism and their counterparts in the arabidopsis genome
| Enzyme | Yeast orthologue | MIPS code | Homology | Functional characterization status |
| Serine pamitoyltransferase (SPT) | LCB1* LCB2* | At4g36480 At3g48780 At5g23670 | +++ +++ +++ | Chimeras of the yeast and arabidopsis LCB2 genes complement the lcb2Δ null mutant |
| 3‐Ketosphingosine reductase | TSC10* | At3g06060 At5g19200 | + + | ND |
| Sphinganine C‐4 hydroxylase | SUR2 | At1g14290 At1g69640 | +++ +++ | Arabidopsis At1g14290 complements the yeast sur2Δ mutant (Sperling et al., 2000) |
| Fatty acyl‐CoA carrier | ACB1 | At1g31812 | ND | |
| Ceramide synthase | LAC1 | At1g1358 | ++ | ND |
| LAG1 | At3g19260 | ++ | ||
| Golgi copper transporter | CCC2 | At1g63440 | ++ | ND |
| At5g44790 | + | |||
| Components of elongase complex required for VLCFA synthesis | ELO1ELO2/FEN1ELO3/SUR4 | At3g06460 At3g06470 At1g75000 | +++ +++ ++ | ND |
| Probable condensing enzymes (3‐keto‐acyl synthases) | At4g36830 | ++ | ||
| Component of elongase complex required for VLCFA synthesis 3‐Keto reductase | YBR159w | At1g67730 At1g24470 | ++ ++ | Heterologous expression of At1g67730 in yeast complements the ybr159Δ mutant (Han et al., 2002) |
| Component of elongase complex required for VLCFA synthesis | TSC13* | At3g55360 | +++ | Heterologous expression of At3g55360 in yeast complements the tsc13‐1 mutant (Gable et al., 2004) |
| Trans 2,3‐enoyl reductase | ||||
| VLCFA α‐Hydroxylase | SCS7/FAH1 | At2g34770 At4g20870 | +++ +++ | At2g34770 complements the scs7Δ yeast mutant (Mitchell and Martin, 1997) |
| LCB‐1‐phosphate lyase | DPL1 | At1g27980 | +++ | ND |
| LCB‐1‐phosphate‐phosphatases | LCB3 | At3g58490 | ++ | ND |
| LCB kinases | LCB4 LCB5 | At4g21540 At5g23450 | ++ ++ | At5g23450 (AtLCBK1) was shown to phosphorylate sphinganine in E. coli extracts (Nishiura et al., 2000) |
| Ceramidases | YDC1 | At4g22330 | ++ | ND |
| YPC1 |
The status as to functional characterization of the arabidopsis orthologues is indicated.
An approximate indication as to the level of homology between the yeast and arabidopsis sequences is indicated (+++, >60 % similarity; ++, >40 % similarity; +, >20 % similarity).
All arabidopsis genes are identified by their MIPS code number (http://mips.gsf.de/proj/thal/db/index.html), where the first digit indicates the chromosome on which the gene is present, and the subsequent five‐digit number is the unique identifier for each ORF.
ND, Not determined.
* Yeast genes which are essential (i.e. lethal on disruption).
PRIMARY CERAMIDE SYNTHESIS
LCB synthesis
Serine palmitoyltransferase (SPT) catalyses the first step of sphingolipid synthesis, the condensation of serine with palmitoyl CoA to form 3‐ketosphinganine (3‐KS) and the byproduct, CO2. In yeast, two related proteins encoded by the LCB1 and LCB2 genes, heterodimerize to form the active SPT enzyme (Gable et al., 2002). A third gene, TSC3, encodes an 80 amino acid protein that associates with the Lcb1p/Lcb2p heterodimer. Although Tsc3p is not essential for SPT activity, it stimulates it several‐fold (Gable et al., 2000). The 3‐KS produced by SPT is reduced to sphinganine by 3‐KS reductase via an NADPH‐dependent reaction catalysed, encoded by the TSC10 gene (Beeler et al., 1998). The SUR2 gene encodes the enzyme that converts sphinganine to 4‐hydroxysphinganine (Haak et al., 1997). Thus, the pathway for primary LCB (i.e. sphinganine) synthesis in yeast comprises just two enzyme activities but (at least) four genes. It is also likely that additional activities, such as the acyl‐CoA binding protein encoded by the ACB1 gene are likely to be involved in LCB synthesis (and fatty acid elongation). Deletion of the ACB1 gene results in altered organellar morphology in yeast, as well as decreased levels of both LCBs and VLCFAs (Gaigg et al., 2001)
Arabidopsis has two genes predicted to encode Lcb2p subunits of SPT, and one gene predicted to encode an Lcb1p subunit. SPT is a member of a small subfamily of pyridoxal 5′‐phosphate (PLP) enzymes that all catalyse the condensation of an amino acid with an acyl‐CoA. There is significant homology between enzymes of this subfamily in the region containing the signature PLP‐binding site including a lysine residue that forms a Schiff’s base with PLP. Tamura and coworkers have reported that heterologous expression of the arabidopsis LCB2‐like open reading frame (ORF) At5g23670 confers sphinganine synthesis in Escerichia coli, indicating that it is sufficient for SPT activity and that (perhaps unexpectedly) there is a 3‐ketoreductase activity in E. coli capable of reducing the non‐native 3‐KS (Tamura et al., 2000). Expression of the same arabidopsis gene restored sphinganine synthesis to an S. cerevisiae mutant lacking SPT activity (Tamura et al., 2001). Biochemical characterization of the plant enzyme reveals many commonalities with its mammalian and yeast counterparts: SPT activity from squash requires pyridoxal 5′‐phosphate as a cofactor, exhibits a strong preference for palmitoyl‐CoA and exhibits an apparent Km for serine of 1·8 mm. Enzyme activity is localized in the endoplasmic reticulum and is inhibited by known mechanism‐based inhibitors of the enzyme from other sources (Lynch and Fairfield, 1993).
There are two uncharacterized arabidopsis genes with weak (20 % identity, 40 % similarity) homology to the yeast TSC10 3‐ketoreductase gene (Table 1). The large number of short‐chain dehydrogenase/reductases‐related sequences present in the arabidopsis genome (149, as annotated by EMBL‐EBI InterPro database) highlights the need for functional characterization of ORFs. The SUR2 gene encodes the enzyme that converts sphinganine to 4‐hydroxysphinganine; interestingly, the sur2Δ null mutant is viable, although the ceramides and IPCs in the mutant contain sphinganine rather than 4‐hydroxysphinganine as the LCB (Haak et al., 1997). Arabidopsis also contains two genes with homology to the S. cerevisiae SUR2 gene (Table 1). Both have been expressed in yeast and found to restore 4‐hydroxysphinganine synthesis in the yeast sur2Δ mutant, confirming that both ORFs encode a C‐4 sphinganine hydroxylase activity; however, the specific substrate (sphinganine or acyl‐sphinganine) for these two gene products was not determined (Sperling et al., 2001). An arabidopsis orthologue of ACB1 is present in the genome, and currently awaits further characterization.
The direct hydroxylation of sphinganine in corn has been demonstrated in vivo and in vitro (Wright et al., 2003), a reaction common to plants and fungi, but apparently not mammals. Sphinganine hydroxylase (Sur2p) activity utilizes either NADH or NADPH as substrate (suggesting that either NADH cytochrome b5 reductase or NADPH cytochrome P450 reductase may serve as electron donors to the reaction via cytochrome b5) and is localized to the endoplasmic reticulum.
VLCFA synthesis
Synthesis of very long‐chain fatty acids (VLCFAs) requires the microsomal chain‐elongating enzyme system, known as elongase (Fig. 2). The ELO genes (ELO1, ELO2 and ELO3 in yeast) encode a family of homologous proteins required for the condensation step of fatty acid elongation. Although the ELO proteins may comprise a novel family of 3‐keto‐acyl synthases, it has not been conclusively shown that they directly catalyse the condensation reaction. Alternatively, the ELO proteins could modify the substrate preferences of an as yet unidentified condensing enzyme. However, since the yeast genome does not contain homologues of the characterized 3‐keto‐acyl synthases (e.g. FAE1), it is clear that the condensing activity of the elongase system is via a novel activity, most likely the ELO proteins per se. ELO1 is required for converting myristate to palmitate; it is not an essential gene in yeast as the soluble fatty acid synthase enzyme (FAS) also generates palmitate. However, the ability to rescue yeast cells lacking FAS with exogenous myristate requires Elo1p, and thus the fasΔelo1Δ double mutant is unable to grow if myristate is the sole fatty acid in the medium (Toke and Martin, 1996; Dittrich et al., 1998). Elo2p and Elo3p are required for VLCFA (>C18) synthesis (Oh et al., 1997). Elo2p is responsible for the majority of C16→C24 synthesis, while Elo3p (which can also elongate C16→C24) is essential to elongate C24→C26. The elo2Δ mutant displays low levels of VLCFAs, while the elo3Δ mutant accumulates significant levels of C22–24, but no C26 fatty acids. Disruption of both genes precludes VLCFA synthesis and the elo2Δelo3Δ double mutant is inviable. The YBR159 gene encodes the major β‐keto reductase of the elongase system (Beaudoin et al., 2002; Han et al., 2002). The gene is not essential since there is low residual β‐keto reductase activity in the mutant, possibly attributed to Ayr1p, but the ybr159Δ null mutant grows very slowly and is temperature‐sensitive (Han et al., 2002). The TSC13 gene encodes the enoyl reductase enzyme that catalyses the last step in each cycle of C2 elongation (Kohlwein et al., 2001). The TSC13 gene is essential for viability, but a temperature sensitive (ts)‐allele (tsc13‐1) has been reported (Beeler et al., 1998). The Tsc13p, Elo3p and Ybr159p proteins co‐immunoprecipitate indicating that the elongase enzymes are organized in a complex (Kohlwein et al., 2001; Han et al., 2002). The gene encoding the dehydratase activity of the elongase system remains to be identified. It is interesting to note that the ybr159Δ mutant accumulates high levels of 3‐hydroxy‐acyl intermediates, suggesting that the either the stability of the dehydratase and/or its association with the elongase complex is compromised when Ybr159p is missing.
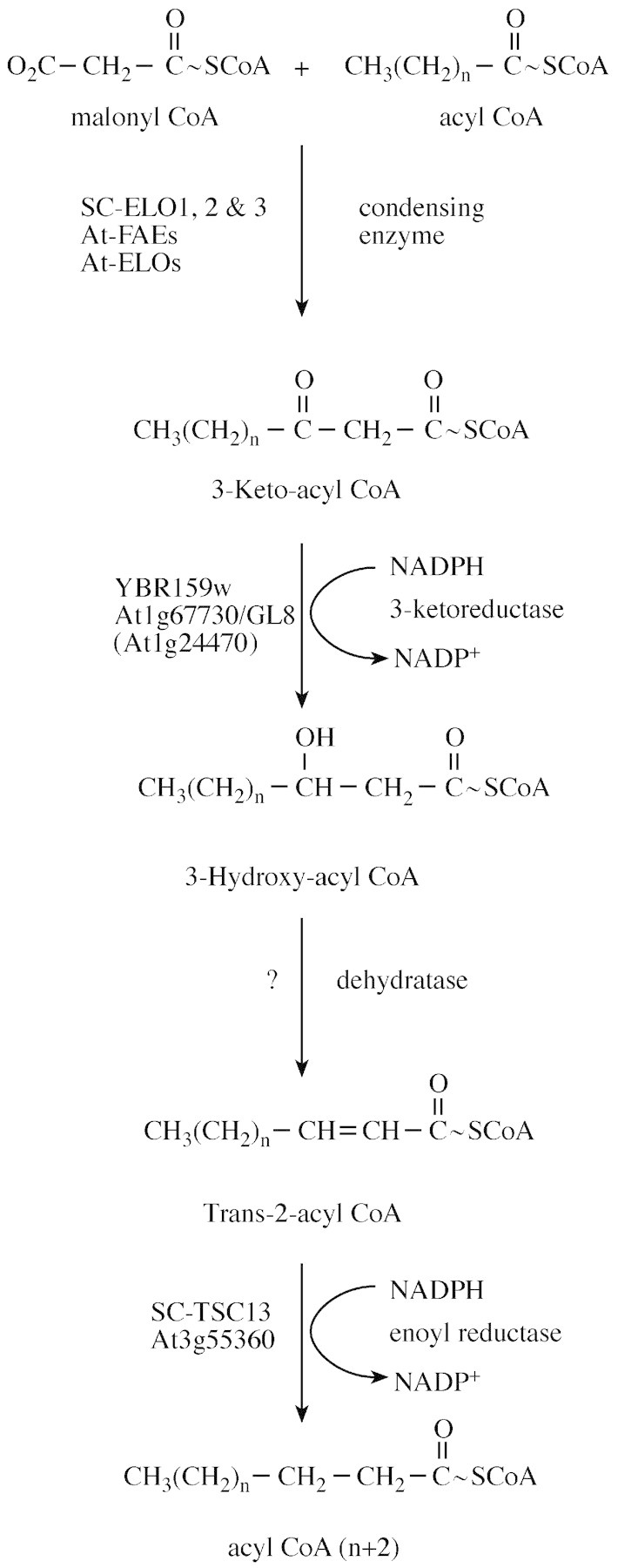
Fig. 2. Microsomal fatty acid elongation and genes involved in this process. Genes from either yeast or arabidopsis are annotated in the four sequential biochemical steps required for the C2 elongation of an acyl‐CoA substrate. The molecular identity of the third reaction, the dehydratase, is currently unknown.
There are functionally defined arabidopsis homologues for several of the elongase components (Table 1). The arabidopsis YBR159 homologue At1g67730 complements the ybr159Δ mutant (Han et al., 2002), and is orthologous to the maize gene Glossy8, which has been shown to be required for cuticular wax synthesis (Xu et al., 2002). Recent work on the biochemical function of the maize GL8 protein has confirmed a role in very long‐chain fatty acyl CoA elongation, with antibodies raised against GL8 inhibiting in vitro stearoyl‐CoA elongation. Interestingly, there is another arabidopsis ORF, At1g24470, which shows (reduced) similarity to YBR159 that is about 70 % similar to At1g67730. Since the enzyme activity encoded by YBR159/GL8 is also a member of the large short‐chain dehydrogenase/reductase superfamily (see above) it remains to be determined if this second arabidopsis ORF is also a reductase involved in fatty acid elongation. We have observed recently that the AT‐TSC13 homologue At3g55360 will complement the temperature‐sensitive and VLCFA synthesis phenotypes of the tsc13‐1 mutant (Gable et al., 2004). Perhaps surprisingly, we have also found that heterologous expression of the arabidopsis FAE1 gene rescues the lethality of the elo2Δelo3Δ double mutant (our unpublished results). The fatty acid profiles of the rescued strain are similar to those of an elo3Δ single mutant. This is consistent with the results of Jaworski and coworkers who showed that Fae1p prefers saturated C18 and C20 substrates (Ghanevati and Jaworski, 2002). It will be interesting to test whether Fae1p physically associates with the other components of the yeast elongase. However, the rescue of elo2Δelo3Δ by this structurally unrelated plant enzyme activity implies some commonality in elongase interactions.
One important point of note is that yeast mutants with defects in VLCFA synthesis (such as elo2Δ, elo3Δ, tsc13‐1 and ybr159Δ) accumulate high levels of free LCB, perhaps reflecting the reduced pool of VLCFAs available for ceramide synthesis (i.e. acylation to LCBs) (Kohlwein et al., 2001; Han et al., 2002). In addition, whereas wild‐type yeast has exclusively C26‐ceramide, the elongase mutants accumulate C16‐ceramide, presumeably as a compensatory response. It remains to be determined if complementation of these yeast mutants with the arabidopsis orthologues results in the full reversal of these pleiotropic effects, and it also will be important to determine if similar metabolic plasticity is observed in arabidopsis plants with disruptions in endogenous sphingolipid biosynthetic genes.
SYNTHESIS, MODIFICATION AND METABOLISM OF CERAMIDE
Formation of ceramide by an acyl‐CoA dependent sphinganine acyltransferase (SAT) (i.e. ceramide synthase) activity has been demonstrated in plant microsomes (Lynch, 1993, 2000). d‐Erythro isomers of sphinganine and sphingosine serve as substrates for the reaction, but 4‐hydroxysphinganine and the threo isomer of sphinganine do not. The relative activities of SAT in different plant tissues using acyl‐CoA molecules varying in chain length from 16 to 24 carbons parallel the distribution of hydroxy‐acyl chains of glucosylceramides isolated from the respective tissues. Unsaturated acyl‐CoA does not serve as substrate for the plant enzyme, nor do α‐hydroxy fatty acyl‐CoA molecules. The plant enzyme activity is localized in the endoplasmic reticulum and is inhibited by nanomolar concentrations of fumonisin B1, resulting in an accumulation of sphinganine and 4‐hydroxysphinganine in plant tissues treated with the toxin (Abbas et al., 1994; Wright et al., 2003).
Two homologous genes, LAG1 and LAC1 are required for the acyl‐CoA‐dependent synthesis of ceramide in yeast (Guillas et al., 2001; Schorling et al., 2001). Neither gene is essential, but the double disruptant grows poorly. Surprisingly, although the mutant makes very little ceramide, it is viable. There are also two homologous genes, YDC1 and YPC1, which encode ceramidases (Mao et al., 2000a, b). Though presumed to be involved in ceramide breakdown, Ypc1p and Ydc1p do have free fatty acid‐dependent ceramide synthase activity in vitro, thus serving as an additional (acyl CoA‐independent) route for ceramide synthesis. Note that ceramide synthesis in vitro utilizing free fatty acid rather than acyl‐CoA has been detected in plant tissues (Lynch, 2000). Although the properties of this reaction appear distinct from those reported for plant ceramidase (Lynch, 2000), it is likely that this mechanism for ceramide formation reflects a reversal of ceramidase activity.
The SCS7 gene encodes the enzyme that hydroxylates the α‐carbon of the VLCFA of ceramide (Haak et al., 1997; Mitchell and Martin, 1997; Dunn et al., 1998). The sur2Δ mutant cells accumulate ceramides and IPCs that contain unhydroxylated VLCFAs, indicating that Scs7p‐dependent hydroxylation of the fatty acid is in turn sensitive to the hydroxylation state of the LCB, and therefore that the substrate of Scs7p is most likely ceramide, with 4‐hydroxysphinganine‐ceramides preferred over sphinganine‐ceramides (Haak et al., 1997). There are presumptive arabidopsis homologues of LAG1/LAC1, YPC1/YDC1 (Table 1), though these ORFs remain to be functionally characterized. A LAG1‐homologue has been shown to mediate pathogen resistance in tomato (Brandwagt et al., 2000). One arabidopsis SCS7 orthologue has been functionally characterized in yeast and shown to partially restore the levels of ceramide α‐hydroxylation in a scs7Δ mutant (Mitchell and Martin, 1997). Intriguingly, this arabidopsis enzyme lacks the N‐terminal cytochrome b5 domain present in the yeast gene, indicating that unlike ‘front‐end’ fatty acid desaturases, this N‐terminal extension is not obligatory for enzyme function. The role of the cytochrome b5 electron transport chain in sphingolipid metabolism remains to be determined in higher plants, though the LCB Δ8‐desaturase of plants also contains an N‐terminal cytochrome b5 domain (discussed below).
An additional gene in yeast (CCC2) has been shown to be required for the synthesis of ceramides containing dihydroxylated VLCFAs (Fu et al., 1995; Beeler et al., 1997). Ccc2p encodes a membrane transporter responsible for Cu2+ accumulation in the Golgi (Yuan et al., 1995), suggesting that the hydroxylase that generates the dihydroxy VLCFAs resides in the Golgi and is Cu2+‐dependent. Two possible arabidopsis candidates are present in the genome, though neither of these ORFs have been functionally characterized. In yeast, ceramide is converted to IPC by Aur1p, which utilizes PI as the phosphoinositol donor. IPC synthase activity has been characterized in wax bean microsomes and detected in a variety of other plant tissues (Bromley et al., 2003). As in yeast, PI serves as the phosphoinositol donor and different ceramide species (including fluorescent NBD‐C6 ceramide) serve as substrate for the in vitro reaction. The enzyme activity is most closely associated with the Golgi apparatus, and is inhibited by aureobasidin A and rustmicin, two potent inhibitors of the fungal enzyme. The AUR1 gene is essential (Heidler and Radding, 1995; Hashida‐Okado et al., 1996; Nagiec et al., 1997), suggesting that IPCs are necessary for viability, although the possibility that elevated ceramide levels cause the death of aur1Δ mutant cells has not been excluded. However, there is no obvious AUR1 orthologue present in the arabidopsis genome (for discussion, see below; also see Table 3). The synthesis of GlcCer utilizing either UDP‐glucose (Nakayama et al., 1995) or steryl glucoside (Lynch et al., 1997) as glucose donor has been demonstrated using plant membrane preparations. While the former activity is similar to that reported in mammalian systems, the latter activity is consistent with other roles for steryl glucoside in plants. Expression of a glucosylceramide synthase gene from cotton in S. cerevisiae (which naturally lacks steryl glucoside as well as GlcCer) did not result in GlcCer synthesis (Leipelt et al., 2001) but expression in a double mutant of Pichia pastoris, lacking both glucosylceramide synthase and sterol glucosyltransferase activities, resulted in the formation of both GlcCer and steryl glucoside in vivo (Hillig et al., 2003).
Table 3.
Yeast genes which are involved in sphingolipid metabolism but lack obvious orthologues in arabidopsis
| Enzyme activity | Yeast gene | Comments |
| SPT‐associated protein | TSC3 | Orthologues may be present in the arabidopsis genome but undetected due to small size of ORF (∼80 residues) |
| IPC synthase | AUR1 | Recent data indicates IPC synthase in plants (like yeast) is AurB‐sensitive. However, no obvious AUR1 orthologues are currently visible in the arabidopsis genome, even though inositol‐containing sphingolipids are prevalent in plants |
| Mannosylation of IPC | CSG1/2 | Definitive identification of mannosylated sphingolipids in plants is currently lacking |
| M(IP)2C synthase | IPT1 | The yeast IPT1 gene is structurally related to AUR1. No obvious homologue is visible |
| SUR7 family of genes | SUR7/YNL194c/YDL222w | These genes appear to specific to yeast, and may be involved in aspects of sphingolipid LCB homeostasis |
| Complex sphingolipase | ICS1 | The presence of higher plant sphingolipases has not been tested |
Synthesis and metabolism of LCB‐P
In yeast, LCBs can be phosphorylated to form LCB‐1‐P by Lcb4p or Lcb5p, with Lcb4p being responsible for the majority (>95 %) of the LCB kinase activity (Nagiec et al., 1998). Once formed, the LCB‐Ps can be dephosphorylated by the Lcb3p phosphatase (Mandala et al., 1998; Mao et al., 1999). Alternatively, LCB‐Ps can be cleaved at the C2–3 C‐C bond by the LCB‐P lyase (Dpl1p) to form a fatty aldehyde and ethanolamine‐phosphate (Saba et al., 1997). Cells lacking either the LCB3 gene (Mandala et al., 1998; Mao et al., 1999a; Skrzypek et al., 1999) or the DPL1 gene (Saba et al., 1997) accumulate LCB‐Ps, but the single mutants are viable. However, the lcb3Δdpl1Δ double mutant is lethal, apparently due to excessive LCB‐1‐P since deletion of the LCB4 gene suppresses the lethality (Kim et al., 2000). Presumptive orthologues for DPL1, LCB3 and LCB4/5 are present in the arabidopsis genome (Table 1), and the partial characterization of an arabidopsis LCB kinase has been published (Nishiura et al., 2000), though the enzyme appears to be less closely related to the yeast genes than the two plants orthologues listed in Table 1. Sphinganine kinase activity (Crowther and Lynch, 1997) and sphingosine phosphorylation (Coursol et al., 2003) have been reported in plant tissues.
PLANT‐SPECIFIC SPHINGOLIPID GENES
In addition to the actual or presumptive arabidopsis orthologues of yeast sphingolipid biosynthetic enzymes, the greater complexity of plant sphingolipids requires the presence of additional enzyme activities not present in S. cerevisiae (see Table 2 for details). Some of these are relatively well defined (such as the sphingolipid‐Δ8‐desaturases), whereas others have only recently been identified (such as the dihydroceramide/dihydrosphingosine Δ4‐desaturase and glucosylceramide synthase). There is also the large plant‐specific FAE1‐like family of genes involved in lipid metabolism, which encodes condensing enzymes involved in VLCFA synthesis. The FAE1‐like gene family has (at least) 20 members in arabidopsis (Table 2). Fae1p is a condensing enzyme required for the synthesis of erucic acid (22:1, n‐9) in arabidopsis, via two sequential rounds of C2 elongation of oleate (Millar and Kunst, 1997). Genetic studies have demonstrated the importance of FAE1‐like genes to arabidopsis development, emphasizing how fundamental lipid metabolism is in plants (Todd et al., 1999; Yephremov et al., 1999; Pruitt et al., 2000; Schreiber et al., 2000; Puyaubert et al., 2001; Roscoe et al., 2001; Rossak et al., 2001). However, the actual role of fatty acyl elongation in these processes is not yet clear and the precise enzymatic functions and substrates of most of the FAE1 gene products are not known. In addition, it is unknown whether is it is an FAE1 homologue or ELO homologue that is responsible for the synthesis of the VCLFA components of sphingolipids. Studies on the FAE1 homologues have been undertaken using the heterologous expression in yeast (Rossak et al., 2001; Blacklock and Jaworski, 2002; Ghanevati and Jaworski, 2002; Katavic et al., 2002). Our recent finding that Fae1p can substitute for the unrelated Elo2p/Elo3p enzymes (also thought to be condensing enzymes of VLCFA synthesis) and thereby restore viability and sphingolipid synthesis to the elo2Δelo3Δ mutant demonstrates that Fae1p‐derived VLCFAs can be channelled into sphingolipids in yeast, and raises the possibility that Fae1p (and the proteins encoded by the FAE‐1 like ORFs) interacts with the other elongase components (Tsc13p and Ybr159p). For example, the arabidopsis FAE1‐like gene HIC (Table 1) has been shown to be involved in a gene involved in the signal transduction pathway responsible for controlling stomatal numbers at elevated CO2 (Gray et al., 2000), raising the possibility of sphingolipid metabolism being involved in another aspect of guard cell biology. Clearly the FAE1‐like gene family is of great interest, but the apparent gene redundancy makes in planta characterization (either by insertional inactivation or gene silencing) a daunting task. Perhaps what makes the FAE1‐like family of condensing enzymes intriguing is that this gene family appears to be exclusive to higher plants. This could represent an adaptive strategy (reflected in chemical diversity of, for example, cuticular waxes and membrane lipids) to deal with the sessile nature of plants and their interactions with a range of biotic and abiotic stresses.
Table 2.
Arabidopsis genes known to be involved in sphingolipid metabolism but absent from yeast (the status of functional characterization of these genes is indicated)
| Enzyme | MIPS code | Functional characterization |
| LCB Δ4‐desaturase | At4g04930 | Presumptive activity, though expression in yeast fails to confirm desaturase substrate |
| LCB Δ8‐desaturases | At2g46210 At3g61580 | Heterologous expression of either ORF in yeast results in the synthesis of t18:1 Δ8‐LCBs. Enzymes are stereo‐unselective, generating cis/trans double bonds |
| Glucosyl‐ceramide synthase (GCS) | At2g19880 | Presumptive activity, though expression in yeast unsuccessful |
| Ceramide kinase | At5g51290 | Corresponds to ACD5 (accelerated cell death) gene, encoding a lipid kinase with in vitro specificity for short‐chain ceramides (Liang et al., 2003) |
| FAE1‐like family Microsomal condensing enzymes some of which may be involved in sphingolipid metabolism | At1g01120 At1g04220 At1g07720 At1g19440 | Several of these ORFs have been functionally characterized and shown to be involved in the synthesis of very long‐chain fatty acids. These include components of waxes (KCS1, CUT1), triacylglycerols (FAE1) or currently undefined lipids |
| At1g25450 | ||
| At1g68530 | ||
| At1g71160 | ||
| At2g15090 | ||
| At2g16280 | ||
| At2g26250 | ||
| At2g26640 | ||
| At2g28630 | ||
| At2g46720 | ||
| At3g10280 | ||
| At3g52160 | ||
| At4g34250 | ||
| At4g34510 | ||
| At4g34520 | ||
| At5g04530 | ||
| At5g43760 | ||
| At5g49070 |
Higher plants are distinct from animals and S. cerevisiae in that they contain two distinct enzymes, which introduce double bonds into the sphingolipid LCB (Fig. 3). These are the cytochrome b5‐fusion protein sphingolipid Δ8‐desaturase and the (non‐fusion) sphingolipid Δ4‐desaturase (Napier et al., 1999; Sperling et al., 2003). Neither enzyme activity is found in S. cerevisiae, whereas the sphingolipid Δ4‐desaturase appears ubiquitous in animals and serves as the key biosynthetic step in the synthesis of sphingosine. The precise substrate (complex sphingolipid, ceramide or LCB) of the sphingolipid Δ8‐desaturase remains to be defined, though it is clear that Δ8‐desaturated LCBs (primarily C18 phytosphingosine) are major components of the higher plant LCB complement. It has also been demonstrated recently that the sphingolipid Δ8‐desaturase is capable of using dihydroxy LCB substrates (Michaelson et al., 2002), whereas previously only trihydroxy LCBs had been demonstrated to act as a substrate for this enzyme (Sperling et al., 2000); this has implications for the synthesis of di‐unsaturated LCBs.
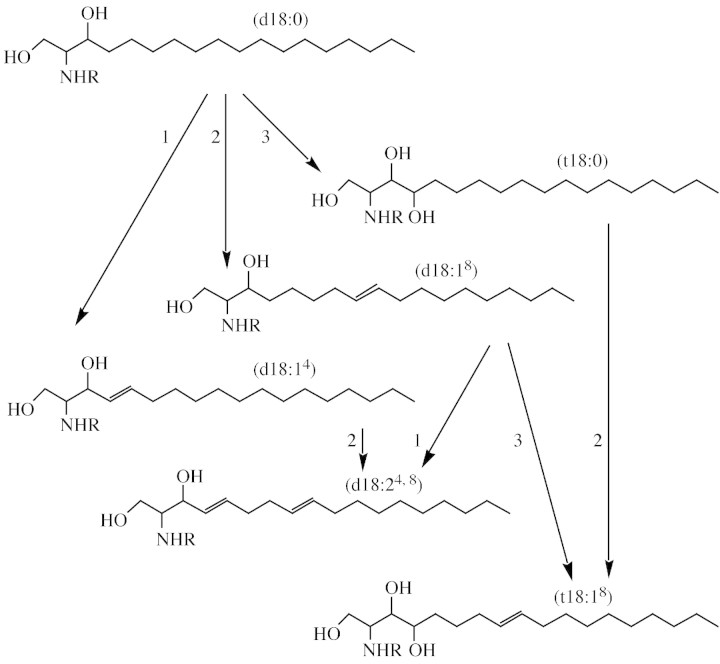
Fig. 3. Long‐chain base heterogeneity in plants is mediated by three different enzyme activities. Modifications to sphingolipid LCBs are mediated by three enzymes, as indicated above. These enzymes are (1) LCB Δ4‐desaturase, (2) LCB Δ8‐desaturase, (3) LCB C4‐hydroxylase (this latter enzyme is encoded by the orthologous SUR2 gene in yeast). The LCB Δ8‐desaturase appears to be the key determinant of enhanced heterogeneity, since not only is this enzyme stereo‐unselective (yielding both cis and trans double bonds at the Δ8 position), it also recognizes both di‐ and trihydroxy LCBs (d18:0 and t18:0) as substrates for desaturation. Allowing for the cis or trans orientation of the Δ8 double bond, nine different C18 LCBs are possible in plant sphingolipids. The d18:24,8 LCBs appear to be enriched in GlcCers, whereas t18:18 LCBs are likely to be major components of IPCs.
One intriguing observation regarding the higher plant sphingolipid Δ8‐desaturases is that they have a high level of sequence identity to the (rarely occurring) higher plant fatty acid Δ6‐desaturase, the enzyme responsible for the synthesis of γ‐linolenic acid (GLA) from linoleic acid (Sayanova et al., 1997). Only a few plant species synthesize Δ6‐desaturated fatty acids such as GLA and the distribution of this unusual trait is dispersed amongst unrelated taxa. This has led us and others to suggest that there is a paralogous relationship between the higher plant fatty acid Δ6‐ and sphingolipid Δ8‐desaturases, and that the rare occurrence of the fatty acid Δ6‐desaturase reflects mutations in the ubiquitous sphingolipid desaturase (Napier et al., 2003; Sperling et al., 2003). Such a concept also helps to explain the very low level of sequence identity between plant and animal fatty acid Δ6‐desaturases (Napier et al., 2003). It is likely, therefore, that the plant sphingolipid desaturase and the animal Δ6‐desaturase evolved from a single ancestral progenitor, and that the presence of higher plant Δ6‐desaturases represents an example of (pseudo‐) convergent evolution. However, the physiological role of the sphingolipid Δ8‐desaturase and the LCBs resulting from this enzyme remain to be determined. That the enzyme generates both cis and trans stereoisomers at the C‐8 position also has implications for the functionality of the desaturated LCB (and any resulting metabolites, e.g. LCB‐1‐P), as well as increasing the heterogeneity of plant LCBs two‐fold. Previously, it has been hypothesized that the presence of high levels of cis‐Δ8‐desaturated LCBs is correlated with a chilling‐tolerance trait in a range of plant species (Imai et al., 1997), though expanding datasets have called this initial proposal into question.
The second enzyme that results in the introduction of a double bond into the LCB is the recently identified sphingolipid Δ4‐desaturase (Napier et al., 2002; Ternes et al., 2002; Garton et al., 2003). This enzyme is responsible for the synthesis of sphingosine in animals, but this LCB is generated by the specific conversion of dihydroceramide (acyl‐sphinganine) to ceramide (acyl‐sphingosine) via the introduction of a trans double bond at the C‐4 position. Thus, the substrate for the Δ4‐desaturase is not the free LCB dihydrosphingosine but the acylated form (i.e. dihydroceramide), with free sphingosine being produced by subsequent deacylation of the resultant ceramide. The dihydroceramide Δ4‐desaturase is not a cytochrome b5 fusion desaturase and defines a new class of lipid desaturase (Napier et al., 2002); moreover, the dihydroceramide Δ4‐desaturase is stereo‐specific for the trans configuration. Saccharomyces cerevisiae does not synthesize Δ4‐desaturated LCBs and does not contain an orthologue of the dihydroceramide desaturase. Fission yeast does contain this activity and the functional characterization of the Schizosaccharomyces pombe sphingolipid Δ4‐desaturase has recently demonstrated it to be a non‐essential gene (Garton et al., 2003). Although the presence of presumptive orthologues of the sphingolipid Δ4‐desaturase are readily detectable as EST sequences in higher plant sequencing projects, no functional characterization of this enzyme has been reported.
Attempts to heterologously express either an arabidopsis or tomato presumptive orthologue has failed to reconstitute any Δ4‐desaturase activity (our unpublished data; Sperling and Heinz, 2003). Whether this reflects an unknown (plant‐specific) aspect of Δ4‐desaturation is currently unclear, though it might be predicted (on the basis of previous data) that the substrate for higher plant dihydroceramide desaturase is N‐acyl‐d18:1 Δ8. This would explain the non‐functionality of the plant enzyme in S. cerevisiae sur2Δ cells (which lack the Δ8‐desaturase). However, a sphingolipid Δ4‐desaturase from Candida albicans has been functionally expressed in the same sur2Δ background, resulting in the synthesis of sphingosine ((Beckmann et al., 2003). This implies that the C. albicans Δ4‐desaturase does not require N‐acyl‐Δ8‐desaturated sphinganine as its sole substrate, but instead can use N‐acyl‐sphinganine even though C. albicans contains Δ8‐desaturated LCBs (Sperling and Heinz, 2003). Thus, this fungal sphingolipid Δ4‐desaturase may be distinct from the higher plant forms of the enzyme. On the other hand, it has recently been reported that another fungus, Pischa pastoris, contains a sphingolipid Δ8‐desaturase which specifically synthesizes the trans isomer of Δ8‐desaturated sphinganine (i.e. d18:1Δ8t) (Sperling and Heinz, 2003). The authors of that study highlighted the observation that such a Δ8‐activity was consistent with the prevalent occurrence of dienine LCBs in the cerebrosides of P. pastoris, and implying Δ4‐desaturation occurred (at least to some extent) on the N‐acyl Δ8‐desaturated sphinganine. It is clear that the subtleties of plant and fungal sphingolipid desaturation are only starting to be unravelled.
As discussed above, the reported presence of the ceramide metabolite sphingosine‐1‐phosphate has generated considerable interest as a signalling molecule in higher plants. Although the most abundant LCBs in leaf tissues of many higher plants are Δ8‐desaturated C18 trihydroxy bases, other LCBs are also present. These include the above‐mentioned Δ4‐desaturated dihydroxy base sphingosine, which in total LCB extracts of arabidopsis is present at <1 %. Also present in this plant are Δ4‐, Δ8‐desaturated dihydroxy LCBs, again at very low levels, though this LCB may be quite abundant in some plant species such as tomato (Imai et al., 1997). As discussed above, the Δ8‐desaturation of both di‐ and trihydroxy LCBs implies that this reaction may precede the introduction of the Δ4 double bond and/or Δ4‐hydroxylation. However, the substrate for Δ8‐desaturation (i.e. free LCB versus ceramide) is currently unknown but most likely follows acylation.
Whilst a number of fungal orthologues of the sphingolipid Δ8‐desaturase have been recently identified which have a defined (trans) stereochemistry (Sperling and Heinz, 2003), the basis for this selectivity, and the stereo‐unselectivity of the higher plant enzymes remains to be determined. Recently, we (L.V.M. and J.A.N., unpublished) isolated a higher plant sphingolipid Δ8‐desaturase which displays stereo‐selectivity, and it is hoped that this sequence will help identify amino acid determinants for this specificity. Some fungi also introduce a methyl branch into dienine LCBs, producing a 9‐methyl, Δ4, Δ8‐desaturated LCB, though the presence of such branched LCBs have not been reported in higher plants (Sperling and Heinz, 2003). This fungal‐specific C‐9 methyl group appears to play a role in the perception of pathogens by plants.
YEAST SPHINGOLIPID GENES NOT PRESENT IN ARABIDOPSIS
Several genes involved in yeast sphingolipid biosynthesis are absent from the arabidopsis genome (see Table 3 for details). The most notable of these are the yeast genes involved in the synthesis of inositolphosphorylceramide (IPC) and its subsequent modifications. These genes are: (1) AUR1, an essential gene required for the synthesis of IPC and probably encoding the IPC synthase activity (Hashida‐Okado et al., 1996); (2) CSG1 and CSG2, required for the addition of mannose to IPC to form mannoseinositolphosphorylceramide (MIPC) (Beeler et al., 1994, 1997); and (3) IPT1, an inositolphosphotransferase which is required for the synthesis of mannosediinositolphosphorylceramide [M(IP)2C] from MIPC (Dickson et al., 1997). Additionally, the SUR7 small gene family that is present in yeast and appears to play a role in defining the LCB composition and hydroxylation status of IPC (Young et al., 2002) is absent from arabidopsis, as is any orthologue of the ISC1 gene that encodes a complex sphingolipase (Sawai et al., 2000). The small polypeptide encoded by the yeast gene TSC3, required for maximal activity of SPT (Gable et al., 2000), is apparently absent from the arabidopsis genome, though this may reflect the problems in predicting small ORFs in complex genomes.
As discussed above, higher plants synthesize both inositol‐containing sphingolipids and glucosylceramides. Previously, it was considered that the glucosylceramide content of plants was greater than that of (glycosyl)‐IPC sphingolipids, since these complex polar IPC derivatives were not readily recovered using standard lipid extraction procedures, though it now seems likely that the reverse is true (Sperling and Heinz, 2003). Given both the importance and prevalence of inositol‐containing sphingolipids in plants, the absence of an AUR1 IPC synthase might indicate that these sphingolipids are synthesized by an alternative enzyme activity; this may take the form of a remodelase type of enzyme (as proposed by Reggiori and Conzelmann, 1997) or a novel process. However, very recent data indicate that a higher plant IPC synthase activity is Aur‐sensitive, indicating some degree of commonality with the Aur1p activity (Bromley et al., 2003).
PLANT SPHINGOLIPID METABOLISM: QUESTIONS STILL TO BE ADDRESSED
Whilst the combination of bioinformatics and heterologous functional expression have helped identify a number of arabidopsis ORFs involved in sphingolipid metabolism, it is clear that many fundamental questions still remain to be answered. The list below should be considered as a starting point, rather than definitive.
Some specific questions that are still outstanding:
•What is the role of the increased LCB heterogeneity (underpinned by the presence of two LCB desaturases and one hydroxylase) present in plants?
•What are the substrates for these LCB modifying enzymes (complex sphingolipids, ceramides or free LCBs)?
•How are the complex sphingolipids synthesized in the apparent absence of the AUR1/IPT1 pathway?
•How/why do GlcCer and IPC have different LCB compositions and how is this regulated?
Other, broader questions include:
•What (if any) is the role of sphingolipids in plant GPI‐anchored proteins?
•Do plant sphingolipids play a role in plant vesicular trafficking?
•Are sphingolipids a component of plant lipid rafts and, if so, in what form?
•How is sphingolipid homeostasis maintained, and what regulates cross‐talk with other lipid components?
•Do plant sphingolipids (or their metabolites) modulate gene expression?
Clearly, further questions will also arise as studies on plant sphingolipid metabolism progress. A good example is the emerging role of the phosphorylated LCB sphingosine‐1‐phosphate in Ca2+‐mediated guard cell closure (Ng et al., 2001). However, as discussed above, the endogenous levels of sphingosine (as opposed to Δ4‐desaturated dienines) in total sphingolipid extracts from a range of plant species are consistently very minor components (unlike the situation in animals). Moreover, studies in animal systems have indicated that the S‐1‐P signal is transduced by a family of G‐protein coupled receptors (GPCRs); such GPCRs are not present in higher plants, though recent data (Coursol et al., 2003) raises the possibility of plants signalling via a heterotrimeric G‐protein. Thus, it should be clear that, far from conforming to the animal paradigm for S‐1‐P signalling, plants should (perhaps rather obviously) be considered different.
Perhaps the most exciting possibility regarding LCB‐1‐P signalling in plants is that the majority of the (more abundant) LCBs have yet to be tested for activity (either as a kinase substrate or as a phosphorylated signal), raising the possibility of novel LCB‐1‐P signalling molecules. Such experiments have not yet been carried out due to the (commercial) non‐availability of these plant‐specific LCBs and LCB‐1‐Ps, though the phosphorylation of an endogenous (unidentified) plant‐specific LCB has been observed (Coursol et al., 2003). In that latter respect, it is very likely that more sensitive analytical techniques will need to be developed, since most mass spectrometry protocols are unable to provide unambiguous data on the regio‐ and stereo‐specificity of double bonds (the very source of plant LCB heterogeneity).
It is also quite possible that endogenous synthesis of minor LCBs (such as sphingosine) is restricted to a very limited number of tissues or cell types, resulting in the localized elevated levels of this LCB not reflected in total plant analysis. It remains to be determined by what process free LCBs are mobilized from sphingolipids, and how the phosphorylation/dephosphorylation and subsequent catabolism is regulated. In animal systems, a ‘sphingolipid rheostat’ model has been proposed by Spiegel and colleagues in which the S‐1‐P phosphohydrolase (orthologous to the yeast gene LCB3) plays a central role in ceramide homeostasis (Le Stunff et al., 2002). Current plant research in this area is lacking, though the emergence of roles in apoptosis for both free LCBs and ceramides (Spassieva et al., 2002) make such models both applicable and appealing. The recent identification of a ceramide kinase ACD5 which is involved in programmed cell death in arabidopsis adds additional impetus to this topic, though the endogenous substrate of the ACD5 kinase remains to be determined (Liang et al., 2003)
CONCLUSIONS
Comparative genomics has revealed candidates for many sphingolipid genes in arabidopsis (Tables 1 and 2), and heterologous expression of several plant lipid genes in yeast has led to determination of their functions (Gachotte et al., 1996; Cahoon and Shanklin, 2000; Leipelt et al., 2000; Sperling et al., 2000, 2001; Zank et al., 2000, 2002; Cahoon et al., 2001, 2002; Leipelt et al., 2001; Mekhedov et al., 2001; Sayanova et al., 2001; Blacklock and Jaworski, 2002; Ghanevati and Jaworski, 2002; Han et al., 2002). Tools for analysing the function of arabidopsis genes using reverse genetics have been developed and are now widely available, as are inducible promoters for the ‘switchable’ modulation of gene expression. Perhaps more importantly, robust and sensitive analytical techniques have started to be developed to allow the determination of plant sphingolipid composition. Thus, the combination of reverse genetics and MS‐ and NMR‐based structural analysis should provide definitive data as to the function of candidate sphingolipid biosynthetic genes in arabidopsis. Functional characterization in a heterologous system (such as yeast) also provides a more rapid confirmation of enzyme activity. An additional tool now becoming available is the profiling analysis of total lipids, which serves to profile the entire complement of lipids, not just a particular sub‐class such as sphingolipids. Such analysis may allow inferences as to the homeostasis and/or remodelling between different lipid species. Finally, NMR protocols for semi‐anonymous analysis of the entire metabolome have been developed for arabidopsis, allowing pleiotropic or unexpected changes in global metabolism to be identified (Ward et al., 2003).
Clearly, the stage is set for identifying the precise functions of the putative arabidopsis sphingolipid genes, for elucidating many unresolved aspects of the sphingolipid pathway in plants and, most significantly, for determining the roles of sphingolipids in plants. The outcomes of such research are likely to widen our understanding of plant metabolism, as well as broaden our knowledge of fundamental plant processes.
ACKNOWLEDGEMENTS
Rothamsted Research receives grant‐aided support from the Biotechnology and Biological Sciences Research Council (BBSRC), UK. T.M.D. and D.V.L. are supported by the National Science Foundation (NSF), USA.
Received: 28 October 2003; Returned for revision: 11 December 2003; Accepted: 6 January 2004 Published electronically: 22 March 2004
References
- AbbasH,Tanaka T, Duke SO, Porter JK, Wray EM, Hodges L, Sessions AE, Wang E, Merrill Jr AH, Riley RT.1994. Fumonisin‐ and AAL‐toxin‐induced disruption of sphingolipid metabolism with accumulation of free sphingoid bases. Plant Physiology 106: 1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BaldwinTC, Handford MG, Yuseff MI, Orellana A, Dupree P.2001. Identification and characterization of GONST1, a golgi‐localized GDP‐mannose transporter in Arabidopsis. Plant Cell 13: 2283–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BeaudoinF, Gable K, Sayanova O, Dunn T, Napier JA.2002. A Saccharomyces cerevisiae gene required for heterologous fatty acid elongase activity encodes a microsomal beta‐keto‐reductase. Journal of Biological Chemistry 277: 11481–11488. [DOI] [PubMed] [Google Scholar]
- BeckmannC, Rattke J, Sperling P, Heinz E, Boland W.2003. Stereochemistry of a bifunctional dihydroceramide delta 4‐desaturase/hydroxylase from Candida albicans; a key enzyme of sphingolipid metabolism. Organic and Biomolecular Chemistry 1: 2448–54. [DOI] [PubMed] [Google Scholar]
- BeelerT, Bacikova D, Gable K, Hopkins L, Johnson C, Slife H, Dunn T.1998. The Saccharomyces cerevisiae TSC10/YBR265w gene encoding 3‐ketosphinganine reductase is identified in a screen for temperature‐sensitive suppressors of the Ca2+‐sensitive csg2D mutant. Journal of Biological Chemistry 273: 30688–30694. [DOI] [PubMed] [Google Scholar]
- BeelerTJ, Fu D, Rivera J, Monaghan E, Gable K, Dunn TM.1997. SUR1 (CSG1/BCL21), a gene necessary for growth of Saccharomyces cerevisiae in the presence of high Ca2+ concentrations at 37 degrees C, is required for mannosylation of inositolphosphorylceramide. Molecular and General Genetics 255: 570–579. [DOI] [PubMed] [Google Scholar]
- BeelerT, Gable K, Zhao C, Dunn T.1994. A novel protein, CSG2p, is required for Ca2+ regulation in Saccharomyces cerevisiae Journal of Biological Chemistry. 269: 7279–7284. [PubMed] [Google Scholar]
- BilleJ, Weiser T, Bentrup FW.1992. The lysolipid sphingosine modulates pyrophosphatase activity in tonoplast vesicles and isolated vacuoles from a heterotrophic cell suspension culture of Chenopodium rubrum Physiologia Plantarum 84: 250–256. [Google Scholar]
- BlacklockBJ, Jaworski JG.2002. Studies into factors contributing to substrate specificity of membrane‐ bound 3‐ketoacyl‐CoA synthases. European Journal of Biochemistry 269: 4789–4798. [DOI] [PubMed] [Google Scholar]
- BoggsJ.1987. Lipid intermolecular hydrogen bonding: influence on structural organization and membrane function. Biochimica et Biophysica Acta 906: 353–404. [DOI] [PubMed] [Google Scholar]
- BornerGH, Lilley KS, Stevens TJ, Dupree P.2003. Identification of glycosylphosphatidylinositol‐anchored proteins in Arabidopsis. A proteomic and genomic analysis. Plant Physiology 132: 568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BrandwagtBF, Mesbah LA, Takken FL, Laurent PL, Kneppers TJ, Hille J, Nijkamp HJ.2000. A longevity assurance gene homolog of tomato mediates resistance to Alternaria alternata f. sp. lycopersici toxins and fumonisin B1. Proceedings of the National Academy of Science of the USA 97: 4961–4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BrodersonP, Pike HM, Olszak B, Skov S, Odum N, Jorgensen LB, Brown RE, Mundy J.2002. Knockout of Arabidopsis accelerated‐cell‐death 11 encoding a sphingosine transfer protein causes activation of programmed cell death and defense. Genes and Development 16: 490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BromleyPE, Li YO, Murphy SM, Sumner CM, Lynch DV.2003. Complex sphingolipid synthesis in plants: characterization of inositolphosphorylceramide synthase activity in bean microsomes. Archives of Biochemistry and Biophysics 417: 219–226. [DOI] [PubMed] [Google Scholar]
- CahoonEB, Ripp KG, Hall SE, Kinney AJ.2001. Formation of conjugated Δ8, Δ10‐double bonds by Δ12‐oleic‐acid desaturase‐related enzymes: biosynthetic origin of calendic acid. Journal of Biological Chemistry 276: 2637–2643. [DOI] [PubMed] [Google Scholar]
- CahoonEB, Ripp KG, Hall SE, McGonigle B.2002. Transgenic production of epoxy fatty acids by expression of a cytochrome P450 enzyme from Euphorbia lagascae seed. Plant Physiology 128: 615–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CahoonEB, Shanklin J.2000. Substrate‐dependent mutant comple mentation to select fatty acid desaturase variants for metabolic engineering of plant seed oils. Proceedings of the National Academy of Science of the USA 97: 12350–12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CoursolS, Fan LM, Le Stunff H, Spiegel S, Gilroy S, Assmann SM.2003. Sphingolipid signalling in Arabidopsis guard cells involves heterotrimeric G proteins. Nature 423: 651–654. [DOI] [PubMed] [Google Scholar]
- CrowtherGJ, Lynch DV.1997. Characterization of sphinganine kinase activity in corn shoot microsomes. Archives of Biochemistry and Biophysics 337: 284–290. [DOI] [PubMed] [Google Scholar]
- DharmawardhaneS, Rubinstein B, Stern AI.1989. Regulation of transplasmalemma electron transport in oat mesophyll cells by sphingoid bases and blue light. Plant Physiology 89: 1345–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DicksonRC1998. Sphingolipid functions in Saccharomyces cerevisiae: comparison to mammals. Annual Reviews of Biochemistry 67: 27–48. [DOI] [PubMed] [Google Scholar]
- DicksonRC, Nagiec EE, Wells GB, Nagiec MM, Lester RL.1997. Synthesis of mannose‐(inositol‐P)2‐ceramide, the major sphingolipid in Saccharomyces cerevisiae, requires the IPT1 (YDR072c) gene. Journal of Biological Chemistry 272: 29620–29625. [DOI] [PubMed] [Google Scholar]
- DittrichF, Zajonc D, Huhne K, Hoja U, Ekici A, Greiner E, Klein H, Hofmann J, Bessoule JJ, Sperling P, Schweizer E1998. Fatty acid elongation in yeast – biochemical characteristics of the enzyme system and isolation of elongation‐defective mutants. European Journal of Biochemistry 252: 477–485. [DOI] [PubMed] [Google Scholar]
- DunnTM., Haak D, Monaghan E, Beeler TJ.1998. Synthesis of monohydroxylated inositolphosphorylceramide (IPC‐C) in Saccharo myces cerevisiae requires Scs7p, a protein with both a cytochrome b5‐like domain and a hydroxylase/desaturase domain. Yeast 14: 311–321. [DOI] [PubMed] [Google Scholar]
- FuD, Beeler TJ, Dunn TM.1995. Sequence, mapping and disruption of CCC2, a gene that cross‐complements the Ca(2+)‐sensitive phenotype of csg1 mutants and encodes a P‐type ATPase belonging to the Cu(2+)‐ATPase subfamily. Yeast 11: 283–292. [DOI] [PubMed] [Google Scholar]
- GableK, Garton S, Napier JA, Dunn TM2004. Functional characterisation of the Arabidopsis thaliana ortholog of Tsc13p, the enoyl reductase of the yeast microsomal fatty acid elongating system. Journal of Experimental Botany (in press). [DOI] [PubMed] [Google Scholar]
- GableK, Han G, Monaghan E, Bacikova D, Natarajan M, Williams R, Dunn TM.2002. Mutations in the yeast LCB1 and LCB2 genes, including those corresponding to the hereditary sensory neuropathy type I mutations, dominantly inactivate serine palmitoyltransferase. Journal of Biological Chemistry 277: 10194–10200. [DOI] [PubMed] [Google Scholar]
- GableK, Slife H, Bacikova D, Monaghan E, Dunn TM.2000. Tsc3p is an 80‐amino acid protein associated with serine palmitoyltransferase and required for optimal enzyme activity. Journal of Biological Chemistry 275: 7597–7603. [DOI] [PubMed] [Google Scholar]
- GachotteD, Husselstein T, Bard M, Lacroute F, Benveniste P.1996. Isolation and characterization of an Arabidopsis thaliana cDNA encoding a delta 7‐sterol‐C‐5‐desaturase by functional comple mentation of a defective yeast mutant. Plant Journal 9: 391–398. [DOI] [PubMed] [Google Scholar]
- GaiggB, Neergaard TB, Schneiter R, Hansen JK, Faergeman NJ, Jensen NA, Andersen JR, Friis J, Sandhoff R, Schroder HDet al.2001. Depletion of acyl‐coenzyme A‐binding protein affects sphingolipid synthesis and causes vesicle accumulation and membrane defects in Saccharomyces cerevisiae. Molecular Biology of the Cell 12: 1147–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GartonS, Michaelson LV, Beaudoin F, Beale MH, Napier JA.2003. The dihydroceramide desaturase is not essential for cell viability in Schizosaccharomyces pombe FEBS Letters 538: 192–196. [DOI] [PubMed] [Google Scholar]
- GhanevatiM, Jaworski JG.2002. Engineering and mechanistic studies of the Arabidopsis FAE1 beta‐ketoacyl‐CoA synthase, FAE1 KCS. European Journal of Biochemistry 269: 3531–3539. [DOI] [PubMed] [Google Scholar]
- GrayJE, van der Lee FM, Bahrami AR, Sijmons PC, Woodward FI, Schuch W, Hetherington AM.2000. The HIC signalling pathway links CO2 perception to stomatal development. Nature 408: 713–716. [DOI] [PubMed] [Google Scholar]
- GuillasI, Kirchman PA, Chuard R, Pfefferli M, Jiang JC, Jazwinski SM, Conzelmann A.2001. C26‐CoA‐dependent ceramide synthesis of Saccharomyces cerevisiae is operated by Lag1p and Lac1p. EMBO Journal 20: 2655–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HaakD, Gable K, Beeler T, Dunn T.1997. Hydroxylation of Saccharomyces cerevisiae ceramides requires Sur2p and Scs7p. Journal of Biological Chemistry 272: 29704–29710. [DOI] [PubMed] [Google Scholar]
- HanG, Gable K, Kohlwein SD, Beaudoin F, Napier JA, Dunn TM.2002. The Saccharomyces cerevisiae YBR159w gene encodes the 3‐ketoreductase of the microsomal fatty acid elongase. Journal of Biological Chemistry 277: 35440–35449. [DOI] [PubMed] [Google Scholar]
- HaschkeH, Kaiser G, Martinoia E, Hammer U, Teucher T, Dorne AJ, Heinz E.1990. Lipid profiles of leaf tonoplasts from plants with different CO2 fixation mechanisms. Botanica Acta 103. [Google Scholar]
- Hashida‐OkadoT, Ogawa A, Endo M, Yasumoto R, Takesako K, Kato I.1996. AUR1, a novel gene conferring aureobasidin resistance on Saccharomyces cerevisiae: a study of defective morphologies in Aur1p‐depleted cells. Molecular and General Genetics 251: 236–244. [DOI] [PubMed] [Google Scholar]
- HeidlerSA, Radding JA.1995. The AUR1 gene in Saccharomyces cerevisiae encodes dominant resistance to the antifungal agent aureobasidin A (LY295337). Antimicrobial Agents and Chemo therapy 39: 2765–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HilligI, Leipelt M, Ott C, Zähringer U, Warnecke D, Heinz E.2003. Formation of glucosylceramide and sterol glucoside by a UDP‐glucose‐dependent glucosylceramide synthase from cotton expressed in Pichia pastoris. FEBS Letters 553: 365–369. [DOI] [PubMed] [Google Scholar]
- ImaiH, Ohnishi M, Hotsubo K, Kojima M, Ito S.1997. Sphingoid base composition of cerebrosides from plant leaves. Bioscience Biotechology and Biochemistry 61: 351–353. [Google Scholar]
- ImaiH, Ohnishi M, Kinishita M, Kojima M, Ito S.1995. Structure and distribution of cerebroside containing unsaturated hydroxy fatty acids in plant leaves. Bioscience Biotechology and Biochemistry 59: 1309–1313. [Google Scholar]
- KarlssonK.1982. Glycosphingolipids and surface membranes. In: Chapman D, ed. Biological membranes New York: Academic Press, 1–74. [Google Scholar]
- KatavicV, Mietkiewska E, Barton DL, Giblin EM, Reed DW, Taylor DC.2002. Restoring enzyme activity in nonfunctional low erucic acid Brassica napus fatty acid elongase 1 by a single amino acid substitution. European Journal of Biochemistry 269: 5625–5631. [DOI] [PubMed] [Google Scholar]
- KimS, Fyrst H, Saba J.2000. Accumulation of phosphorylated sphingoid long chain bases results in cell growth inhibition in Saccharomyces cerevisiae Genetics 156: 1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KohlweinSD, Eder S, Oh CS, Martin CE, Gable K, Bacikova D, Dunn T2001. Tsc13p is required for fatty acid elongation and localizes to a novel structure at the nuclear‐vacuolar interface in Saccharomyces cerevisiae Molecular and Cellular Biology 21: 109–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LiangH, Yao N, Song JT, Luo S, Lu H, Greenberg JT2003. Ceramides modulate programmed cell death in plants. Genes and Development 17: 2636–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le StunffH, Peterson C, Liu H, Milstien S, Spiegel S.2002. Sphingosine‐1‐phosphate and lipid phosphohydrolases. Biochimica et Biophysica Acta 1582: 8–17. [DOI] [PubMed] [Google Scholar]
- LeipeltM, Warnecke DC, Hube B, Zahringer U, Heinz E.2000. Characterization of UDP‐glucose:ceramide glucosyltransferases from different organisms. Biochemical Society Transactions 28: 751–752. [PubMed] [Google Scholar]
- LeipeltM, Warnecke D, Zahringer U, Ott C, Muller F, Hube, Heinz E.2001. Glucosylceramide synthases, a gene family responsible for the biosynthesis of glucosphingolipids in animals, plants, and fungi. Journal of Biological Chemistry 276: 33621–33629. [DOI] [PubMed] [Google Scholar]
- LesterRL, Dickson RC.1993. Sphingolipids with inositolphosphate‐containing head groups. Advances in Lipid Research 26: 253–274. [PubMed] [Google Scholar]
- LynchD.1993. Sphingolipids. In: Moore TS Jr, ed. Lipid metabolism in plants Boca Raton, FL: CRC Press, 285–308. [Google Scholar]
- LynchD, Caffrey M, Hogan JL, Steponkus PL.1992. Calorimetric and x‐ray diffraction studies of rye glucocerebroside mesomorphism. Biophysical Journal 610: 1289–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LynchD, Cahoon EB, Fairfield SR, Tannishtha R.1990. Glycosphingolipids of plant membranes. In: Quinn PJ, Harwood, JL, eds. Plant lipid biochemistry, structure and utilization London: Portland Press, 47–52. [Google Scholar]
- LynchDV, Dunn TM.2004. An introduction to plant sphingolipids. New Phytologist 161: 677–702. [DOI] [PubMed] [Google Scholar]
- LynchDV, Fairfield SR.1993. Sphingolipid long‐chain base synthesis in plants: characterization of serine palmitoyltransferase activity in squash fruit microsomes. Plant Physiology 103: 1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LynchDV, Criss AK, Lehoczky JL, Bui VT.1997. Ceramide glucosylation in bean hypocotyl microsomes: evidence that steryl glucoside serves as glucose donor. Archives of Biochemistry and Biophysics 340: 311–6. [DOI] [PubMed] [Google Scholar]
- MandalaSM, Thornton R, Tu Z, Kurt MB, Nickels J, Broach J, Menzeleev R, Spiegel S.1998. Sphingoid base 1‐phosphate phosphatase: a key regulator of sphingolipid metabolism and stress response. Proceedings of the National Academy of Science of the USA 95: 150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MaoC, Ruijuan X, Bielawska A, Obeid L.2000a. Cloning of an alkaline ceramidase from Saccharomyces cerevisiae An enzyme with reverse (CoA‐independent) ceramide synthase activity. Journal of Biological Chemistry 275: 6876–6884. [DOI] [PubMed] [Google Scholar]
- MaoC, Saba JD, Obeid LM.1999. The dihydrosphingosine‐1‐phosphate phosphatases of Saccharomyces cerevisiae are important regulators of cell proliferation and heat stress responses. Biochemical Journal 342: 667–675. [PMC free article] [PubMed] [Google Scholar]
- MaoC, Xu R, Bielawska A, Szulc ZM, Obeid LM.2000b. Cloning and characterization of a Saccharomyces cerevisiae alkaline ceramidase with specificity for dihydroceramide. Journal of Biological Chemistry 275: 31369–31378. [DOI] [PubMed] [Google Scholar]
- MekhedovS, Cahoon EB, Ohlrogge J.2001. An unusual seed‐specific 3‐ketoacyl‐ACP synthase associated with the biosynthesis of petroselinic acid in coriander. Plant Molecular Biology 47: 507–518. [DOI] [PubMed] [Google Scholar]
- MichaelsonLV, Longman AJ, Sayanova O, Stobard AK, Napier JA.2002. Isolation and characterization of a cDNA encoding a Δ8 sphingolipid desaturase from Aquilegia vulgaris Biochemical Society Transactions 30: 1073–1075. [DOI] [PubMed] [Google Scholar]
- MillarAA, Kunst L.1997. Very‐long‐chain fatty acid biosynthesis is controlled through the expression and specificity of the condensing enzyme. Plant Journal 12: 121–31. [DOI] [PubMed] [Google Scholar]
- MitchellAG, Martin CE.1997. Fah1p, a Saccharomyces cerevisiae cytochrome b5 fusion protein, and its Arabidopsis thaliana homolog that lacks the cytochrome b5 domain both function in the alpha‐hydroxylation of sphingolipid‐associated very long chain fatty acids. Journal of Biological Chemistry 272: 28281–28288. [DOI] [PubMed] [Google Scholar]
- MoritaN, Ngazato H, Okuyama H, Kim Y, Thompson Jr GR.1996. Evidence for a glycosylinositolphospholipid‐anchored alkaline phosphatase in the aquatic plant Spirodela oligorrhiza. Biochimica et Biophysica Acta 1290: 53–62. [DOI] [PubMed] [Google Scholar]
- NagiecMM, Nagiec EE, Baltisberger JA, Wells GB, Lester RL, Dickson RC.1997. Sphingolipid synthesis as a target for antifungal drugs. Complementation of the inositol phosphorylceramide synthase defect in a mutant strain of Saccharomyces cerevisiae by the AUR1 gene. Journal of Biological Chemistry 272: 9809–9817. [DOI] [PubMed] [Google Scholar]
- NagiecMM, Skrzypek M, Nagiec EE, Lester RL, Dickson RC.1998. The LCB4 (YOR171c) and LCB5 (YLR260w) genes of Saccharomyces encode sphingoid long chain base kinases. Journal of Biological Chemistry 273: 19437–19442. [DOI] [PubMed] [Google Scholar]
- NakayamaM, Kojima M, Ohnishi M, Ito S.1995. Enzymatic formation of plant cerebroside: properties of UDP‐glucose: ceramide glucosyltransferase in radish seedlings. Bioscience Biotechology and Biochemistry 59: 1882–1886. [DOI] [PubMed] [Google Scholar]
- NapierJA, Michaelson LV, Dunn TM.2002. A new class of lipid desaturase central to sphingolipid biosynthesis and signalling. Trends in Plant Science 7: 475–478. [DOI] [PubMed] [Google Scholar]
- NapierJA, Michaelson LV, Sayanova O.2003. The role of cytochrome b5 fusion desaturases in the synthesis of polyunsaturated fatty acids. Prostaglandins; Leukotrienes and Essential Fatty Acids 68: 135–143. [DOI] [PubMed] [Google Scholar]
- NapierJA, Sayanova O, Sperling P, Heinz E.1999. A growing family of cytochrome b5 fusion desaturases. Trends in Plant Science 4: 2–4. [Google Scholar]
- NgCK, Hetherington AM.2001. Sphingolipid‐mediated signalling in plants. Annals of Botany 88: 957–965. [Google Scholar]
- NgCK, Carr K, McAinsh MR, Powell B, Hetherington AM.2001. Drought‐induced guard cell signal transduction involves sphingosine‐1‐phosphate. Nature 410: 596–599. [DOI] [PubMed] [Google Scholar]
- NishiuraH, Tamura K, Morimoto Y, Imai H.2000. Characterization of sphingolipid long‐chain base kinase in Arabidopsis thaliana Biochemical Society Transactions 28: 747–748. [PubMed] [Google Scholar]
- NorbergP, Nilsson R, Nyiredy S, Liljenberg C.1996. Glucosyl ceramides of oat root plasma membranes – physicochemical behavior in natural and in model systems. Biochimica et Biophysica Acta 1299: 80–86. [DOI] [PubMed] [Google Scholar]
- OhCS, Toke DA, Mandala S, Martin CE.1997. ELO2 and ELO3, homologues of the Saccharomyces cerevisiae ELO1 gene, function in fatty acid elongation and are required for sphingolipid formation. Journal of Biological Chemistry 272: 17376–17384. [DOI] [PubMed] [Google Scholar]
- PruittRE, Vielle‐Calzada JP, Ploense SE, Grossniklaus U, Lolle SJ.2000. FIDDLEHEAD, a gene required to suppress epidermal cell interactions in Arabidopsis, encodes a putative lipid biosynthetic enzyme. Proceedings of the National Academy of Science of the USA 97: 1311–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PuyaubertJ, Garbay B, Costaglioli P, Dieryck W, Roscoe TJ, Renard M, Cassagne C, Lessire R.2001. Acyl‐CoA elongase expression during seed development in Brassica napus Biochimica et Biophysica Acta 1533: 141–152. [DOI] [PubMed] [Google Scholar]
- ReggioriF, Conzelmann A.1998. Biosynthesis of inositol phosphoceramides and remodeling of glycosylphosphatidylinositol anchors in Saccharomyces cerevisiae are mediated by different enzymes. Journal of Biological Chemistry 273: 30550–30559. [DOI] [PubMed] [Google Scholar]
- RoscoeTJ, Lessire R, Puyaubert J, Renard M, Delseny M.2001. Mutations in the fatty acid elongation 1 gene are associated with a loss of beta‐ketoacyl‐CoA synthase activity in low erucic acid rapeseed. FEBS Letters 492: 107–111. [DOI] [PubMed] [Google Scholar]
- RossakM, Smith M, Kunst L.2001. Expression of the FAE1 gene and FAE1 promoter activity in developing seeds of Arabidopsis thaliana Plant Molecular Biology 46: 717–725. [DOI] [PubMed] [Google Scholar]
- SabaJD, Nara F, Bielawska A, Garrett S, Hannun YA.1997. The BST1 gene of Saccharomyces cerevisiae is the sphingosine‐1‐phosphate lyase. Journal of Biological Chemistry 272: 26087–26090. [DOI] [PubMed] [Google Scholar]
- SawaiH, Okamoto Y, Luberto C, Mao C, Bielawska A, Domae N, Hannun YA.2000. Identification of ISC1 (YER019w) as inositol phosphosphingolipid phospholipase C in Saccharomyces cerevisiae Journal of Biological Chemistry 275: 39793–8. [DOI] [PubMed] [Google Scholar]
- SayanovaO, Beaudoin F, Libisch B, Castel A, Shewry PR, Napier JA.2001. Mutagenesis and heterologous expression in yeast of a plant Δ6‐fatty acid desaturase. Journal of Experimental Botany 52: 1581–1585. [DOI] [PubMed] [Google Scholar]
- SayanovaO, Smith MA, Lapinskas P, Stobart AK, Dobson G, Christie WW, Shewry PR, Napier JA.1997. Expression of a borage desaturase cDNA containing an N‐terminal cytochrome b5 domain results in the accumulation of high levels of Δ6‐desaturated fatty acids in transgenic tobacco. Proceedings of the National Academy of Science of the USA 94: 4211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SchorlingS, Vallee B, Barz WP, Riezman H, Oesterhelt D.2001. Lag1p and Lac1p are essential for the ccyl‐CoA‐dependent ceramide synthase reaction in Saccharomyces cerevisae Molecular Biology of the Cell 12: 3417–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SchreiberL, Skrabs M, Hartmann K, Becker D, Cassagne C, Lessire R.2000. Biochemical and molecular characterization of corn (Zea mays L.) root elongases. Biochemical Society Transactions 28: 647–649. [PubMed] [Google Scholar]
- SherrierDJ, Prime TA, Dupree P.1999. Glycosylphosphatidylinositol‐anchored cell‐surface proteins from Arabidopsis. Electrophoresis 20: 2027–2035. [DOI] [PubMed] [Google Scholar]
- SkrzypekMS, Nagiec MM, Lester RL, Dickson RC.1999. Analysis of phosphorylated sphingolipid long‐chain bases reveals potential roles in heat stress and growth control in Saccharomyces. Journal of Bacteriology 181: 1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SpassievaSD, Markham JE, Hille J.2002. The plant disease resistance gene Asc‐1 prevents disruption of sphingolipid metabolism during AAL‐toxin‐induced programmed cell death. Plant Journal 32: 561–572. [DOI] [PubMed] [Google Scholar]
- SperlingP, Heinz E.2003. Plant sphingolipids: structural diversity, biosynthesis, first genes and functions. Biochimica et Biophysica Acta 1632: 1–15. [DOI] [PubMed] [Google Scholar]
- SperlingP, Blume A, Zahringer U, Heinz E.2000. Further characterization of Δ8‐sphingolipid desaturases from higher plants. Biochemical Society Transactions 28: 638–641. [PubMed] [Google Scholar]
- SperlingP, Ternes P, Moll H, Franke S, Zahringer U, Heinz E.2001. Functional characterization of sphingolipid C4‐hydroxylase genes from Arabidopsis thaliana. FEBS Letters 494: 90–94. [DOI] [PubMed] [Google Scholar]
- SperlingP, Ternes P, Zank T, Heinz E.2003. The evolution of desaturases. Prostaglandins, Leukotrienes and Essential Fatty Acids 68: 73–95. [DOI] [PubMed] [Google Scholar]
- SteponkusPL, Lynch D.1989. Freeze/thaw‐induced destabilization of the plasma membrane and the effects of cold acclimation. Journal of Bioenergetics and Biomembranes 21: 21–41. [DOI] [PubMed] [Google Scholar]
- SvetekJYM, Nothnagel EA1999. Presence of glycosylphosphatidyl inositol lipid anchor on rose arabinogalactan proteins. Journal of Biological Chemistry 274: 14724–14733. [DOI] [PubMed] [Google Scholar]
- TamuraK, Nishiura H, Mori J, Imai H.2000. Cloning and characterization of a cDNA encoding serine palmitoyltransferase in Arabidopsis thaliana Biochemical Society Transactions 28: 745–747. [PubMed] [Google Scholar]
- TamuraK, Mitsuhashi N, Hara‐Nishimura I, Imai H.2001. Characterization of an Arabidopsis cDNA encoding a subunit of serine palmitoyltransferase, the initial enzyme in sphingolipid biosynthesis. Plant and Cell Physiology 42: 1274–1281. [DOI] [PubMed] [Google Scholar]
- TavernierE, Le Quoc D, Le Quoc K.1993. Lipid composition of the vacuolar membrane of Acer pseudoplatanus cultured cells. Biochimica et Biophysica Acta 167: 242–247. [DOI] [PubMed] [Google Scholar]
- TernesP, Franke S, Zahringer U, Sperling P, Heinz E.2002. Identification and characterization of a sphingolipid Δ4‐desaturase family. Journal of Biological Chemistry 277: 25512–25518. [DOI] [PubMed] [Google Scholar]
- ThompsonGA, Okuyama H.2000. Lipid‐linked proteins of plants. Progress in Lipid Research 39: 19–39. [DOI] [PubMed] [Google Scholar]
- ThompsonG, Morita N, Okuyama H, Kim Y, Ko Y‐G, Hung C‐Y.1995. On the presence of glycosylphosphatidylinositol‐anchored proteins in plants. In: Kader J‐C, Mazliak P, eds. Plant lipid metabolism Dordrecht: Kluwer Academic Publishers, 227–229. [Google Scholar]
- ToddJ, Post‐Beittenmiller D, Jaworski JG.1999. KCS1 encodes a fatty acid elongase 3‐ketoacyl‐CoA synthase affecting wax biosynthesis in Arabidopsis thaliana Plant Journal 17: 119–130. [DOI] [PubMed] [Google Scholar]
- TokeDA, Martin CE.1996. Isolation and characterization of a gene affecting fatty acid elongation in Saccharomyces cerevisiae Journal of Biological Chemistry 271: 18413–18422. [DOI] [PubMed] [Google Scholar]
- UemuraM, Steponkus PL.1994. A contrast of the plasma membrane lipid composition of oat and rye leaves in relation to freezing tolerance. Plant Physiology 104: 479–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UemuraM, Joseph RA, Steponkus PL.1995. Cold acclimation of Arabidopsis thaliana (effect on plasma membrane lipid composition and freeze‐induced lesions). Plant Physiology 109: 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UmemuraK, Ogawa N, Yamauchi T, Iwata M, Shimura M, Koga J.2000. Cerebroside elicitors found in diverse phytopathogens activate defense responses in rice plants. Plant and Cell Physiology 41: 676–683. [DOI] [PubMed] [Google Scholar]
- WangH, Li J, Bostock RM, Gilchrist DG.1996. Apoptosis: a functional paradigm for programmed plant cell death induced by a host selective phytotoxin and invoked during development. Plant Cell 8: 375–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WardJL, Harris C, Lewis J, Beale MH.2003. Assessment of 1H NMR spectroscopy and multivariate analysis as a technique for metabolite fingerprinting of Arabidopsis thaliana Phytochemistry 62: 949–57 [DOI] [PubMed] [Google Scholar]
- WorrallD, Ng C, Hetherington AM.2003. Sphingolipids, new players in plant signaling. Trends in Plant Science 8: 317–320. [DOI] [PubMed] [Google Scholar]
- WrightBS, Snow JW, O’Brien TC, Lynch DV.2003. Synthesis of 4‐hydroxysphinganine and characterization of sphinganine hydroxylase activity in corn. Archives of Biochemistry and Biophysics 415: 184–192. [DOI] [PubMed] [Google Scholar]
- XuX, Dietrich CR, Lessire R, Nikolau BJ, Schnable PS.2002. The endoplasmic reticulum‐associated maize GL8 protein is a component of the acyl‐coenzyme A elongase involved in the production of cuticular waxes. Plant Physiology 128: 924–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YephremovY, Wisman E, Huijser P, Huijser C, Wellesen K, Saedler H.1999. Characterization of the fiddlehead gene of arabidopsis reveals a link between adhesion response and cell differentiation in the epidermis. Plant Cell 11: 2187–2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YoungME, Karpova TS, Brugger B, Moschenross DM, Wang GK, Schneiter R, Wieland FT, Cooper JA.2002. The Sur7p family defines novel cortical domains in Saccharomyces cerevisiae, affects sphingolipid metabolism, and is involved in sporulation. Molecular and Cellular Biology 22: 927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YoshidaS. Uemura M.1986. Lipid composition of plasma membranes and tonoplasts isolated from etiolated seedlings of mung bean (Vigna radiata L.). Plant Physiology 82: 807–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YuanDS, Stearman R, Dancis A, Dunn T, Beeler T, Klausner RD.1995. The Menkes/Wilson disease gene homologue in yeast provides copper to a ceruloplasmin‐like oxidase required for iron uptake. Proceedings of the National Academy of Science of the USA 92: 2632–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZankTK, Zahringer U, Beckmann C, Pohnert G, Boland W, Holtorf H, Reski R, Lerchl J, Heinz E.2002. Cloning and functional characterisation of an enzyme involved in the elongation of Δ6‐polyunsaturated fatty acids from the moss Physcomitrella patens Plant Journal 31: 255–268. [DOI] [PubMed] [Google Scholar]
- ZankTK, Zahringer U, Lerchl J, Heinz E.2000. Cloning and functional expression of the first plant fatty acid elongase specific for Δ6‐polyunsaturated fatty acids. Biochemical Society Transactions 28: 654–658. [PubMed] [Google Scholar]