Abstract
• Background and aims The lip structure of six Brazilian and one Asiatic species of Bulbophyllum with wind‐assisted fly pollination (B. involutum, B. ipanemense and B. weddellii) and non‐wind‐assisted fly pollination (B. epiphytum, B. glutinosum, B. regnellii and B. rothschildianum) was studied to investigate the presence of secretory tissues related to these pollination mechanisms.
• Methods The lip study was carried out through scanning electron microscopy (lip surface) and light microscopy (anatomical features).
• Key Results In most of the species studied, the osmophores (odour glands) were located in the lobes and in the upper surface of the lip callus. Differences in the lip structure were observed between the two groups (the presence of a nectary and the extent of osmophore surface), depending on the mechanism of pollination. Nectaries were found in the cavity callus in B. ipanemense, B. involutum and B. weddellii, even though their pollinators were presumably attracted by the instinct to oviposit.
• Conclusions These findings corroborate the hypothesis that, because pollination in these species is dependent on an unpredictable external factor (wind), nectar is necessary to keep the insect in the flower for a long period. Despite the occurrence of a liquid‐like nectar in the flowers of B. epiphytum, B. glutinosum, B. regnelli and B. rothschildianum, no anatomical evidence for nectaries was found in the lips of these species, although a similar structure may occur in another region of the flowers. This observation agrees with the fact that pollination by lip movement in the latter species requires only gravity, with no additional mechanism being needed to keep the flies in the flower.
Key words: Anatomy, Bulbophyllum, nectary, Orchidaceae, osmophores, pollination, scanning electron microscopy
INTRODUCTION
Bulbophyllum is a myophilous, pantropical orchid genus containing over 1000 species, most of them found in Asia (Vermeulen, 1991; Dressler, 1993). About 55 species occur in Brazil (Pabst and Dungs, 1975, 1977).
The lip of Bulbophyllum flowers is trilobed and usually fleshy, with a large callus. This lip is movable and is articulated with the column foot at 0–90° angle to the column. In some species, the lip is moved by the wind, and some authors have suggested that this lip movement is an important mechanism by which fly‐pollinated flowers attract insects (quick lip movements would mimic flies shaking their wings and would attract other insects; Barth, 1985; Meve and Liede, 1994; Borba and Semir, 1998).
In addition to visual attraction, the movable lip of Bulbophyllum species plays an essential role in the pollination of this genus. Ridley (1890) described the basic pollination mechanism based on the Asiatic B. macranthum. In this sequence of events, the fly lands on the hinged lip, causing the lip to move down. When the fly crawls up the lip and passes the balance point, the lip returns to its original position, pressing the fly against the column. This mechanism, in which lip movement is caused by the weight of the insect, has been observed in several other forest species of Bulbophyllum (van der Pijl and Dodson, 1966; Jones and Gray, 1976; Braga, 1977; Christensen, 1994). Preliminary investigations, including a morphological analysis, indicate that this mechanism occurs in most Brazilian forest species, such as in the complexes represented by B. glutinosum and B. regnellii (e.g. Verola, 2002), and also in most Asiatic species. Variations of this basic mechanism have been described by Verola (2002) for B. epiphytum and allied species and for B. insectiferum.
Another pollination mechanism, in which wind is necessary to move the lip and then press the insect against the column, was observed and described in detail by Sazima (1978) and Borba and Semir (1998) for B. involutum, B. ipanemense and B. weddellii, species found in open areas of south‐eastern Brazil. These authors suggested that, although the flowers of these Bulbophyllum species produce nectar, the flies are attracted by their instinct for oviposition (only female flies visit the plants). Borba and Semir (1998) hypothesized that because pollination by the wind‐assisted mechanism is dependent on an unpredictable external factor (winds ranging from 1·0–1·5 m s–1), nectar is necessary to keep the insect in the flower for a long period. Conversely, the primary mechanism of lip movement in the genus requires only gravity, and nothing is needed to keep the fly in the flower.
The aim of this work was to investigate the lip anatomy of seven species of Bulbophyllum with two distinct pollination mechanisms: B. epiphytum, B. glutinosum, B. regnellii and B. rothschildianum, which present the insect weight mechanism and its variations, and B. involutum, B. ipanemense and B. weddellii, which present the wind‐assisted mechanism. Specifically, we addressed the following questions. In which lip region is fragrance produced and how is this region characterized? What structure on the base of the lip is responsible for providing the flies with a reward and can this structure be considered a nectary? Does lip anatomy reflect species groups based on the mechanism of pollination?
MATERIALS AND METHODS
Plant material
We collected flowers at anthesis in the morning from plants growing in a glasshouse at the Universidade Estadual de Campinas (UNICAMP). Voucher specimens were deposited in the herbaria of the Universidade Estadual de Campinas (UEC: B. regnellii Rchb.f.—E. L. Borba et al. 587, B. weddellii (Lindl.) Rchb. f.—E. L. Borba 151, B. involutum Borba, Semir & F. Barros—E. L. Borba 150, B. ipanemense Hoehne—E. L. Borba 177 and B. epiphytum (Barb.Rodr.) Cogn.—E. L. Borba & C.F. Verola, no number, UEC 122327) and the Escola Superior de Agricultura Luiz de Queiroz/USP (ESA: B. glutinosum (Barb.Rodr.) Cogn.—no collector, in Coll. Brieger 9811).
Scanning electron microscopy (SEM)
Flower lips were preserved in FAA (formalin–acetic acid–ethanol) and stored in 70 % alcohol. After dehydration in an ethanol–acetone series followed by critical‐point drying in a Balzers CPD 030 apparatus, the specimens were mounted on aluminium stubs with colloidal carbon and coated with gold in a Balzers SCD 050 sputter coater for 280 s (Tucker, 1993). The samples were observed with a DSM 940A (Zeiss) scanning electron microscope operated at 10 kV, and photomicrographs were taken using a Sinar 67 camera.
Light microscopy
To prepare permanent slides, the labella (Fig. 1) were removed from flowers and fixed in Karnovsky solution for 24 h (Karnovsky, 1965), then dehydrated in alcohol series, embedded in historesin (Gerrits, 1991), and sectioned transversely and longitudinally. Sections (5–8 µm) were stained with 0·05 % toluidine blue (O’Brien et al., 1964) and mounted in Permount resin. To investigate the presence of lipids, labella were fixed in buffered formalin and then post‐fixed in buffered osmium tetroxide, dehydrated in an alcohol series, embedded in historesin and stained with Sudan Black B (Stern et al., 1986). Polysaccharides were detected by staining with ruthenium red. For all staining procedures, appropriate controls were run simultaneously. Photomicrographs were obtained using an Olympus BX50 microscope with the same optical settings for all photographs.
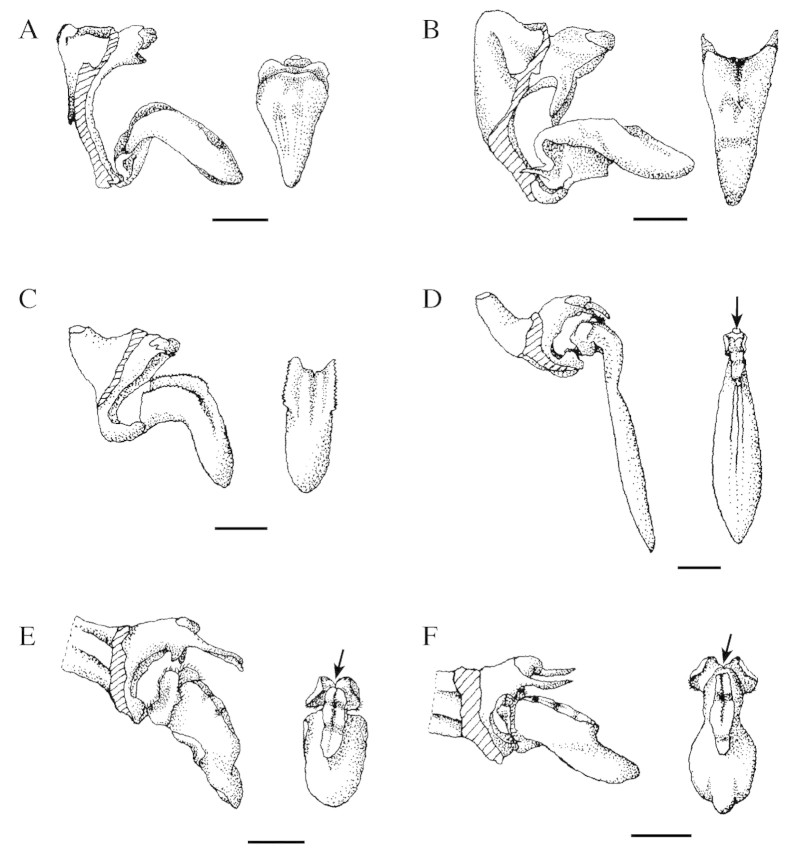
Fig. 1. Lateral view of lip and column (left) and upper view of lip (right) of Bulbophyllum species. (A) B. regnellii; (B) B. glutinosum; (C) B. epiphytum; (D) B. weddellii; (E) B. involutum; (F) B. ipanemense. The arrows indicate the cavity formed in the lip callus. Scale bars: A, B, E and F = 2 mm; C and D = 1 mm. Drawings by Patrícia L. Ribeiro.
RESULTS
In most of the Bulbophyllum species studied, fragrance was produced by osmophores located in the lobes and on the adaxial surface of the lip callus. These osmophores were similar in shape (Figs 1 and 2 and Table 1). In addition to the papillose osmophores present on the callus surface, a nectary formed by an epithelial layer and two or three adjacent layers was found in the cavity of the callus in B. ipanemense (Figs 1F and 3A, B and E), B. involutum (Figs 1E and 3C, D and F) and B. weddellii (Fig. 1D), as shown by SEM (Table 1) and light microscopy, and confirmed by histochemical tests.

Fig. 2. Osmophores on the lip surface of Bulbophyllum species. (A and B) B. ipanemense: (A) lip lobe; (B) callus adaxial surface. (C and D) Callus adaxial surface of B. involutum: (C) scanning electron micrograph; (D) histological section. (E) Scanning electron micrograph of the callus adaxial surface of B. rothschildianum. (F) Histological section of a region between the callus cavity and the epidermis of the adaxial surface of B. regnellii. Scale bar: A, B, E = 8.5 µm; C = 13 µm; D = 22 µm; F = 43 µm.
Table 1.
Lip features according to position in some Bulbophyllum species
| Callus | |||||
| Species | Adaxial surface | Abaxial surface | Cavity | Lobe surface | Limb surface |
| B. epiphytum | Papillate | Papillate with a stronger cutinization | Absent | Papillate | Flat |
| B. involutum | Papillate with unicellular trichomes | Flat with a stronger cutinization | Papillate with conspicuous subjacent tissue | Papillate with unicellular trichomes | Papillate with unicellular trichomes |
| B. ipanemense | Papillate with unicellular trichomes | Flat with a stronger cutinization | Flat with conspicuous subjacent tissue | Papillate with unicellular trichomes | Flat |
| B. glutinosum | Papillate | Flat | Absent | Papillate | Flat |
| B. regnellii | Papillate | Flat | Absent | Papillate | Flat |
| B. rothschildianum | Papillate with unicellular trichomes | Flat | Absent | Papillate | Flat |
| B. weddellii | Papillate with unicellular trichomes | Papillate | Papillate with conspicuous subjacent tissue | Papillate with unicellular trichomes | Flat |

Fig. 3. Nectary in the lip cavity of Bulbophyllum: (A, B and E) B. ipanemense; (C, D and F) B. involutum. (A) Scanning electron micrograph showing flat epithelial cells. (B) Histological section; note the droplets in the epithelial cell cytoplasm (arrow). (C) Scanning electron micrograph showing papillose epithelial cells. (D) Histological section showing papillose epithelial cells and subjacent layers. (E) Scanning electron micrograph showing a region of osmophores (o) and nectary cells (n) in the cavity. (F) Histological section showing the transition between osmophores (o) and nectary (n). Note the densely staining cytoplasm of the epithelial cells. Scale bars: A = 7.0 µm; B, D, F = 43 µm; C = 13 µm; E = 8.5 µm.
In B. ipanemense (Fig. 2A and B), B. involutum (Fig. 2C and D) and B. weddellii the papillose epidermal cells were densely stained, with basal nuclei and many droplets, as well as amyloplasts. Abaxial epidermal cells of the callus were flat and heavily cutinized. The trichomes were unicellular and contained many droplets, and those located on the lateral lobes were longer. The epithelial cells of the cavity were palisade‐like and densely stained and had central nuclei. The subjacent tissue was two‐ or three‐layered and consisted of compact parenchyma cells with many cytoplasmic granules; these cells were smaller than the parenchyma cells occurring in the centre of the lip. Idioblasts with raphide‐type crystals were observed in the central parenchyma of the callus. In B. weddellii, crystalliferous idioblasts were also present on the lateral lobes. The central parenchyma of lip limb was spongy. Collateral vascular bundles were observed in the centre of all lip regions, but were more numerous in the limb. There were no fibres delimiting the vascular bundle.
In B. epiphytum, B. glutinosum, and B. regnellii (Fig. 2F), the papillose epidermal cells had basal nuclei and many lipid droplets. In B. rothschildianum, unicellular trichomes were observed (Fig. 2E) in addition to papillae, the latter being smaller and less variable in size than those occurring in B. ipanemense, B. involutum and B. weddellii. Idioblasts with raphides crystals occurred in all parenchyma cells of the lip. Collateral vascular bundles were observed in the centre of all lip regions. No similar cavity formed by the lobes was found in these species. The papillose epidermal cells were strongly cutinized in B. epiphytum.
All droplets in the epidermal cell cytoplasm stained darkish with osmium tetroxide–Sudan Black B, including those in the cavity cells of B. involutum, B. ipanemense and B. weddellii. The staining properties indicated that the contents of the epidermal cells of the lip cavity were either osmiophilic or lipophilic while those of the lip surface were osmiophilic. Granules in subjacent tissues stained positively with ruthenium red, indicating that they were amyloplasts. In B. regnellii, idioblasts in the lobes were greenish when stained with toluidine blue and reddish when stained with Sudan Black B, indicating that their content was probably phenolic.
DISCUSSION
The lip surface of Bulbophyllum species is secretory, based on (a) the presence of a small quantity of fluid secreted by the lip in plants growing in a glasshouse and an odour of acid released by the flowers at anthesis (Borba and Semir, 1998; Silva et al., 1999) and (b) anatomical evidence of lip secretion after fresh sectioning and a lipid‐specific test, which indicated the presence of osmophores distributed on the lip surface (all Bulbophyllum species) and also an epithelial nectary located in the lip cavity (B. involutum, B. ipanemense and B. weddellii). Since no pores or stomata were found in the nectar‐secreting epithelium, the mechanism of nectar secretion remains unknown.
Two heterogenous fragrance centres have been reported in Bulbophyllum ornatissimum: the moveable lip produces a trimethylamine‐like odour while the upper groove secretes simultaneously a viscous nectar (Vogel, 1990). In other Bulbophyllum species (B. barbigerum, B. cerambix, B. distans, B. grandiflorus and B. triclavigerum), the fragrance organs were located in the tepals or sepals (Vogel, 1990).
The occurrence of osmophores and nectaries was most likely related to the pollination mechanisms in Bulbophyllum. Pollinators (flies) are attracted by volatile oils secreted by the ventral lip surface (Silva et al., 1999), walk on the lip and feed on nectar released in the lip cavity (Borba and Semir, 1998). As shown here, there were some differences in the lip structure (the presence of a nectary and the extent of osmophore surface) among the groups considered, depending on the mechanism of pollination (Table 1).
In the group containing B. involutum, B. ipanemense and B. weddellii, the pollination mechanism is dependent on an unpredictable external factor (wind of a precise velocity). Because of this, there is a need for some means of keeping the insect in the flower in the right position for a longer period. This attraction is provided by the nectar secreted by the epithelial nectary in the base of the lip. The larger osmophore surface in these species (osmophores consisting of trichomes and larger papillae) also contributes to maintaining the flies in the limb. Even the spongy parenchyma found in the lip limb appears to be involved in pollination, since any movement of the narrow lip by the wind will force the fly towards the column (Borba and Semir, 1998).
Despite the presence of nectar in the flowers of B. epiphytum, B.glutinosum, B. regnellii and B. rothschildianum, no histological evidence for a nectary was found. However, a nectary may occur in another region of the flower in these species. The papillose epidermal cells with lipidic droplets present on all lip surfaces probably have a secretory function.
Flies (Tachinidae and Sciaridae) are attracted to the flowers of B. epiphytum, B. glutinosum and B. regnellii to feed on the nectar that accumulates at the base of the lip (Verola, 2002). According to Borba and Semir (1998), the presence of nectar is unrelated to the attraction of female Milichiidae flies to the flowers of B. involutum, B. ipanemense and B. weddellii, but is important for the pollination mechanism to work. These authors suggested that pollinators are attracted by the instinct to oviposit (odour and colours) but do not lay their eggs in the flower, possibly because the females realize they have not landed in an adequate place for oviposition.
Although only a few species were studied here, the homogeneous organization of the lip surface found in Bulbophyllum species indicated that this structure is a highly conservative character in the genus. This contrasts with some Maxillaria species, for example, in which the lip surface is either homogeneous or heterogeneous. This variation was considered by Davies and Winters (1998) to be a useful taxonomic character for species identification.
ACKNOWLEDGEMENTS
The authors thank Adriane C. S. Sprogis and Antonia M. F. Lima (SEM Laboratory/IB, UNICAMP) for technical assistance, Sandra Maria Carmello‐Guerreiro for allowing the use of equipment in the Plant Anatomy Laboratory at the UNICAMP, Patrícia L. Ribeiro for the drawings in Fig. 1, Cássio van den Berg and Stephen Hyslop for the English review. E.L.B. is supported by a grant from CNPq (PQ‐2C).
Received: 10 September 2003; Returned for revision: 7 December; Accepted: 6 January 2004 Published electronically: 5 March 2004
References
- BarthFG.1985.Insects and flowers: the biology of a partnership. Princeton: Princeton University Press. [Google Scholar]
- BorbaEL, Semir J.1998. Wind‐assisted fly pollination in three Bulbophyllum (Orchidaceae) species occurring in the Brazilian campos rupestres. Lindleyana 13: 203–218. [Google Scholar]
- BragaPIS.1977. Aspectos biológicos das Orchidaceae de uma campina na Amazônia Central. Acta Amazonica 7 (Suppl. 2): 1–89. [Google Scholar]
- ChristensenDE.1994. Fly pollination in the Orchidaceae. In: Arditti J, ed. Orchid biology: reviews and perspectives, Vol. VI New York: John Wiley and Sons, 415–454. [Google Scholar]
- DaviesKL, Winters C.1998. Ultrastructure of the lip epidermis in selected Maxillaria species (Orchidaceae). Botanical Journal of the Linnean Society 126: 349–361. [Google Scholar]
- DresslerRL.1993.Phylogeny and classification of the orchid family. Portland: Dioscorides Press. [Google Scholar]
- GerritsPO.1991.The application of glycol methacrylate in histotechnology: some fundamental principles. Department of Anatomy and Embryology, State University of Groningen, Netherlands. [Google Scholar]
- JonesDL, Gray B.1976. The pollination of Bulbophyllum longiflorum Thouars. American Orchid Society Bulletin 45: 15–17. [Google Scholar]
- KarnovskyMJ.1965. A formaldehyde‐glutaraldehyde fixative of high osmalarity for use in electron microscopy. Journal of Cell Biology 27: 137A–138A. [Google Scholar]
- MeveU, Liede S.1994. Floral biology and pollination in stapeliads – new results and a literature review. Plant Systematics and Evolution 192: 99–116. [Google Scholar]
- O’BrienTP, Feder N, McCully ME.1964. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 59: 368–373. [Google Scholar]
- PabstGFJ, Dungs F.1975.Orchidaceae Brasiliensis, Vol. 1. Hildesheim: Brücke‐Verlag Kurt Schmersow. [Google Scholar]
- PabstGFJ, Dungs F.1977.Orchidaceae Brasiliensis, Vol. 2. Hildesheim: Brücke‐Verlag Kurt Schmersow. [Google Scholar]
- RidleyHN.1890. On the method of fertilization in Bulbophyllum macranthum and allied orchids. Annals of Botany 4: 327–336. [Google Scholar]
- SazimaM.1978. Polinização por moscas em Bulbophyllum warmingianum Cogn. (Orchidaceae), na Serra do Cipó, Minas Gerais, Brasil. Revista Brasileira de Botânica 1: 133–138. [Google Scholar]
- SilvaUF, Borba EL, Semir J, Marsaioli AJ.1999. A simple solid injection device for the analyses of Bulbophyllum (Orchidaceae) volatiles. Phytochemistry 50: 31–34. [Google Scholar]
- SternWL, Curry KJ, Whitten WM.1986. Staining fragrance glands in orchid flowers. Bulletin of the Torrey Botanical Club 113: 288–297. [Google Scholar]
- TuckerS.1993. Floral ontogeny in Sophoreae (Leguminosae, Papilionoideae). I. Myroxylon (Myroxylon group) and Castano spermum (Angylocalyx group). American Journal of Botany 80: 65–75. [Google Scholar]
- van der PijlL, Dodson CH.1966.Orchid flowers: their pollination and evolution. Coral Gables: University of Miami Press. [Google Scholar]
- VermeulenJJ.1991. Orchids of Bornio. Vol. 2 – Bulbophyllum. Kew: Royal Botanic Gardens. [Google Scholar]
- VerolaCF.2002.Biologia floral e sistemas de reprodução em espécies de Bulbophyllum (Orchidaceae) ocorrentes em mata de galeria, campo rupestre e floresta estacional. Masters Dissertation, Universidade Estadual de Campinas, Brazil. [Google Scholar]
- VogelS 1990The role of scent glands in pollination. New Delhi: Amerind Publishing. [Google Scholar]