Abstract
• Background and Aims The increase of molecular data and the resulting insights into legume systematics make the search for new morphological characters and a careful re‐investigation of already stated characters necessary. Bracteoles are small, reduced leaves borne close to the base of lateral branches. Although they seem unimportant in older buds, they have an ecological function in protecting the sepal primordia. Furthermore, a morphogenetic function in mediating the onset of sepal initiation is suspected in the literature. The occurrence of bracteoles varies within Papilionoideae, and their distribution is used in legume systematics. But this is open to criticism, because there is a tendency to use ‘absent’ for ‘caducous’. Thus attention here was paid to the initiation of bracteoles as well as to the sequence of sepal initiation.
• Methods The floral development of 30 taxa out of 15 tribes of Papilionoideae was investigated using scanning electron microscopy (SEM).
• Key Results In five taxa the bracteoles initiated, but suppressed early. Furthermore, a broad variability of sepal initiation was found. Besides the widely stated unidirectional pattern, modified unidirectionality, tendencies towards whorled, fully whorled, bidirectional and successive initiation of sepals were all found.
• Conclusion Initiated but suppressed bracteoles are presented as a ‘new’ character in Papilionoideae. Considering the presence of bracteoles as a plesiomorphy, their suppression can be seen as a step towards completely reduced bracteoles. The remarkable variability of the sequence of sepal initiation questions the widely stated unidirectionality of organ initiation in Papilionoideae. The different modes of sepal initiation are deducible from the helical pattern of some caesalpinioids, which is seen as a developmental link of the flowers of Papilionoideae and Caesalpinioideae. The bidirectional sepal initiation is possibly a consequence of the morphogenetic function of bracteoles, although bidirectionality is not found in all taxa with reduced bracteoles.
Key words: Bracteoles, Caesalpiniaceae, Caesalpinioideae, floral development, Fabaceae, Faboideae, Leguminosae, Papilionoideae, plastochron, sepals
INTRODUCTION
Papilionoideae are, with approx. 12 000 species, the largest subfamily of Leguminosae. They are widely distributed from rainforests to the edges of dry and cold deserts, and they play an important role in human nutrition as well as in soil fertilization. The increase of molecular data and the resulting new insights into legume systematics make the search for new morphological characters, and a careful reinvestigation of already stated characters, necessary (e.g. Crisp et al., 2000; Pennington et al., 2000, 2001). A broad investigation was initiated into the floral development of 30 taxa out of 15 tribes, focussing on the initiation of bracteoles and on the sequence of sepal initiation.
Floral bracteoles were first mentioned by de Candolle (1813), and Eames (1961) defines them as small, reduced leaves borne close to the base of lateral branches. Although bracteoles seem unimportant in older buds and at anthesis, Endress (1994) mentions two important functions in early floral ontogeny. (1) A morphogenetic function: as the two first organs at the floral axis they mediate the onset of the spiral of the calyx; and (2) an ecological function: they are protective organs for the floral apex and sepal primordia (see also Endress, 1987).
The occurrence of bracteoles varies within Papilionoideae (cf. Tucker, 1987), and the character ‘bracteoles present versus absent’ is used frequently in cladistic analyses of the subfamily (e.g. Crisp and Weston, 1987, 1995; Lavin, 1987, 1995; van Wyk and Schutte, 1989; Sousa and Rudd, 1993; Breteler, 1995; Schrire, 1995; Tucker and Douglas, 1994; Barker et al., 2000; van der Bank et al. 2002). Tucker (1987) states that the verification of the occurrence of bracteoles could be useful, because there is a tendency to use ‘absent’ for ‘caducous’, which could lead to erroneous conclusions. For the decision as to whether or not bracteoles are initiated, a careful investigation of the floral primordium is necessary.
In contrast to the variable occurrence of bracteoles, in papilionoids the initiation of sepals is said to be almost uniformly unidirectional from the abaxial to the adaxial side of the flower (Tucker, 1984, 1987, 2003). Exceptions are only rarely reported (e.g. Tucker and Stirton, 1991; Klitgaard, 1999). Considering the above‐cited morphogenetic function of bracteoles, additional attention was paid to the sequence of sepal initiation.
The aim of this study is to present initiated but early suppressed bracteoles as a ‘new’ character in Papilion oideae, and to show a broad variability in sepal initiation. This variability can be derived from the helical pattern of Caesalpinioideae. A possible morphogenetical function of bracteoles as well as systematic aspects of the presented characters are discussed.
MATERIALS AND METHODS
Material
SEM micrographs are shown for Baptisia australis R. Br.: Prenner 249, cult. Austria, Hortus Botanicus Graecensis (HBG); Dorycnium germanicum Rouy: Prenner 288, cult. HBG; Ebenus cretica L.: Prenner 440, Greece, Crete, Psiloritis; Laburnum alpinum J. Presl.: Prenner 408, cult. HBG; Lathyrus latifolius L.: Prenner 272, cult. HBG; Kennedia nigricans Lindl.: Prenner 491, cult. HBG; Petteria ramentacea (Sieber) Presl: Prenner 183, cult. HBG; Thermopsis lanceolata R. Br.: Prenner 242, cult. HBG. Voucher specimens have been deposited in the herbarium of the Institute of Botany, Karl‐Franzens‐University Graz (GZU), and liquid‐preserved collections are held by the author.
Scanning electron microscopy (SEM)
For SEM, young inflorescences and flower buds of different sizes and ages were collected, immediately fixed in FAA (5 parts formalin : 5 parts 100 % acetic acid : 90 parts 70 % ethyl alcohol) and stored in 70 % ethyl alcohol. Floral parts were dissected in alcohol under a Zeiss stereomicroscope. The specimens were dehydrated in formalindimethylacetal (FDA) for at least 24 h and critical‐point dried with liquid CO2 in a Polaron 7010 CPD. The dried specimens were mounted with nail polish on aluminium stubs, on which dissection was completed. The buds were coated with gold in an Agar sputter‐coater. SEM studies were done with a Philips XL 30 ESEM at 20 kV at the Institute of Plant Physiology, Karl‐Franzens‐University Graz. The micrographs were saved as TIF files and labelling was done with Adobe Photoshop 6.0.
Depending on the investigated species 60 to 180 pictures were taken and analysed. Complete ontogenetic series of three species are shown in Prenner (2003a, b, 2004). Ontogenetic series of the other species will be published elsewhere.
Interpretation of the SEM micrographs
For an accurate interpretation of the SEM micrographs, great attention was paid to ensure that separated flowers and floral primordia were investigated in exact frontal view, in which all organs are clearly visible. Furthermore, an attempt was made to find as many different developmental stages as possible. Under optimal conditions, only stages in which the organ just becomes visible were analysed. If such exact developmental stages were lacking, the size of the organ was used to interpret the time of organ initiation. But it should be emphasized that this could easily lead to erroneous interpretations, since the growth rate can vary notably. Therefore, such data were treated with caution. Analysis of tilted specimens and/or side views of specimens were avoided whenever possible, in order to assure an accurate analysis.
Terminology and systematic treatment
The terminology used refers to Tucker (1987). ‘Adaxial’ means the upper side of the flower, which is closest to the inflorescence axis, and ‘abaxial’ means the lower side of the flower, which is closest to the subtending bract. The tribal classification of the mentioned taxa follows Polhill (1994).
RESULTS
Bracteoles initiated but suppressed
Bracteoles are initiated to the left and right of the floral primordium and are clearly discernable before the first floral organ becomes visible (Fig. 1A). The protuberances remain small during initiation of the first sepals (Fig. 1B), and they disappear completely in the course of floral development (Fig. 1C). Hence they are no longer visible at anthesis. This character was observed in the Thermopsideae Baptisia australis (Fig. 1A–C) and Thermopsis lanceolata (Fig. 1D–F), in Kennedia nigricans (Fig. 1G–I; Phaseoleae–Kennediinae), and in Petteria ramentacea (Fig. 1J–L; Genisteae). In Ebenus cretica (Hedysareae) bracteoles are initiated before the floral apex becomes visible, and they are discernable to the left and right of the floral bract (Fig. 2A). These small protuberances disappear early and are no longer visible when the first sepals are formed (Fig. 2B, C).
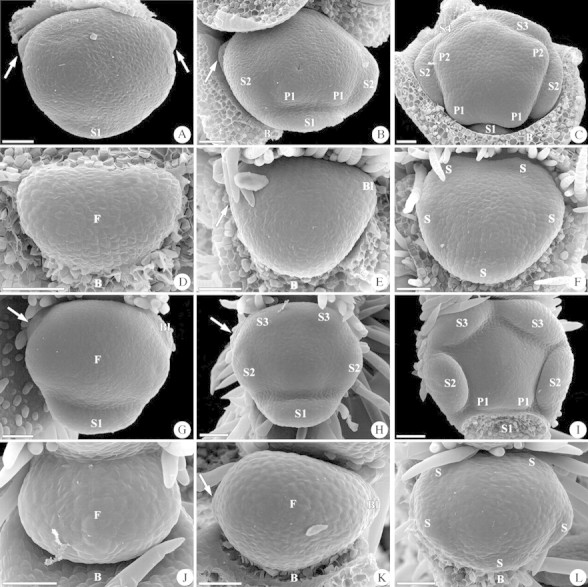
Fig. 1. Initiation of bracteoles and sepals in Baptisia australis, Thermopsis lanceolata, Kennedia nigricans and Petteria ramentacea. (A–C) Baptisia australis. (A) To the left and right of the floral primordium bracteoles are visible as two shallow protuberances (arrows). The first sepal is initiated in an abaxial position (S1). (B) The bracteoles remain small (arrow). The two lateral sepals (S2) and the two abaxial petals (P1) are formed. (C) The bracteoles are no longer visible. The two adaxial sepals arise in succession (S3, S4) and the two lateral petals are formed (P2). (D–F) Thermopsis lanceolata. (D) Floral primordium in the axil of a floral bract. (E) To the left and right of the floral primordium bracteoles become visible as two shallow protuberances (arrow, Bl). (F) No remnants of the initiated bracteoles are visible and all five sepals are formed simultaneously (S). (G–I) Kennedia nigricans. (G) Two bracteoles are initiated to the left and right of the floral primordium (arrow, Bl), and the first sepal is formed in an abaxial position. (H) On the left side of the young flower one bracteole remains visible (arrow). The two lateral and the two adaxial sepals are formed in a unidirectional manner. (I) No bracteoles are visible and the sepals enlarge (abaxial sepal removed). The two abaxial petals become visible. (J–L) Petteria ramentacea. (J) Floral primordium in the axil of a bract. (K) Two bracteoles are initiated to the left and right of the floral primordium (arrow, Bl). (L) The sepals are formed in a simultaneous whorl and no bracteole is visible. Scale bar = 50 µm in all images. Abbreviations: B = bract; Bl = bracteole; C = carpel; F = floral apex; P = petal; S = sepal (numbered in order of their appearance). All micrographs show the buds with the subtending bract orientated abaxially (lowermost).
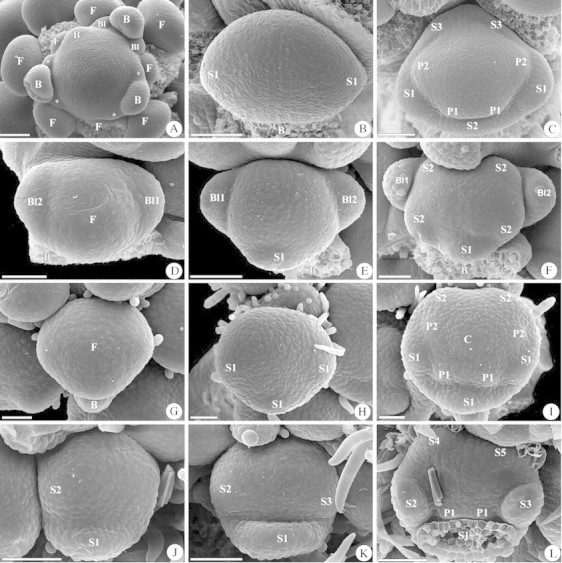
Fig. 2. Initiation of bracteoles and sepals in Ebenus cretica, Laburnum alpinum, Lathyrus latifolius and Dorycnium germanicum. (A–C) Ebenus cretica. (A) Apex of the inflorescence with formation of bracteoles (Bl and asterisks) laterally of the bracts. (B) No bracteoles are visible, and the lateral sepals are formed first. (C) All sepals are visible and numbered in order of their appearance. (D–F) Laburnum alpinum. (D) Initiation of bracteoles laterally on the floral apex. (E) The bracteoles enlarge and the abaxial sepal is formed. (F) Ongoing enlargement of sepals and simultaneous initiation of the lateral and the adaxial sepals. (G–I) Lathyrus latifolius. (G) Floral apex in the axil of a bract. No bracteoles are formed. (H) Simultaneous initiation of the abaxial and the two lateral sepals. (I) Simultaneous initiation of the two adaxial sepals and unidirectional initiation of the two abaxial and the lateral petals. (J–L) Dorycnium germanicum. (J) Initiation of the abaxial sepal, followed by the lateral sepal to its left. No bracteoles are formed. (K) The second lateral sepal is formed to the right of the abaxial sepal. (L) The two adaxial sepals are formed in succession, and the two abaxial petals become visible. Scale bar = 100 µm in (A); scale bar = 50 µm in (B–L). See Fig. 1 for abbreviations.
Bracteoles initiated and fully developed
This character was found in 16 species, and as an example Laburnum alpinum is shown (Fig. 2D–F). The bracteoles are initiated to the left and right of the floral primordium (Fig. 2D), and they enlarge parallel to the initiation of the sepals (Fig. 2E, F). Thus, they have to be removed for the analysis of later developmental stages.
Bracteoles entirely suppressed
No bracteoles were found in ten species, which is illustrated for Lathyrus latifolius (Fig. 2G–I) and Doryc nium germanicum (Fig. 2J–L). In both taxa the abaxial sepals are the first organs initiated on the floral primordium (see below).
Sepal initiation
Six different patterns of sepal initiation were found (Fig. 3), which are shown in the following sections by means of selected examples.
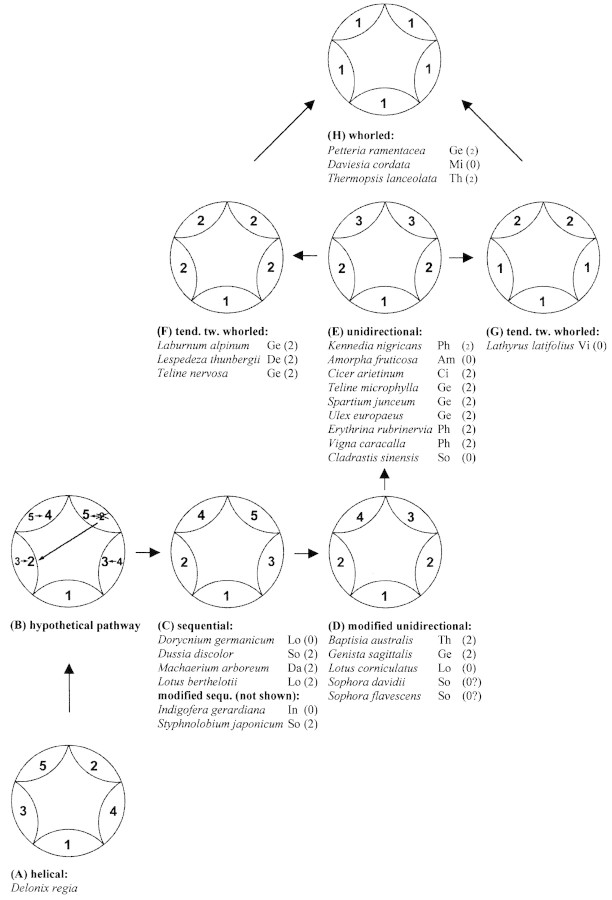
Fig. 3. Hypothetical pathway from the helical direction of sepal formation in Delonix regia (Caesalpinioideae; after Endress, 1994) to the whorled pattern in (H) as endpoint. (A) Helical pattern of sepal initiation in Delonix regia. (B) Hypothetic pathway (original helix in small numbers; crossed number = delay in the time of initiation). Sepal two on the adaxial right side is delayed (crossed) and instead sepal three in the lateral left position is initiated (following the originally helical pattern; long arrow). Now the original helix is continued, and sepal four is initiated in lateral right position, which is followed by sepal five (adaxially left). Finally, the delayed sepal two is initiated in the adaxial right position. (C) Sequential sepal initiation. (D) Modified unidirectional sepal initiation: due to harmonization of the plastochrons between sepal two and three, the unidirectional tendency is strengthened and only the adaxial sepals arise in succession. (E) Unidirectional pattern of sepal initiation with ongoing harmonization of the adaxial sepals. (F) Tendency towards whorled initiation with harmonization of the lateral and the adaxial sepals. (G) Tendency towards whorled sepal initiation with harmonization of the abaxial and the two lateral sepals. (H) Whorled sepal formation as endpoint of the harmonization of plastochrons among the sepals. Abbreviations: Am = Amorpheae, Ci = Cicereae, Da = Dalbergieae, De = Desmodieae, Ge = Genisteae, In = Indigofereae, Lo = Loteae, Mi = Mirbelieae, Ph = Phaseoleae, So = Sophoreae, Th = Thermopsideae, Vi = Vicieae; (2) = two well‐developed bracteoles; (2) = bracteoles initiated but suppressed; (0) = no bracteoles discernable.
Sepal initiation modified unidirectional.
In Baptisia australis the initiation starts in a unidirectional manner in that the abaxial sepal is formed first (Fig. 1A), and the two lateral sepals arise simultaneously (Fig. 1B). The modification is found in the two adaxial sepals, which are formed successively (Fig. 1C). Modified unidirectionality is also found in Genista sagittalis, Lotus corniculatus, Sophora davidii and S. flavescens (Fig. 3D).
Sepal initiation whorled.
In Thermopsis lanceolata and Petteria ramentacea all sepals are formed in a simultaneous whorl (Fig. 1F, L). Whorled sepal initiation is also found in Daviesia cordata (Fig. 3H).
Sepal initiation unidirectional.
In Kennedia nigricans sepal formation is unidirectional from the abaxial to the adaxial side. The abaxial sepal is initiated first (Fig. 1G), followed by the two lateral sepals, and by the two adaxial sepals (Fig. 1H, I). Unidirectional sepal initiation was found in nine of the studied taxa (Fig. 3E).
Sepal initiation bidirectional.
In Ebenus cretica sepal initiation is bidirectional, with the two lateral sepals formed first (Fig. 2B). The abaxial sepal is initiated next and the two adaxial sepals arise at the end (Fig. 2C). The same mode of sepal formation is found in Crotalaria pallida. In Galega officinalis this mode is modified in that the two adaxial sepals are formed successively.
Tendencies towards whorled sepal initiation.
In Laburnum alpinum calyx initiation starts with the abaxial sepal (Fig. 2E), and the remaining four sepals arise simultaneously (Fig. 2F). This is seen as a tendency towards whorled initiation, which is also found in Lespedeza thunbergii and Teline nervosa (Fig. 3F). Another tendency towards whorled sepal initiation can be found in Lathyrus latifolius, in which the abaxial and the two lateral sepals are formed simultaneously (Fig. 2H), while the two adaxial sepals arise later (Figs 2I, 3G).
Sepal initiation sequential.
In Dorycnium germanicum all five sepals are formed sequentially, beginning with the abaxial sepal, which is followed either by the lateral left (Fig. 2J) or by the lateral right sepal (not shown). The second lateral sepal arises next (Fig. 2K), and the two adaxial sepals are formed finally in succession (Fig. 2L). Further examples of successive sepal initiation have been found in Dussia discolor, Machaerium arboreum, Lotus berthelotii, Indigofera gerardiana and Styphnolobium japonicum (Fig. 3C).
DISCUSSION
Bracteoles initiated but suppressed
In the present study initiated but early suppressed bracteoles are shown as a ‘new’ character in Papilionoideae. Early suppressed bracteoles were found in Petteria ramentacea (Genisteae), Ebenus cretica (Hedysareae), Kennedia nigricans (Phaseoleae–Kennediinae), Baptisia australis (Thermopsideae) and Thermopsis lanceolata (Thermopsideae).
Petteria ramentacea (Genisteae).
The observations presented here on bracteoles in Petteria ramentacea are in contrast to those by Polhill (1976), Bisby (1981), and Tucker (1987). Bracteoles are not present in mature flowers. However, bracteoles are initiated, stop growing early, and are no longer visible at maturity. Thus the occurrence of bracteoles can be confirmed in a modified sense. van Wyk and Schutte (1989) state for Melolobium, Polhillia and Argyrolobium brevicalyx that bracteoles are ‘+/– absent’, which should be checked by use of SEM. Considering that the Argyrolobium group is classified either in Crotalarieae or in Genisteae (e.g. Polhill, 1968, 1981d; Bisby, 1981; van Wyk and Schutte, 1989, 1995), it seems important to know whether bracteoles are present or absent.
Ebenus cretica (Hedysareae).
Hutchinson (1964) mentions the presence of inconspicuous bracteoles in Ebenus, and Polhill (1981b) of small bracteoles in Hedysareae. However, in E. cretica bracteoles were discernable neither in the mature flowers nor at the time of calyx initiation. They are ‘initiated but early suppressed’.
Kennedia nigricans (Phaseoleae–Kennediinae).
Bract eoles occur in the larger part of Phaseoleae. They are absent in Neorautanenia, Alepidocalyx and Alistilus of the subtribe Phaseolinae according to Baudet (1978), and in Amphicarpa of the subtribe Glycininae, in all species except Adenodolichos of the subtribe Cajaninae, and in the subtribe Kennediinae according to Lackey (1981). However, in Kennedia nigricans at the very beginning of the floral development bracteole primordia are clearly discernable, but disappear at the time of sepal formation. Lackey (1977) suggests a distinct position of Kennediinae due to the absence of bracteoles, the prominent aril and the geographical isolation. For a clarification of the occurrence of bracteoles in Kennediinae further genera should be investigated.
Baptisia australis and Thermopsis lanceolata (Therm opsideae).
In Thermopsideae only Pickeringia is said to have minute bracteoles (Hutchinson, 1964; Tucker, 1987), while they are lacking in the remaining genera. In contrast, in the present study it was found that in B. australis and T. lanceolata two bracteoles are initiated at the floral primordium stage, but these are suppressed early in floral development. Doyle et al. (2000) mention uncertainties in the classification of Thermopsideae (see also Käss and Wink, 1995; Crisp et al., 2000). Thus a clarification of the presence or absence of bracteoles in the remaining genera Ammopiptanthus, Anagyris and Piptanthus is desirable.
Bracteoles developed and bracteoles absent
The findings of developed, alternatively absent bracteoles largely confirm indications from the literature (e.g. Hutchinson, 1964; Polhill, 1976, 1981a, c, 1982; Baudet, 1978; Bisby, 1981; Lackey, 1981; Kupicha, 1981; Tucker, 1987; Schrire, 1995; Crisp and Weston, 1987, 1995; Crisp, 1995). Nonetheless some outstanding results should be mentioned, as detailed below.
Cicereae.
Hutchinson (1964) mentions the absence of bracteoles in Cicer, and Tucker (1987) cites Cicereae as having bracteoles which are converted into spines. However, according to the observations reported here, bracteoles are clearly visible on the mature flower of C. arietinum. Spines, which occur in some species of Cicer, are seen as the sterile outgrowth of the reduced inflorescence rather than as converted bracteoles (cf. Wydler, 1860).
Sophoreae.
Bracteoles were found in Dussia discolor and Styphnolobium japonicum, while in Cladrastis sinensis, Sophora davidii and S. flavescens no bracteoles were observable. These findings agree with Sousa and Rudd (1993), who mention bracteolate flowers for Styphnolobium and ebracteolate flowers for Sophora s.s. The absence of bracteoles in C. sinensis, and their presence in D. discolor, confirms Hutchinson (1964).
Amorpheae.
Statements on the occurrence of bracteoles within this tribe are somewhat inconsistent (e.g. Hutchinson, 1964; Tucker, 1987). No bracteoles were found in Amorpha fruticosa.
Systematic treatment of initiated but suppressed bracteoles
While in Mimosoideae bracteoles are lacking, they are frequently found in the polyphyletic ‘Caesalpinioideae’, in which bracteoles are absent only in more derived lineages (Herendeen et al., 2003). Considering the ancestors of Papilionoideae within progenitors of Caesalpinioideae (e.g. Doyle et al., 2000; Bruneau et al., 2001; Wojciechowski, 2003), the occurrence of bracteoles can be seen as a plesiomorphic character in Papilionoideae. Consequently, the character ‘initiated but suppressed’ is seen as a step towards fully absent bracteoles. Initiated but suppressed bracteoles are found in Thermopsideae, Genisteae, Hedysareae and Phaseoleae. Hence the character is scattered almost throughout the papilionoid phylogeny, which is a hint for convergent reduction of bracteoles. Nonetheless, at lower taxonomic levels the occurrence of initiated but suppressed bracteoles could be a useful character, and the study of ‘ebracteolate’ taxa could bring new insights to legume evolution.
Sepal initiation variable
Considering that unidirectional organ formation is said to be the rule in Papilionoideae (e.g. Tucker, 1984, 1987, 1989, 2003; Endress, 1994; Erbar and Leins, 1997), the variability in sepal initiation is unexpected. Besides the unidirectional pattern sensu Tucker (1984), the following were found in this present study: modified unidirectionality, tendencies towards whorled and whorled initiation, bidirectional, and sequential formation of all sepals. Considering that in Caesalpinioideae helical sepal initiation is common (cf. Tucker, 1989, 2003; Endress, 1994 in Delonix regia; G. Prenner, unpubl. res.), and that Papilionoideae are nested within this subfamily (cf. Doyle et al., 2000), a model has been generated to deduce the variability of sepal initiation from the helical pattern of caesalpinioids. Evidence for helical sepal initiation has already been found in Psoralea pinnata (Tucker and Stirton, 1991) and Dalbergia brasiliensis (Klitgaard, 1999).
Both helices to the right and to the left occur. For the interpretation of the different developmental patterns, a helix to the left is chosen as a basis (Fig. 3A). The first modification of the helix is that the adaxial right sepal, which would arise after the abaxial sepal, is delayed in the time of its initiation. Continuing the helix, despite of this sepal, the lateral left sepal is formed next. According to the ongoing helical sequence, the second lateral sepal now appears, which is followed by the adaxial left sepal. Finally the delayed sepal arises, resulting in a sequential organ formation from the abaxial to the adaxial side of the flower (Fig. 3B). This pattern is found in Dorycnium germanicum, Dussia discolor, Machaerium arboreum and Lotus berthelotii × maculatus (Fig. 3C). Indigofera gerardiana and Styphnolobium japonicum deviate from this pattern in that the adaxial sepals are initiated in reversed sequence.
In Caesalpinioideae the lateral sepals are initiated in very short succession. The plastochron between these organs decreases (= harmonizes) until they arise simultaneously. As a last reminder of the originally helical initiation, the adaxial sepals are initiated in succession (Tucker et al., 1985). The same pattern was found in Genista sagittalis, Lotus corniculatus (Prenner, 2003a), Sophora davidii, Sophora flavescens and Baptisia australis (Figs 1C, 3D).
Unidirectional sepal initiation sensu Tucker (1984) is the result of equalization of the plastochrons of the adaxial sepals, and was found in nine species out of five tribes (Fig. 3E).
Assuming that the ongoing process of harmonization of the plastochrons is inherent, the developmental pattern ‘tendencies towards whorled initiation’ can be derived from the unidirectional pattern. Harmonization occurs on either the adaxial or the abaxial side of the flower. Adaxial harmonization is found in Lespedeza thunbergii, Laburnum alpinum and Teline nervosa, in which the lateral and the adaxial sepals are formed simultaneously (Figs 2F, 3F). Abaxial harmonization occurs in Lathyrus latifolius. Here the adaxial sepal and the two lateral sepals arise simultaneously (Figs 2H, 3G; for the complete ontogenetic sequence see Prenner, 2003b).
Due to further harmonization, the simultaneous or whorled pattern results. This is found in Petteria ramentacea, Daviesia cordata (Prenner, 2004) and Thermopsis lanceolata (Figs 1F, L, 3H).
The sequence presented here (sequential → modified unidirectional → unidirectional → tendencies towards whorled → whorled) should be seen as a hypothetical pathway, which needs to be tested on the basis of an enlarged developmental data matrix.
Bidirectional sepal initiation and the morphogenetic function of bracteoles
In Crotalaria pallida, Ebenus cretica and Galega officinalis sepal initiation is bidirectional with the two lateral sepals formed first. The abaxial sepal is initiated next, and finally the adaxial sepals arise either simultaneously or in succession. This uncommon pattern corresponds to the results of Breindl (1934) in Vaccinium rollisoni and Limnanthes douglasii, in which the first two sepals have a lateral position if the bracteoles are lacking or small. Referring to the morphogenetic function of bracteoles, Endress (1994, p. 97) states that ‘as the two first organs at the floral axis they mediate the onset of the spiral of the calyx’. In Galega officinalis bracteoles are lacking, in Ebenus cretica the bracteoles are of the type ‘initiated but suppressed’ and in Crotalaria pallida the bracteoles remain minute. In these taxa the loss or reduction of bracteoles could have influenced the order of sepal initiation. However, in contrast to this hypothesis other investigated taxa with reduced or lost bracteoles do not show bidirectional sepal formation. Hence further observations are necessary for a clarification of this uncommon pattern in Papilionoideae.
Systematic interpretation of the diversity of sepal initiation
Analogous to initiated but suppressed bracteoles, the big diversity of sepal initiation does not show a clear line within Papilionoideae. The different modes seem to be scattered throughout the papilionoid phylogeny. Two different modes of sepal initiation (unidirectional and tendency towards whorled initiation) within the genus Teline corroborate this, and are evidence for a lability of these patterns, which is also found in the petal whorl and in the two stamen whorls (G. Prenner, unpubl. res.). While Tucker and Douglas (1994) highlight the discrete position and monophyly of Papilionoideae on the basis of the analysis of previous ontogenetic characters, the present study corroborates Wojciechowski (2003, p. 9), who suggests that ‘papilionoids are only weakly differentiated molecularly from their caesalpinioid sister groups’. In fact, the derivation from the helical pattern of caesalpinioids and the broad variability of sepal initiation link the papilionoid flower more closely with the caesalpinioid flower than it was thought before (e.g. Tucker and Douglas, 1994; Tucker, 2003).
CONCLUSIONS
In the present paper ‘initiated but suppressed’ bracteoles are shown as a ‘new’ character in Papilionoideae. Furthermore, a remarkable variability of the sequence of sepal initiation was found, which can be derived from the helical pattern of caesalpinioids. With this result the widely stated unidirectionality of Papilionoideae is questioned, and a link between the flowers of Papilionoideae and Caesalpinioideae is shown. Bidirectional sepal initiation is possibly a consequence of the morphogenetic function of bracteoles, although bidirectionality is not found in all taxa with reduced bracteoles. Clarification and a detailed phylogenetic analysis of the presented characters based on a broadened data matrix could allow new insights into legume systematics.
ACKNOWLEDGEMENTS
I thank Herwig Teppner for valuable discussions on the topic, for instruction in the field of plant systematics and for supervising the PhD thesis of which this study is a part; Helmut Mayrhofer, head of the Institute of Botany, Karl‐Franzens‐University Graz, for providing the facilities for the study; Edith Stabentheiner for the opportunity to carry out the SEM studies in her lab; Elisabeth Baloch for comments on the manuscript; Peter K. Endress and Peter Leins for very helpful suggestions which improved the manuscript; and Pramodchandra Harvey for assistance with the English language.
Received: 1 October 2003; Returned for revision: 18 December 2003; Accepted: 14 January 2004 Published electronically: 16 March 2004
References
- BarkerNP, Schrire BD, Kim J‐H.2000. Generic relationships in the tribe Indigofereae (Leguminosae: Papilionoideae) based on sequence data and morphology. In: Herendeen PS, Bruneau A, eds. Advances in legume systematics part 9 Kew: Royal Botanic Gardens, 311–337. [Google Scholar]
- BaudetJC.1978. Prodrome d’une classification générique des Papilionaceae‐Phaseoleae. Bulletin du Jardin Botanique National de Belgique 48: 183–220. [Google Scholar]
- BisbyFA.1981. Genisteae (Adans.) Benth. (1865). In: Polhill RM, Raven PH, eds. Advances in legume systematics part 1 Kew: Royal Botanic Gardens, 409–425. [Google Scholar]
- BreindlM.1934. Zur Kenntnis der Baumechanik des Blütenkelches der Dikotyledonen. Botanisches Archiv 36: 191–268. [Google Scholar]
- BretelerFJ.1995. The boundary between Amherstieae and Detarieae (Caesapinioideae). In: Crisp M, Doyle J, eds. Advances in legume systematics part 7. Kew: Royal Botanic Gardens, 53–61. [Google Scholar]
- BruneauA, Forest F, Herendeen PS, Klitgaard BB, Lewis GP.2001. Phylogenetic relationships in the Caesalpinioideae (Leguminosae) as inferred from chloroplast trnL intron sequences. Systematic Botany 26: 487–514. [Google Scholar]
- de CandolleAP.1813.Théorie élémentaire de la botanique. Paris: Deterville. [Google Scholar]
- CrispMD.1995. Contributions towards a revision of Daviesia (Fabaceae: Mirbelieae), III. A synopsis of the genus. Australian Systematic Botany 8: 1155–1249. [Google Scholar]
- CrispMD, Weston PH.1987. Cladistics and legume systematics, with an analysis of Bossiaeeae, Brongniartieae and Mirbelieae (Papilionoideae, Leguminosae). In: Stirton CH, ed. Advances in legume systematics part 3 Kew: Royal Botanic Gardens, 65–130. [Google Scholar]
- CrispMD, Weston PH.1995. Mirbelieae. In: Crisp M, Doyle J. eds. Advances in legume systematics part 7 Kew: Royal Botanic Gardens, 245–282. [Google Scholar]
- CrispMD, Gilmore S, van Wyk B‐E.2000. Molecular phylogeny of the genistoid tribes of papilionoid legumes. In: Herendeen PS, Bruneau A, eds. Advances in legume systematics part 9 Kew: Royal Botanic Gardens, 249–276. [Google Scholar]
- DoyleJJ, Chapill JA, Bailey CD, Kajita T.2000. Towards a comprehensive phylogeny of legumes: evidence from rbcL sequences and non‐molecular data. In: Herendeen PS, Bruneau A, eds. Advances in legume systematics part 9 Kew: Royal Botanic Gardens, 1–20. [Google Scholar]
- EamesAJ.1961. Morphology of the angiosperms. New York: McGraw‐Hill Book Comp. [Google Scholar]
- EndressPK.1987. Floral phyllotaxis and floral evolution. Botanische Jahrbücher für Systematik und Pflanzengeographie 108: 417–438. [Google Scholar]
- EndressPK.1994.Diversity and evolutionary biology of tropical flowers. Cambridge tropical biology series. Cambridge: Cambridge University Press. [Google Scholar]
- ErbarC, Leins P.1997. Different patterns of floral development in whorled flowers, exemplified by Apiaceae and Brassicaceae. International Journal of Plant Science 158 (6 Supplement): 49–64. [Google Scholar]
- HerendeenPS, Bruneau A, Lewis GP.2003. Phylogenetic relationships in caesalpinioid legumes: A preliminary analysis based on morphological and molecular data. In: Klitgaard BB, Bruneau A, eds. Advances in legume systematics part 10 Kew: Royal Botanic Gardens, 37–62. [Google Scholar]
- HutchinsonJ.1964.The genera of flowering plants part 1. Oxford: Clarendon Press. [Google Scholar]
- KässE, Wink M.1995. Molecular phylogeny of the Papilionoideae (Family Leguminosae): rbcL gene sequence versus chemical taxonomy. Botanica Acta 108: 149–162. [Google Scholar]
- KlitgaardBB.1999. Floral ontogeny in tribe Dalbergieae (Leguminosae: Papilionoideae): Dalbergia brasiliensis, Machaerium villosum s.l., Platymiscium floribundum and Pterocarpus rotundifolius Plant Systematics and Evolution 219: 1–25. [Google Scholar]
- KupichaFK.1981. Vicieae (Adams.) DC. (1825), nom. conserv. prop. In: Polhill RM, Raven PH, eds. Advances in legume systematics part 1 Kew: Royal Botanic Gardens, 377–381. [Google Scholar]
- LackeyJA.1977. A revised classification of the tribe Phaseoleae (Leguminosae: Papilionoideae), and its relation to canavanin distribution. Botanical Journal of the Linnean Society 74: 163–178. [Google Scholar]
- LackeyJA.1981. Phaseoleae DC. (1825). In: Polhill RM, Raven PH, eds. Advances in legume systematics part 1 Kew: Royal Botanic Gardens, 301–327. [Google Scholar]
- LavinM.1987. A cladistic analysis of the tribe Robinieae (Papilionoideae, Leguminosae). In: Stirton CH, ed. Advances in legume systematics part 3 Kew: Royal Botanic Gardens, 31–64. [Google Scholar]
- LavinM.1995. Tribe Robinieae and allies: model groups for assessing early Tertiary northern latitude diversification of tropical legumes. In: Crisp M, Doyle J, eds. Advances in legume systematics part 7 Kew: Royal Botanic Gardens, 141–160. [Google Scholar]
- PenningtonRT, Klitgaard BB, Ireland H, Lavin M.2000. New insights into floral evolution of basal Papilionoideae from molecular phylogenies. In: Herendeen PS, Bruneau A, eds. Advances in legume systematics part 9 Kew: Royal Botanic Gardens, 233–248. [Google Scholar]
- PenningtonRT, Lavin M, Irelnad H, Klitgaard B, Preston J, Hu J‐H.2001. Phylogenetic relationships of basal papilionoid legumes based upon sequences of the chloroplast trnL intron. Systematic Botany 26: 537–556. [Google Scholar]
- PolhillRM.1968.Argyrolobium Eckl. & Zeyh. (Leguminosae) in tropical Africa. Kew Bulletin 22: 145–168. [Google Scholar]
- PolhillRM.1976. Genisteae (Adans.) Benth. and related tribes (Leguminosae). Botanical Systematics 1: 143–368. [Google Scholar]
- PolhillRM.1981a. Dalbergieae Bronn ex DC. (1825). In: Polhill RM, Raven PH, eds. Advances in legume systematics part 1 Kew: Royal Botanic Gardens, 233–242. [Google Scholar]
- PolhillRM.1981b. Hedysareae DC. (1825). In: Polhill RM, Raven PH, eds. Advances in legume systematics part 1 Kew: Royal Botanic Gardens, 367–370. [Google Scholar]
- PolhillRM.1981c. Loteae DC. (1825). In: Polhill RM, Raven PH, eds. Advances in legume systematics part 1 Kew: Royal Botanic Gardens, 371–374. [Google Scholar]
- PolhillRM.1981d. Crotalarieae (Benth.) Hutch. (1964). In: Polhill RM, Raven PH, eds. Advances in legume systematics part 1 Kew: Royal Botanic Gardens, 399–402. [Google Scholar]
- PolhillRM.1982. Crotalaria in Africa and Madagascar Rotterdam: AA Balkema, 1–389. [Google Scholar]
- PolhillRM.1994. Complete synopsis of legume genera. In: Bisby FA, Buckingham J, Harborne JB, eds. Phytochemical dictionary of the Leguminosae part 1. New York: Chapman and Hall. [Google Scholar]
- PrennerG.2003a. A developmental analysis of the inflorescence and the flower of Lotus corniculatus (Fabaceae‐Loteae). Mitteilungen des Naturwissenschaftlichen Vereins für Steiermark 133: 99–107. [Google Scholar]
- PrennerG.2003b. Floral ontogeny in Lathyrus latifolius (Fabaceae–Vicieae). Phyton (Horn, Austria) 43: 392–400. [Google Scholar]
- PrennerG.2004. Floral development in Daviesia cordata (Leguminosae: Papilionoideae: Mirbelieae) and its systematic implications. Australian Journal of Botany (in press). [Google Scholar]
- SchrireBD.1995. Evolution of the tribe Indigofereae (Leguminosae–Papilionoideae). In: Crisp M, Doyle J, eds. Advances in legume systematics part 7 Kew: Royal Botanic Gardens, 161–244. [Google Scholar]
- SousaM, Rudd VE.1993. Revision del genero Styphnolobium (Leguminosae: Papilionoideae: Sophoreae). Annals of the Missouri Botanical Garden 80: 270–283. [Google Scholar]
- TuckerSC.1984. Unidirectional organ initiation in leguminous flowers. American Journal of Botany 71: 1139–1148. [Google Scholar]
- TuckerSC.1987. Floral initiation and development in legumes. In: Stirton CH, ed. Advances in legume systematics part 3 Kew: Royal Botanic Gardens, 83–239. [Google Scholar]
- TuckerSC.1989. Evolutionary implications of floral ontogeny in legumes. In: Stirton CH, Zarucchi JL, eds. Advances in legume biology Monographs in Systematic Botany from the Missouri Botanical Garden 29: 59–75. [Google Scholar]
- TuckerSC.2003. Floral development in legumes. Plant Physiology 131: 911–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TuckerSC, Stein OL, Derstine KS.1985. Floral development in Caesalpinia (Leguminosae). American Journal of Botany 72: 1424–1434. [Google Scholar]
- TuckerSC, Stirton CH.1991. Development of the cymose inflorescence, cupulum and flower of Psoralea pinnata (Leguminosae: Papilionoideae: Psoraleeae). Botanical Journal of the Linnean Society 106: 209–227. [Google Scholar]
- TuckerSC, Douglas AW.1994. Ontogenetic evidence and phylogenetic relationships among basal taxa of legumes. In: Ferguson IK, Tucker SC, eds. Advances in legume systematics part 6 Kew: Royal Botanic Gardens, 11–32. [Google Scholar]
- van der BankM, Chase MW, van Wyk B‐E, Fay MF, van der Bank FH, Reeves G, Hulme A.2002. Systematics of the tribe Podalyrieae (Fabaceae) based on DNA, morphological and chemical data. Botanical Journal of the Linnean Society 139: 159–170. [Google Scholar]
- van WykB‐E, Schutte AL.1989. Taxonomic relationships amongst some genera of Leguminosae tribe Crotalarieae and Argyrolobium (Genisteae). Kew Bulletin 44: 397–423. [Google Scholar]
- van WykB‐E, Schutte AL.1995. Phylogenetic relationships in the tribes Podalyrieae, Liparieae and Crotalarieae. In: Crisp M, Doyle JJ, eds. Advances in legume systematics part 7 Kew: Royal Botanic Gardens, 283–308. [Google Scholar]
- WojciechowskiMF.2003. Reconstructing the phylogeny of legumes (Leguminosae): An early 21st century perspective. In: Klitgaard BB, Bruneau A, eds. Advances in legume systematics part 10 Kew: Royal Botanic Gardens, 5–35. [Google Scholar]
- WydlerH.1860. Kleine Beiträge zur Kenntnis einheimischer Gewächse. Flora (Regensburg) 43: 83–96. [Google Scholar]