Abstract
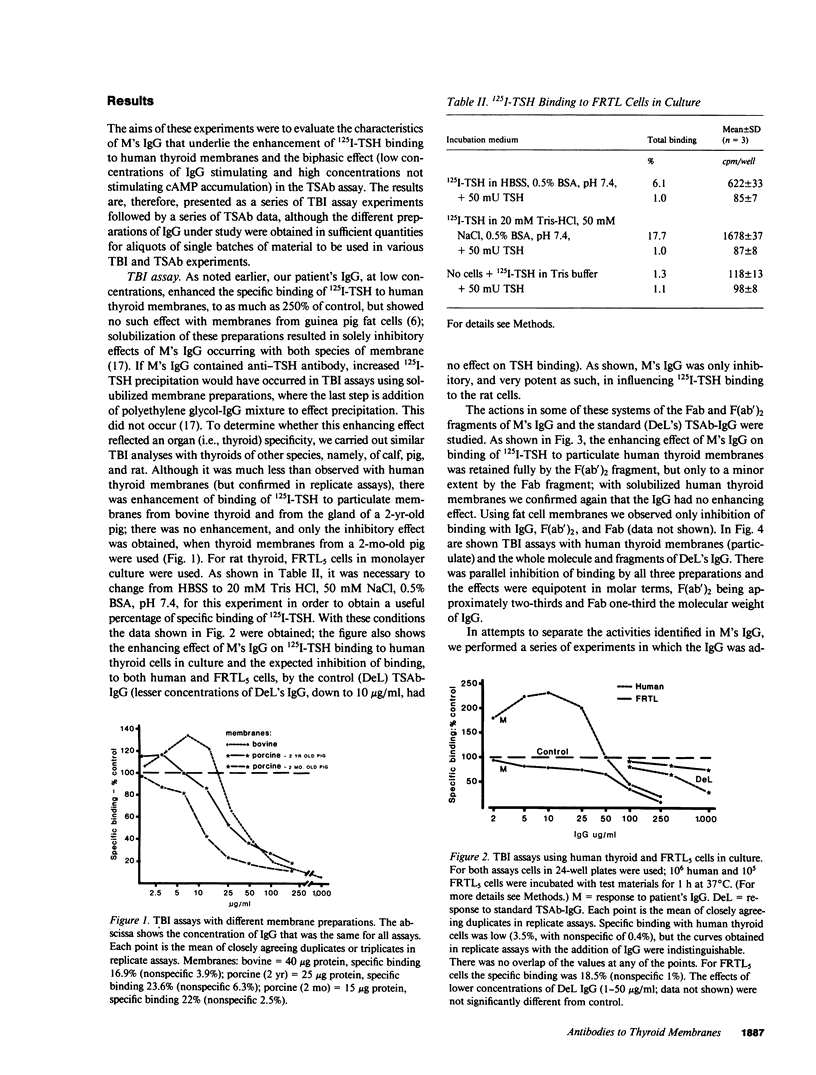
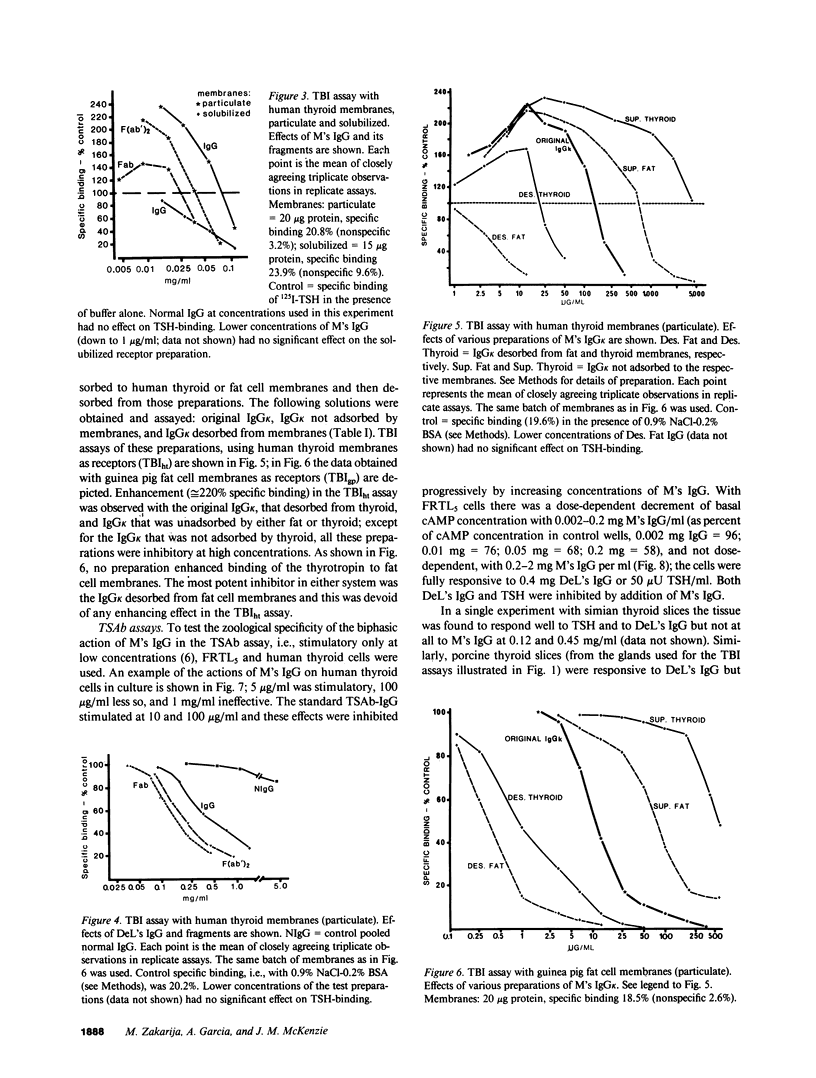
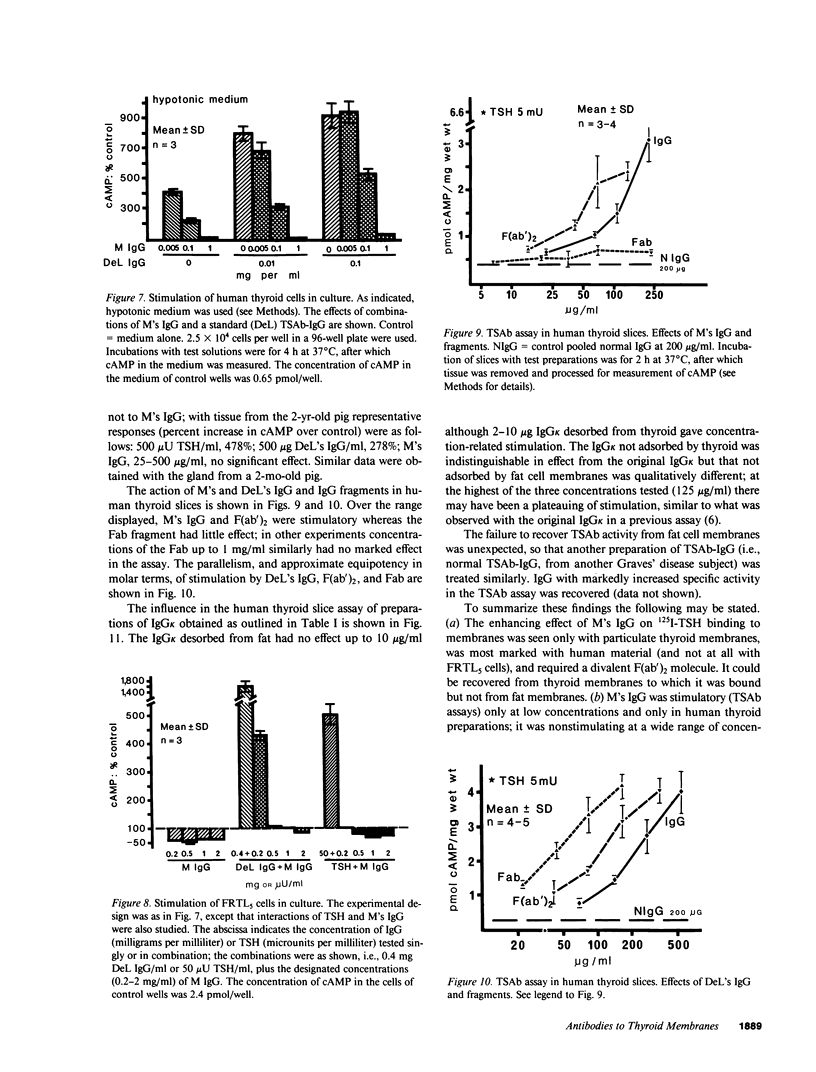
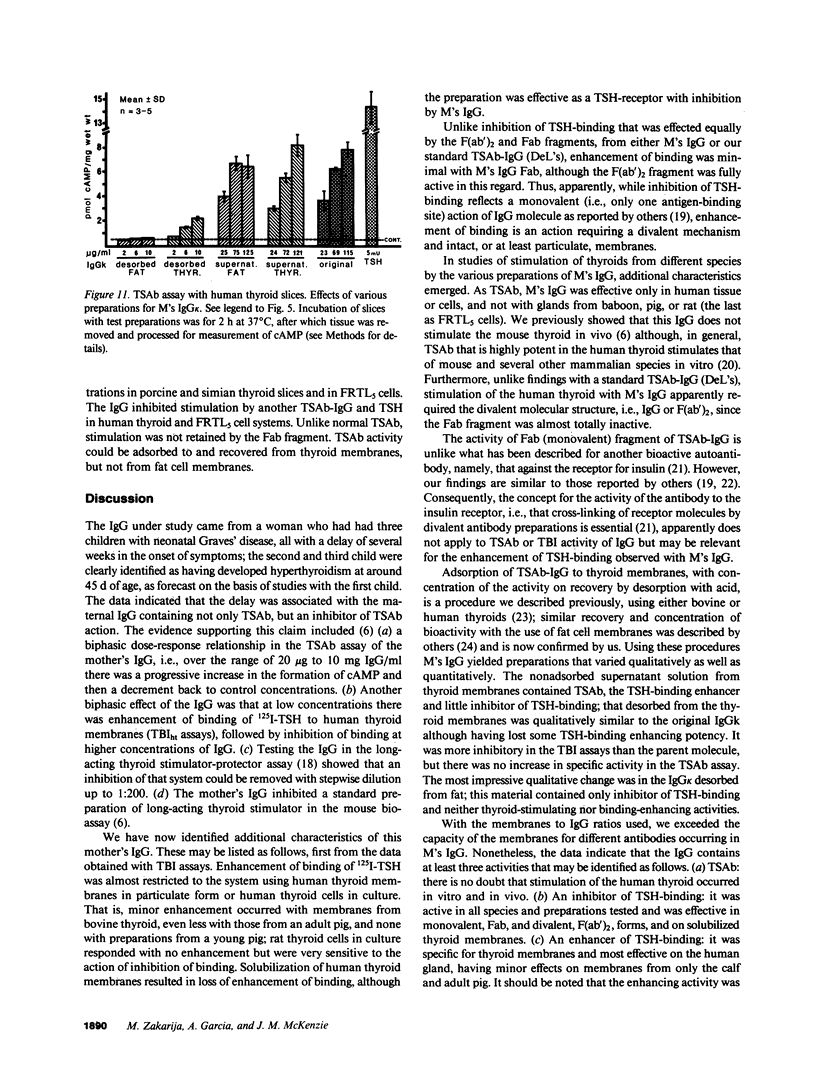
Immunoglobulin G was obtained from the serum of a woman who had given birth to three children with a delayed onset of hyperthyroidism; the clinical events were due to the coexistence of thyroid-stimulating antibody (TSAb) and an inhibitor of TSAb in the maternal serum. The current studies explore the possible existence of additional thyroid membrane-directed antibodies. Human thyroid slices, cells in monolayer culture, and functioning rat thyroid cells (FRTL5), with measurement of cyclic AMP concentration, were used for TSAb assays. Assays of the inhibition of binding of 125I-thyrotropin (TSH) to its receptor used human thyroid and FRTL5 cells, and human thyroid and guinea pig fat cell membranes as receptors. All activities were associated with IgG kappa. Fractions of IgG kappa obtained by adsorption to and the desorption from human thyroid and guinea pig fat cell preparations and F(ab')2 and Fab fragments of the parent IgG were tested. Results indicated that there were three activities in the IgG, namely, TSAb; an inhibitor of TSH-binding that was active in all species and preparations tested, and was effective as Fab and F(ab')2 on both particulate and solubilized thyroid membranes; and an enhancer of TSH-binding (e.g., approximately equal to 220% increase in binding) that was relatively specific for human thyroid membranes only in particulate form, was not adsorbed by fat, and was active as F(ab')2, but minimally as Fab. The concept is developed that dilution of the total IgG, experimentally in vitro or by metabolic clearance in vivo in neonates, determines the effect on either thyroid stimulation or TSH-binding. The incidence of such multiple antibodies and their interaction remains to be determined.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambesi-Impiombato F. S., Parks L. A., Coon H. G. Culture of hormone-dependent functional epithelial cells from rat thyroids. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3455–3459. doi: 10.1073/pnas.77.6.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Endo K., Amir S. M., Ingbar S. H. Development and evaluation of a method for the partial purification of immunoglobulins specific for Graves' disease. J Clin Endocrinol Metab. 1981 Jun;52(6):1113–1123. doi: 10.1210/jcem-52-6-1113. [DOI] [PubMed] [Google Scholar]
- FRANKLIN E. C. Structural units of human 7S gamma globulin. J Clin Invest. 1960 Dec;39:1933–1941. doi: 10.1172/JCI104218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R., Baird K. L., Jarrett D. B., Flier J. S. Direct demonstration that receptor crosslinking or aggregation is important in insulin action. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4209–4213. doi: 10.1073/pnas.75.9.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishihara M., Nakao Y., Baba Y., Matsukura S., Kuma K., Fujita T. Interaction between thyroid-stimulating immunoglobulins and thyrotropin receptors in fat cell membranes. J Clin Endocrinol Metab. 1979 Nov;49(5):706–711. doi: 10.1210/jcem-49-5-706. [DOI] [PubMed] [Google Scholar]
- Koizumi Y., Zakarija M., McKenzie J. M. Solubilization, purification, and partial characterization of thyrotropin receptor from bovine and human thyroid glands. Endocrinology. 1982 Apr;110(4):1381–1391. doi: 10.1210/endo-110-4-1381. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McKenzie J. M., Zakarija M. Assays of thyroid-stimulating antibody. Methods Enzymol. 1985;109:677–691. doi: 10.1016/0076-6879(85)09123-6. [DOI] [PubMed] [Google Scholar]
- Rapoport B., Filetti S., Takai N., Seto P., Halverson G. Studies on the cyclic AMP response to thyroid stimulating immunoglobulin (TSI) and thyrotropin (TSH) in human thyroid cell monolayers. Metabolism. 1982 Nov;31(11):1159–1167. doi: 10.1016/0026-0495(82)90168-8. [DOI] [PubMed] [Google Scholar]
- Rapoport B., Greenspan F. S., Filetti S., Pepitone M. Clinical experience with a human thyroid cell bioassay for thyroid-stimulating immunoglobulin. J Clin Endocrinol Metab. 1984 Feb;58(2):332–338. doi: 10.1210/jcem-58-2-332. [DOI] [PubMed] [Google Scholar]
- Smith B. R., Hall R. Thyroid-stimulating immunoglobulins in Graves' disease. Lancet. 1974 Aug 24;2(7878):427–431. doi: 10.1016/s0140-6736(74)91815-7. [DOI] [PubMed] [Google Scholar]
- Smith B. R. Immunology of the thyrotropin receptor. Immunol Commun. 1976;5(5):345–360. doi: 10.3109/08820137609033853. [DOI] [PubMed] [Google Scholar]
- Vitti P., Rotella C. M., Valente W. A., Cohen J., Aloj S. M., Laccetti P., Ambesi-Impiombato F. S., Grollman E. F., Pinchera A., Toccafondi R. Characterization of the optimal stimulatory effects of graves' monoclonal and serum immunoglobulin G on adenosine 3',5'-monophosphate production in fRTL-5 thyroid cells: a potential clinical assay. J Clin Endocrinol Metab. 1983 Oct;57(4):782–791. doi: 10.1210/jcem-57-4-782. [DOI] [PubMed] [Google Scholar]
- Zakarija M. Immunochemical characterization of the thyroid-stimulating antibody (TSAb) of Graves' disease: evidence for restricted heterogeneity. J Clin Lab Immunol. 1983 Feb;10(2):77–85. [PubMed] [Google Scholar]
- Zakarija M., McKenzie J. M. Adsorption of thyroid-stimulating antibody (TSAb) of Graves' disease by homologous and heterologous thyroid tissue. J Clin Endocrinol Metab. 1978 Oct;47(4):906–908. doi: 10.1210/jcem-47-4-906. [DOI] [PubMed] [Google Scholar]
- Zakarija M., McKenzie J. M., Banovac K. Clinical significance of assay of thyroid-stimulating antibody in Graves' disease. Ann Intern Med. 1980 Jul;93(1):28–32. doi: 10.7326/0003-4819-93-1-28. [DOI] [PubMed] [Google Scholar]
- Zakarija M., McKenzie J. M., Munro D. S. Immunoglobulin G inhibitor of thyroid-stimulating antibody is a cause of delay in the onset of neonatal Graves' disease. J Clin Invest. 1983 Oct;72(4):1352–1356. doi: 10.1172/JCI111091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakarija M., McKenzie J. M. Pregnancy-associated changes in the thyroid-stimulating antibody of Graves' disease and the relationship to neonatal hyperthyroidism. J Clin Endocrinol Metab. 1983 Nov;57(5):1036–1040. doi: 10.1210/jcem-57-5-1036. [DOI] [PubMed] [Google Scholar]
- Zakarija M., McKenzie J. M. Thyroid-stimulating antibody (TSAb) of Graves' disease. Life Sci. 1983 Jan 3;32(1-2):31–44. doi: 10.1016/0024-3205(83)90171-6. [DOI] [PubMed] [Google Scholar]
- Zakarija M., McKenzie J. M. Zoological specificity of human thyroid-stimulating antibody. J Clin Endocrinol Metab. 1978 Aug;47(2):249–254. doi: 10.1210/jcem-47-2-249. [DOI] [PubMed] [Google Scholar]



