Abstract
• Background and Aims Andromonoecy, as a breeding system, has generated a considerable body of theory in terms of sexual selection, but extended records comparing the performance of pollen grains from staminate versus hermaphrodite flowers are still sparse. The objective in this study was to elucidate the role of staminate flowers in the andromonoecious breeding system of olive (Olea europaea).
• Methods To determine the meaning of staminate flowers, an evaluation was made of resource allocation to, and phenology of, staminate and hermaphrodite flowers in the cultivar ‘Mission’, and a comparison was made of the male function between both kinds of flowers.
• Key Results Dry weight of hermaphrodite flowers was 19 % greater than dry weight of staminate flowers arising in comparable positions of the panicle. This difference was mainly due to pistil and petal weight; there were no significant differences in stamen weight. There were no significant differences between staminate and hermaphrodite flowers in either amount of pollen per anther, or pollen quality as determined by pollen viability, germinability or ability to fertilize other flowers. There was no significant link between gender and time of anthesis. However, position of the flower within the panicle correlated with time of anthesis and gender. Flowers at the apex and at primary pedicels tended to be hermaphrodite and open earlier, whereas flowers arising in secondary pedicels were mainly staminate and were commonly the last to reach anthesis.
• Conclusions It is proposed that the main advantage provided by production of staminate flowers in olive is to enhance male fitness by increasing pollen output at the whole plant level, although a relict function of attracting pollinators cannot be completely discarded.
Key words: Andromonoecy, olive, Olea europaea, male function, wind pollination, pistil abortion
INTRODUCTION
Olive (Olea europaea) is andromonoecious, i.e. individual trees bear both hermaphrodite and staminate flowers. Hermaphrodite flowers consist of a small, greenish calyx, four white petals, two stamens with large anthers, and a pistil composed of a bilobulate stigma, a short style and a bilocular ovary with four ovules. Staminate flowers result from pistil abortion at varying stages of gynoecium differentiation and possess a non‐functional, rudimentary pistil. Although self‐fertilization is not totally precluded, cross‐pollination results in earlier and greater levels of fertilization (Cuevas, 1992). Olive is an evergreen tree cultivated from ancient times in the Mediterranean basin for its oil and fruit. Its wild relatives, known as oleaster forms, are generally indistinguishable from feral types and constitute a common component of the Mediterranean vegetation (Zohary and Hopf, 1993). Cultivated varieties are propagated clonally and very often a single genotype is planted in orchards.
The purpose of this study was to elucidate the role of staminate flowers in the breeding system of olive. Andromonoecious breeding systems have been studied primarily in herbaceous species (Dulberger et al., 1981; Bertin, 1982; Emms, 1993; Diggle, 1994; Huang, 2003). Olive is a long‐lived woody perennial where the phenology of floral induction and differentiation (Fernández‐Escobar et al., 1992) and pistil abortion (Cuevas et al., 1999) are well understood, thus it presents an attractive system for expanding our understanding of andromonoecious breeding systems.
Uriu (1953) demonstrated the role of nutrition in olive pistil abortion. His observations that high leaf/flower ratios and nitrogen fertilization promote hermaphrodite flower production agree with the trend toward femaleness in other andromonoecious plants growing under favourable environmental conditions (Primack and Lloyd, 1980; Solomon, 1985; Emms, 1993). Hence, the high variation observed in olive in the proportion of staminate flowers among years, trees, branches, shoots and even inflorescences in the same shoot (Brooks, 1948) may be part of an overall reproductive strategy that adjusts maternal investment in sex expression in response to available resources and environmental conditions.
If nutritional deficiencies lead to increased pistil abortion and formation of staminate flowers, the nutritional deficit might also affect pollen production or pollen quality. If this is the case, staminate flowers would be considered a consequence of the nutritional deficit and the perceived andromonoecy merely a process of incomplete flower abortion. If, on the contrary, staminate flowers benefit by reallocating resources saved by pistil abortion, we would expect more pollen grains or superior performance from pollen produced by staminate flowers compared with hermaphrodite flowers. In this case the condition could be interpreted as an early step toward monoecy. To understand the role of staminate flowers in olive, we compared phenology, resource allocation to floral parts, and pollen output and performance in staminate versus hermaphrodite flowers.
Floral induction in olive occurs during the summer prior to bloom (Fernández‐Escobar et al., 1992), although flower primordia are not evident until March (Hartmann, 1951). Blooming occurs in mid spring (typically May) and lasts for about 2 weeks (Griggs et al., 1975). A single adult tree may bear as many as 500 000 flowers; only about 1·2 % of these set fruit (Martin, 1990) due to intense abscission of young fruits as a result of competition for maternal resources (Rallo and Fernández‐Escobar, 1985). Staminate and hermaphrodite flowers develop simultaneously within the same panicle. Panicles develop in the axils of leaves on the previous year’s growth. Bees occasionally collect olive pollen, but wind is the primary pollination vector (Morettini, 1972; Griggs et al., 1975). The fruit, a single‐seeded drupe with high oil content, is dispersed by birds (Herrera, 1982). Cultivated varieties, such as ‘Mission’ used in this work, have been selected for such traits as fruit size or oil content, not for mating system characteristics.
MATERIALS AND METHODS
Trees of Olea europaea L. ‘Mission’ used in this work were growing in an experimental orchard at the University of California, Davis (N 38°32′41′′, W 121°44′21′′).
Inflorescence architecture and phenology
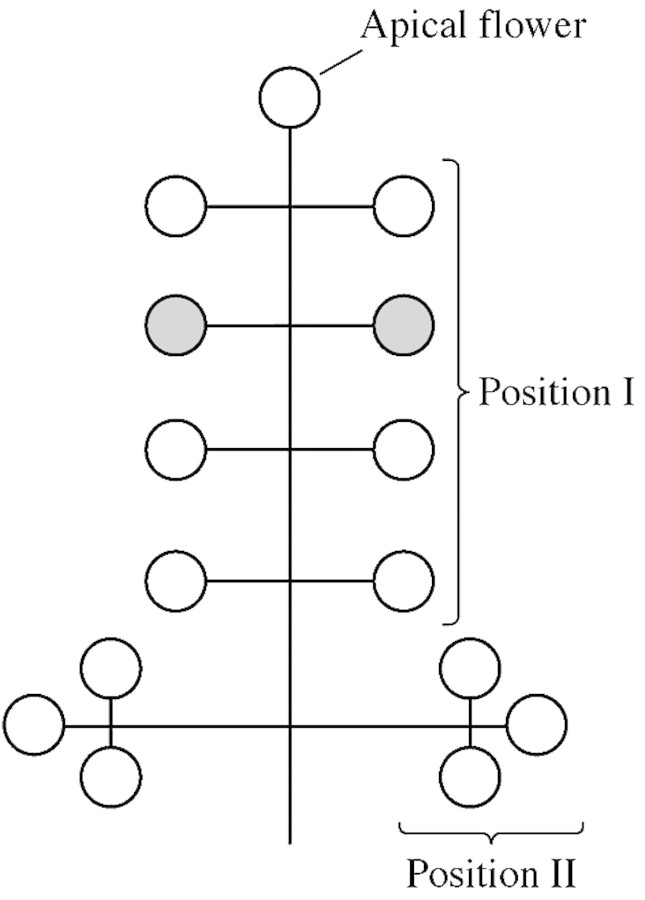
In order to determine inflorescence structure, a total of 40 inflorescences from four trees were sampled, their flowers counted and the gender assessed in flowers arising in three positions of the inflorescence: the apex, primary pedicels (position I) and secondary pedicels of the panicle (position II; Fig. 1). The single apical flower, three flowers in position I and three in position II were examined in each inflorescence. The position and gender of the first and the last flowers to reach anthesis were determined on an additional set of 80 inflorescences distributed around the tree in four trees.
Fig. 1. Inflorescence structure of olive ‘Mission’ panicle. Shaded circles represent the position of the flowers sampled for pollen performance.
Resource allocation pattern
A total of 40 flowers from four trees were gathered at bloom just before anther dehiscence, dissected into stamens, pistil and corolla, and dried at 65 °C for 72 h. In order to minimize the effect of inflorescence nutrition on the flower types, the flowers were sampled from opposite positions within the panicle in primary pedicels (Fig. 1). The same procedure was followed on another set of 40 staminate flowers arising at position II of the same inflorescences. The relationship between fresh and dry weight of flowers was determined in another set of 20 flowers. ANOVA and correlation tests were conducted.
Pollen performance
Hermaphrodite and staminate flowers arising at opposite positions within a panicle (Fig. 1) were collected and their anthers allowed to dehisce at room temperature. The pollen was collected and stored at 4 °C until further evaluation. Pollen grain number was calculated for 20 flowers (five each from four trees) of each type. The anthers were dried at 60 °C, crushed and then hydrolyzed with 8 n NaOH for 3 h at 60 °C, which digested most of the flower tissue and allowed pollen grains to be counted under a microscope. NaOH was replaced with distilled water and pollen grain number in every flower was determined from five 10 µL aliquots.
Equatorial diameter of 100 fully hydrated pollen grains was measured under a microscope using a digitizer tablet interfaced to a desktop computer and the program SigmaScan 1.0 (Jandell Scientific, Corte Madera, CA, USA). Pollen viability was determined using fluorescein diacetate (Heslop‐Harrison and Heslop‐Harrison, 1970, Pinney and Polito, 1990) on six samples of at least 100 pollen grains for each pollen type. Pollen germination was evaluated on three fields, containing over 100 pollen grains each, in each of three different Petri dishes for each pollen type after 24 h in pollen germination medium consisting of 0·8 % agar, 15 % sucrose, 100 ppm boric acid and 60 ppm tetracycline (Pinney and Polito, 1990). A pollen grain was considered germinated when its pollen tube length was at least equal to the pollen diameter. Pollen tube growth in vitro was measured at 4, 8 and 24 h after dusting rehydrated pollen on the pollen germination medium. Pollen tube length was measured for 200 pollen tubes per flower type and time. Pollen ability to effect fertilization was evaluated on flowers of the compatible cultivar ‘Sevillano’ (Cuevas and Polito, 1997). Fresh pollen from each flower type was applied to the stigmas of newly opened receptive ‘Sevillano’ flowers with a fine paint brush. Twenty flowers per flower type were sampled at 5 and 10 d after pollination, and the rate and timing of fertilization were determined using aniline blue fluorescence staining technique (Martin, 1959). The fertilization rate was estimated as the proportion of flowers with a pollen tube entering at least one of the four ovules (Cuevas et al., 1994). ANOVA and Chi‐square analyses were conducted to determine differences between flower types.
RESULTS
Inflorescence architecture and phenology
The most common structure for the panicle is represented in Fig. 1. Inflorescences comprise a mean number of 17 flowers, with small differences among trees. The mean proportion of hermaphrodite flowers was 53·4 %, ranging from 31·4–75·7 % among trees. The probability of developing as hermaphrodite was 0·85 for apical flowers, 0·65 for flowers at position I and 0·31 for position II. Inflorescences with all inspected flowers hermaphrodite, or all staminate, were not uncommon (25 and 12·5 %, respectively).
Chi‐square tests reveal no significant relation between gender and time of anthesis for either first (P = 0·91, flowers in position I) or last (P = 0·82 and P = 0·43 for flowers in position I and II, respectively) blooming flowers. However, flower position within the panicle affected both time of anthesis and gender. Flowers originating in primary pedicels were always the first to reach anthesis (Table 1), and 65·8 % of these were hermaphrodite. The last flower to open originated at secondary pedicels in 79 % of the cases and in position I for 20 % of cases, most commonly the flowers arising immediately basal to the apical flower. In only 1 % of panicles was the apical flower the last to open. The last flower to open was hermaphrodite in 40·9 % of the cases (Table 1). As a result of the differential distribution of male and hermaphrodite flowers within the panicle, male flowers tended to bloom later than neighbouring hermaphrodite flowers. However, with only one exception, all flowers in a given inflorescence reached anthesis within a 2‐d period. Mean daily temperature during the sampling period ranged between 15·1 and 23·7 °C.
Table 1.
Relationship among flower positions in the panicle and phenology
| First to open | Last to open | |
| Apical | 0 | 1 (0, 1) |
| Position I | 76 (50, 26) | 16 (11, 5) |
| Position II | 0 | 59 (15, 44) |
Values in parentheses indicate the gender of the flowers (hermaphrodite, staminate). Number of inflorescences sampled = 76 (20 for each of four trees, four missing).
Resource allocation
Mean dry weight of hermaphrodite flowers borne in position I was 19 % greater than staminate flowers arising in opposite positions within a panicle, and 41 % greater than staminate flowers borne in secondary pedicels. This difference was primarily due to pistil dry weight, although significant differences were also evident in corolla dry weight (Table 2). Small, non‐significant differences occurred for stamen dry weight. Staminate flowers developing in secondary pedicels were the lightest, although dry weight of the stamens did not vary with staminate flowers in position I or with hermaphrodite flowers. Pistil weight was highly variable even within each flower type, while stamen dry weight barely changed. Flower fresh and dry weights were linearly correlated (r2 = 0·93).
Table 2.
Dry weight of floral parts in hermaphrodite and staminate flowers borne in primary and secondary pedicels of the olive panicle
| Stamens (mg) | Pistil (mg) | Petals (mg) | Total (mg) | |
| Hermaphrodite position I | 1·343a | 0·533a | 1·709a | 3·585 |
| Staminate position I | 1·322a | 0·194b | 1·498b | 3·014 |
| Staminate position II | 1·277a | 0·097c | 1·162c | 2·536 |
Different letters within a column indicate significant differences at P = 0·05, Tukey’s test.
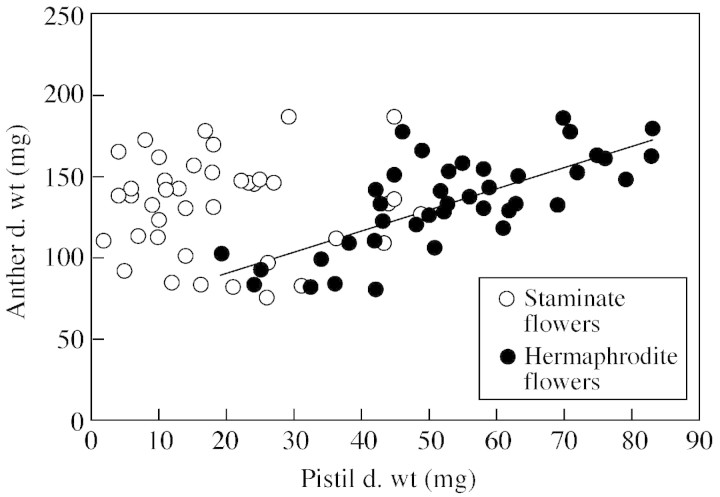
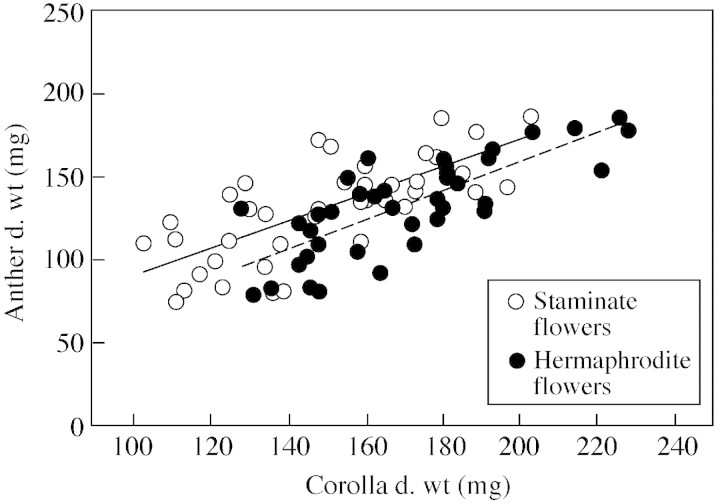
A positive linear relation (P < 0·001) was detected in hermaphrodite flowers between stamen and pistil dry weight, but not for staminate flowers (P = 0·95; Fig. 2). Stamen and petal biomass were linearly related (P < 0·001) in both flower types (Fig. 3). Pistil and petal dry weight were significantly correlated (P < 0·001) in hermaphrodite flowers.
Fig. 2. Dry weights of anthers (ADW) and pistils (PDW) of staminate and hermaphrodite flowers of olive ‘Mission’. For hermaphrodite flowers ADW = 1·317PDW + 64·159, r2 = 0·55, P < 0·001.
Fig. 3. Dry weights of anthers (ADW) and petals (CDW) of staminate and hermaphrodite flowers of olive ‘Mission’. For staminate flowers ADW = 0·805CDW + 11·520, r2 = 0·53, P < 0·001. For hermaphrodite flowers ADW = 0·862CDW – 12·968, r2 = 0·59, P < 0·001.
Pollen performance
No significant differences were found in any of the tests conducted for pollen performance either in vitro or in vivo (Table 3). Mean pollen grain number per flower fluctuated between 91 967 ± 4178 in staminate flowers and 96 194 ± 4405 in hermaphrodite flowers. In preliminary experiments the previous year following the same procedure with a smaller sample, we calculated 137 000 ± 3110 pollen grains in staminate flowers versus 139 800 ± 11 840 in hermaphrodite flowers. No interaction was detected between trees and flower type. Since olive flowers contain four ovules (although only one becomes a seed), the pollen/ovule ratio is around 24 000 : 1. Mean equatorial diameter of the pollen grain ranged between 28·69 ± 0·19 µm in staminate flowers and 29·08 ± 0·23 µm in hermaphrodite flowers (Table 3).
Table 3.
Pollen performance in hermaphrodite and staminate flowers of Olea europaea, ‘Mission’
| Floral type | |||
| Parameter | Staminate | Hermaphrodite | P |
| Pollen grains per flower | 91 967 ± 4178 | 96 194 ± 4405 | 0·72 |
| Pollen diameter (µm) | 28·69 ± 0·19 | 29·08 ± 0·23 | 0·18 |
| Pollen viability (%) | 83·4 ± 1·0 | 82·1 ± 1·8 | 0·54 |
| Pollen germination (%) | 74·8 ± 2·2 | 75·4 ± 2·7 | 0·77 |
| Fertilization at 5 d (%) | 30·0 | 25·0 | 0·72 |
| Fertilization at 10 d (%) | 35·0 | 35·0 | 1 |
Mean values ± s.e.
When needed, percentages were arcsine transformed prior to analyses.
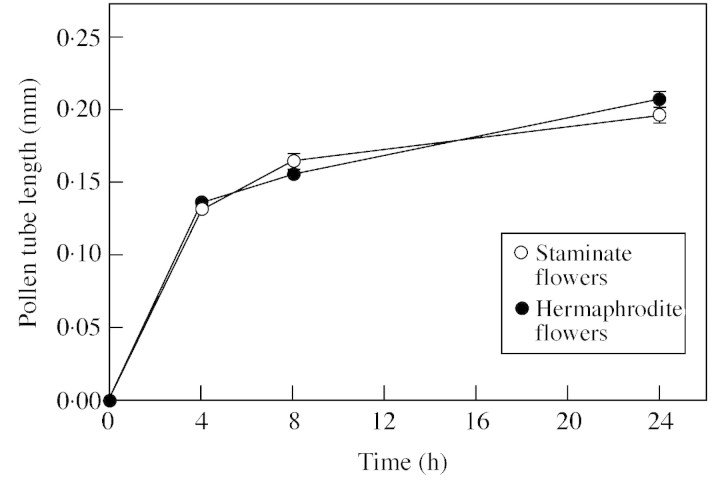
Pollen viability and germination were similar in both flower types. Viability of pollen as determined by FDA test averaged 83·4 ± 1·0 % for staminate flowers and 82·1 ± 1·8 % for hermaphrodite flowers, whereas pollen germination in vitro averaged 74·8 ± 2·2 % in staminate flowers and 75·4 ± 2·7 % in hermaphrodite flowers. Pollen tube growth rate in vitro was similar for both flower types and declined with time (Fig. 4). No significant differences were found for any of the incubation periods (P = 0·38, P = 0·13 and P = 0·15 for 4, 8 and 24 h, respectively). No effect of the pollen origin could be detected in either the timing or the rate of fertilization on ‘Sevillano’ flowers (Table 3). Similar results (data not shown) were also obtained the previous year in preliminary experiments using ‘Manzanillo’ as the pollen recipient.
Fig. 4. Pollen tube length in vitro after 4, 8 and 24 h. Data points represent means of 200 pollen tubes. There are no significant differences for any incubation period.
DISCUSSION
Theory interprets andromonoecy as a strategy to maximize fitness by optimal allocation of reproductive resources to male and female functions (Bertin, 1982). When the optimum number of staminate flowers is greater than that of pistils, andromonoecious plants may enhance fitness by increasing the number of staminate flowers without increasing resource expenditure in pistil development (Lloyd, 1979; Primack and Lloyd, 1980; Stephenson and Bertin, 1983). In olive, fitness can be enhanced through the male function of staminate flowers, which weigh 19 % less than hermaphrodite flowers in comparable positions of the panicle of ‘Mission’, and 41 % less when borne in secondary pedicels. Some savings may also result from avoiding expenditure in early development of surplus fruits (Rallo and Fernández‐Escobar, 1985; Cuevas et al., 1995). Heavier hermaphrodite flowers in andromonoecious plants are, therefore, the rule. A notable exception recently reported in Sagittaria guyanensis has been interpreted as an unusual reversion to andromonoecy from the widespread monoecy in the genus (Huang, 2003).
Labile gender expression has been proposed by Bertin (1982) as an advantage of andromonoecy to cope with unpredictable resource availability; therefore, sex ratio in andromonoecious plants is commonly variable among individuals and years, reflecting environmental and nutritional changes (Primack and Lloyd, 1980; Solomon, 1985; Diggle, 1993). This phenomenon is well characterized for olive cultivars. The proportion of roughly 1 : 1 staminate : hermaphrodite flowers found in this study fits in the range of 38–68 % found by Brooks (1948) for the same cultivar during three consecutive years in several locations in California. Plasticity in sex expression in andromonoecious plants is due to changes in the proportion of flowers developing a functional pistil. Such decisions take place independently within each floral primordium at various stages during flower development (Emms, 1993; Diggle, 1994; Ito and Kikuzawa, 2000). We have previously shown that pistil abortion in olive may occur throughout the period of pistil differentiation (Cuevas et al., 1999). In a few cases, pistil size in staminate flowers is very similar to the size achieved in hermaphrodite flowers, but the pistil is not functional because the stigma papillae are collapsed (Cuevas et al., 1999). Different pistil sizes among staminate flowers, a commonly documented phenomenon in andromonoecious plants (Solomon, 1985; May and Spears, 1988; Diggle 1994; Ito and Kikuzawa, 2000), might then be interpreted as the results of early and late pistil abortion.
All flowers in andromonoecious plants are initiated as bisexual, although spatial patterns within inflorescences and plants are often reported (Primack and Lloyd, 1980; Solomon, 1986; Emms, 1993; Diggle, 1994; Spalik and Woodell 1994). Highlighting a dependence of pistil abortion on resource availability, we too found that hermaphrodite flowers tend to develop at the apex and primary pedicel of the panicle, whereas male flowers have a tendency to arise in the more poorly nurtured secondary pedicels where fruit set is unlikely. However, panicles occur with all flowers male or hermaphrodite, which implies that the fate of a given floral meristem in ‘Mission’ is not fixed and gender cannot be definitively assigned to any particular position of the inflorescence.
Although staminate flowers develop in less‐favoured positions, the results indicate that their function is not negatively affected by the factors that lead to pistil abortion. The results also show, however, that pollen grains from staminate flowers do not benefit by reallocating resources saved by pistil abortion. Since resources for sexual reproduction are finite, it is sometimes assumed on theoretical grounds that an increase in resources allocated to male function is realized at the expense of resources allocated to female function and vice versa (Charnov, 1982; Sutherland and Delph, 1984). Such a trade‐off between male and female organs does not occur within olive flowers (Table 2). Higher resource allocation to pistils occurs in hermaphrodite flowers, but stamen dry weight does not increase in staminate flowers in response to reduced allocations to pistils. On the contrary, the positive correlation among petal, stamen and pistil dry weight found in hermaphrodite flowers (Figs 2 and 3) suggests that conditions favouring pistil development also positively affect stamen and petal resource investment. Patterns of resource allocation to floral organs in other andromonoecious taxa also indicate that plants do not reallocate resources from pistil to stamens in staminate flowers (Lovett Doust and Harper 1980; Emms, 1993).
A plausible explanation for the observed lack of effect of pistil abortion on male function may be the differential developmental timing between stamens and pistils in olive flowers. Most pistil abortion occurs at 4 weeks before bloom, by which time stamen development is well advanced (Uriu, 1953; Cuevas et al., 1999). Thus, we might expect pistil abortion to have little influence on the male component of flowers. Flower number is determined well before the period of pistil abortion. Therefore, saved resources cannot be reallocated to produce additional staminate flowers. The same situation applies to other andromonoecious taxa with synchronous blooming (Spalik and Woodell, 1994).
In accordance with equal investment in male organs, pollen produced by staminate and hermaphrodite olive flowers does not differ in amount, size or quality (Table 2 and Fig. 4). Similar results were found for Solanum carolinense, in which neither in vitro pollen germination, pollen number, pollen mass, nor the number and mass of the seeds fathered differed between pollen from male versus hermaphrodite flowers (Solomon, 1985; 1986). Likewise, pollen germination and pollen size do not differ between functionally staminate and hermaphrodite flowers of other Solanum species (Baksh and Iqbal, 1978; Baksh et al., 1978). In contrast, long‐styled flowers of Solanum marginatum have higher pollen grain number than short‐styled (functionally male) flowers, but the same pollen viability (Dulberger et al., 1981). Emms (1993) found slightly higher pollen viability in male flowers of Zigadenus paniculatus, although the author attributed little biological significance to this small difference.
Our results therefore indicate that staminate flowers are not a non‐functional by‐product, but that they play a key role in the breeding system of olive. The consensus theory is that in an andromonoecious species, staminate flower production would be advantageous if fitness enhancement compensates for the cost of production. Staminate flowers may enhance plant fitness in at least two ways: they can augment the attractiveness of the plant to pollinators, thus ensuring that its flowers receive enough pollen, and they can enhance pollen donation to fertilize other flowers. Since biotic pollination vectors do not play a significant role in olive (Morettini, 1972; Griggs et al., 1975), only enhancement of pollen donation seems applicable. It should be noted, however, that most genera of Oleaceae are entomophilous, and the acquisition of anemophily is thought to be a recent evolutionary event in the family (Proctor and Yeo, 1972). Therefore, attraction to pollinators functioning as a relict character for the staminate flowers cannot be completely discarded.
Nevertheless, pollen donation does seem to be the primary role for olive staminate flowers. A plant can increase pollen donation by affecting the phenology of the male phase, or by increasing the total number of pollen grains per plant. Stephenson and Bertin (1983) indicate that staminate flower phenology may affect pollen donation success not only by extending the overall length of the male phase, but also by earlier flowering of the staminate flowers. Hermaphrodite olive flowers tend to bloom earlier (Brooks, 1948; this study), although our results demonstrate that early blooming is not inherent to hermaphrodite flowers, but is associated with their better‐nurtured positions within the panicle. Later blooming of staminate flowers has often been reported in other andromonoecious taxa (Primack and Lloyd, 1980; Emms, 1993; Diggle, 1994). Thus, the most obvious advantage provided by staminate flower production in olive is the increase in the number of pollen grains available to achieve fertilization. This would be particularly important under anemophily and, as pointed out by Stephenson and Bertin (1983), in species experiencing intense male competition in which the likelihood of paternity is influenced by the quantity of pollen produced.
In theory, olive could increase pollen output by producing more pollen grains per hermaphrodite flower or by producing staminate flowers serving solely the male function. Conditions favouring andromonoecy versus hermaphroditism have been extensively discussed by Bertin (1982) and modelled by Spalik (1991). Many of the required conditions (savings in staminate flowers, large fruits and very large number of flowers, resource limitation in female function and temporal decline in fruit set), but not all (cost of petals is high even in staminate flowers) are met in olive. Emms (1993) emphasizes the spatial and temporal components of andromonoecy in Zigadenus paniculatus, an insect‐pollinated lily. However, both spatial and temporal consequences might differ for animal and wind‐pollinated plants. In olive, staminate flowers are produced once optimum resource investment in female function is reached. Fine tuning by the maternal plant is suggested by the occurrence of pistil abortion at various stages of flower development (Cuevas et al., 1999). Thus, staminate flowers are developed in more poorly nurtured positions of the panicle where fruit set is unlikely, but male function is unaffected. Such placement in olive has minor temporal consequences, such as a slight delay in bloom. A male bias in late blooming flowers of andromonoecious plants is predicted to cope with more intense male competition at the end of the bloom period (Brunet and Charlesworth, 1995).
In summary, the similar pollen quantity and quality of staminate and hermaphrodite flowers is consistent with andromonoecy being an evolutionarily stable strategy in olive, and an increase in pollen production at the plant level is proposed as the likely role for staminate flowers in olive. However, more phylogenetic work is needed to fully understand the evolutionary forces behind andromonoecy in Olea europaea.
ACKNOWLEDGEMENTS
This research was partly funded by project AGF98‐0802‐C02‐02. J.C. was supported by a grant from the Spanish Ministry of Education and Science. We thank K. Pinney for her valuable help in the field and laboratory.
Supplementary Material
Received: 13 October 2003; Returned for revision: 20 November 2003; Accepted: 9 January 2004 Published electronically: 22 March 2004
References
- BakshS, IqbalM.1978. Floral features of Solanum macranthum Dunn. with special reference to stylar heteromorphism and intercrossability. Flora 167: 423–431. [Google Scholar]
- BakshS, IqbalM, Jamal A.1978. Breeding system of Solanum integrifolium Poir. with an emphasis on sex potential and intercrossability. Euphytica 27: 811–815. [Google Scholar]
- BertinRI.1982. The evolution and maintenance of andromonoecy. Evolutionary Theory 6: 25–32. [Google Scholar]
- BrooksRM.1948. Seasonal incidence of perfect and staminate olive flowers. Proceedings of the American Society for Horticultural Science 52: 213–218. [Google Scholar]
- BrunetJ, Charlesworth D.1995. Floral sex allocation in sequentially blooming plants. Evolution 49: 70–79. [DOI] [PubMed] [Google Scholar]
- CharnovEL.1982.The theory of sexual allocation. Princeton, NJ: Princeton University Press. [Google Scholar]
- CuevasJ.1992.Incompatibilidad polen‐pistilo, procesos gaméticos y fructificación de cultivares de olivo (Olea europaea L.). PhD Thesis. University of Córdoba, Spain. [Google Scholar]
- CuevasJ, Pinney K, Polito VS.1999. Flower differentiation, pistil development and pistil abortion in olive (Olea europaea L.). Acta Horticulturae 474: 293–296. [Google Scholar]
- CuevasJ, Polito VS.1997. Compatibility relationships in ‘Manzanillo’ olive. Horticultural Science 32: 1056–1058. [Google Scholar]
- CuevasJ, Rallo L, Rapoport HF.1994. Staining procedure for the observation of olive pollen tube behavior. Acta Horticulturae 356: 264–267. [Google Scholar]
- CuevasJ, Rallo L, Rapoport HF.1995. Relationships among reproductive processes and fruitlets abscission in Arbequina olive. Advances in Horticultural Science 9: 92–96. [Google Scholar]
- DigglePK.1993. Developmental plasticity, genetic variation, and the evolution of andromonoecy in Solanum hirtum (Solanaceae). American Journal of Botany 80: 967–973. [Google Scholar]
- DigglePK.1994. The expression of andromonoecy in Solanum hirtum (Solanaceae): phenotypic plasticity and ontogenic contingency. American Journal of Botany 81: 1354–1365. [Google Scholar]
- DulbergerR, Levy A, Palevitch D.1981. Andromonoecy in Solanum marginatum Botanical Gazette 142: 259–266. [Google Scholar]
- EmmsSK.1993. Andromonoecy in Zigadenus paniculatus (Liliaceae): spatial and temporal patterns of sex allocation. American Journal of Botany 80: 914–923. [Google Scholar]
- Fernández‐EscobarR, Benlloch M, Navarro C, Martin GC.1992. The time of floral induction in the olive. Journal of the American Society for Horticultural Science 117: 304–307. [Google Scholar]
- GriggsWH, Hartmann HT, Bradley MV, Iwakiri BT, Whisler JE.1975. Olive pollination in California. California Agricultural Experimental Station Bulletin 869. [Google Scholar]
- HartmannHT.1951. Time of floral differentiation of the olive in California. Botanical Gazette 112: 323–327. [Google Scholar]
- HerreraCM.1982. Breeding systems and dispersal‐related maternal reproductive effort of Southern Spanish bird‐dispersed plants. Evolution 36: 1299–1314. [DOI] [PubMed] [Google Scholar]
- Heslop‐HarrisonJ, Heslop‐Harrison Y.1970. Evaluation of pollen viability by enzymatically induced fluorescence: intracellular hydrolysis of fluorescein diacetate. Stain Technology 45: 115. [DOI] [PubMed] [Google Scholar]
- HuangS‐Q.2003. Flower dimorphism and the maintenance of andromonoecy in Sagittaria guyanensis ssp. lapula (Alismataceae). New Phytologist 157: 357–364. [DOI] [PubMed] [Google Scholar]
- ItoE, Kikuzawa K.2000. Differentiation of the timing of flower abortion in Tilia japonica Plant Species Biology 15: 179–186. [Google Scholar]
- LloydDG.1979. Parental strategies of angiosperms. New Zealand Journal of Botany 17: 595–606. [Google Scholar]
- Lovett DoustJ, Harper JL.1980. The resource costs of gender and maternal support in an andromonoecious umbellifer, Smyrnium olusatrum L. New Phytologyst 85: 251–264. [Google Scholar]
- MartinFW.1959. Staining and observing pollen tubes in the style by means of fluorescence. Stain Technology 34: 125–128. [DOI] [PubMed] [Google Scholar]
- MartinGC.1990. Olive flower and fruit population dynamics. Acta Horticulturae 286: 141–153. [Google Scholar]
- MayPG, Spears EE.1988. Andromonoecy and variation in phenotypic gender of Passiflora incarnata (Passifloraceae). American Journal of Botany 75: 1830–1841. [Google Scholar]
- MorettiniA.1972.Olivicoltura. Rome: REDA. [Google Scholar]
- PinneyK, Polito VS.1990. Olive pollen storage and in vitro germination. Acta Horticulturae 286: 207–210. [Google Scholar]
- PrimackRB, Lloyd DG.1980. Andromonoecy in the New Zealand montane shrub manuka, Leptospermun scoparium (Myrtaceae). American Journal of Botany 67: 361–368. [Google Scholar]
- ProctorM, Yeo P.1972.The pollination of flowers. New York: Taplinger. [Google Scholar]
- RalloL, Fernández‐Escobar R.1985. Influence of cultivar and flower thinning within the inflorescence on competition among olive fruits. Journal of the American Society for Horticultural Science 110: 303–308. [Google Scholar]
- SolomonBP.1985. Environmentally influenced changes in sex expression in an andromonoecious plant. Ecology 66: 1321–1332. [Google Scholar]
- SolomonBP.1986. Sexual allocation and andromonoecy: resource investment in male and hermaphrodite flowers of Solanum carolinense (Solanaceae). American Journal of Botany 73: 1215–1221. [Google Scholar]
- SpalikK.1991. On evolution of andromonoecy and ‘overproduction’ of flowers: a resource allocation model. Biological Journal of the Linnean Society 42: 325–336. [Google Scholar]
- SpalikK, Woodell SRJ.1994. Regulation of pollen production in Anthriscus sylvestris, an andromonoecious species. International Journal of Plant Science 155: 750–754. [Google Scholar]
- StephensonAG, Bertin, RI.1983. Male competition, female choice and sexual selection in plants. In: Real L, ed. Pollination Biology London: Academic Press, 109–149. [Google Scholar]
- SutherlandS, Delph LF.1984. On the importance of male fitness in plants: patterns of fruit set. Ecology 65: 1093–1104. [Google Scholar]
- UriuK.1953.Pistil abortion in the olive (Olea europaea L.) as influenced by certain physiological conditions. PhD Thesis, University of California, USA. [Google Scholar]
- ZoharyD, Hopf M.1993.Domestication of plants in the Old World. Oxford: Clarendon Press. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.