Abstract
• Background and Aims The interaction between the gall‐forming grapevine parasite, phylloxera, and the susceptible grapevine species Vitis vinifera was investigated.
• Methods Phylloxera and grapevines were cocultivated using both potted and micropropagated grapevines. Development of nodosities on primary roots was studied by microscopy and histochemistry, and nodosities were analysed for biochemical changes and changes in gene expression.
• Key Results Within a nodosity, phylloxera fed at a site in the root cortex. Nodosity development was characterized by swelling of the root tissue distal to the feeding site, lack of development of a suberized endodermis, and starch and amino acid accumulation, and was eventually followed by root necrosis. No evidence of a defence response was observed in pre‐necrotic nodosities, but defence‐type responses were observed in tissue adjacent to necrotic regions. Changes in gene expression were not detected by northern hybridization using DNA probes encoding a range of V. vinifera transcripts.
• Conclusions Nodosities on V. vinifera potentially function as nutrient reservoirs, and defence responses to phylloxera attack were not detected.
Key words: Daktulosphaira vitifoliae, phylloxera, Vitis vinifera, grapevine, nodosity, gall formation
INTRODUCTION
Grape phylloxera (Daktulosphaira vitifoliae Fitch) is an aphid‐like gall‐forming parasite of grapevines (Vitis spp.). It forms pouch‐like leaf galls, and/or superficial root galls on its natural hosts, American Vitis species, and forms damaging root galls on the European grape Vitis vinifera L. The fleshy galls formed on primary roots by swelling of the root cortex are called ‘nodosities’. Galls that form on the roots of V. vinifera are metabolically active organs suited to the nutritional requirements of phylloxera, an obligate biotroph on this genus, and can support populations with high reproductive rates. Phylloxera infestation is capable of destroying the root system of V. vinifera vines (Granett et al., 2001).
A few studies of the interaction between phylloxera and grapevine roots have described anatomical changes in the root during formation of nodosities (Niklowitz, 1954; Hofmann, 1957; Forneck et al., 2002) and investigated the biochemical (phenolic acids, sugars) composition of nodosities (Denisova, 1965; Sobetskiy and Derzhavina, 1973). In particular, the detailed anatomical study of nodosity formation by Niklowitz (1954) provides an excellent background to current observations of the interaction.
New technologies offer the potential to obtain more detailed knowledge of the interaction between phylloxera and the grapevine. The present study investigates the interaction between a susceptible grapevine, V. vinifera ‘Shiraz’, and two isolates of phylloxera, VWL‐1 and SRU‐1 (Corrie et al., 1997). These isolates are genetically distinct, but the interaction of both with V. vinifera may be considered ‘compatible’ (i.e. phylloxera are able to form galls and reproduce on the grapevine roots; Kellow et al., 2002). Microscopic and biochemical methods were selected to investigate the role of nodosities as nutrient sinks, and the defence response, if any, of V. vinifera roots to phylloxera attack. Northern blot hybridization, using a range of cDNA probes, was used for preliminary screening of gene expression patterns in uninfested roots compared with nodosities.
MATERIALS AND METHODS
Co‐cultivation of phylloxera with grapevines
Phylloxera (Daktulosphaira vitifoliae) isolate VWL‐1 was collected from own‐rooted V. vinifera. ‘Cabernet Sauvignon’ vines at Brown Brother’s Whitlands vineyard in the King Valley, Victoria, then maintained on excised roots of V. vinifera using methods described by Granett et al. (1985). SRU‐1 phylloxera were collected from leaf galls on ‘Schwarzmann’ (V. riparia × V. rupestris) vines at Campbell’s vineyard, Rutherglen, Victoria, and applied directly to experimental (V. vinifera) grapevines. These two strains of phylloxera were selected as they were readily available and had both been genetically characterized (Corrie et al., 1997).
Potted grapevines (V. vinifera ‘Shiraz’ accession 12/BVRC‐12‐C12A obtained from the Riverland Vine Improvement Association) were propagated from rootlings in a steam‐sterilized potting mix of 2 parts peat: 3 parts coarse sand: 4 parts perlite, in 17·5 cm diameter pots and inoculated with phylloxera infested root pieces (2 × 5 cm) or leaf material (5–10 leaf galls). Infested vines were maintained in a shadehouse. To keep the potting mix temperature at approximately 25 °C, pots were placed in pits filled with moist sand, or were wrapped in aluminium foil to minimize radiant heat from the sun. (Potting mix temperature in ‘untreated’ pots reached temperatures of 32–37 °C during the day.) Vines were watered by drip irrigation, and sprayed on alternate fortnights to control fungal disease and some insect pests with propiconazole (0·01 % v/v) and B.t. toxin (0·1 % w/v) suspension, respectively. Two‐spotted mites were controlled with predatory mites. Uninoculated vines were propagated and maintained under similar conditions in a separate phylloxera‐free shadehouse. Nodosities were harvested 6 weeks following inoculation.
All co‐cultivations were carried out in the summer, within the phylloxera quarantine district of Rutherglen, Victoria, Australia and root material was disinfested by freezing or chemical fixation, before transport to The University of Adelaide, Glen Osmond, South Australia for analysis.
Microscopy
Uninfested primary roots and nodosities formed at root tips by either VWL‐1 or SRU‐1 phylloxera isolates were harvested and most were cut into 1 cm lengths, fixed in FAA (5 % formaldehyde, 5 % propionic acid, 90 % ethanol) for at least 48 h, and dehydrated through an alcohol series (ethanol 2 h, propanol 2 h, butanol 2 h). The hydrated material was embedded in glycol methacrylate [GMA; 93 % 2‐hydroxyethyl methacrylate, 7 % polyethylene glycol 400, 0·6 % (w/v) benzoyl peroxide] as follows: 1 part butanol: 1 part GMA for 2 h, GMA for 48 h, fresh GMA for 48 h, then polymerized in fresh GMA in sealed gelatine capsules in an oven at 60 °C for 48 h. Sections 4 µm thick were cut on a Reichart‐Jung Supercut microtome fitted with glass knives. Some nodosities with isolate SRU‐1 were cut into 2‐mm lengths, fixed for 48 h in 1·25 % glutaraldehyde, 4 % paraformaldehyde, 4 % sucrose, pH 7·2, then washed twice for 30 min in phosphate buffered saline (PBS) with 4 % sucrose. These samples were post‐fixed in 1 % (w/v) OsO4 in PBS for 2 h, then dehydrated through an alcohol series (70 % ethanol 3 × 20 min, 90 % ethanol 3 × 20 min, 95 % ethanol 3 × 20 min, 100 % ethanol 3 × 20 min, 100 % ethanol 1 × 60 min). The dehydrated material was embedded in Procure araldite‐epoxy resin as follows: 1 part ethanol: 1 part resin for 8 h, 100 % resin 3 × 8 h, then polymerized in fresh araldite‐epoxy resin in a vacuum oven at 70 °C. Sections 0·5 µm thick were cut on a Reichart‐Jung Ultracut microtome fitted with a glass knife.
Staining of sections embedded in GMA with periodic acid/Schiff’s reagent (BDH, Poole, UK) with a Toluidine Blue O counterstain (PAS/TBO) was according to O’Brien and McCully (1981). The presence of starch was confirmed by PAS staining with no TBO counterstain. For staining with TBO alone, GMA sections were stained in 0·05 % (w/v) TBO pH 4·5, while araldite‐epoxy resin sections were stained in 0·5 % (w/v) TBO pH 11·1. Sudan Black staining of GMA sections was according to Bronner (1975). Stained sections were mounted in Surgipath mounting medium. For examination of autofluorescence, unstained GMA sections were mounted in either 70 % (v/v) glycerol or Surgipath mounting medium and observed under UV excitation (365 nm excitation, 400 nm barrier filters). All photographs were taken using an Olympus BH‐2 compound photomicroscope with a BH2‐RFCA excitation cube for fluorescence microscopy.
For the study of nodosity development, nodosities with isolate VWL‐1 were sorted into four categories according to the developmental stages (instars) of the phylloxera present. Stages two and four (Fig. 1) were selected as being representative of an immature (with first and second instar phylloxera) and a mature nodosity (with adult phylloxera), respectively. Sections were cut from at least three nodosities of each stage. For the histochemical studies, at least three uninfested roots were sectioned 0·5–1·0 cm behind the root tip. Thus the distance from the root tip was the same for sections of uninfested roots and nodosities. At least ten sections were observed for each replicate and each histological method.
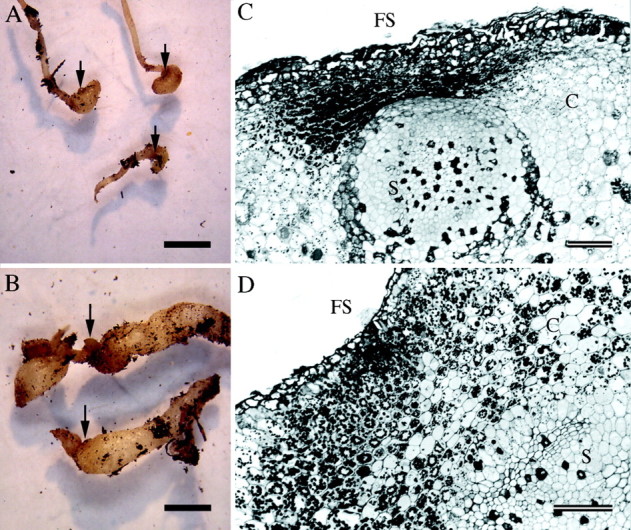
Fig. 1. Development and anatomy of nodosities induced by isolate VWL‐1. (A, B) Stage two and stage four nodosities, respectively. Scale bars = 2 mm. (C, D) Transverse sections though stage two and stage four nodosities, respectively, stained with PAS/TBO. Amyloplasts are evident in the cortex (C) between the feeding site (FS) and stele (S). Scale bars = 100 µm.
Starch content of roots and nodosities
Root tips harvested from uninfested vines, and nodosities harvested from vines infested with phylloxera isolate VWL‐1, were quickly immersed in liquid nitrogen. Uninfested root tips and nodosities at developmental stages two and four were selected from pooled samples for determination of starch content using an enzymatic assay modified from Burton et al. (2002). Frozen root tissue (100 mg), ground to a fine powder in a mortar and pestle, was mixed with 1·5 mL 80 % ethanol, incubated on ice for 20 min and then centrifuged at 3000 g for 10 min, and the supernatant discarded. The remaining pellet was washed twice by resuspending in 2 mL 80 % ethanol and centrifuging as above. After resuspending the pellet in 2 mL H2O, the total volume of the suspension was measured, then duplicate 0·5 mL aliquots were placed in screw‐capped eppendorf tubes and the starch solubilized by autoclaving at 121 °C for 15 min. To each tube the following were added: 0·5 mL 100 mM sodium acetate pH 5·2, 2 µL α‐amylase (18 000 U mL–1, Boehringer, Mannheim, Germany), 8 µL amyloglucosidase (at 14 U mL–1, Sigma, St Louis, MO, USA), and incubated at 37 °C for 4 h yielding ‘digested starch’. The resultant glucose concentration was determined spectrophotometrically by mixing 25 µL ‘digested starch’ in a 1 mL cuvette with the following: 925 µL buffer mix (100 mM Bicine pH 7·7, 5 mM MgCl2, 0·5 mM NAD), 50 µL 20 mM ATP, 2·5 mL hexokinase (200 U mL–1, Boehringer, Mannheim, Germany). The optical density (OD) was measured at 340 nm and then 2·5 µL glucose 6‐phosphate dehydrogenase (1000 U mL–1, Sigma St Louis, MO, USA) was added to the cuvette. After mixing, the OD was monitored at 340 nm until stable. The concentration of glucose (µmol per 25 µL ‘digested starch’) was calculated as ΔOD340 × 6·22–1 (extinction coefficient for reduction of NAD+ to NADH 6·22 mM–1 cm–1). The concentration of starch in the initial samples was then calculated on the basis of 1 µmol glucose being released from 162 µg starch, with appropriate dilution factors taken into account.
Free amino acid and amide content of roots and nodosities
Root tips and stage four nodosities from vines infested with phylloxera isolate VWL‐1 were harvested in the same manner as for starch assays, with each tissue type sampled in triplicate, and the means presented. Frozen root tissue (0·5 g) was ground to a fine powder in a mortar and pestle and placed in a 2 mL eppendorf tube with the following added: 600 µL methanol, 250 µL chloroform, 150 µL de‐ionized water, 100 µL 1 % (w/v) homoarginine hydrochloride in water (internal standard). The tubes were mixed for 20 min, then centrifuged at 13 000 g at 4°C for 5 min and the aqueous phase transferred to a fresh tube. From this, a 100 µL aliquot was transferred to a fresh tube and diluted 1 : 5 in 0·0131 % (w/v) carboxymethyl cysteine (internal standard) in borate buffer (pH 8·5), then centrifuged at 13 000 g for 30 min. The final supernatant was analysed by reversed phase HPLC at the Australian Wine Research Institute (AWRI), Urrbrae, Australia as described in Stines et al. (2000).
Northern blot analysis of gene expression
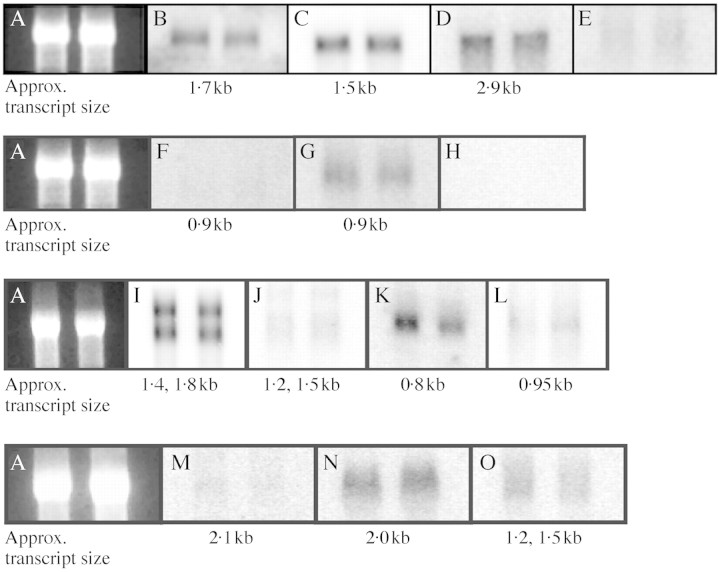
Northern blot analyses were carried out using total RNA extracted from uninfested root tips or nodosities from vines infested with phylloxera isolate VWL‐1. Root tips or nodosities were harvested and immediately frozen in liquid nitrogen. Total RNA was extracted using methods described by Davies and Robinson (1996). RNA was separated in denaturing formaldehyde–agarose gels and blotted onto nylon membranes (Hybond N, Amersham) using standard protocols (Ausubel et al., 1999). Northern hybridization was carried out as follows: membranes were prehybridized in 5–10 mL 5 × SSC, 0·5 % (v/v) SDS, 5× Denhardts reagent, 100 µg mg–1 freshly denatured, sheared salmon testes DNA (Sigma) for 1 h at 65 °C. cDNA probes labelled with α‐32P dCTP (Geneworks, Adelaide, Australia) using a Megaprime labelling kit (Amersham) were added directly to the prehybridization solution and hybridized at 65 °C overnight. Hybridized blots were washed twice in 2× SSC, once in 1× SSC then once in 0·1× SSC as described in Tattersall et al. (1997). Hybridized blots were exposed and viewed using a Molecular Dynamics phosphorimager, and images converted to TIFF files for preparation of figures. The cDNA clones used as probes are listed in Table 1. In all cases the cDNA sequences were extracted from their plasmid vectors and purified by agarose gel electrophoresis prior to labelling.
Table 1.
cDNA clones used as probes in northern blot hybridizations
| cDNA | Clone/Genbank accession no. | Putative function or closest homologue in databases; previous reports of expression pattern in grapevine roots (see references) | Source/reference |
| Stilbene synthase VvStSy | pSV696/X76892 | Catalyses synthesis of resveratrol and derivatives; induced in cell cultures by fungal elicitation; expression in grapevine roots unknown, but constitutive in grapevine seedlings | H. Kindl, University of Marburg, Germany. (Melchior and Kindl, 1991; Sparvoli et al., 1994) |
| Chalcone synthase VvCHS | pBS305/X75969 | First dedicated enzyme in flavonoid synthesis pathway; expression in grapevine roots unknown | F. Sparvoli, Universita degli Studi di Milano, Milan, Italy. (Sparvoli et al., 1994; Boss et al., 1996) |
| Phenylalanine ammonia‐lyase VvPAL | pBS204/X75967 | Key regulatory enzyme in phenylpropanoid pathway; upregulated in V. vinifera on fungal elicitation; expression in grapevine roots unknown, but constitutive in grapevine seedlings | F. Sparvoli, Universita degli Studi di Milano, Milan, Italy. (Sparvoli et al., 1994; Boss et al., 1996) |
| Polyphenol oxidase GPO1 | pID96/A27657 | Polyphenol oxidase from grape berries; expressed at high levels in young grapevine roots, but not mature tissues | I. Dry, CSIRO, Adelaide, Australia. (Dry and Robinson, 1994) |
| Thaumatin‐like protein VvTL1 | pTL3/AF003007 | Thaumatin‐like protein; not expressed in grapevine roots | D. Tattersall, University of Adelaide, Adelaide, Australia. (Tattersall et al., 1997) |
| Thaumatin‐like protein VvTL2 | Y10992 | Thaumatin‐like protein. Partial homology to osmotin‐like protein from V. vinifera; expression in roots unknown | I. Dry, CSIRO, Adelaide, Australia. (Jacobs et al., 1999) |
| PR‐4 protein VvPR4a | pWIN52/AF061329 | PR4‐like protein; not expressed in grapevine roots | D. Tattersall, University of Adelaide, Adelaide, Australia. (D. Tattersall, pers. comm.) |
| Grip 15 | AJ237984 | Partial homology to nodule cell wall protein from Maakia amurensis; not expressed in grapevine roots | C. Davies, CSIRO, Adelaide, Australia. (Davies and Robinson, 2000) |
| Grip 28 | AJ237985 | Similarity to pectin methylesterases; partial homology to protein from alfalfa nodules; not expressed in grapevine roots | C. Davies, CSIRO, Adelaide, Australia. (Davies and Robinson, 2000) |
| Grip 31 | AJ237986 | Partial homology to protein in young nodule pericycle and in older senescent nodule cells in Alnus glutinosa; expressed in grapevine roots at a low level | C. Davies, CSIRO, Adelaide, Australia. (Davies and Robinson, 2000) |
| Grip 68 | AJ237987 | Partial homology to Grip 31, homology to protein from potato stolon tip; expressed in grapevine root at a low level | C. Davies, CSIRO, Adelaide, Australia. (Davies and Robinson, 2000) |
| Grip 21 | AJ237988 | Related to proteins produced in glucose starvation response in maize roots; expressed in grapevine root at a low level | C. Davies, CSIRO, Adelaide, Australia. (Davies and Robinson, 2000) |
| Sucrose transporter 1/2 VvSuc12 | AF021809 | Sucrose transporter from grape berries; homology to maize sucrose transporter; expression in grapevine roots unknown | C. Davies, CSIRO, Adelaide, Australia. (Davies et al., 1999) |
| Sucrose transporter 2/7 VvSuc27 | AF021810 | Sucrose transporter from grape berries; homology to maize sucrose transporter; expression in grapevine roots unknown | C. Davies, CSIRO, Adelaide, Australia. (Davies et al., 1999) |
All clones are from Vitis spp.
RESULTS
Nodosity development and anatomy
Phylloxera‐induced nodosities developed immediately behind the root tip, in or near the zone of elongation. Examples representative of VWL‐1‐induced nodosities, stages two and four, including transverse sections, are shown in Fig. 1. All sections of stage two nodosities showed that the root tissues were differentiated with a recognizable primary root anatomy (Fig. 1C). Cells of the cortex and stele adjacent to the feeding site were flattened in a radial direction, while those distal to the feeding site were rounded. There was little evidence of hypertrophy of cortical cells distal to the feeding site, with root swelling in the cortex more likely to be caused by increased cell division. In sections of some stage two nodosities, the endodermis was poorly defined, indicating a partial failure to differentiate, while in sections of other stage two nodosities, the endodermis was clearly defined. More mature, stage four, nodosities (Fig. 1B, D) had clearly differentiated vascular tissues in the stele indicating ‘normal’ development of root tissue. Nodosities induced by isolate SRU‐1 showed similar morphology (Kellow, 2000). Nodosities eventually became necrotic, and while the cause was unknown, it was not due to fungal infection, as it occurred in aseptic tissue culture cocultivations.
Location of feeding sites
Transverse sections through a stage four (mature) SRU‐1‐induced nodosity in GMA illustrate how the stylet penetrated through the cortical cells and terminated in a single cell, from which the phylloxera was presumably feeding (Fig. 2A, B). Observations of sections of four nodosities indicated that the stylet tip was usually located four to five cell layers below the epidermis, and was never observed within the stele. It was not clear, at this level of resolution, whether the stylet tip actually penetrates the cytoplasm or the vacuole of the putative feeding site cell. Cells immediately surrounding those penetrated by the stylet appeared unaffected.
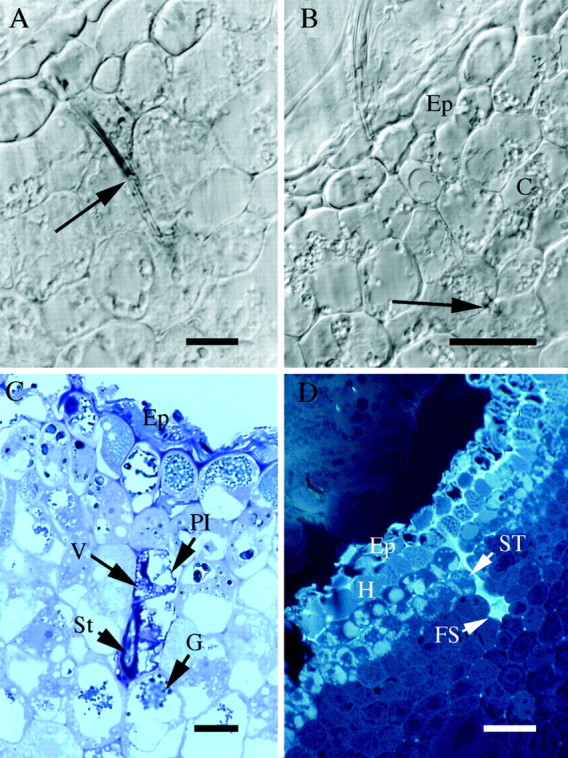
Fig. 2. Location of feeding sites in nodosities induced by isolate SRU‐1 (A–C) or VWL‐1 (D). (A) 4 µm transverse section in GMA of nodosity at point of stylet penetration (arrow), viewed with Nomarski differential interference contrast optics. Scale bar = 10 µm. (B) As for (A), adjacent section showing stylet tip (arrow). The stylet penetrates through the epidermis (Ep) and several cell layers into the cortex (C) of the root, but does not reach the stele. Scale bar = 20 µm. (C) 0·5 µm transverse section in epoxy resin, stained with TBO, showing epidermis (Ep); collapsed plasmalemma (Pl); vesicles (V); granular structures (G); section cuts obliquely through stylet (St). Scale bar = 10 µm. (D) 4 µm transverse section in GMA viewed under UV (365 nm) excitation, showing stylet track of feeding phylloxera. Epidermis (Ep); hypodermis (H); stylet track (ST); feeding site (FS). Scale bar = 50 µm.
TBO staining of sections in epoxy resin of stage four nodosities induced by isolate SRU‐1 showed that cells through which the stylet had passed were plasmolysed. Figure 2C shows a typical example of cells where the plasmalemma has collapsed inward from the cell wall and the cytoplasm is dark and irregular with vesicles apparent. In another section from the same nodosity as shown in Fig. 2C, a stylet‐penetrated cell one to two layers deeper in the cortex appeared to have even less cytoplasm remaining (Kellow, 2000). In all sections examined, most of the cells surrounding those penetrated by the stylet appeared unaffected, containing lightly stained cytoplasm of more regular appearance, and one or more vacuoles, some of which are heavily stained. The exceptions were those cells towards which the stylet was directed, which also contained some granular structures.
Stylet tracks (the remainder of stylet sheaths left behind when the aphid withdraws its stylet; Miles, 1999) were viewed by autofluorescence under UV excitation. In V. vinifera stage four nodosities induced by both phylloxera isolates, only a single, unbranched stylet track was visible at any one feeding site and no other evidence of multiple penetration points formed by a single insect was observed (e.g. Fig. 2D).
Histochemistry of root responses to phylloxera feeding
Sudan Black staining demonstrated the presence of suberin lamellae in all walls of endodermal cells in uninfested roots (Fig. 3A) but, in three out of three nodosities sectioned, no suberin was detected in the endodermis (Fig. 3B). Suberization of epidermal or cortical cells, which could indicate the activation of a defence response (Boubals, 1966; King and Rilling, 1991), was never observed at active feeding sites. Similarly, no autofluorescent compounds were detected in pre‐necrotic (healthy) nodosities other than those already present in uninfested roots (Fig. 2D). Not even very localized accumulation of autofluorescent compounds was observed at the feeding site.
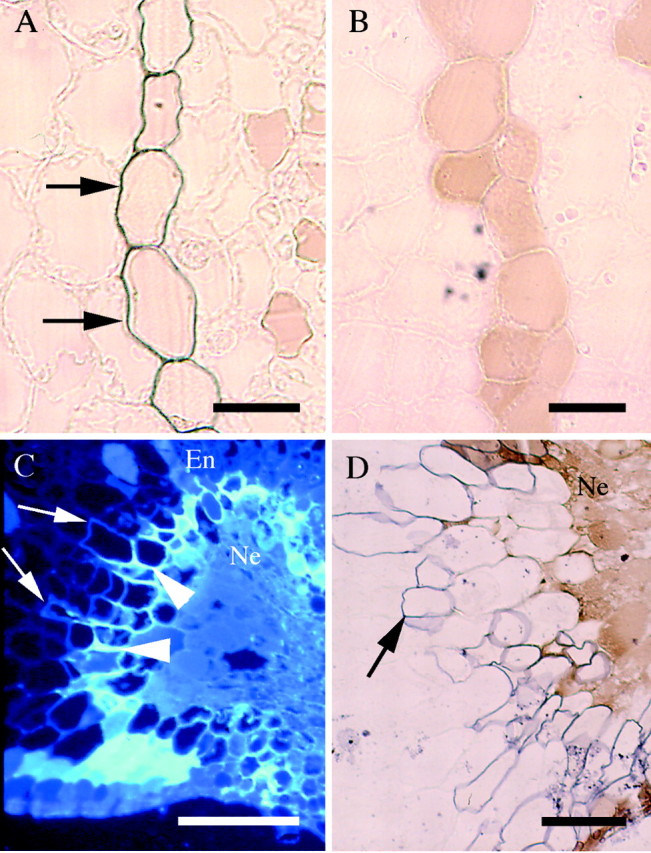
Fig. 3. Histochemistry of root response to phylloxera feeding. Shown are transverse sections stained with Sudan Black (A, B, D), or observed under UV (365 nm) excitation (C). (A) An uninfested root endodermis with ‘state two’ suberization (arrows). Scale bar = 20 µm. (B) A nodosity induced by isolate VWL‐1 where the endodermis has no detectable suberization. Scale bar = 20 µm. (C) A nodosity induced by isolate SRU‐1 with necrotic region (Ne) surrounded by blue‐white fluorescence (arrowhead) and royal blue fluorescence, which is indicative of suberin (arrow). Cells in the necrotic region were filled with phenolic compounds. This region extends beyond the endodermis (En). Scale bar = 100 µm. (D) As for (C), showing suberization (arrow) of healthy tissue surrounding necrotic region (Ne). Scale bar = 50 µm.
In a stage four nodosity showing early signs of necrosis, observation under UV excitation showed accumulation of a compound emitting bright blue‐white autofluorescence in tissue immediately adjacent to the necrotic region of the cortex (Fig. 3C). Suberin lamellae, detected by both royal blue autofluorescence (Fig. 3C), and by Sudan Black stain (Fig. 3D), were deposited in walls of the cell layers immediately outside this necrotic region.
Accumulation of starch, free amino acids and amides
Starch accumulation, in the form of amyloplasts, was evident in the cortex of PAS/TBO stained VWL‐1‐induced nodosities adjacent to phylloxera feeding sites (Fig. 1C, D). Amyloplasts were also seen in SRU‐1‐induced nodosities and confirmed by PAS staining (Kellow, 2000). In uninfested roots, amyloplasts were much smaller and scattered throughout the root cortex (Kellow, 2000). The concentration of starch in stage two nodosities formed by VWL‐1 phylloxera, as measured by an enzymatic assay, was at least ten times that of uninfested root tips (Table 2). The concentration of starch in stage two nodosities indicates a relatively early onset of starch accumulation.
Table 2.
Concentration of starch in uninfested roots or nodosities of V. vinifera ‘Shiraz’
| Starch concentration µg mg–1 f. wt (mean ± range)* | ||
| Tissue sample | Rep. 1 | Rep. 2 |
| Uninfested root | 1·80 ± 0·19 | 2·89 ± 0·38 |
| Immature nodosities (stage two) | 30·25 ± 2·56 | 31·55 ± 7·19 |
| Mature nodosities (stage four) | 19·72 ± 0·68 | 26·63 ± 1·32 |
* Each replicate represents individual tissue samples, each result being the mean of duplicate assays on these samples.
The total concentration of free amino acids and amides in VWL‐1‐induced nodosities was greater than that in uninfested roots (Table 3). There were also changes in the relative amounts of particular compounds, with the nodosity : root ratio varying from 0·70 (ornithine) to 26·66 (histidine). Aspartic acid constituted 49 % of the total amino nitrogen pool in uninfested roots, but only 17 % of the total pool in nodosities. All other compounds except ornithine were increased in absolute concentration in nodosities. In particular, glutamine increased from 14 % to 31 % of the total pool, to become the predominant form of free amino nitrogen.
Table 3.
Free amino acids and amides in uninfested roots and nodosities of V. vinifera ‘Shiraz’
| Uninfested roots | Nodosities | ||||
| Mean conc. ± s.d. (µg g–1 f. wt) | % of total* | Mean conc. ± s.d. (µg g–1 f. wt) | % of total* | Ratio nodosity: root** | |
| Aspartic acid | 653·64 ± 36·12 | 49·75 | 581·39 ± 62·68 | 17·60 | 0·89 |
| Glutamic acid | 209·15 ± 39·20 | 15·92 | 448·68 ± 50·28 | 13·58 | 2·15 |
| Hydroxyproline | 6·66 ± 1·47 | 0·51 | 20·08 ± 1·95 | 0·61 | 3·02 |
| Asparagine | 36·07 ± 16·38 | 2·75 | 212·89 ± 37·67 | 6·44 | 5·90 |
| Glutamine | 180·83 ± 43·15 | 13·76 | 1016·88 ± 447·69 | 30·78 | 5·62 |
| Serine | 22·76 ± 2·52 | 1·73 | 84·35 ± 22·01 | 2·55 | 3·71 |
| Histidine | 1·28 ± 0·70 | 0·10 | 34·13 ± 22·17 | 1·03 | 26·66 |
| Glycine | 7·09 ± 0·51 | 0·54 | 14·80 ± 1·72 | 0·45 | 2·09 |
| Threonine | 12·15 ± 2·73 | 0·92 | 81·42 ± 23·34 | 2·46 | 6·70 |
| Alanine | 47·32 ± 9·62 | 3·60 | 143·33 ± 63·95 | 4·34 | 3·03 |
| GABA | 50·57 ± 13·60 | 3·85 | 87·30 ± 20·36 | 2·64 | 1·73 |
| Proline | 25·18 ± 3·39 | 1·92 | 154·51 ± 84·85 | 4·68 | 6·14 |
| Tyrosine | 8·42 ± 0·45 | 0·64 | 32·49 ± 3·60 | 0·98 | 3·86 |
| Arginine | 9·72 ± 0·49 | 0·74 | 139·28 ± 70·72 | 4·22 | 14·33 |
| Isoleucine | 10·07 ± 1·73 | 0·77 | 60·48 ± 14·28 | 1·83 | 6·01 |
| Leucine | 2·45 ± 1·82 | 0·19 | 8·21 ± 1·75 | 0·25 | 3·35 |
| Valine | 7·40 ± 1·21 | 0·56 | 32·77 ± 7·06 | 0·99 | 4·43 |
| Methionine | 7·35 ± 0·56 | 0·56 | 42·76 ± 8·71 | 1·29 | 5·82 |
| Phenylalanine | 6·28 ± 0·92 | 0·48 | 87·51 ± 20·21 | 2·65 | 13·93 |
| Ornithine | 3·54 ± 2·58 | 0·27 | 2·47 ± 2·02 | 0·07 | 0·70 |
| Lysine | 6·00 ± 5·22 | 0·46 | 18·39 ± 6·04 | 0·56 | 3·07 |
| Total | 1313·90 ± 111·89 | 100·00 | 3304·13 ± 861·78 | 100·00 | 2·51 |
* Concentration of each amino acid/amide as % of total pool.
** Ratio of concentration in nodosities to that in uninfested roots.
Northern blot hybridization analysis
The results of the northern blot hybridizations using the cDNA probes listed in Table 1 are shown in Fig. 4. Hybridizing transcripts were readily detected in both uninfested roots and VWL‐1‐induced nodosities using cDNA probes for stilbene synthase (StSy; Fig. 4B), chalcone synthase (CHS; Fig. 4C), PAL (Fig. 4D), Grip 15 (Fig. 4I), Grip 28 (Fig. 4J), Grip 68 (Fig. 4L), VvSUC27 (Fig. 4N) and Grip 21 (Fig. 4O). VvTL2 (Fig. 4G) transcripts were present at lower levels, and polyphenol oxidase (PPO; Fig. 4E), VvTL1 (Fig. 4F), VvPR4a (Fig. 4H) and Grip 31 (Fig. 4K) transcripts were not detected in either tissue type. VvSUC12 also showed little to no expression in uninfested roots or nodosities (Fig. 4M). In no case was any observable difference apparent in steady state levels of hybridizing transcripts in RNA from nodosities when compared with that from uninfested roots.
Fig. 4. Northern hybridization of RNA of V. vinifera ‘Shiraz’ roots and nodosities induced by isolate VWL‐1 with defence‐related and other cDNA probes (described in Table 1). For each hybridization, the left lane is 10 µg uninfested root RNA, the right lane 10 µg nodosity RNA; each row represents one RNA gel. (A) Ethidium bromide‐stained lanes show relatively even loading of RNA on each of four gels. (B) Stilbene synthase probe cDNA clone pSV696. (C) Chalcone synthase probe cDNA clone pBS305. (D) Phenylalanine ammonia lyase probe cDNA clone pBS204. (E) Polyphenol oxidase probe cDNA clone pID96. (F) Thaumatin‐like protein probe cDNA clone VvTL1. (G) Thaumatin‐like protein probe cDNA clone VvTL2. (H) PR‐4 type protein probe cDNA clone VvPR4a. (I) Nodule cell wall‐related protein cDNA clone Grip 15 (J) Nodule cell wall‐related protein cDNA clone Grip 28. (K) Nodule pericycle‐related protein cDNA clone Grip 31. (L) Nodule pericycle‐related protein cDNA clone Grip 68. (M) Sucrose transporter cDNA clone VvSUC12. (N) Sucrose transporter cDNA clone VvSUC27. (O) Sucrose starvation‐related protein cDNA clone Grip 21.
DISCUSSION
This study set out to investigate changes in primary root tissue following infestation by phylloxera and formation of phylloxera‐induced galls, or ‘nodosities’. These nodosities are nutrient reservoirs from which grape phylloxera, obligate biotrophs of grapevines, are able to obtain all their nutritional requirements. The feeding site of phylloxera within nodosities was confirmed by observation of stylet tracks in parenchyma cells in the outer region of the root cortex. Most aphids feed directly from phloem, but there are a number of aphid groups (e.g. adelgids) that feed from parenchyma (reviewed by Pollard, 1973). Phylloxera appears to fall into this category of feeders.
The insect appeared to feed from one cell, or possibly a column of adjacent cells progressing successively deeper towards the stele. Few cells near the stylet track appeared to be changed or damaged other than those through which the stylet had passed, which had collapsed plasmalemma, dark staining indicating an accumulation of tannins (or other polyphenolics), and vesicles in their cytoplasm. Changes described here are similar to those reported by Niklowitz (1954); in particular the collapsed plasmalemma and vesicles, which Niklowitz described as ‘bubble filled looking structures’.
Without ultrastructural studies it was not possible to determine whether or not the stylet penetrated the plasmalemma of the cell from which it was actively feeding, or only penetrated the cell wall. Phylloxera’s feeding may be comparable with that of the ring nematode Criconomella xenoplax. This nematode also feeds on root cortical cells through a stylet, but does not penetrate the plasmalemma of its food cell (Hussey et al., 1992). Rather, the aperture of the stylet is closely appressed to the plasmalemma forming a small hole through which the nematode feeds.
Only single unbranched stylet tracks were observed at any phylloxera feeding site. This is in contrast with feeding sites on resistant rootstocks, where multiple and branched stylet tracks, presumably representing aborted feeding attempts, were observed in regions in which a single insect had been probing (Kellow, 2000). Thus, on susceptible vines, phylloxera appear to be predominantly sedentary feeders, gaining sufficient nutrition from a single feeding site to support the growth and development of an individual (and the production of 100–200 eggs).
Although little is known about the mechanism of gall induction by insects, it is believed they result from the injection of some inductive agent, possibly IAA or its precursors, by the gall‐forming insect (reviewed by Miles, 1990). Artificial injection of secondary grapevine roots with IAA has produced swellings superficially similar to tuberosities (phylloxera‐induced galls on secondary roots; Granett, 1990), however these swellings failed to attract phylloxera as tuberosities do. This may be because they were not functioning as nutrient sinks.
The accumulation of starch in the cortex of nodosities is consistent with their function as a nutrient sink. This accumulation would require both enhanced import of sucrose and its subsequent conversion to starch, which is normally rapidly turned over in growing primary roots (Farrar, 1991). Root galls formed by the endoparasitic nematode Nacobbus aberrans (false root‐knot nematode) also accumulate large quantities of starch (Jones and Payne, 1977). The starch in these galls declines during the reproductive phase of the nematodes, suggesting its role as an energy reserve for the nematodes. Other gall‐forming nematodes (e.g. root‐knot and cyst nematodes) induce only minor starch accumulation in plant roots; however, these nematodes create much more elaborate and specialized feeding sites (reviewed by Sijmons et al., 1994).
Starch accumulation in nodosities could result from slowed root growth, from a direct effect on the pathways of sucrose uptake and starch synthesis, and/or from an indirect effect of other physiological changes within the root cortex. Starch synthesis follows a complex pathway (reviewed by Ziegler, 1991, and Atwell et al., 1999, pp. 182–185) that is not yet fully understood. Sucrose, the main solute transported in the phloem (Atwell et al., 1999, p.167), is imported into starch‐storing sink tissues along a negative concentration gradient. In the cytoplasm it is metabolized by sucrose synthase (Zrenner et al., 1995; Koch, 1996) to UDP‐glucose, the substrate for starch synthesis (Sung et al., 1989; Patrick, 1990; Wang et al., 1993). The regulation of the enzymes in this pathway is under complex control and it is possible that starch accumulation in nodosities could be precipitated, via changes in gene expression, due to a high level of sucrose import.
Nodosities contained higher levels of free amino nitrogen compounds, in particular glutamine, compared with uninfested roots. The high concentrations of amides in particular are likely to result from increased phloem unloading and would be expected in active sink tissues, as amides are thought to become the prominent form of nitrogen transport into plant tissues during periods of nitrogen assimilation (Pate, 1975). The total concentration of soluble protein was increased in nodosities, while the protein profile as assessed by SDS–PAGE was not significantly altered (Kellow, 2000), indicating that the increase in free amino acid content in nodosities is not due to enhanced proteolysis or net tissue breakdown. Increases in the amino nitrogen content have also been reported in phylloxera‐induced leaf galls when compared with uninfested leaves (Sobetskiy and Derzhavina, 1973). Ryan et al. (2000), however, were unable to demonstrate a change in free amino acid levels that was positively correlated with phylloxera infestation of excised secondary roots.
Accumulation of starch and amino acids implies enhanced transport of solutes through plasmodesmata and/or via an apoplastic route from the phloem into the root cortex, or slowed growth. This study cannot determine which mechanism(s) are involved but has shown that nodosities possess some structural features which might facilitate solute transport into the cortex. First, cells of the root cortex and stele adjacent to the feeding site do not expand radially, meaning the phylloxera feeding site remains close to the phloem. Secondly, transport of nutrients to the feeding site would be facilitated by the development of apparently normal vascular tissue in nodosities. Finally, and importantly, the endodermis in nodosities lacks the suberin lamellae detectable in uninfested roots.
Non‐suberization of the endodermis may enable enhanced apoplastic and/or symplastic transport of solutes from the stele to the root cortex throughout nodosity development. The endodermis in primary roots of dicotyledons normally goes through two states of suberization as the root differentiates (Peterson and Enstone, 1996). State two suberization was present in uninfested grapevine roots at an equivalent distance from root tip as nodosities described here. State two endodermis has all walls thickened with suberin lamellae, resulting in constriction of plasmodesmata and consequently reduced symplastic, as well as apoplastic, transport of solutes to the root cortex (Warmbrodt, 1985). In some cases phylloxera feeding also appeared to inhibit further differentiation of the endodermis (also noted by Petri, 1907, cited in Niklowitz, 1954). The mechanism by which endodermal differentiation is inhibited is unknown. It may simply result from the prevention of root elongation subsequent to nodosity formation, but this does not appear to affect the differentiation of vascular tissues which continue to develop in nodosities.
No localized defence responses were observed histochemically at any of the phylloxera feeding sites in this study, in contrast with responses seen in roots of resistant grapevines (Kellow, 2000). The results of the northern blot hybridizations, which included several defence‐related cDNAs as probes, further suggest the lack of a defence response. It seems likely that unless a systemic response is expected, for example a defence response in a resistant variety, any changes in gene expression may be only transient or localized to the site of attack, such that northern blot hybridization analyses of RNA or total soluble protein extracts prepared from total gall tissue (Kellow, 2000) are not likely to detect them.
The accumulation of blue‐white autofluorescent material surrounding the necrotic region in decaying nodosities was similar to that reported by Dai et al. (1995) in resistant grapevine leaves following infection with Plasmopara viticola (downy mildew), indicating a defence‐type response that might include the accumulation of trans‐resveratrol. Similarly, the deposition of suberin lamellae in the walls of cells surrounding a necrotic region in a decaying nodosity is reminiscent of the suberized wound periderm which develops in secondary roots of resistant grapevines (Boubals, 1966; King and Rilling, 1991). Clearly, however, neither of the responses observed in decaying nodosities was strong enough, or rapid enough to protect the root from either phylloxera attack or further nodosity decay.
These results suggest that the roots of V. vinifera are capable of defence‐type responses, but that they do not exhibit them in response to phylloxera attack, either because they do not recognize it as a pest, or because immediate, localized defence responses are somehow suppressed. Vitis vinifera is susceptible to many pathogens, and often shows little or no induced response to their attack (Langcake, 1981; Calderón et al., 1992; Dai et al., 1995). It is possible that the stylet sheath (a lipoprotein‐rich matrix secreted by aphids during stylet penetration) could ‘disguise’ phylloxera and thus avoid activation of a defence response (Miles, 1999). The induction of nodosity formation clearly indicates, however, that V. vinifera does at least in some way recognize and react to the presence of this pest.
ACKNOWLEDGEMENTS
We thank Sarah Hetherington and Angela Corrie (Agriculture Victoria, Australia) for technical assistance; Brown Brothers and Campbell’s wineries for access to their vineyards; Kay Denyer (John Innes Institute, UK) for the starch assay method; Chris Davies (CSIRO) and David Tattersall (The University of Adelaide) for cDNA clones used in Northern hybridizations; Michael Bayly (Museum of New Zealand) and Eileen Scott (The University of Adelaide) for valuable comments on the manuscript. This research was funded by The Cooperative Research Centre for Viticulture, and conducted with cooperation from Agriculture Victoria, Rutherglen.
Supplementary Material
Received: 11 July 2003; Returned for revision: 17 October 2003; Accepted: 23 January 2004 Published electronically: 24 March 2004
References
- AtwellB, Bieleski R, Eamus D, Kriedmann P.1999.Plants in action. Adaptation in nature, performance in cultivation. South Yarra, Melbourne: Macmillan Education Australia. [Google Scholar]
- AusubelFM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, and Struhl K.1999.Current protocols in molecular biology. New York: John Wiley & Co. [Google Scholar]
- BossPK, Davies C, Robinson S.1996. Analysis of the expression of anthocyanin pathway genes in developing V vinifera (L. cv Shiraz) grape berries and the implications for pathway regulation. Plant Physiology 111: 1059–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BoubalsD.1966. Étude de la distribution et des causes de la resistance au phylloxera radicole chez les Vitacées. Annales d‘Amelioration des Plantes 16: 145–184. [Google Scholar]
- BronnerR.1975. Simultaneous demonstration of lipids and starch in plant tissues. Stain Technology 50: 1–4. [DOI] [PubMed] [Google Scholar]
- BurtonRA, Jenner H, Carrangis L, Fahy B, Fincher GB, Hylton C, Laurie DA, Parker M, Waite D, van Wegen S, Verhoeven T, Denyer K.2002. Starch granule initiation and growth are altered in barley mutants that lack isoamylase activity. Plant Journal 31: 97–112. [DOI] [PubMed] [Google Scholar]
- CalderónAA, Zapata JM, Pedreño MA, Muñoz R, Barceló AR.1992. Levels of 4‐hydroxystilbene‐oxidizing isoperoxidases related to constitutive disease resistance in in vitro‐cultured grapevine. Plant Cell, Tissue and Organ Culture 29: 63–70. [Google Scholar]
- CorrieAM, Buchanan G, van Heeswijck R.1997. DNA typing of populations of phylloxera (Daktulosphaira vitifoliae (Fitch)) from Australian vineyards. Australian Journal of Grape and Wine Research 3: 50–56. [Google Scholar]
- DaiGH, Andary C, Mondolot‐Cosson L, Boubals D.1995. Histochemical studies on the interaction between three species of grapevine, Vitis vinifera, V rupestris and V rotundifolia and the downy mildew fungus Plasmopara viticola Physiological and Molecular Plant Pathology 46: 177–188. [Google Scholar]
- DaviesC, Robinson SP.1996. Sugar accumulation in grape berries. Plant Physiology 111: 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaviesC, Wolf T, Robinson SP.1999. Three putative sucrose transporters are differentially expressed in grapevine tissues. Plant Science 147: 93–100. [Google Scholar]
- DaviesC, Robinson SP.2000. Differential screening indicates a dramatic change in mRNA profiles during grape berry ripening: Cloning and characterisation of cDNAs encoding putative cell wall and stress response proteins. Plant Physiology 122: 803–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DenisovaTV.1965. The phenolic complex of vine roots infested by phylloxera as a factor in resistance. Vestnik Selskokhoziaistvennoi Nauki 10: 114–118. [Google Scholar]
- DryI, Robinson, S.1994. Molecular cloning and characterisation of grape berry polyphenol oxidase. Plant Molecular Biology 26: 495–502. [DOI] [PubMed] [Google Scholar]
- FarrarJF.1991. Starch turnover: its role in source‐sink relations and a comparison with the role of sucrose. In: Bonnemann JL, Delrot S, Lucas WJ, Dainty J, eds. Recent advances in phloem transport and assimilate compartmentation Nantes, France: Ouest Editions, 213–223. [Google Scholar]
- ForneckA, Kleinmann S, Blaich R, Anvari SF.2002.Histochemistry and anatomy of phylloxera (Daktulosphaira vitifoliae) nodosities on young roots of grapevine (Vitis spp). Vitis 41: 93–97. [Google Scholar]
- GranettJ.1990. Comparison of swellings caused by indoleacetic acid and tuberosities induced by grape phylloxera. Journal of Economic Entomology 83: 494–499. [Google Scholar]
- GranettJ, Walker MA, Kocsis L, Omer AD.2001. Biology and management of grape phylloxera. Annual Review of Entomology 46: 387–412. [DOI] [PubMed] [Google Scholar]
- GranettJ, Timper P, Lider LA.1985. Grape phylloxera (Daktulosphaira vitifoliae) (Homoptera: Phylloxeridae) biotypes in California. Journal of Economic Entomology 78: 1463–1467. [Google Scholar]
- HofmannEL.1957. Die histologie de nodositaten verschiedener rebensorten bie reblausbefall. Vitis 1: 125–141. [Google Scholar]
- HusseyRS, Mims CW, Wescott SWI.1992. Ultrastructure of root cortical cells parasitized by the ring nematode Criconomella xenoplax Protoplasma 167: 55–65. [Google Scholar]
- JacobsAK, Dry IB, Robinson SP.1999. Induction of different pathogenesis‐related cDNAs in grapevine infected with powdery mildew and treated with ethephon. Plant Pathology 18: 325–336. [Google Scholar]
- JonesMGK, Payne HL.1977. The structure of syncytia induced by the phytoparasitic nematode Nacobbus aberrans in tomato roots, and the possible role of plasmodesmata in their nutrition. Journal of Cell Science 23: 299–313. [DOI] [PubMed] [Google Scholar]
- KellowAV.2000.A study of the interaction between susceptible and resistant grapevines and phylloxera. PhD Thesis, The University of Adelaide, Adelaide, Australia. [Google Scholar]
- KellowAV, McDonald G, Corrie AM, van Heeswijck R.2002.In vitro assessment of grapevine resistance to two populations of phylloxera from Australian vineyards. Australian Journal of Grape and Wine Research 8: 109–116. [Google Scholar]
- KingPD, Rilling G.1991. Further evidence of phylloxera biotypes: variations in the tolerance of mature grapevine roots related to the geographical origin of the insect. Vitis 30: 233–244. [Google Scholar]
- KochKE.1996. Carbohydrate‐modulated gene expression in plants. Annual Review of Plant Physiology 47: 509–540. [DOI] [PubMed] [Google Scholar]
- KochKE, Nolte KD, Duke ER, McCarthy DR, Avigne WT.1992. Sugar levels modulate differential expression of maize sucrose synthase genes. The Plant Cell 4: 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LangcakeP.1981. Disease resistance of Vitis spp. and the production of the stress metabolites resveratrol, ϵ‐viniferin, α‐viniferin and pterostilbene. Physiological Plant Pathology 18: 213–226. [Google Scholar]
- MilesPW.1990. Aphid salivary secretions and their involvement in plant toxicoses. In: Campbell RK, Eikenbary RD, eds. Aphid–plant genotype interactions Amsterdam: Elsevier, 131–147. [Google Scholar]
- MilesPW.1999. Aphid saliva. Biological Review 74: 41–85. [Google Scholar]
- NiklowitzW.1954. Histologische studien an reblausgallen und reblausabwehrnekrosen. Phytopathology Z. 24: 299–340. [Google Scholar]
- O’BrienTP, McCully ME.1981.The study of plant structure. Principles and selected methods. Melbourne, Australia: Termacarphi Pty, Ltd. [Google Scholar]
- PateJS.1975. Exchange of solutes between phloem and xylem and circulation in the whole plant. In: Zimmermann MH, Milburn JA, eds. Transport in plants. Phloem transport. Berlin: Springer‐Verlag, 1. [Google Scholar]
- PatrickJW.1990. Sieve element unloading: cellular pathway, mechanism and control. Physiologia Plantarum 78: 298–308. [Google Scholar]
- PetersonCA, Enstone DE.1996. Functions of passage cells in the endodermis and exodermis of roots. Physiologia Plantarum 97: 592–598. [Google Scholar]
- PollardDG.1973. Plant penetration by feeding aphids (Hemiptera, Aphidea) a review. Bulletin of Entomological Research 62: 631–714. [Google Scholar]
- RyanFJ, Omer AD, Aung LH, Granett J.2000. Effects of infestation by grape phylloxera on sugars, free amino acids, and starch of grapevine roots. Vitis 39: 175–176. [Google Scholar]
- SijmonsPC, Atkinson HJ, Wyss U.1994. Parasitic strategies of root nematodes and associated host cell responses. Annual Review of Phytopathology 32: 235–259. [Google Scholar]
- SobetskiyLA, Derzhavina MA.1973. A contribution to the study of the physiology of the feeding of the vine phylloxera, Viteus vitifolii Fitch (Homoptera, Phylloxeridae). Entomological Revue 52: 357–361. [Google Scholar]
- SparvoliF, Martin C, Scienza A, Gavazzi G, Tonelli C.1994. Cloning and molecular analysis of structural genes involved in flavonoid and stilbene biosynthesis in grape (Vitis vinifera L.). Plant Molecular Biology 24: 743–755. [DOI] [PubMed] [Google Scholar]
- StinesAP, Grubb J, Gockowiak H, Henschke PA, Høj PB, van Heeswijck R.2000. Proline and arginine accumulation in developing berries of Vitis vinifera L. in Australian vineyards: influence of vine cultivar, berry maturity and tissue type. Australian Journal of Grape and Wine Research 6: 150–158. [Google Scholar]
- SungS‐JS, Xu D‐P, Black CC.1989. Identification of actively filling sucrose sinks. Plant Physiology 89: 1117–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TattersallDB, van Heeswijck R, Høj PB.1997. Identification and characterization of a fruit‐specific, thaumatin‐like protein that accumulates at very high levels in conjunction with the onset of sugar accumulation and berry softening in grapes. Plant Physiology 114: 759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WangF, Sanz A, Brenner ML, Smith A.1993. Sucrose synthase, starch accumulation, and tomato fruit sink strength. Plant Physiology 101: 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WarmbrodtRD.1985. Studies on the root of Zea mays L. – structure of the adventitious roots with respect to phloem unloading. Botanical Gazette 146: 169–180. [Google Scholar]
- WilliamsonVM, Hussey RS.1996. Nematode pathogenesis and resistance in plants. The Plant Cell 8: 1735–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZieglerP.1991. Starch metabolism in plants: an overview. In: Bonnemann JL, Delrot S, Lucas WJ, Dainty J, eds. Recent advances in phloem transport and assimilate compartmentation Nantes, France: Ouest Editions, 197–203. [Google Scholar]
- ZrennerR, Salanouba TM, Willmitzer L, Sonnewald U.1995. Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants (Solanum tuberosum L.). The Plant Journal 7: 97–107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.