Abstract
• Background and Aims An unusual form of pollen tube growth was observed for several Conospermum species (family Proteaceae). The rate of pollen tube growth, the number of tubes to emerge and the ultrastructure of these tubes are given here.
• Methods Pollen was germinated in vitro in different sucrose concentrations and in the presence of calcium channel blockers, and tube emergence and growth were recorded on a VCR. Measurements were taken of the number of tubes to emerge and rate of tube emergence. Pollen behaviour in vivo was also observed. The ultrastructure of germinated and ungerminated pollen was observed using TEM.
• Key Results After 10 s to 3 min in germination medium, up to three pollen tubes emerged and grew at rates of up to 55 µm s–1; the rate then slowed to around 2 µm s–1, 30 s after the initial growth spurt. Tubes were observed to grow in pulses, and the pulsed growth continued in the presence of calcium channel blockers. Optimal sugar concentration for pollen germination was 300 g L–1, in which up to 81 % of pollen grains showed fast germination. Germination and emergence of multiple tubes were observed in sucrose concentrations of 100–800 g L–1. The vegetative and generative nuclei moved into one of the tubes. Multiple tubes from a single grain were observed on the stigma. Under light microscopy, the cytoplasm in the tube showed a clear region at the tip. The ultrastructure of C. amoenum pollen showed a bilayered exine, with the intine being very thick at the pores, and elsewhere having large intrusions into the plasma membrane. The cytoplasm was dense with vesicles packed with inner tube cell wall material. Golgi apparatus producing secretory vesicles, and mitochondria were found throughout the tube. The tube wall was bilayered; both layers being fibrous and loosely packed.
• Conclusions It is proposed that, for Conospermum, initial pollen tube wall constituents are manufactured and stored prior to pollen germination, and that tube extension occurs as described in the literature for other species, but at an exceptionally fast rate.
Key words: Pollen germination rate, multiple pollen tubes, pollen ultrastructure, calcium channel blockers, smokebush
INTRODUCTION
The rate at which pollen tubes elongate varies widely between species and also between in vivo and in vitro conditions. Some of the fastest recorded in vivo speeds are around 1·8 µm s–1 (evening primrose) to 2·7 µm s–1 (maize) (Stanley, 1971; Barnabas and Fridvalszky, 1984). Growth rates in vitro are slower, for example Zea mays at 1·14 µm s–1 and Ornithogalum virens at 0·25 µm s–1 (Heslop‐Harrison and Heslop‐Harrison, 1984; Stepka et al., 2000).
The in vitro conditions required for pollen germination vary between species, but in general the optimum temperatures range from 20 to 30 °C, and the medium composition includes boron (Vasil, 1960), calcium and a carbon source such as sucrose, lactose or raffinose (Stanley and Linskens, 1974; Cheng and McComb, 1992). Pollen tubes growing in vitro usually do not reach the length that would be required to effect fertilization in nature. This suggests that in vitro optimal growth conditions are not met, and the role of the style is more than just a tract to the ovary.
When a pollen grain lands on a compatible stigma in nature, it hydrates and germinates to produce a pollen tube. Active cytoplasmic streaming carries the contents of the cytoplasm towards the tip of the tube, and the tube grows by tip extension. A clear zone at the apical region of the tip does not participate in cytoplasmic streaming; its function and contents are a topic of controversy. It is thought that Golgi and secretory vesicles transport and deposit polymerized and esterified pectins to the growing tube wall, allowing extension by the tip (Pierson et al., 1994; Lin and Yang, 1997; Franklin‐Tong, 1999).
Guidance of the pollen tube down the style involves mechanical, chemical and physical cues that are not yet fully understood. It is believed that calcium gradients play important roles (Pierson et al., 1996; Holdaway‐Clarke et al., 1997). Pollen tube growth in vitro is usually unidirectional but can be influenced by calcium, glucose and water (Mascarenhas, 1993; Lush et al., 1998). Pollen tubes are usually straight and unbranched, unless there is an incompatible pollination which may result in a range of abnormalities (Williams et al., 1982).
The pollen tube wall consists of cellulose, callose and pectins (Heslop‐Harrison, 1987). Callose and cellulose surround the tube and plugs are deposited, concentrating cytoplasm at the growing tip (Ferguson et al., 1998). The growing tip itself is covered only by the outer fibrillar layer, the structure of which remains undefined, but is known to contain pectin (Heslop‐Harrison, 1987; Stepka et al., 2000).
This paper reports that Conospermum pollen cultured in vitro produces multiple pollen tubes from a single grain, and that they grow at growth rates up to 20‐fold faster than any previously reported growth rates.
MATERIALS AND METHODS
Pollen germination tests
Several blue‐flowered Conospermum species were used: C. amoenum Meisn. subsp. amoenum, C. spectabile E.M. Benn., C. eatoniae E. Pritz., C. caeruleum R. Br. subsp caeruleum, C. brownii Meisn., and the white‐flowered C. incurvum. All flowers with the exception of C. spectabile were collected from the Department of Agriculture, Western Australia research station at Medina, Western Australia (32°13′S, 115°48′E). Conospermum spectabile flowers were collected from the Stirling Range National Park, also in Western Australia (34°24′S, 118°09′E).
In vitro: optimal sucrose concentration
Fertile anthers were collected from mature C. amoenum flowers prior to dehiscence. Pollen from two or three flowers was mixed into a drop of pollen germination medium on a slide. Pollen germination medium consisted of 0·01 m H3BO3, 0·03 m CaCl2.H2O in distilled water and sucrose ranging in concentration from 100 to 1000 g L–1. Counts of germinated pollen were made using light microscopy after a 5–10 min germination period in the dark at room temperature. Approximately 200 pollen grains per slide were counted, four slides per sucrose concentration being examined. Slides were examined for periods of up to 24 h.
In addition, pollen was germinated on cellophane as described by Alexander and Ganeshan (1989), using the pollen germination medium described above. Pollen germination was monitored every 3–6 h for 24 h to observe if any pollen which did not initially germinate at a rapid speed did so slowly over the 24‐h period.
The percentage pollen germination at different sucrose concentrations for C. amoenum was compared using analysis of variance (ANOVA) after arcsin transformation of percentages using STATISTICA (StatSoft, 1999).
Pollen tube growth rate
Pollen from several Conospermum species was collected and germinated in pollen germination medium with 500 g L–1 sucrose on a slide as described above. At least four slides were examined per species. Germination was immediately recorded with a VHS video recorder using ×4 and ×10 magnification. For C. amoenum, the rate of growth, length and numbers of pollen tubes were determined from the videotape using appropriate scales. The time between germination of multiple tubes was also recorded and the average length of pollen tubes was calculated. For all species, the rate of pollen tube growth (µm s–1) was measured at 2, 5, 10 and 30 s following tube emergence. STATISTICA (StatSoft, 1999) enabled data to be analysed using a repeated measures analysis of variance. Factors analysed were species, the number of tubes produced (one, two or three) and time (the repeated measures factor). Separate analyses were performed for pollen that produced one, two or three tubes. Greenhouse–Geisser epsilons (∈) were used to modify degrees of freedom for all main effects and interactions involving the repeated measures factor, to protect against the possibility of a violation of the sphericity assumption.
Effect of sucrose
Pollen from C. amoenum was germinated on slides using pollen germination medium as described previously, but varying sucrose concentrations from 100 to 1000 g L–1. Germination was recorded using a VHS video recorder as described above. The rate of pollen tube growth of multiple tubes was measured for 4–10 grains per concentration. Data were analysed using repeated measures ANOVA as described above.
Effect of calcium channel blockers
Pollen from C. amoenum was germinated in pollen germination medium (see above) with 500 g L–1 sucrose, and aluminium chloride or lanthanum chloride at concentrations ranging from 0 to 300 µm. Stock solutions of the channel blockers were prepared in distilled water and stored at 4 °C. Germination was recorded immediately using a VHS video recorder, and growth rate of pollen tubes was determined at 2, 5, 10 and 30 s following tube emergence. In addition, tube length was measured in each video frame (24 frames per second) for the first 5 s for at least four grains per treatment. Data were analysed using repeated measures ANOVA as described above.
Observations of cytoplasm and nuclei movement
Cytoplasmic movement was observed under UV and light microscopy. To observe the vegetative and generative nuclei in C. amoenum, pollen from two or three flowers was placed in a drop of 1 µg ml–1 DAPI (a′‐6‐diamidino‐2‐phenylindole.2HCl) in 0·05 m citrate‐phosphate buffer (pH 4) and left for 1–2 h in a humid container in the dark at room temperature (Vergne et al., 1987). Locations of the nuclei were observed using UV microscopy. In some experiments fluorescein diacetate (FDA) was added to the germination medium: 100 µL of a stock solution of 0·5 % FDA was added to 5 mL of germination medium (Widholm, 1972).
In vivo
Open, untriggered C.amoenum flowers were triggered with forceps, and pollinated with self‐ and cross‐pollen; flowering stems were then covered with crispy‐wrap bread bags. After 5 d, flowers were removed, fixed and stained according to Oddie and McComb (1998), and styles viewed under light and fluorescent microscopy.
Ultrastructure
Pollen from C. amoenum was collected from recently opened flowers prior to anther dehiscence. Pollen from five flowers was collected in a 1·5‐mL Eppendorf tube. Fixation and sectioning of pollen and pollen tubes followed the method of Ferguson et al. (1998) with some modifications. Pollen was fixed immediately in 1 mL Karnovsky’s fixative (4 % paraformaldehyde, 0·1 % gluteraldehyde in 50 mm Na‐phosphate buffer at pH 7·2) with the addition of 500 g L–1 sucrose. Samples were kept at room temperature for up to 3 h, then overnight at 4 °C. Tubes were centrifuged at 4000 g for 3 min, and the supernatant carefully removed. Pellets were washed twice in Na‐phosphate buffer by resuspension and centrifugation at 4000 g for 3 min. Pollen was then treated in 1 % OsO4 in half‐strength Na‐phosphate buffer for 1 h and washed in distilled water three times following centrifugation at 4000 g for 2 min. Pellets were resuspended in 10 mL of 10 % agar dissolved in distilled water: 1·5 mL was added to each Eppendorf tube to resuspend the pellet, which was then added to the remaining 8·5 mL in a 10‐cm Petri dish and swirled to give an even distribution of pollen and pollen with tubes. Agar was allowed to set at room temperature. Petri dishes were examined under a dissecting microscope to locate pollen or germinated pollen, and these were excised with a scalpel. Excised agar sections were dehydrated through a series of 30–100 % acetone before embedding in Spurr’s resin (Spurr, 1969) using a series of infiltration steps of 10–100 % resin for 2 h each, and a final infiltration overnight in 100 % resin.
RESULTS
Pollen germination in vitro: optimal sucrose concentration
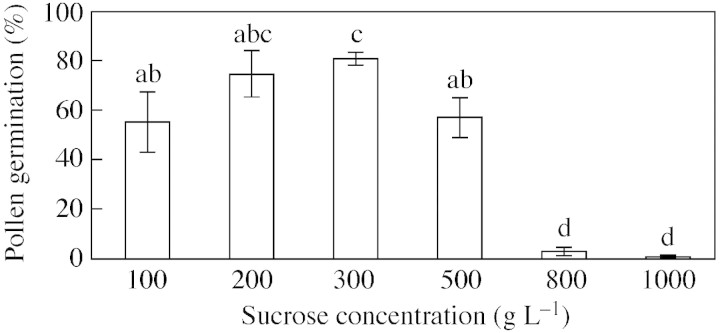
Conospermum pollen is triporate and, when cultured in vitro up to three pollen tubes can develop from one grain. Pollen germination in vitro was observed in media with sucrose concentrations ranging from 100 to 1000 g L–1 (Fig. 1). For C. amoenum germination percentages varied significantly across sucrose concentrations, as shown by ANOVA (n = 5278, F5,18 = 32·83, P < 0·001) with optimum germination at 300 g L–1. The speed of hydration and germination was very fast as some pollen tubes emerged within s of being placed in the germination solution. When pollen from C. amoenum was placed on cellophane wetted with 300 g L–1 sucrose, some grains germinated rapidly, and the remainder did not do so over a subsequent 24‐h period. Pollen was also observed to germinate in water at a rapid rate. In several cases the pollen tubes emerged and elongated rapidly, then burst, with the cytoplasm streaming out of the burst tip.
Fig. 1. Germination of C. amoenum pollen 15–30 min after being placed in pollen germination medium containing different sucrose concentrations. Bars show standard errors. Columns with the same letter are not significantly different.
Pollen tube growth rate
Although the optimal sucrose concentration for pollen germination was 300 g L–1 in C. amoenum, most experiments used 500 g L–1 sucrose as at this concentration germination was often delayed a few more seconds, enabling positioning of the slides under the microscope before the first tube emerged.
The sequence of C. amoenum pollen tube growth in vitro in 500 g L–1 sucrose for up to 101 s is shown in Fig. 2. In the first 2 s after emerging, pollen tubes grew at up to 55 µm s–1 and showed growth pulses. Tubes continued to grow after 30 s but at a slow rate, and the initial tube(s) continued to grow slowly as the second or third tube emerged. Of the C. amoenum pollen grains measured in 500 g L–1 sucrose (n = 477), 15 % produced a single tube, 24 % produced two tubes and 46 % produced three tubes. The time between the emergence of subsequent tubes showed that the second pollen tube emerged 1–189 s after the first tube (mean 54 s); the third tube emerged 2–106 s (mean 43 s) after the second.
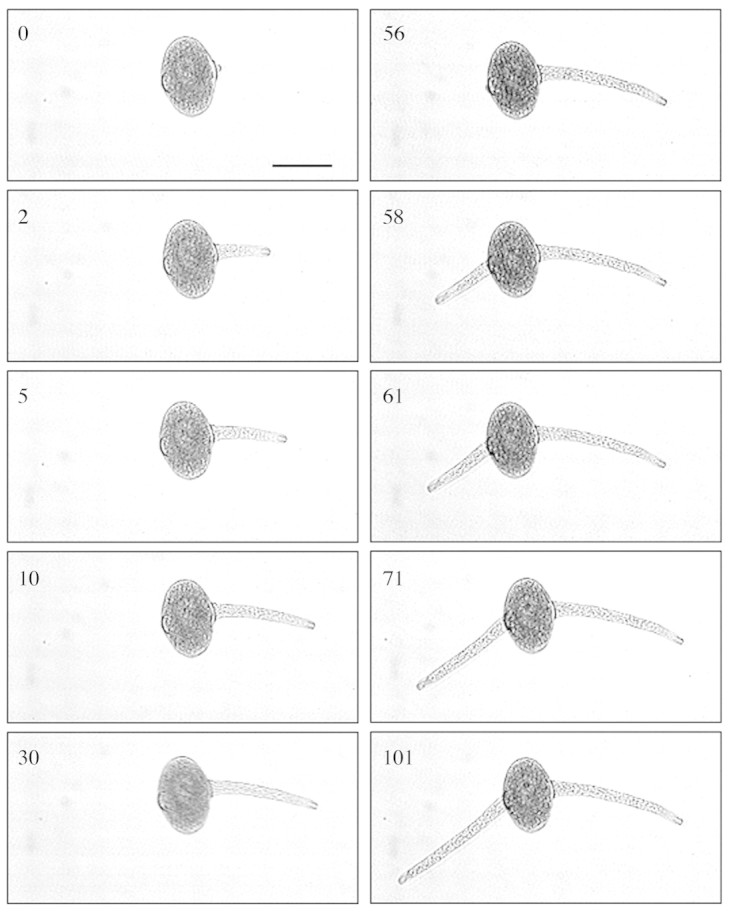
Fig. 2.Conospermum amoenum pollen tube growth up to 101 s after initial germination in 500 g L–1 sucrose. Pictures taken from a video recording of pollen germination. Bar = 75 µm.
Pollen germination data for grains that produced one, two and three tubes were analysed separately. When growth rates of the first, second and third tubes were compared, they were found to be very similar, particularly over the first 2 s (Table 1). Conospermum spectabile (n = 15) and C. incurvum (n = 7) also produced tubes with high growth rates and the mean growth rates were not significantly different between these species (P = 0·806).
Table 1.
Mean growth rate of C. amoenum, C. spectabile and C. incurvum pollen tubes in grains producing three tubes, in the first 5 s after tube emergence (time 0) in 500 g L–1 sucrose
| Growth rate (µm–1) | ||||||
| 0–2 s | 2–5 s | |||||
| Species | Tube 1 | Tube 2 | Tube 3 | Tube 1 | Tube 2 | Tube 3 |
| C. amoenum | 38·1 (4·00) | 36·6 (5·31) | 39·1 (3·87) | 12·5 (1·95) | 3·1 (1·04) | 13·9 (3·67) |
| C. spectabile | 38·4 (2·18) | 32·7 (2·16) | 45·8 (5·59) | 3·0 (0·79) | 7·6 (2·32) | 12·5 (2·29) |
| C. incurvum | 48·8 (8·12) | 49·8 (11·34) | 39·4 (9·71) | 10·6 (4·95) | 10·8 (6·13) | 8·3 (2·76) |
Standard error of the means is given in parenthesis.
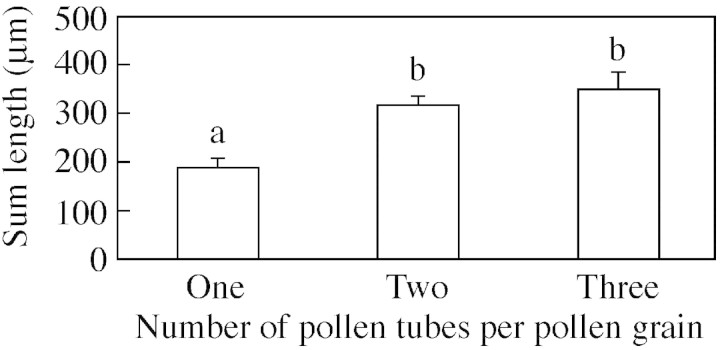
The total length of all pollen tubes produced was calculated for C. amoenum grains that produced one, two and three tubes in 500 g L–1 sucrose (n = 8) (Fig. 3), and varied significantly as shown by ANOVA (F2,42 = 4·40, P = 0·018). An LSD test indicated that grains producing a single tube had a pollen tube length significantly shorter than the total length achieved by those that produced two (P = 0·013) or three (P = 0·006) tubes. There was no significant difference in total pollen tube length between grains producing two or three tubes (P = 0·43).
Fig. 3. Total lengths of pollen tubes emerging from pollen grains producing one, two or three tubes. Data combined for C. amoenum and C. spectabile. Bars show standard errors. Columns with the same letter are not significantly different.
Effect of sucrose
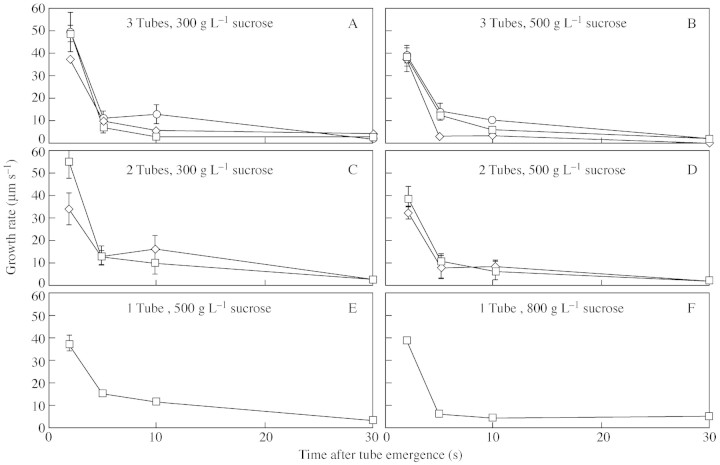
An analysis was made of the growth rates of the first, second and third tubes to emerge, in different sucrose concentrations, but it was not possible to obtain a complete set of data. Although multiple tubes emerged from pollen grains across a range of sucrose from 100 to 1000 g L–1 (Fig. 4), in less than 300 g L–1 tubes burst within 1 min. Too few grains produced a single tube in 300 g L–1 sucrose to enable collection of sufficient data at this concentration. At 800 g L–1 sucrose, multiple tubes were rarely produced. A multiple analysis of variance showed that multiple tubes usually grew at similar rates even in different sucrose concentrations, but that there was a significant decrease in growth (P < 0·001) over the first 30 s.
Fig. 4. Examples of the effect of sucrose on C. amoenum pollen tube growth rate. (A) Grains that produced three tubes in 300 g L–1 sucrose (n = 13). (B) Grains that produced three tubes in 500 g L–1 sucrose (n = 4). (C) Grains that produced two tubes in 300 g L–1 sucrose (n = 6). (D) Grains that produced two tubes in 500 g L–1 sucrose (n = 3). (E) Grains that produced a single tube in 500 g L–1 sucrose (n = 5). (F) Grains that produced a single tube in 800 g L–1 sucrose (n = 11). Pollen tube 1 (squares); pollen tube 2 (diamonds); pollen tube 3 (circles). Bars show standard error.
Effect of calcium channel blockers
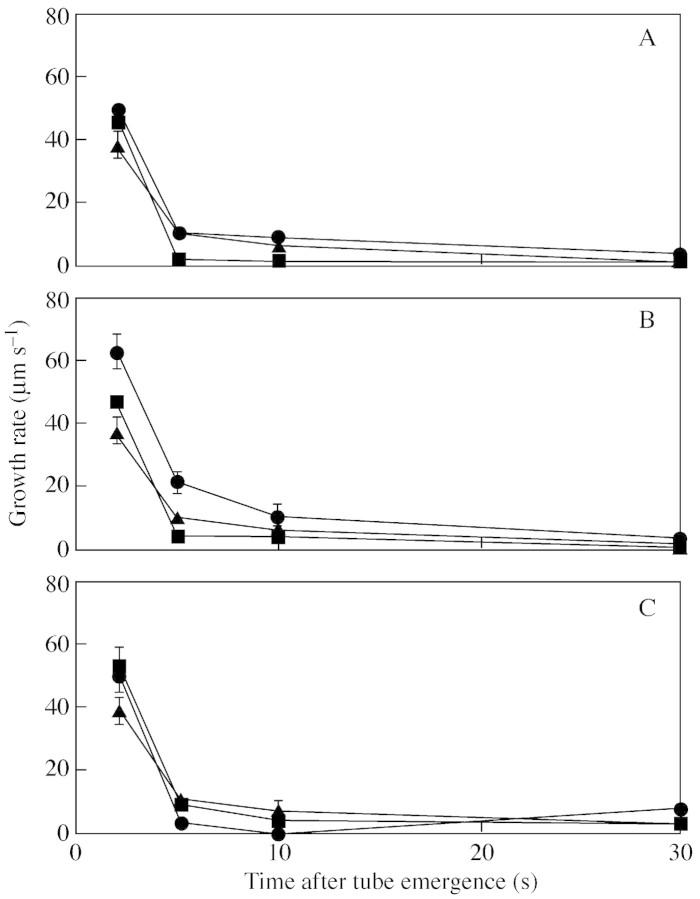
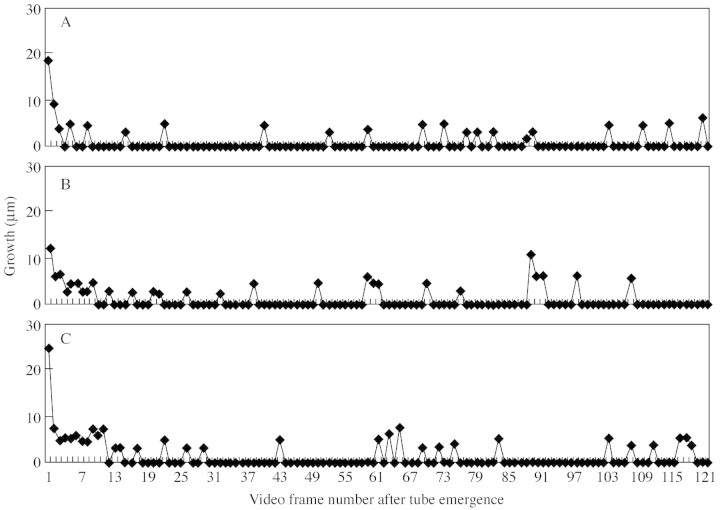
Concentrations of 80–150 µm of AlCl3 and LaCl3 induced no obvious change in pollen tube growth from the control (Fig. 5), and there were no significant differences between concentrations of either channel blocker. A closer investigation of the first 5 s of pollen tube growth by measuring growth per video frame (24 frames per second) of four pollen grains revealed no change to the irregular pulsing growth of the tubes (Fig. 6). In the absence of calcium channel blockers, the mean number of pulses in 30 s was 14·8; 15·8 for AlCl3 and 13·0 for LaCl3. The time between pulses was 0·3 s for the control AlCl3 treatment, and 0·2 s for LaCl3. Pollen germination solution with 300 µm of both AlCl3 and LaCl3 resulted in the bursting of C. amoenum pollen grains.
Fig. 5. Effect of calcium channel blockers on C. amoenum pollen tube growth in pollen germination medium with 500 g L–1 sucrose, in grains producing two tubes. Growth of first tube to emerge is shown. (A) Channel blockers at 80 µm; (B) channel blockers at 100 µm; (C) channel blockers at 150 µm. Bars show standard error. Aluminium chloride (squares); lanthanum chloride (circles); control (absence of blockers) (triangles).
Fig. 6. An example of the pulsing growth of a C. amoenum pollen tube in germination medium containing 500 g L–1 sucrose in the presence of (A) no calcium channel blockers, (B) aluminium chloride, (C) lanthanum chloride. There were 24 frames per second.
Cytoplasmic and nuclei movement
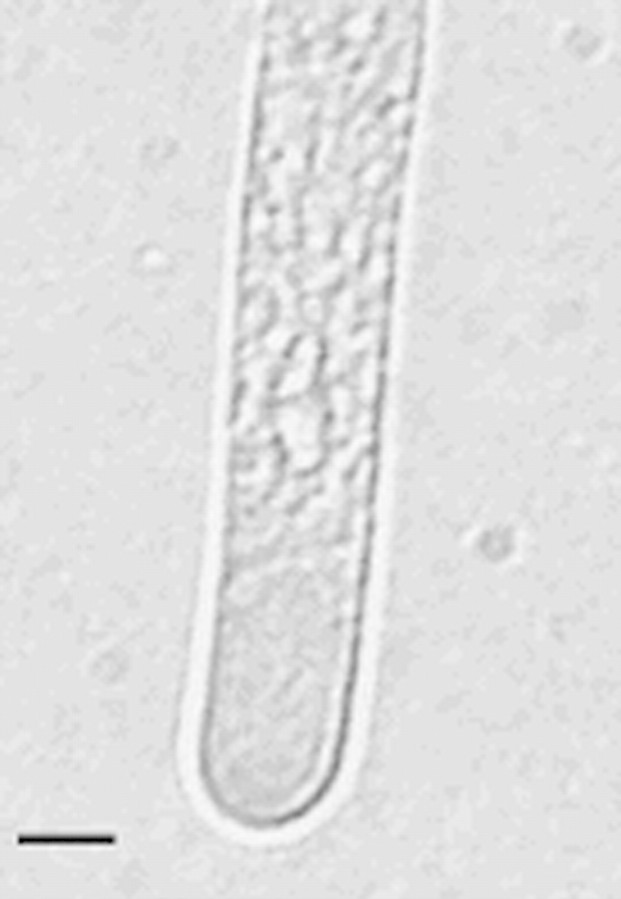
Pollen tubes had a distinct clear tip region (Fig. 7). Staining with FDA and illuminating using fluorescence clearly showed the cytoplasm flowing from the pollen grain into each pollen tube. In some cases a reversal of the flow could also be seen. This was also visible on the video footage. DAPI staining revealed that the pollen had the normal number of nuclei and that the vegetative and generative nuclei moved into one of the tubes.
Fig. 7. Clear tip region of C. amoenum pollen tube taken from a video recording of pollen germinating in 500 g L–1 sucrose. Bar = 5 µm.
In vivo
Styles viewed under fluorescent microscopy revealed pollen tubes extending down the style towards the ovary. Pollen on the stigma germinated with multiple tubes in both self‐ and cross‐pollination. These multiple tubes could only be seen under light microscopy, and the absence of fluorescence suggested that no callose plugs were formed at this stage. Only one pollen tube from a grain appeared to enter the style.
Ultrastructure
Pollen grains of Conospermum had two distinct layers, a bilayered exine, and a thick intine. The ectexine and endexine appeared smooth, dense and homogeneous, and were of identical electron densities. A vacuolated area separated the two, the ectexine being discontinuous in places. The intine ranged between 2·5 and 12·0 µm thick. The inner surface of the intine was not smooth but had large intrusions into the cytoplasm and around the pores (Fig. 8).
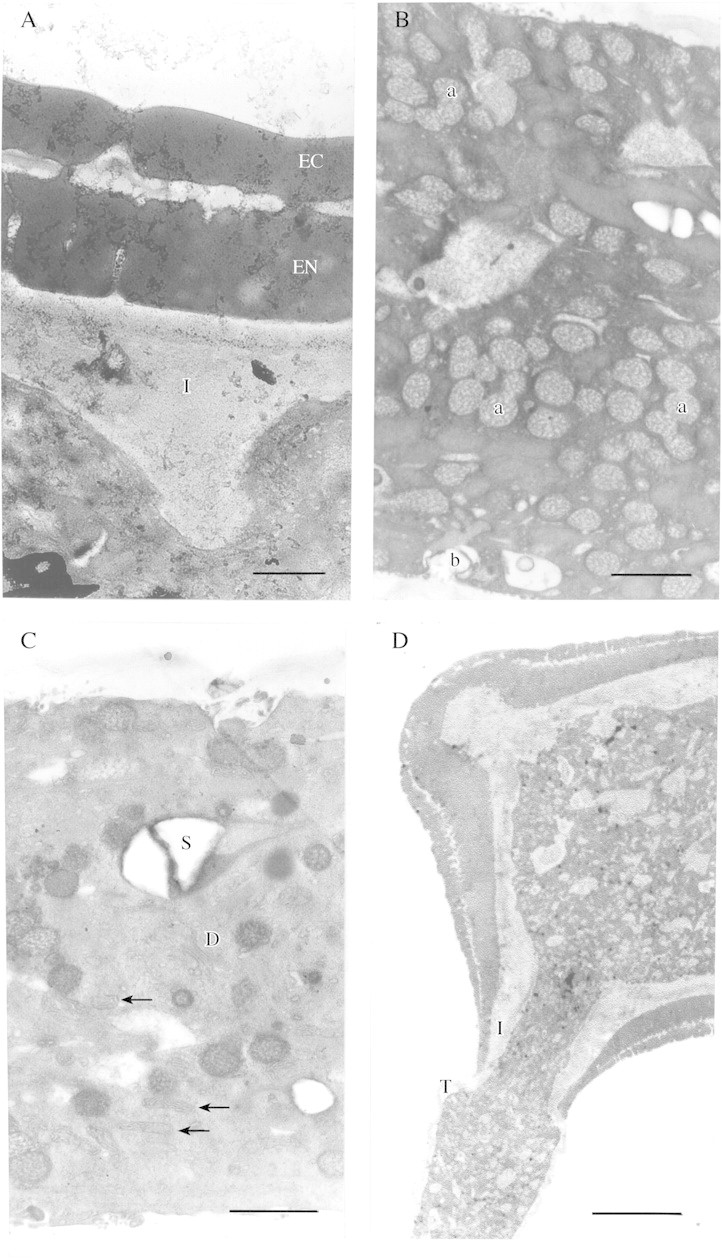
Fig. 8. (A) Conospermum amoenum pollen grain showing smooth homogenous ectexine (EC) and endexine (EN), and intine (I) with intrusions into the cytoplasm. Bar = 1 µm. (B) An abundance of Golgi vesicles in the pollen tube in various states of fusion with each other (a) and the plasma membrane (b). Bar = 1 µm. (C) Cytoplasm of pollen tube showing a dictyosome with secretory vesicles attached (D), many mitochondria (arrows) and a large starch grain (S). Bar = 1 µm. (D) Germinating C. amoenum pollen grain. Note the tube wall (T) appears continuous with the pollen grain intine (I). Bar = 10 µm.
The cytoplasmic contents of the vegetative cell appeared similar to other species with some starch storage evident, but also with very high numbers of Golgi vesicles distributed throughout the pollen grain and tube (Fig. 8B). The abundant Golgi vesicles appeared to be carrying the inner callose/cellulose cell wall material, and could be seen in various states of fusion with each other, and in contact with the pollen tube membrane (Fig. 8B). They ranged in size from approx. 0·17 to 0·42 µm. Large vacuoles were also observed that appeared to be filled with cell wall material and were up to 3·6 µm in diameter.
Mitochondria, Golgi apparatus, endoplasmic reticulum and lipids were also present. Figure 8C from a region midway along the tube shows a Golgi apparatus with five cisternae and two secretory vesicles present on the cis‐face, and many mitochondria. Sections taken from the point of emergence from the grain to approximately the subapical region showed similar cytoplasmic contents, and no zonation was evident (Fig. 8D). Callose plugs were not deposited at this early stage of growth and the tube was not vacuolated near the grain in tubes up to 300 µm long. Sections taken near the tip showed abundant smaller secretory vesicles of 55 nm diameter, Golgi vesicles fusing with each other, and many mitochondria (Fig. 9A)
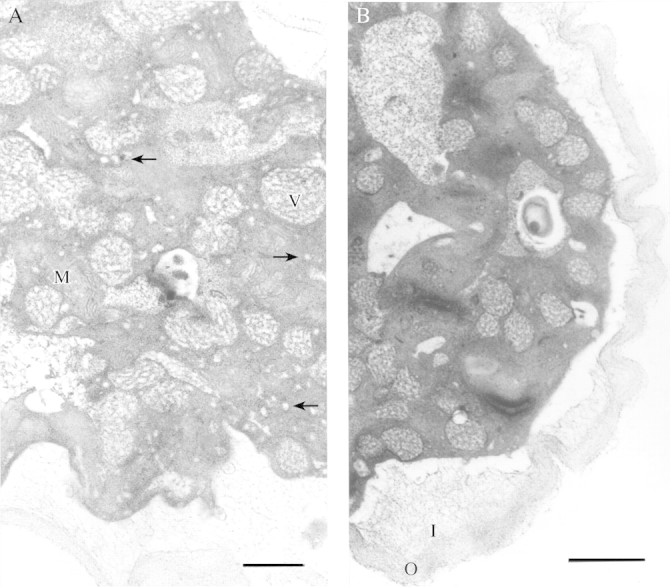
Fig. 9. (A) Pollen tube near the tip. Secretory vesicles are numerous (arrows) as are mitochondria (M). Larger Golgi vesicles are also present (V). Bar = 1 µm. (B) Transverse section of a pollen tube showing a wrinkled pectic outer layer of the cell wall (O) and loosely bound fibrils of the inner callose/cellulosic tube wall (I). Bar = 1 µm.
The wall of the pollen tube was bilayered, and appeared continuous with the intine of the grain. The outer layer consisted of a loosely bound matrix of fibrous, textured material. The inner layer was less dense with dispersed fibrils (Fig. 9A and B).
DISCUSSION
When placed in in vitro culture solution with appropriate sucrose content, pollen of most species rehydrates then germinates from a single pore over a period of 2–24 h. The pollen tube extends at a rate typically less than 1 µm s–1 and stops growing at variable lengths. This type of ‘normal’ germination was never observed in Conospermum. The speed of germination and the rate of pollen tube growth at up to 55 µm s–1 greatly exceeds previously reported rates in vitro or in vivo (Heslop‐Harrison and Heslop‐Harrison, 1984; Stepka et al., 2000). Conospermum pollen grains were able to produce up to three pollen tubes that displayed similar rates of growth, and achieved similar lengths. Observations of multiple tube emergence have been reported previously (Sniezko, 1997), but are uncommon.
Most observations of pollen in vitro report bursting of grains without germination in water or low concentrations of sucrose, and of optimal germination percentages in concentrations of approx. 200–300 g L–1. Optimal conditions for germination of Conospermum were at 300 g L–1 sucrose, which is comparable to other species. Conospermum is unusual in that tubes are still able to germinate rapidly in water and even at up to 1000 g L–1 sucrose, albeit at very low frequency. The osmolarity of the pollen germination solution is usually a key factor in achieving successful germination, and can also be used to manipulate pollen tube growth (Li et al., 1996). Cono spermum tube growth rate was not significantly affected by a change in sucrose concentration from 300 g L–1 to 800 g L–1.
It is widely accepted that pollen tubes grow by tip extension, with the highly active cytoplasmic streaming transporting vesicles and organelles towards the tip to then deposit cell wall components. Conospermum pollen tubes were normal in that a clear zone was observed at the tip, and the cytoplasm showed reverse fountain streaming. The ultrastructure also revealed a normal bilayered tube wall and the cytoplasmic contents necessary for tube extension from the tip. Cytoplasmic zonation away from the tip region was not evident; the organelles appeared fairly evenly distributed throughout the tube, which is similar to grasses such as Secale cereale and Pennisetum typhoideum (Heslop‐Harrison and Heslop‐Harrison, 1982).
Pulsatory growth of pollen tubes was observed in lily by Pierson et al. (1995), and was later shown to be linked to oscillations in concentration of Ca2+ (Holdaway‐Clarke et al., 1997). In the case of Conospermum, growth spurts were observed on the video recording, and when measured more precisely (Fig. 6) showed pulses lasting an average of 0·24–0·26 s in the presence and absence of calcium channel blockers. Other studies using inorganic calcium channel blockers took measurements every 15 s for up to 1 h (Geitmann and Cresti, 1998) and reported that calcium channel blockers prevented pulsatory growth but not tube extension. In Conospermum the growth rate is 20‐fold faster, and the majority of pollen tube growth occurs in the first 30 s. The pulses observed here may not be equivalent to those observed during ‘normal’ pollen tube growth and further investigation, including use of higher concentrations of calcium channel blockers, is required to understand the role calcium plays for Conospermum tube growth.
From the observations made here, it appears that the initial fast growth of the pollen tube could be attributed to the pre‐synthesis of cell wall material in the pollen grain prior to germination. The rate of initial pollen tube growth is so fast it would be difficult for the Golgi and other organelles which normally produce cell wall precursors to manufacture, transport and deposit these components. It is quite possible that the calcium channel blockers did not have time to infiltrate the pollen tube and inhibit pulsatory growth before germination occurred, and as tube wall components were already manufactured the cytoplasm was able to transport them to the tip region for deposition. However, tests with higher levels of the channel blockers would be required to confirm this speculation.
The total length of pollen tube produced from two or three pores is greater than from a single pore (Fig. 3). This suggests either that metabolites in the cytoplasm of the pollen grain are more fully transported and utilized when there are two or three pollen tubes rather than one tube, or that resources available to the grain influences the number of tubes produced. At least in vitro, multiple tubes were more common than single tubes.
The rate of pollen germination and tube growth on the stigma has not been measured, but multiple tubes from a single grain were observed. The function of this is unclear, but it does support the observations in vitro and suggests that multiple tube growth and possibly the rapid growth rate of the tubes are not artefacts of in vitro culture.
Although the biological function of the fast pollen tube growth is obscure, it may reveal important information on how pollen tubes grow and synthesize walls. It is a unique and effective system for obtaining pollen tubes rapidly. Further biochemical and ultrastructural studies are required to understand fully how Conospermum pollen tubes can elongate at such unparalleled speed for a very short period and remain structurally sound.
ACKNOWLEDGEMENTS
Thanks go to Gordon Thomson for technical assistance, Steve Read for advice and Mike Calver for statistical expertise. This study was funded by a postgraduate ARC‐SPIRT grant between Murdoch University, Department of Conservation and Land Management (CALM) and the Western Australian Department of Agriculture.
Supplementary Material
Received: 19 September 2003; Returned for revision: 6 November 2003; Accepted: 3 December 2003; Published electronically: 23 February 2004
References
- AlexanderMP, Ganeshan S.1989. An improved cellophane method for in vitro germination of recalcitrant pollen. Stain Technology 644: 225–257. [DOI] [PubMed] [Google Scholar]
- BarnabasB, Fridvalszky L.1984. Adhesion and germination of differently treated maize pollen grains on the stigma. Acta Botanica Hungary 30: 329–332. [Google Scholar]
- ChengC, McComb JA.1992.In vitro germination of wheat pollen on raffinose medium. New Phytologist 120: 459–462. [Google Scholar]
- FergusonC, Teeri TT, Siika‐aho M, Read SM, Bacic A.1998. Location of cellulose and callose in pollen tubes and grains of Nicotiana tabacum Planta 206: 452–460. [Google Scholar]
- Franklin‐TongVE.1999. Signaling and the modulation of pollen tube growth. Plant Cell 11: 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GeitmannA, Cresti M.1998. Ca2+ channels control the rapid expansions in pulsating growth of Petunia hybrida pollen tubes. Journal of Plant Physiology 152: 439–447. [Google Scholar]
- Heslop‐HarrisonJ.1987. Pollen germination and pollen‐tube growth. International Review of Cytology 107: 1–78. [Google Scholar]
- Heslop‐HarrisonJ, Heslop‐Harrison Y.1982. The growth of the grass pollen tube. 1. Characteristics of the polysaccharide particles (‘‘P‐particles’’) associated with apical growth. Protoplasma 112: 71–80. [Google Scholar]
- Heslop‐HarrisonJ, Heslop‐Harrison Y.1984. The disposition of gamete and vegetative‐cell nuclei in the extending pollen tubes of a grass species, Alopecurus pratensis L. Acta Botanica Neerlandica 33: 131–134. [Google Scholar]
- Holdaway‐ClarkeTL, Feijo JA, Hackett GR, Kunkel JG, Hepler PK.1997. Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell 9: 1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LiY‐Q, Zhang H‐Q, Pierson ES, Huang F‐Y, Linskens HF, Hepler PK, Cresti M.1996. Enforced growth‐rate fluctuation causes pectin ring formation in the cell wall of Lilium longiflorum pollen tubes. Planta 200: 41–49. [Google Scholar]
- LinY, Yang Z.1997. Inhibition of pollen tube elongation by microinjected Anti‐Rop1Ps antibodies suggests a crucial role for Rho‐type GTPases in the control of tip growth. Plant Cell 9: 1647–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LushWM, Grieser F, Wolters‐Arts M.1998. Directional guidance of Nicotiana alata pollen tubes in vitro and on the stigma. Plant Physiology 118: 733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MascarenhasJP.1993. Molecular mechanisms of pollen tube growth and differentiation. Plant Cell 5: 1303–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OddieRLA, McComb JA.1998. Stigma receptivity in Eucalyptus camaldulensis Dehnh. Silvae Genetica 47: 2–3. [Google Scholar]
- PiersonES, Li YQ, Zhan HQ, Willemse MTM, Linskens HF, Cresti M.1995. Pulsatory growth of pollen tubes: investigation of a possible relationship with the periodic distribution of cell wall components. Acta Botanica Neerlandica 44: 121–128. [Google Scholar]
- PiersonES, Miller DD, Callaham DA, Shipley AM, Rivers BA, Cresti M, Hepler PK.1994. Pollen tube growth is coupled to the extracellular calcium ion flux and the intracellular calcium gradient: Effect of BAPTA‐type buffers and hypertonic media. Plant Cell 6: 1815–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PiersonES, Miller DD, Callaham DA, Van Aken J, Hackett GR, Hepler PK.1996. Tip‐localized calcium entry fluctuates during pollen tube growth. Developmental Biology 174: 160–173. [DOI] [PubMed] [Google Scholar]
- SniezkoR.1997. Postulated interaction between branching pollen tubes and ovules in Oenthora hookeri de Vries (Onagraceae). Journal of Plant Research 110: 411–416. [Google Scholar]
- SpurrA.1969. A low viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructural Research 26: 31–43. [DOI] [PubMed] [Google Scholar]
- StanleyRG.1971. Pollen chemistry and tube growth. In: Heslop‐Harrison J, ed. Pollen: development and physiology London: Butterworths, pp. 131–155. [Google Scholar]
- StanleyRG, Linskens HF.1974.Pollen: biology, biochemistry and management. Heidelberg: Springer Verlag. [Google Scholar]
- StatSoft.1999.STATISTICA for Windows (Computer Program Manual). Tulsa, USA: StatSoft Inc. [Google Scholar]
- StepkaM, Ciampolini F, Charzynska M, Cresti M.2000. Localization of pectins in the pollen tube wall of Ornithogalum virens L. Does the pattern of pectin distribution depend on the growth rate of the pollen tube? Planta 210: 630–635. [DOI] [PubMed] [Google Scholar]
- VasilIK.1960. Pollen germination in some Gramineae: Pennisetum typhoideum Nature 187: 1134–1135. [Google Scholar]
- VergneP, Delvallee I, Dumas C.1987. Rapid assessment of microspore and pollen development stage in wheat and maize using DAPI and membrane permeabilization. Stain Technology 62: 299–304. [DOI] [PubMed] [Google Scholar]
- WidholmEG.1972. Fluorescein diacetate for estimating viability of cells. Stain Technology 47: 189. [DOI] [PubMed] [Google Scholar]
- WilliamsEG, Knox RB, Rouse JL.1982. Pollination sub‐systems distinguished by pollen tube arrest after incompatible interspecific crosses in Rhododendron (Ericaceae). Journal of Cell Science 53: 255–277. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.