Abstract
• Background and Aims The number of genera included in Apocynaceae subfamily Periplocoideae is a matter of debate. DNA sequences are used here as an independent dataset to clarify generic relationships and classification of the tuberous periplocoid genera and to address the question of the phylogenetic interpretation of pollinia formation in Schlechterella.
• Methods Representatives of nearly all African and Malagasy genera of Periplocoideae possessing root tubers were analysed using ITS and plastid DNA sequence characters.
• Key Results Sequence data from non‐coding molecular markers (ITS of nrDNA and the trnT‐L and trnL‐F spacers as well as the trnL intron of plastid DNA) give support for a broad taxonomic concept of Raphionacme including Pentagonanthus. Together with Schlechterella, which is sister to Raphionacme, all Raphionacme‐like taxa form a derived monophyletic group of somewhat diverse species. Sister to the Schlechterella/Raphionacme clade is a clade comprising Stomatostemma and the not truly tuberous vine Mondia. In the combined analysis, sister to these two clades combined is a clade formed by Petopentia natalensis and Periploca.
• Conclusions The recent inclusion of the monotypic South African Petopentia in the monotypic Malagasy endemic Ischnolepis is to be rejected. The Malagasy Camptocarpus is sister to the remainder of Periplocoideae in the ITS and combined analyses, and a Malagasy origin for the subfamily is discussed.
Key words: Africa, Apocynaceae, cpDNA, Ischnolepis, Madagascar, nrDNA, Raphionacme, Pentagonanthus, Periplocoideae, Petopentia, root tubers, sequence analysis, Schlechterella, Stomatostemma
INTRODUCTION
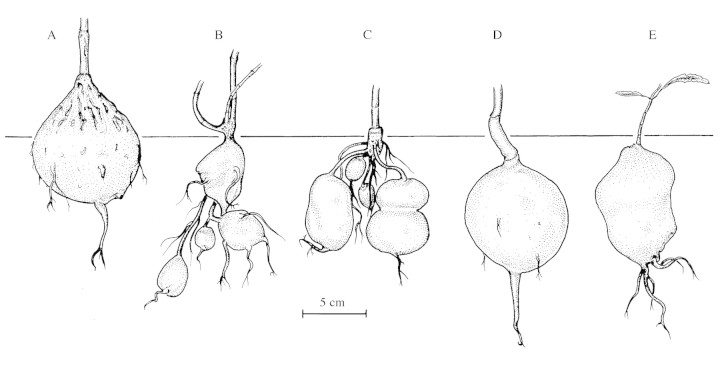
The number of genera to be accepted in Apocynaceae subfamily Periplocoideae is still a matter of debate. Venter and Verhoeven (1997) gave approx. 190 species in 44 genera; a number also relevant for Meve (2002). The number of genera decreased considerably to only 31 in the review paper published in late 2001 (Venter and Verhoeven, 2001). However, since many of the Asiatic genera are poorly known, the true number of genera is still unclear. The final number probably lies between 31 and 44 genera. Apart from lack of information, major differences in taxonomic views still contribute to these discrepancies (cf. new synonymies for Cryptolepis and Raphionacme; Venter and Verhoeven, 1997, 2001), and the tuberous Periplocoideae contribute largely to the changing genus numbers. The geophytic growth form is found in roughly one‐third of the periplocoid genera. Of these, the genera Decalepis Wight & Arn., Ischnolepis Jum. & H.Perrier, Petopentia Bullock, Raphionacme Harv., Sacleuxia Baill., Sarcorrhiza Bullock, Schlechterella K. Schum. and Stomatostemma N.E.Br. only comprise tuberous species. All of them, except for the Indian Decalepis and the Malagasy Ischnolepis, are African (one Raphionacme species is Arabian). Three different types of true root tubers (without involvement of the hypocotyl) can be distinguished: semi‐subterranean tubers formed by the main root (Petopentia; Fig. 1A), subterranean (rarely partly exposed) tubers formed by few to many lateral roots (Ischnolepis, Stomatostemma; Fig. 1B and C), and subterranean tubers formed by the main root (Raphionacme, Schlechterella; Fig. 1D and E). The African Mondia Skeels and the Malagasy Camptocarpus Decne. are incompletely known with regard to this character. Mondia, although described as tuberous by Venter and Verhoeven (2001), is not or not always tuberous in our own observations. In Tanzanian representatives of Mondia whitei (Hook.f.) Skeels we observed woody rootstocks at the most. The reverse situation occurs in the Malagasy endemic Camptocarpus. Whereas Klackenberg (1998) and Venter and Verhoeven (2001) did not report on any thickened roots at all, plants of Camptocarpus mauritianus Decne. grown from seed in the glasshouse in Bayreuth possess some fleshy and swollen (lateral) roots. One non‐tuberous Peri plocoideae from Madagascar, Petopentia Decne., and one from Africa, Periploca L., have been added to the material investigated. Due to the lack of suitable material for sequencing, Sacleuxia (exact tuber shape unknown) and Sarcorrhiza (tubers developing from lateral roots) have not been analysed here. The bark of the tubers seen is usually smooth or nearly so, although rough and scaly in Petopentia (see also caption of Fig. 1).
Fig. 1. Examples of different kind of root tubers in Periplocoideae. (A) Petopentia natalensis: semi‐subterranean root tuber with upper half (third) exposed above ground, bark thick and scaly. (B) Ischnolepis graminifolia: multiple root tubers on primary and secondary roots, bark thin and smooth. (C) Stomatostemma monteiroae: multiple root tubers on primary and secondary roots, bark thin and smooth. (D) Raphionacme vignei E.A. Bruce: root tuber, bark stout but rather smooth. (E) Schlechterella africana: root tuber, bark thin and smooth (illustration slightly magnified in relation to A–D). (A) From ex hort. Bot. Garden Kiel; (B) from Mangelsdorff s. n.; (C) from Albers & Meve 524; (D) from Porembski 503, (E) from Specks 676. All drawn by U. Meve.
Raphionacme, comprising one‐fifth of the species in the subfamily, is a geophytic, erect and sometimes twining herb of tropical and subtropical Africa and Oman. For the most spectacular flowering taxa, Oliver (1887; Chlorocyathus Oliv.) and Bullock (1962a; Pentagonanthus Bullock) founded new genera. However, both these segregates have been synonymized again under Raphionacme (cf. Venter and Verhoeven, 1997). Schlechterella, which superficially looks like a Raphionacme but possesses a corona with a basal ring‐like structure and pollinia instead of loose pollen tetrads (Verhoeven and Venter, 1998), was once partially included in Raphionacme [e.g. Verhoeven and Venter (1994) for Schlechterella abyssinica (Chiov.) Venter & R.L.Verh.]. However, Venter and Verhoeven (1997, 1998, 2001) later treated both pollinia‐bearing species as a separate genus Schlechterella (syn. Triodoglossum Bullock). DNA sequences are used here as an independent dataset to clarify generic relationships and classification of the tuberous periplocoid genera and to address the question of the phylogenetic interpretation of pollinia formation in Schlechterella. Recent molecular studies in Asclepiadeae and Ceropegieae (Liede and Kunze, 2002; Liede and Täuber, 2002; Meve and Liede, 2002) have shown that floral characters are often of limited value in generic circumscription in Asclepiadoideae, because high degrees of parallelism and homoplasy often obliterate phylogenetic relationships. Instead, vegetative characters have been found to be more reliable, including stem succulence in Cynanchum L. (Liede and Kunze, 2002), and stem/stipule characters in Caralluma R.Br. sensu lato (Meve and Liede, 2002). Before taxa of Periplocoideae with differently shaped root tubers are included in Raphionacme (which is characterized by more or less turnip‐shaped tubers, Fig. 1D) as well as herbacous and woody plants, the relationships of these geophytes to each other and to tuberless periplocoids should be assessed.
Similar flowers (and similar stem texture) were used by Venter and Verhoeven (2001) to support the inclusion of the South African Petopentia natalensis (Schltr.) Bullock in the Malagasy genus Ischnolepis. Petopentia is a woody climber with enormous, mostly single tubers (Fig. 1A). Ischnolepis, established for the single species I. graminifolia (Costantin & Gallaud) Klack. is a shrub and not a climber. It possesses numerous underground tubers on lateral roots (Fig. 1B), which can reach a combined mass of 100 kg in mature plants. With Petopentia it shares the reddish, smooth bark of the stems and the large yellow, flat and star‐shaped flowers. However, the tubers and leaf morphology and anatomy of Ischnolepis and Petopentia are different. Molecular data are used to test their congenerity.
MATERIALS AND METHODS
Taxa
The material used in the molecular studies is summarized in Table 1, including voucher specimens, authors of species and donors of material.
Table 1.
Voucher information and EMBL numbers for plant material used in the molecular studies
| EMBL acc. no.: | |||||
| trnT‐L spacer | |||||
| trnL intron | EMBL | ||||
| Species | Origin | Voucher | trnL‐F spacer | acc. ITS no. | |
| Outgroup | South Africa: E Cape, Baviaanskloof | Liede 2931 | UBT | AJ428828 | AJ581692 |
| Secamone alpinii Schult. (Secamonoideae) | AJ428829AJ428830 | ||||
| Ingroup: Periplocoideae | |||||
| Camptocarpus mauritianus Decne. | Madagascar: Toliara | Petignat s. n. | UBT | AJ581794 | AJ581677 |
| AJ581795 | |||||
| AJ581796 | |||||
| Ischnolepis graminifolia (Costantin & Gallaud) Klack. | Madagascar: W Fianarantsoa | Röösli s. n. | UBT | AJ581799AJ581798AJ581797 | AJ581678 |
| Mondia whitei (Hook.f.) Skeels | Tanzania: Kilimanjaro, Msaranga Valley | Liede & Meve 3351 | UBT | AJ581800 | AJ581679 |
| AJ581801 | |||||
| AJ581802 | |||||
| Pentopetia grevei (Baill.) Venter | Madagascar: Ambinanitelo | Mangelsdorff 516 | UBT | AJ581805 | AJ581681 |
| AJ581804 | |||||
| AJ581803 | |||||
| Periploca graeca L. | ex hort. Z | Endress s.n. | Z | AJ581806 | AJ581682 |
| AF102468 | |||||
| AJ581807 | |||||
| Periploca visciformis K.Schum. | Socotra: Wadi Ayhaft | Mies 1444 | UBT | AJ431734 | AJ581683 |
| AJ431735 | |||||
| AJ431736 | |||||
| Petopentia natalensis Bullock | s. loc. | ex hort. Kiel | in cult. UBT | AJ581810 | AJ581684 |
| AJ581809 | |||||
| AJ581808 | |||||
| Raphionacme angolensis (Baill.) N.E.Br. | Tanzania: W Songea | Specks 287 | UBT | AJ431770 | AJ581685 |
| AJ431771 | |||||
| AJ431772 | |||||
| Raphionacme dyeri Retief & Venter | South Africa: KwaZulu‐Natal | Nicholas 2862 | NH | AJ581811 | AJ581686 |
| AJ581812 | |||||
| AJ581813 | |||||
| Raphionacme elata N.E.Br. | South Africa: Durban‐Westville | Borchers 47 | NH | AJ581816 | AJ581687 |
| AJ581815 | |||||
| AJ581814 | |||||
| Raphionacme flanaganii Schltr. | South Africa: Pietermaritzburg | Albers K 1577 | MSUN | AJ581817 | AJ581688 |
| AJ581818 | |||||
| AJ581819 | |||||
| Raphionacme grandiflora N.E.Br. [Pentagonanthus grandiflorus (N.E.Br.) Bullock] | Tanzania: W Songea | Specks 770 | UBT | AJ581822AJ581821AJ581820 | AJ581680 |
| Raphionacme hirsuta (E.Mey.) R.A.Dyer | South Africa: KwaZulu‐Natal | Nicholas 2863 | NH | AJ581823AJ581824AJ581825 | AJ581689 |
| Raphionacme madiensis S.Moore | Tanzania: Dodoma | Specks 682 | UBT | AJ581828 | AJ581690 |
| AJ581827 | |||||
| AJ581826 | |||||
| Schlechterella abyssinica (Chiov.) Venter & R.L.Verh. | Kenya: Furole | Newton 4555 | UBT | AJ581829AJ581830AJ581831 | AJ581691 |
| Stomatostemma monteiroae Oliv. | s. loc. | IPPS 1427 | UBT | AJ431779 | AJ581692 |
| AJ431780 | |||||
| AJ431781 | |||||
| Stomatostemma monteiroae Oliv. | Zimbabwe: NW Hwange NP, Thomson Junction | Albers & Meve 518 | UBT | AJ581834AJ581833AJ581832 | AJ581693 |
Secamone alpinii Schult. (Apocynaceae subfamily Secamonoideae) was selected as the outgroup. Tribes Echiteae and Apocyneae of Apocynaceae subfamily Apocynoideae, equally suitable as outgroups following Potgieter and Albert (2001), were not used because it was not possible to align ITS sequences. In the ingroup, 16 tuberous and non‐tuberous periplocoid species were analysed. Of these, two different specimens of Stomatostemma monteiroae N.E.Br. were included (Table 1). For Periploca graeca L., the published sequence AF102468 (Potgieter and Albert, 2001) was used for the trnL intron. ITS, trnT‐L and trnL‐F spacers of P. graeca were sequenced from the material also used by Potgieter and Albert (2001).
DNA extraction and PCR
DNA was isolated from fresh stem tip tissue according to Doyle and Doyle (1987). PCR primers and the protocol for the plastid trnT‐L and trnL‐F spacers and the trnL intron are from Taberlet et al. (1991). The internal transcribed spacer (ITS) region of nuclear ribosomal DNA was amplified using the flanking primers ITS4 and ITS5 following a slightly modified protocol from Baldwin (1992) as detailed by Meve and Liede (2001a, b). Sequences were obtained on an ABI Prism Model 310 Version 3.0 sequencer. All sequences have been deposited at EMBL Nucleotide Sequence Database (see Table 1 for accession numbers).
Data analysis
Sequences were aligned with Perkin Elmer Sequence Navigator Version 1.0.1 and the alignment was refined manually. Indels were coded as missing characters throughout.
Phylogenetic analysis and tests for clade support were performed using PAUP version 4.0b8a (PPC) (Swofford, 1998). Phylogenies were generated using Fitch parsimony as implemented in PAUP employing branch‐and‐bound search (Hendy and Penny, 1982), with addition sequence set to ‘furthest’. Decay indices (Bremer, 1988; Donoghue et al., 1992) and bootstrap values (Felsenstein, 1985) derived from 1000 replicates (saving a maximum of 100 trees per replicate) were calculated as measures of support for individual clades. Decay analyses were performed with AutoDecay 4.0 (Eriksson, 1998) in combination with the reverse constraint option of PAUP.
A partition homogeneity test (Bull et al., 1993), as included in PAUP version 4.0b8a, showed that the plastid DNA and ITS datasets are concordant (P = 0·27), and they were combined.
RESULTS
The plastid DNA alignment comprises 17 taxa and a total of 1804 characters [879 sequence characters in the trnT‐L spacer (primers a–b), 526 sequence characters in the trnL intron (primers c–d), and 399 sequence characters in the trnL‐F spacer (primers e–f)]; 25 data cells are unknown, the end of the trnL spacer in Periploca graeca, for which the published sequences are shorter than ours. The ITS alignment comprises 17 taxa and 700 sequence characters, ten data cells are unknown, affecting the 18S region of Camptocarpus mauritianus and Raphionacme elata. Both alignments are available from the authors or can be viewed online at http://www.uni‐bayreuth.de/departments/planta2/ or in TreeBASE study (accession number = S956, matrix accession number = M1587; Sanderson, 1994).
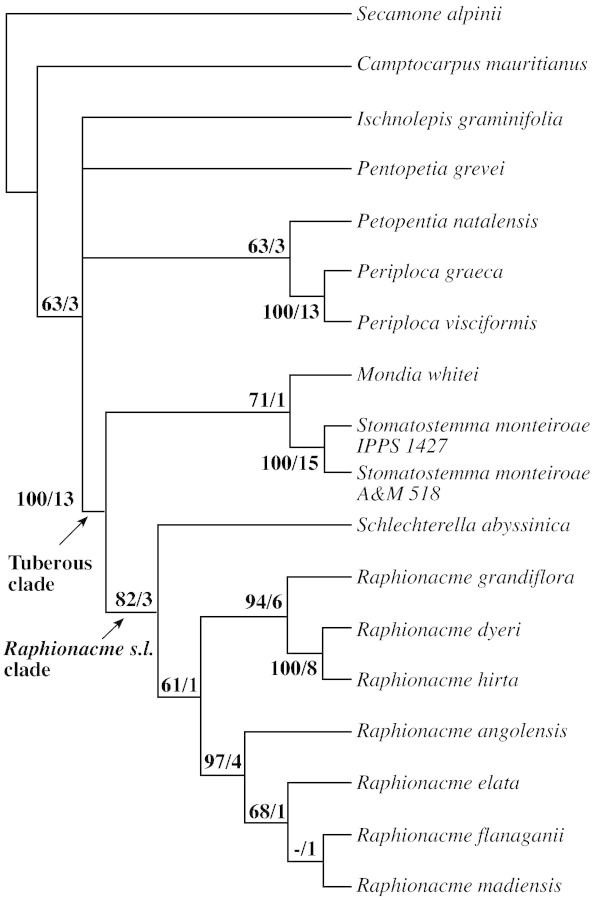
Parsimony analysis of the plastid DNA sequence characters alone (29 parsimony‐informative characters) resulted in one tree of 38 steps (Fig. 2), consistency index (CI) 0·84 (excluding uninformative characters) and retention index (RI) 0·90. Parsimony analysis of the ITS data alone (124 parsimony‐informative characters) resulted in four trees of 274 steps (Fig. 3), CI 0·60 (excluding uninformative characters) and RI 0·68. Parsimony analysis of combined ITS and plastid DNA sequence characters (153 parsimony‐informative characters) resulted in three trees of 318 steps (Fig. 4), CI 0·62 (excluding uninformative characters), RI 0·70.
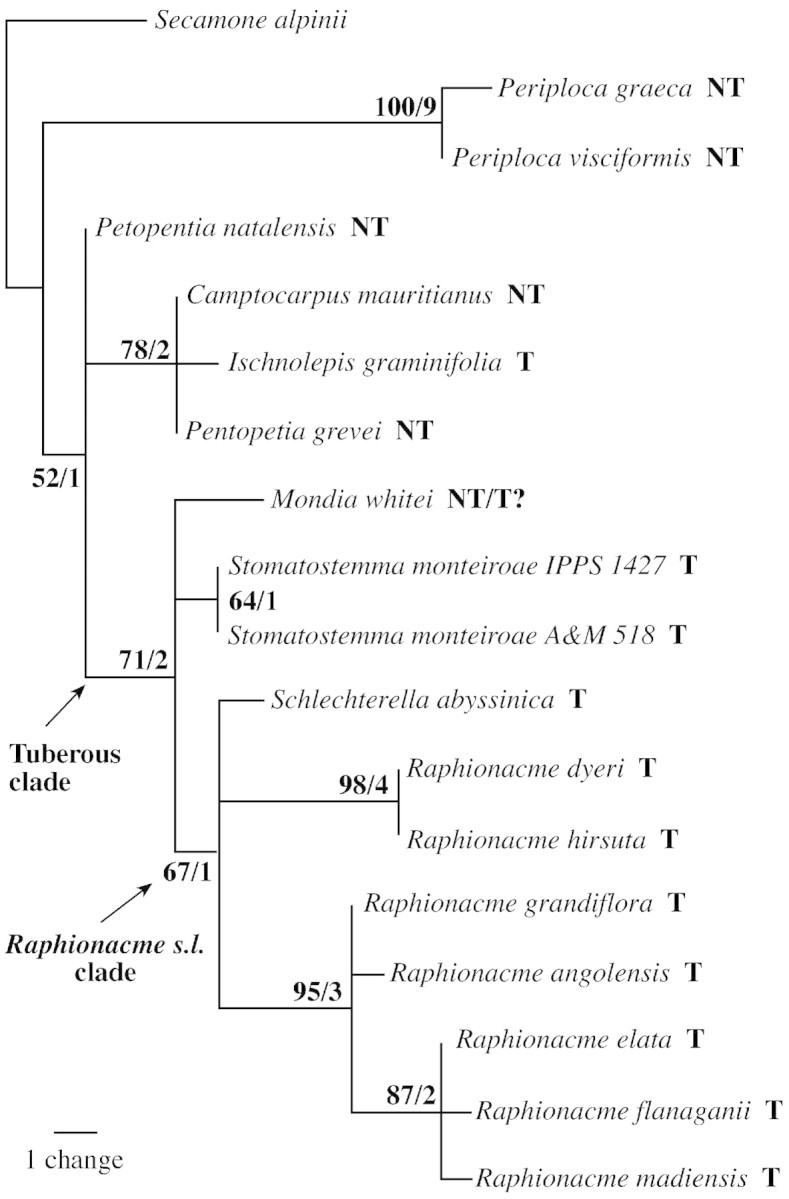
Fig. 2. Single most‐parsimonious tree resulting from branch‐and‐bound analysis of cpDNA data (29 parsimony‐informative characters, tree length = 38 steps, CI = 0·8421, RI = 0·9016, RC = 0·7593 if uninformative characters are excluded; NT = not tuberous, T = tuberous).
Fig. 3. One of the four most‐parsimonious trees resulting from branch‐and‐bound analysis of ITS data (124 parsimony‐informative characters, tree length = 274 steps, CI = 0·5985, RI = 0·6812, RC = 0·4077 if uninformative characters are excluded).
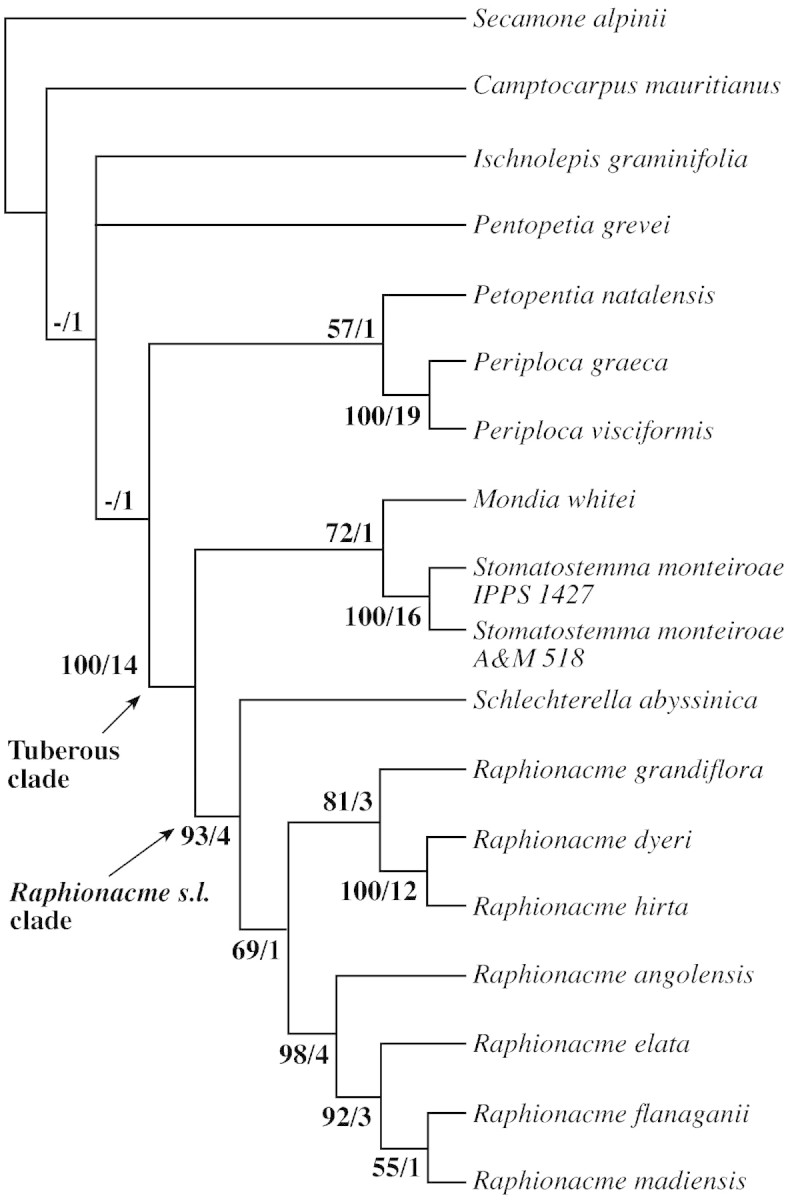
Fig. 4. One of the three most‐parsimonious trees resulting from branch‐and‐bound analysis of combined cpDNA and ITS data (153 parsimony‐informative characters, tree length = 318 steps, CI = 0·6164, RI = 0·9695, RC = 0·4311 if uninformative characters are excluded).
In all analyses, the tuberous African Periplocoideae formed a derived clade with moderate support (bootstrap 71 %) in the plastid DNA analysis (Fig. 2, ‘tuberous clade’), and 100 % bootstrap support in the ITS and the combined analyses (Figs 3 and 4). Only Petopentia did not belong to this clade, despite its tuberous habit. The tuberous African clade comprised two groups: the Mondia/Stomatostemma clade, and the clade containing the Raphionacme relatives. In the latter, Schlechterella was the sister to ‘true’ Raphionacme, while Raphionacme itself was divided into two subclades. One, well supported by ITS data, placed Raphionacme (Pentagonanthus) grandiflora N.E.Br. together with Raphionacme dyeri Retief & Venter and R. hirsuta (E.Mey.) R.A.Dyer (Fig. 3). The other, also well‐supported subclade, comprised four Raphionacme species representing fairly different growth forms.
The remaining taxa were less well resolved than the ‘tuberous clade’. Weakly supported by ITS data, Petopentia was sister to Periploca, while Camptocarpus was sister to all investigated periplocoid taxa in the ITS and combined analyses (Figs 3 and 4). In the plastid DNA dataset, the Malagasy Camptocarpus, Ischnolepis and Pentopetia formed a monophyletic group (Fig. 2).
DISCUSSION
The bitypic Pentagonanthus was separated from Raphionacme because it possesses five spur‐like sacs at the corolla base, a feature to which the genus name refers (Bullock, 1962a). Verhoeven and Venter (1994), however, recognized that each pollen grain in Raphionacme has eight to 16 pores instead of four to six, a character unique in Periplocoideae. These authors then included Pentagonanthus grandiflorus, which also has eight to 16 pores, in Raphionacme. Our molecular data support the conclusion of Verhoeven and Venter (1994) that Pentagonanthus is a member of the Raphionacme group. The close relationship of P. grandiflora to R. dyeri and R. hirsuta (Figs 3 and 4) cannot be explained morphologically. Raphionacme hirsuta and R. dyeri differ from R. grandiflora with respect to tuber shape and size, foliage, corolla and corona structure. This subclade is relatively strongly supported in the ITS and combined analyses (Figs 3 and 4). However, in the analysis of plastid DNA alone, R. grandiflora switches over to the subclade of the genus comprising R. angolensis, R. elata, R. flanagani and R. madiensis (Fig. 2). Schlechterella is sister to the remaining species of Raphionacme according to the nrDNA dataset (Figures 3 and 4), but forms a trichotomy with the R. dyeri/R. hirsuta subclade and the R. grandiflora/R. angolensis/R. elata/R. flanagani/R. madiensis subclade in the plastid analysis (Fig. 2). Since the Raphionacme s.l. clade (including Schlechterella) is monophyletic in both analyses (Figs 2–4), a close relationship of Raphionacme s.s. (with loose pollen tetrads) to Schlechterella (with pollen tetrads agglutinated into pollinia) is likely. Nevertheless, the generic status of Schlechterella should be maintained, because it is well defined morphologically and genetically. Although the character ‘pollen tetrads forming pollinia’ is clearly a derived state in Periplocoideae (cf. also Nilsson et al. 1993), Schlechterella is most probably an early segregate of the Raphionacme group because it shows some characters regarded as ancestral in Raphionacme: the small, edible tubers, the membranous leaves with thin, long petioles, and the membranous, short‐lived flowers (lasting just 1 d at the most, not fleshy and long‐lived as in Raphionacme). Finally, biogeographic factors support this conclusion. Both species of Schlechterella are East African endemics reaching as far north as Ethiopia, where Raphionacme is nearly absent (Venter and Verhoeven, 1988). (North‐) East Africa has been recognized as centre of origin for many asclepiad groups such as Fockeeae (Kunze et al., 1994) or Ceropegieae (Meve and Liede, 2002). There are three current centres of distribution in Raphionacme: west Africa (up to four species per 5° square), east Africa (up to ten species per 5° square) and south‐east Africa (up to seven species per 5° square) (Venter and Verhoeven, 1988). Apart from east Africa, which cannot be separated distinctly from north‐east Africa, the west and the south‐east African centres must be regarded as secondary.
Stomatostemma forms several globoid root tubers per plant. Usually, these tubers are produced on secondary roots. They reach the size and fleshiness of large potatoes (Fig. 1C). This kind of tuber formation is similar to that found in Ischnolepis (Fig. 1B). We cultivated several plants of two different clones of Stomatostemma, which were both investigated (Table 1 and Figs 2–4). All these plants dev eloped the characteristic tubers which, when growing in cultivation, easily break the pot. Venter and Verhoeven (2001) coded Stomatostemma as non‐tuberous, whereas they coded Mondia as tuberous, which could not be confirmed here (see Introduction). Nevertheless, the molecular data point to a close relationship between these two genera (Figs 2–4). Again, the differences in floral morphology between Mondia and Stomatostemma are considerable, so that Venter and Verhoeven (1997) placed them in two different tribes. In this case, even vegetative morphology fails to indicate a relationship, because in addition to the different root systems, Mondia possesses large, slightly coriacous leaves on smooth, stout stems, whereas Stomatostemma has small, membraneous leaves on wiry, rough stems. Only in their pollen morphology are the two genera similar (Verhoeven and Venter, 1993, fig. 1). However, rhomboidal‐to‐tetrahedal pollen tetrads as seen in these genera seem to represent a plesiomorphic rather than an apomorphic state, because this condition is fairly widespread in the other Periplocoideae (Verhoeven and Venter, 1993). The close and well‐supported relationship between Mondia and Stomatostemma was also found by Potgieter and Albert (2001) (‘Stomatostemma monteiri’ instead of S. monteiroae), who analysed a different set of periplocoid genera.
Ischnolepis graminifolia (= I. tuberosa Jum. & H.Perrier) is an erect shrub with linear, hypostomatic leaves arranged in whorls of three (cf. Klackenberg, 1999). In contrast, Petopentia possesses large, ovate, opposite, amphistomatic leaves (Venter et al., 1990). Red, waxy, smooth stems are stressed by Venter and Verhoeven (2001) in support of their merging of the two taxa. However, Ischnolepis has characteristically swollen nodes which are lacking in Petopentia. Finally, reddish stems also occur in other distantly related taxa (e.g. Raphionacme lobulata Venter & R.L.Verh., cf. Venter and Verhoeven, 1988; Sarcorrhiza, Bullock, 1962b). Thus, red stems are of multiple origin in the subfamily, and cannot be used to support a close relationship between Ischnolepis and Petopentia. Ischnolepis graminifolia bears numerous tubers on lateral roots. Again, this is different from the large, usually single tubers of Petopentia natalensis, which are formed by the main root and are partly exposed above ground (Fig. 1). In the flowers, corolla, corona and anther structure are similar, but the shape of the pollen tetrads in the two genera is different. In Petopentia the pollen tetrads are typically elongated, mainly linear (55–90 × 25–40 µm, cf. Nilsson et al., 1993; Verhoeven and Venter, 1994), whereas in Ischnolepis they are rhomboidal (approx. 52 × 40 µm; U. Meve, unpubl. res., from Röösli s.n., UBT). Although such differences do not necessarily exclude a close relationship, they do not constitute a supporting character, as do the number of pores in the Raphionacme complex (Verhoeven and Venter, 2001). Our sequence data show that Ischnolepis is a rather isolated genus, weakly associated with the Malagasy genera Camptocarpus and Pentopetia according to the plastid DNA data (Fig. 2), while Petopentia is equally weakly associated with Periploca, in this case supported by the ITS data (Figs 3 and 4). The relationship of Petopentia to Periploca is unexpected, since the shrubby‐to‐twining, more or less woody Periploca species do not show too many similarities with Petopentia. The mainly northern hemisphere Periploca (14 species) has the widest distribution of all periplocoid genera, being found from the Canary Islands to China (Venter, 1997). In contrast, the single species of Petopentia is restricted to South Africa (Venter et al., 1990), and thus the two distribution areas do not overlap. However, Verhoeven and Venter (1994) reported the peculiar linear to T‐shaped pollen tetrads, which are shared by only Periploca and Petopentia. This observation hints at one putative morphological synapomorphy of a Petopentia/Periploca clade. From this discussion it becomes clear that Ischnolepis and Petopentia cannot be considered congeneric, and Petopentia is reinstated here (see Taxonomy).
In their tribal classification of Periplocoideae, based on floral characters, Venter and Verhoeven (1997) scattered the representatives of our ‘tuberous clade’ throughout the three tribes they recognized. However, as highlighted in the case of Petopentia and Ischnolepis, floral characters are of limited taxonomic value in Periplocoideae, except for pollen and pollinium characters, which are at least of some value. A careful assessment of all available data, especially the vegetative characters, is necessary for an appropriate classification of Periplocoideae. This is not surprising with regard to information presented in several recently published papers in Apocynaceae subfamily Asclepiadoideae (Liede and Kunze, 2002; Liede and Täuber, 2002; Meve and Liede, 2002), and also in, for example, two tribes of Orchidaceae: Pleurothallidinae (Pridgeon et al., 2001) and Oncidiinae (Williams et al., 2001a, b). Morphological similarities, in particular those of floral structures, are often a poor guide to phylogenetic relationships because of the high degree of parallelism and homoplasy brought about by selection for pollinators. For example, the genus Caralluma s.l. in Asclepiadoideae tribe Ceropegieae has been divided into seven genera, although their flowers are so similar in many cases that they cannot be keyed‐out morphologically. However, the classification based on molecular data is supported by stem and leaf characters, and so the taxa can be keyed‐out using these characters (Meve and Liede, 2002).
No tuberous species is known in Apocynaceae subfamily Secamonoideae, whereas in subfamily Asclepiadoideae root tubers as storage organs are widespread. In the Fockeeae, sister group of the remaining Asclepiadoideae, large, more or less woody tubers are the rule, sometimes including the hypocotyl and basal internodes in addition to the main root (cf. Kunze et al., 1994). In tribe Marsdenieae, slightly woody root tubers, sometimes with tuberous secondary roots as well, are restricted to Marsdenia R.Br. (Forster, 1995; Albers and Meve, 2002). In the closely related and more derived Ceropegieae, fleshy, non‐woody hypocotyl tubers, root tubers and/or tuberous secondary roots are restricted to Brachystelma R.Br. and parts of the species‐rich genus Ceropegia L. (Albers and Meve, 2002). In the fourth tribe Asclepiadeae, semi‐subterranean root tubers are found in one species of subtribe Gonolobinae, Matelea cyclophylla (Standl.) Woodson. Subtribe Metastelminae is also poor in tuberous species (e.g. leafy Cynanchum L. from Madagascar with root tubers or tuberous secondary roots; see photographs in Rauh, 1995), whereas in subtribe Asclepiadinae most species have globose, fusiform or napiform root or stem tubers (sometimes with additional secondary tuberous roots; Goyder, 1998, 2001; Albers and Meve, 2002).
Root succulence is undoubtedly an advanced character typically confined to taxa of arid environments or those with at least a seasonal shortage of water. Within each tribe, tubers never occur in the first‐branching groups (except for the generally root‐succulent Fockeeae). This is the case in Cryptolepis R.Br. (Periplocoideae; Potgieter and Albert, 1991; this paper), Gymnema R.Br. and Cionura Griseb. (Marsdenieae), Heterostemma Wight & Arn. and Lepta denia R.Br. (Ceropegieae; Meve and Liede, 2004), and Calotropis R.Br. (Asclepiadeae; Potgieter and Albert, 2001). Clear delimitations between genera on the basis of tuber morphology as in Alstroemeriaceae (Sanso and Xifreda, 2001), for example, are the exception. Only in a few less‐species‐rich genera such as Raphionacme and Stathmostelma does the possession of tubers serve as a reliable generic marker, whereas it is possible to distinguish between at least two main different types of root succulence in Trachycalymma, Brachystelma and Ceropegia (Goyder, 2001; Albers and Meve, 2002). Biogeographically, root tubers are mostly restricted to Africa, which might be highly influenced by the many different arid to semi‐arid habitats and the occurrence of relevant groups such as Periplocoideae, Fockeeae and Ceropegieae.
Chromosomes are often useful in systematics and taxonomy, but are of restricted taxonomic value in Periplocoideae. Apart from some (auto‐)tetraploids with 2n = 44 chromosomes in Periploca and Raphionacme (Albers and Meve, 2001), all investigated periplocoid taxa show the basal chromosome number of x = 11 (2n = 22). However, all ‘Raphionacme‐like taxa’ investigated, eight Raphionacme species, one Schlechterella species (Albers and Meve, 2001), and Raphionacme (Pentagonanthus) grandiflora (U. Meve, unpubl. res.), possess fairly large chromosomes of approx. 1·5 µm length on average, in contrast to Periploca, Petopentia and Stomatostemma, where the chromosomes are only approx. 1 µm long (Albers and Meve, 2001). Ischnolepis graminifolia and Camptocarpus mauritianus also have small chromosomes, approx. 1·10 µm in length (U. Meve, unpubl. res.).
The Malagasy Periplocoideae occupy the first three branches in the subfamily in our combined analysis. This might lead to the idea that Periplocoideae in general could be of Malagasy origin. Of the 32 genera in the subfamily [31 genera sensu Venter and Verhoeven (2001), plus Petopentia, this paper]; however, only five genera are from Madagascar, whereas 15 are found exclusively in mainland Africa. Except for Ischnolepis, no true tubers are found in any Malagasy taxon and other derived characters are also lacking, including epiphytic growth (Africa: Sarcorrhiza; Bullock, 1962b), (sub)succulence (Africa: Epistemma H.Huber, Raphionacme p.p.), pollen tetrads in pollinia (present in seven genera: two in Africa, four in Asia, one in Australia), clear latex (Africa: Raphionacme namibiana Venter & R.L.Verh.) and linear pollen tetrads (Africa: Petopentia; Asia: Periploca). It can be hypothesized, therefore, that Madagascar houses a stock of relatively early diverged taxa. However, by far the largest number of genera (many monotypic) occur in Africa, so the area of origin of Periplocoideae cannot yet be identified.
TAXONOMY
Petopentia reinstated
Petopentia Bullock, Kew Bulletin 10: 362 (1954).
Type species: Petopentia natalensis (Schltr.) Bullock. Basionym: Pentopetia natalensis Schltr., Journal of Botany and Foreign 32: 257 (1894) = Tacazzea natalensis (Schltr.) N.E.Br. in Dyer, Flora Capensis 4(1): 541 (1907).
Type: Republic of South Africa, KwaZulu‐Natal, Isipingo, Wood s.n. (K, holo).
Description and illustration: Venter and Verhoeven (1993).
Number of species: 1 species.
Distribution: Republic of South Africa (KwaZulu‐Natal, Mpumalanga, Eastern Cape).
ACKNOWLEDGEMENTS
Several collectors provided us with valuable plant material; in particular we thank E. and M. Specks, W. Röösli, M. Endress, A. Nicholas, L. E. Newton, B. Mies and R. D. Mangelsdorff.
Received: 28 August 2002; Returned for revision: 4 August 2003; Accepted: 16 January 2004; Published electronically: 23 February 2004
References
- AlbersF, Meve U.2001. A karyological survey of Asclepiadeae, Periplocoideae and Secamonoideae, and evolutionary considerations in Apocynaceae s.l. In Endress M. Stevens WD, eds. Proceedings of the International Botanical Congress (IBC), St Louis, 1999. Annals of the Missouri Botanical Garden 88: 624–656. [Google Scholar]
- AlbersF, Meve U.2002.Illustrated handbook of succulent plants: Asclepiadaceae. Heidelberg: Springer. [Google Scholar]
- BaldwinBG.1992. Phylogenetic utility of the internal transcribed spacers of nuclear ribosomal DNA in plants: an example from the Compositae. Molecular Phylogenetics and Evolution 1: 3–16. [DOI] [PubMed] [Google Scholar]
- BremerK.1988. The limits of amino acid sequence data in angiosperm phylogenetic reconstruction. Evolution 42: 795–803. [DOI] [PubMed] [Google Scholar]
- BullJJ, Huelsenbeck JP, Cunninngham CW, Swofford DL, Waddell PJ.1993. Partitioning and combining data in phylogenetic analysis. Systematic Biology 42: 384–397. [Google Scholar]
- BullockAA.1962a.Pentagonanthus grandiflorus (N.E.Br.) Bullock (Periplocaceae). Hooker’s Icon. Plantarum 36: t. 3583 + text. [Google Scholar]
- BullockAA.1962b.Sarcorrhiza epiphytica Bullock (Periplocaceae). Hooker’s Icon. Plantarum 36: t. 3585 + text. [Google Scholar]
- DonoghueMJ, Olmstead RG, Smith JF, Palmer JD.1992. Phylogenetic relationships of Dipsacales based on rbcL sequences. Annals of the Missouri Botanical Garden 79: 333–345. [Google Scholar]
- DoyleJJ, Doyle JL.1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19: 11–15. [Google Scholar]
- ErikssonT.1998.AutoDecay, 4·0 edn. Distributed by the author, Bergius Foundation, Royal Swedish Academy of Sciences. Stockholm, Sweden. [Google Scholar]
- FelsensteinJ.1985. Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39: 783–791. [DOI] [PubMed] [Google Scholar]
- ForsterPI.1995. Circumscription of Marsdenia (Asclepiadaceae: Marsdenieae) with a revision of the genus in Australia and Papuasia. Australian Systematic Botany 8: 703–933. [Google Scholar]
- GoyderDJ.1998. A revision of the African genus Stathmostelma K. Schum. (Apocynaceae: Asclepiadeae). Kew Bulletin 53: 577–616. [Google Scholar]
- GoyderDJ.2001. A revision of the tropical African genus Trachycalymma (K. Schum.) Bullock (Apocynaceae Asclepia deae). Kew Bulletin 56: 129–161. [Google Scholar]
- HendyMD,Penny D.1982. Branch and bound algorithms to determine minimal evolutionary trees. Mathematical Biosciences 59: 277–290. [Google Scholar]
- KlackenbergJ.1998. Taxonomy and phylogeny of the genus Camptocarpus s.l. (Periplocoideae, Asclepiadaceae). Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzen geographie 120: 45–85. [Google Scholar]
- KlackenbergJ.1999. Revision of the Malagasy genera Pentopetia and Ischnolepis (Apocynaceae s.l. Periplocoideae). Candollea 54: 257–339. [Google Scholar]
- KunzeH, Meve U, Liede S.1994.Cibirhiza albersiana, a new species of Asclepiadaceae, and establishment of the tribe Fockeeae. Taxon 43: 367–376. [Google Scholar]
- LiedeS, Kunze H.2002.Cynanchum and the Cynanchinae (Apocynaceae – Asclepiadeae) – a molecular, anatomical and latex triterpenoid study. Organisms, Diversity & Evolution 2: 239–269. [Google Scholar]
- LiedeS, Täuber A.2002. Circumscription of the genus Cynanchum (Apocynaceae – Asclepiadeae). Systematic Botany 27: 789–800. [Google Scholar]
- MeveU.2002. Species numbers and progress in asclepiad taxonomy. Kew Bulletin 57: 459–464. [Google Scholar]
- MeveU, Liede S.2001a. Inclusion of Tenaris and Macropetalum in Brachystelma (Apocynaceae‐Asclepiadeae‐Ceropegieae) inferred from non‐coding nuclear and chloroplast DNA sequences. Plant Systematics and Evolution 228: 89–105. [Google Scholar]
- MeveU, Liede S.2001b. Reconsideration of the status of Lavrania, Larryleachia and Notechidnopsis (Asclepiadeae‐Ceropegieae). South African Journal of Botany 66: 161–168. [Google Scholar]
- MeveU, Liede S.2002. A molecular phylogeny and generic rearrangement of the stapelioid Ceropegieae (Apocynaceae‐Asclepiadeae). Plant Systematics and Evolution 234: 171–209. [Google Scholar]
- MeveU, Liede S.2004. Subtribal division of Ceropegieae (Apocynaceae‐Asclepiadeae). Taxon, in press. [Google Scholar]
- OliverD.1887. Chlorocyathus monteiroae. Hooker’s Icones Plantarum: t. 1591. [Google Scholar]
- NilssonS, Endress ME, Grafström E.1993. On the relationships of the Apocynaceae and Periplocaceae. Grana Palynologica Suppl. 2: 3–20. [Google Scholar]
- PotgieterK, Albert VA.2001. Phylogenetic relationships within Apocynaceae s.l. based on trnL intron and trnL‐F spacer sequences and propagule characters. In: Endress M, Stevens WD, eds. Proceedings of the International Botanical Congress (IBC), St Louis, 1999. Annals of the Missouri Botanical Garden 88: 623–649. [Google Scholar]
- PridgeonAM, Solano R, Chase MW.2001. Phylogenetic relationships in Pleurothallidinae (Orchidaceae): combined evidence from nuclear and plastid DNA sequences. American Journal of Botany 88: 2286–2308. [PubMed] [Google Scholar]
- RauhW.1995.Succulents and xerophytes of Madagascar, Vol. 1. Strawberry Press. [Google Scholar]
- SandersonMJ, Donoghue MJ, Piel W, Eriksson T.1994. TreeBASE: a prototype database of phylogenetic analyses and an interactive tool for browsing the phylogeny of life. American Journal of Botany 81: 183. [Google Scholar]
- SansoAM, Xifreda CC.2001. Generic delimitation between Alstroemeria and Bomarea (Alstroemeriaceae). Annals of Botany 88: 1050–1069. [Google Scholar]
- SwoffordDL.1998.PAUP*. Phylogenetic analysis using parsimony (*and other methods). Version 4. Sunderland, MA: Sinauer Associates. [Google Scholar]
- TaberletP, Gielly L, Pautou G, Bouvet J.1991. Universal primers for amplification of three non‐coding regions of chloroplast DNA. Plant Molecular Biology 17: 1105–1109. [DOI] [PubMed] [Google Scholar]
- VenterHJT.1997. A revision of Periploca (Periplocaceae). South African Journal of Botany 63: 123–128. [Google Scholar]
- VenterHJT, Verhoeven RL.1988. Phytogeography of Raphionacme Harv. (Periplocaceae). Monograph. Monographs in Systematic Botany from the Missouri Botanical Garden St Louis 25: 687–697. [Google Scholar]
- VenterHJT, Verhoeven RL.1993. A taxonomic account of Stomatostemma South African Journal of Botany 59: 50–56. [Google Scholar]
- VenterHJT, Verhoeven RL.1997. A tribal classification of the Periplocoideae (Apocynaceae). Taxon 46: 705–720. [Google Scholar]
- VenterHJT, Verhoeven RL.1998. A taxonomic revision of Schlechterella (Periplocoideae, Apocynaceae). South African Journal of Botany 64: 350–355. [Google Scholar]
- VenterHJT, Verhoeven RL.2001. Diversity and relationships within Periplocoideae. Annals of the Missouri Botanical Garden 88: 550–568. [Google Scholar]
- VenterHJT, Verhoeven RL, Kotze JDS.1990. The genus Petopentia (Periplocaceae). South African Journal of Botany 56: 393–398. [Google Scholar]
- VerhoevenRL, Venter HJT.1988. Pollen morphology of Raphionacme (Periplocaceae). South African Journal of Botany 54: 123–132. [Google Scholar]
- VerhoevenRL, Venter HJT.1993. Pollen morphology of Curroria, Mondia, Socotranthus and Stomatostemma (Periplocaceae). Bothalia 23: 105–110. [Google Scholar]
- VerhoevenRL, Venter HJT.1994. A preliminary report on the pollen morphology of the Periplocaceae. In: Seyani JH, Chikuni AC, eds. Proceedings of the XIIIth Plenary Meeting AETFAT, Malawi, 2: 1239–1255. [Google Scholar]
- VerhoevenRL, Venter HJT.1998. Pollinium structure in Periplocoideae (Apocynaceae). Grana 37: 1–14. [Google Scholar]
- WilliamsNH, Chase MW, Fulcher T, Whitten WM.2001a. Molecular systematics of the Oncidiinae based on evidence from four DNA sequence regions: expanded circumscriptions of Cyrtochilum, Erycina, Otoglossum, and Trichocentrum and a new genus (Orchidaceae). Lindleyana 16: 113–139. [Google Scholar]
- WilliamsNH, Chase MW, Whitten WM.2001b. Phylogenetic position of Miltoniopsis, Caucaea, a new genus, Cyrtochiloides, and relationship of Oncidium phymatochilum based on nuclear and chloroplast dna sequence data (Orchidaceae: Oncidiinae). Lindleyana 16: 272–285. [Google Scholar]