Abstract
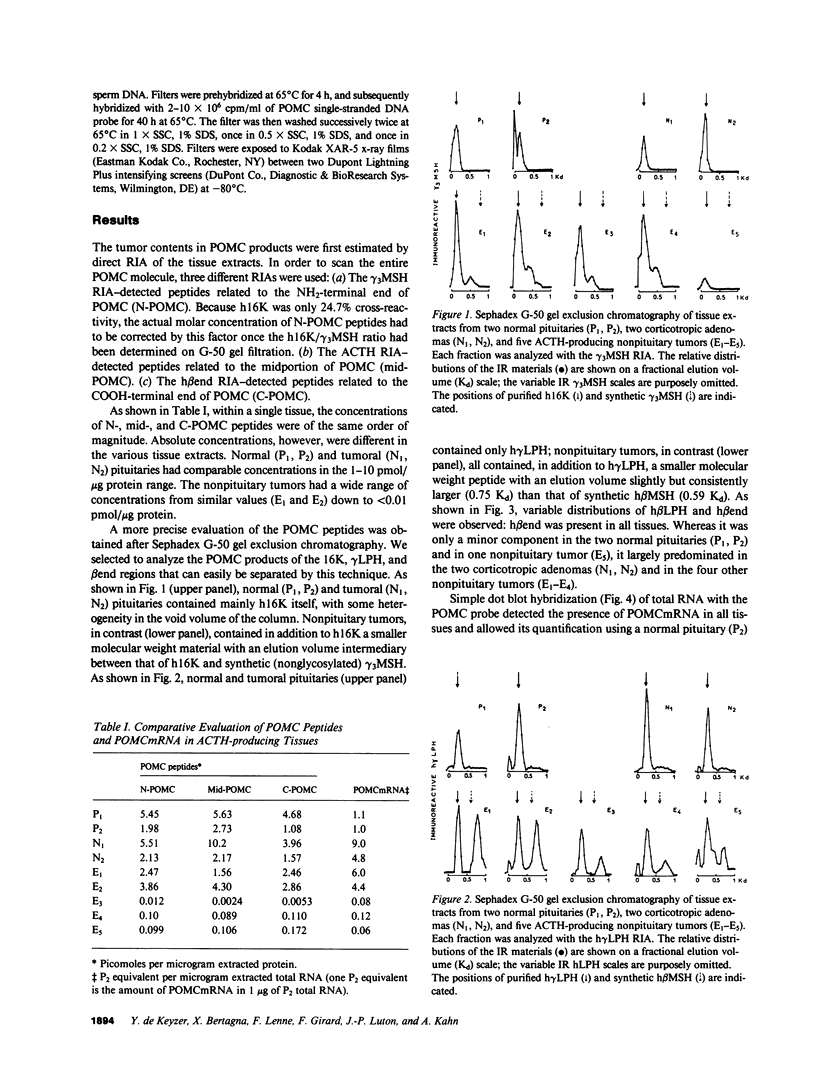
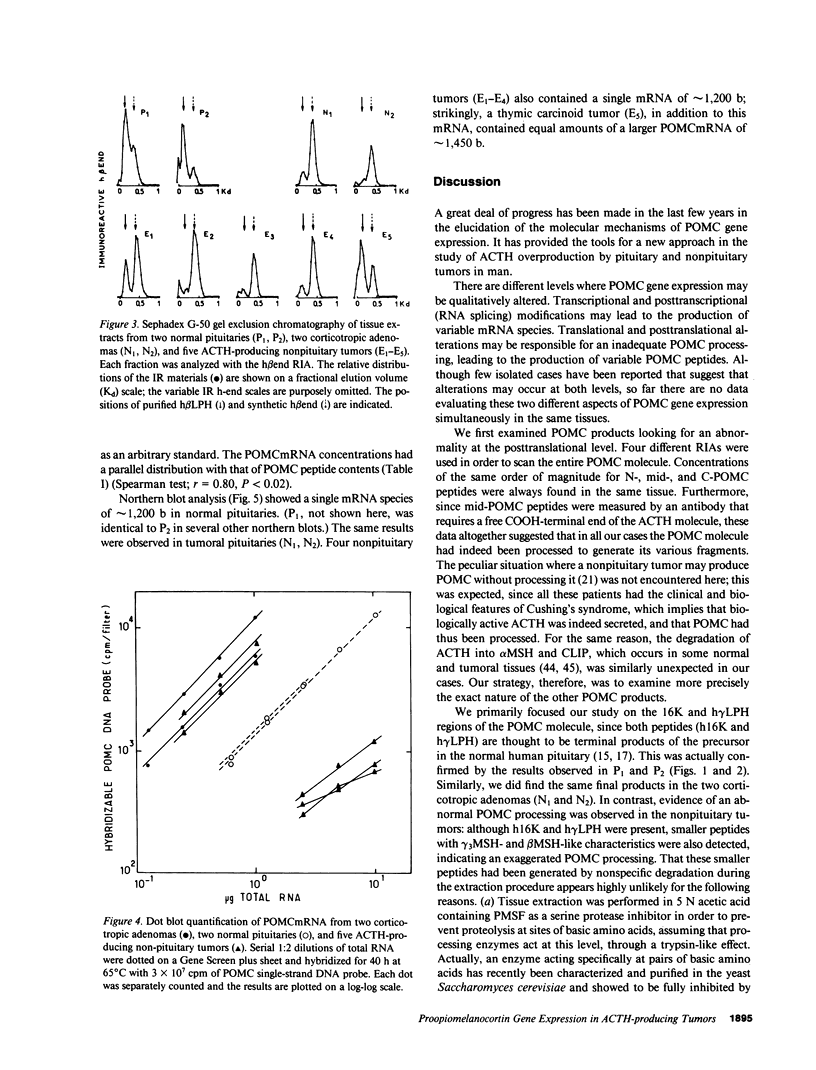
In order to assess the mechanisms of proopiomelanocortin (POMC) gene expression in human ACTH-producing tumors, we performed the simultaneous evaluation of POMC products and messenger RNA (mRNA) in tissue fragments obtained from two corticotropic adenomas, five nonpituitary tumors, and two normal human pituitaries. The POMC products were examined using a combination of gel exclusion chromatography and four different radioimmunoassays directed against gamma 3 melanocyte stimulating hormone (gamma 3MSH), ACTH, gamma-lipotropin (gamma LPH), and beta-endorphin. The POMCmRNA was detected and analyzed by dot and northern blot hybridization using a single-stranded genomic DNA probe corresponding to the coding region of the human POMC gene. Tissue concentrations of POMC products and mRNA showed parallel distributions. Immunoreactive gamma 3MSH and gamma LPH patterns revealed only 16-kD fragment- and gamma LPH-like peptides in normal and tumoral pituitaries; additional gamma 3MSH- and/or beta MSH-like peptides were found in all five nonpituitary tumors. A single POMCmRNA of approximately 1,200 bases (b) was detected in normal and tumoral pituitaries; a single identical POMCmRNA was also found in four nonpituitary tumors. A thymic carcinoid tumor, in addition to the 1,200-b POMCmRNA, contained equal amounts of a second larger POMCmRNA of approximately 1,450 b. It is concluded that POMC gene expression appears qualitatively unaltered in corticotropic adenomas. In nonpituitary tumors, in contrast, abnormal POMC processing is frequent; in addition, an extra POMCmRNA was detected in a thymic tumor with a greater length than the normal mRNA; the mechanisms and pathophysiological implications of these modifications remain to be elucidated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. G., Orwoll E., Kendall J. W., Herbert E., Paxton H. The distribution of forms of adrenocorticotropin and beta-endorphin in normal, tumorous, and autopsy human anterior pituiary tissue: virtual absence of 13K adrenocorticotropin. J Clin Endocrinol Metab. 1980 Aug;51(2):376–380. doi: 10.1210/jcem-51-2-376. [DOI] [PubMed] [Google Scholar]
- Bailey J. M., Davidson N. Methylmercury as a reversible denaturing agent for agarose gel electrophoresis. Anal Biochem. 1976 Jan;70(1):75–85. doi: 10.1016/s0003-2697(76)80049-8. [DOI] [PubMed] [Google Scholar]
- Bertagna X. Y., Stone W. J., Nicholson W. E., Mount C. D., Orth D. N. Simultaneous assay of immunoreactive beta-lipotropin, gamma-lipotropin, and beta-endorphin in plasma of normal human subjects, patients with ACTH/lipotropin hypersecretory syndromes, and patients undergoing chronic hemodialysis. J Clin Invest. 1981 Jan;67(1):124–133. doi: 10.1172/JCI110004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertagna X., Seurin D., Pique L., Luton J. P., Bricaire H., Girard F. Peptides related to the NH2-terminal end of proopiocortin in man. J Clin Endocrinol Metab. 1983 Mar;56(3):489–495. doi: 10.1210/jcem-56-3-489. [DOI] [PubMed] [Google Scholar]
- Boileau G., Barbeau C., Jeannotte L., Chrétien M., Drouin J. Complete structure of the porcine pro-opiomelanocortin mRNA derived from the nucleotide sequence of cloned cDNA. Nucleic Acids Res. 1983 Nov 25;11(22):8063–8071. doi: 10.1093/nar/11.22.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cochet M., Cohen S. N. Structural organization of human genomic DNA encoding the pro-opiomelanocortin peptide. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4890–4894. doi: 10.1073/pnas.77.8.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. L., Mather J. P., Morris P. L., Bardin C. W. Expression of pro-opiomelanocortin-like gene in the testis and epididymis. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5672–5675. doi: 10.1073/pnas.81.18.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelli O., Birnberg N., Herbert E. Detection and quantitation of pro-opiomelanocortin mRNA in pituitary and brain tissues from different species. J Biol Chem. 1982 Jun 25;257(12):6783–6787. [PubMed] [Google Scholar]
- DeBold C. R., Schworer M. E., Connor T. B., Bird R. E., Orth D. N. Ectopic pro-opiolipomelanocortin: sequence of cDNA coding for beta-melanocyte-stimulating hormone and beta-endorphin. Science. 1983 May 13;220(4598):721–723. doi: 10.1126/science.6301015. [DOI] [PubMed] [Google Scholar]
- DeNoto F. M., Moore D. D., Goodman H. M. Human growth hormone DNA sequence and mRNA structure: possible alternative splicing. Nucleic Acids Res. 1981 Aug 11;9(15):3719–3730. doi: 10.1093/nar/9.15.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin J., Goodman H. M. Most of the coding region of rat ACTH beta--LPH precursor gene lacks intervening sequences. Nature. 1980 Dec 11;288(5791):610–613. doi: 10.1038/288610a0. [DOI] [PubMed] [Google Scholar]
- Eberwine J. H., Roberts J. L. Analysis of pro-opiomelancortin gene structure and function. DNA. 1983;2(1):1–8. doi: 10.1089/dna.1.1983.2.1. [DOI] [PubMed] [Google Scholar]
- Eipper B. A., Mains R. E. Structure and biosynthesis of pro-adrenocorticotropin/endorphin and related peptides. Endocr Rev. 1980 Winter;1(1):1–27. doi: 10.1210/edrv-1-1-1. [DOI] [PubMed] [Google Scholar]
- Jingami H., Nakanishi S., Imura H., Numa S. Tissue distribution of messenger RNAs coding for opioid peptide precursors and related RNA. Eur J Biochem. 1984 Aug 1;142(3):441–447. doi: 10.1111/j.1432-1033.1984.tb08306.x. [DOI] [PubMed] [Google Scholar]
- Kahn A., Cottreau D., Daegelen D., Dreyfus J. C. Cell-free translation of messenger RNAs from adult and fetal human muscle. Characterization of neosynthesized glycogen phosphorylase, phosphofructokinase and glucose phosphate isomerase. Eur J Biochem. 1981 May;116(1):7–12. doi: 10.1111/j.1432-1033.1981.tb05293.x. [DOI] [PubMed] [Google Scholar]
- MEADOR C. K., LIDDLE G. W., ISLAND D. P., NICHOLSON W. E., LUCAS C. P., NUCKTON J. G., LUETSCHER J. A. Cause of Cushing's syndrome in patients with tumors arising from "nonendocrine" tissue. J Clin Endocrinol Metab. 1962 Jul;22:693–703. doi: 10.1210/jcem-22-7-693. [DOI] [PubMed] [Google Scholar]
- Messing J., Crea R., Seeburg P. H. A system for shotgun DNA sequencing. Nucleic Acids Res. 1981 Jan 24;9(2):309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Matsuo H. A novel protease from yeast with specificity towards paired basic residues. Nature. 1984 Jun 7;309(5968):558–560. doi: 10.1038/309558a0. [DOI] [PubMed] [Google Scholar]
- NELSON D. H., MEAKIN J. W., THORN G. W. ACTH-producing pituitary tumors following adrenalectomy for Cushing's syndrome. Ann Intern Med. 1960 Mar;52:560–569. doi: 10.7326/0003-4819-52-3-560. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Inoue A., Kita T., Nakamura M., Chang A. C., Cohen S. N., Numa S. Nucleotide sequence of cloned cDNA for bovine corticotropin-beta-lipotropin precursor. Nature. 1979 Mar 29;278(5703):423–427. doi: 10.1038/278423a0. [DOI] [PubMed] [Google Scholar]
- Oates E., Herbert E. 5' sequence of porcine and rat pro-opiomelanocortin mRNA. One porcine and two rat forms. J Biol Chem. 1984 Jun 25;259(12):7421–7425. [PubMed] [Google Scholar]
- Orth D. N., Nicholson W. E., Mitchell W. M., Island D. P., Liddle G. W. Biologic and immunologic characterization and physical separation of ACTH and ACTH fragments in the ectopic ACTH syndrome. J Clin Invest. 1973 Jul;52(7):1756–1769. doi: 10.1172/JCI107357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintar J. E., Schachter B. S., Herman A. B., Durgerian S., Krieger D. T. Characterization and localization of proopiomelanocortin messenger RNA in the adult rat testis. Science. 1984 Aug 10;225(4662):632–634. doi: 10.1126/science.6740329. [DOI] [PubMed] [Google Scholar]
- Pique L., Jegou S., Bertagna X., Javoy-Agid F., Seurin D., Proeschel M. F., Girard F., Agid Y., Vaudry H., Luton J. P. Pro-opiomelanocortin peptides in the human hypothalamus: comparative study between normal subjects and Parkinson patients. Neurosci Lett. 1985 Mar 15;54(2-3):141–146. doi: 10.1016/s0304-3940(85)80069-0. [DOI] [PubMed] [Google Scholar]
- Pullan P. T., Clement-Jones V., Corder R., Lowry P. J., Besser G. M., Rees L. H. ACTH LPH and related peptides in the ectopic ACTH syndrome. Clin Endocrinol (Oxf) 1980 Nov;13(5):437–445. doi: 10.1111/j.1365-2265.1980.tb03409.x. [DOI] [PubMed] [Google Scholar]
- Ratcliffe J. G., Scott A. P., Bennett H. P., Lowry P. J., McMartin C., Strong J. A., Walbaum P. R. Production of a corticotrophin-like intermediate lobe peptide and of corticotrophin by a bronchial carcinoid tumour. Clin Endocrinol (Oxf) 1973 Jan;2(1):51–55. doi: 10.1111/j.1365-2265.1973.tb03484.x. [DOI] [PubMed] [Google Scholar]
- Roberts J. L., Seeburg P. H., Shine J., Herbert E., Baxter J. D., Goodman H. M. Corticotropin and beta-endorphin: construction and analysis of recombinant DNA complementary to mRNA for the common precursor. Proc Natl Acad Sci U S A. 1979 May;76(5):2153–2157. doi: 10.1073/pnas.76.5.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. G., Amara S. G., Roos B. A., Ong E. S., Evans R. M. Altered expression of the calcitonin gene associated with RNA polymorphism. Nature. 1981 Mar 5;290(5801):63–65. doi: 10.1038/290063a0. [DOI] [PubMed] [Google Scholar]
- Scott A. P., Lowry P. J. Adrenocorticotrophic and melanocyte-stimulating peptides in the human pituitary. Biochem J. 1974 Jun;139(3):593–602. doi: 10.1042/bj1390593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A. P., Lowry P. J., Ratcliffe J. G., Rees L. H., Landon J. Corticotrophin-like peptides in the rat pituitary. J Endocrinol. 1974 Jun;61(3):355–367. doi: 10.1677/joe.0.0610355. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Rochemont J., Hamelin J., Lis M., Chrétien M. Primary structure of the major human pituitary pro-opiomelanocortin NH2-terminal glycopeptide. Evidence for an aldosterone-stimulating activity. J Biol Chem. 1981 Aug 10;256(15):7977–7984. [PubMed] [Google Scholar]
- Simon M. P., Besmond C., Cottreau D., Weber A., Chaumet-Riffaud P., Dreyfus J. C., Trépat J. S., Marie J., Kahn A. Molecular cloning of cDNA for rat L-type pyruvate kinase and aldolase B. J Biol Chem. 1983 Dec 10;258(23):14576–14584. [PubMed] [Google Scholar]
- Steenbergh P. H., Höppener J. W., Zandberg J., Roos B. A., Jansz H. S., Lips C. J. Expression of the proopiomelanocortin gene in human medullary thyroid carcinoma. J Clin Endocrinol Metab. 1984 May;58(5):904–908. doi: 10.1210/jcem-58-5-904. [DOI] [PubMed] [Google Scholar]
- Suda T., Abe Y., Demura H., Demura R., Shizume K., Tamahashi N., Sasano N. ACTH, beta-LPH and beta-endorphin in pituitary adenomas of the patients with Cushing's disease: activation of beta-LPH conversion to beta-endorphin. J Clin Endocrinol Metab. 1979 Sep;49(3):475–477. doi: 10.1210/jcem-49-3-475. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Hakamata Y., Watanabe Y., Kikuno R., Miyata T., Numa S. Complete nucleotide sequence of the human corticotropin-beta-lipotropin precursor gene. Nucleic Acids Res. 1983 Oct 11;11(19):6847–6858. doi: 10.1093/nar/11.19.6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Nicholson W. E., Orth D. N. The nature of the immunoreactive lipotropins in human plasma and tissue extracts. J Clin Invest. 1978 Jul;62(1):94–104. doi: 10.1172/JCI109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada T., Nakai Y., Jingami H., Imura H., Taii S., Nakanishi S., Numa S. Identification of the mRNA coding for the ACTH-beta-lipotropin precursor in a human ectopic ACTH-producing tumor. Biochem Biophys Res Commun. 1981 Jan 30;98(2):535–540. doi: 10.1016/0006-291x(81)90873-1. [DOI] [PubMed] [Google Scholar]
- Tsukada T., Watanabe Y., Nakai Y., Imura H., Nakanishi S., Numa S. Repetitive DNA sequences in the human corticotropin-beta-lipotrophin precursor gene region: Alu family members. Nucleic Acids Res. 1982 Mar 11;10(5):1471–1479. doi: 10.1093/nar/10.5.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda M., Takeuchi T., Abe K., Miyakawa S., Ohnami S., Yanaihara N. beta-Melanocyte-stimulating hormone immunoreactivity in human pituitaries and ectopic adrenocorticotropin-producing tumors. J Clin Endocrinol Metab. 1980 Mar;50(3):550–556. doi: 10.1210/jcem-50-3-550. [DOI] [PubMed] [Google Scholar]
- Uhler M., Herbert E. Complete amino acid sequence of mouse pro-opiomelanocortin derived from the nucleotide sequence of pro-opiomelanocortin cDNA. J Biol Chem. 1983 Jan 10;258(1):257–261. [PubMed] [Google Scholar]
- Whitfeld P. L., Seeburg P. H., Shine J. The human pro-opiomelanocortin gene: organization, sequence, and interspersion with repetitive DNA. DNA. 1982;1(2):133–143. doi: 10.1089/dna.1.1982.1.133. [DOI] [PubMed] [Google Scholar]
- Wilson R. E., Orth D. N., Nicholson W. E., Mount C. D., Bertagna X. Y. Human gamma-lipotropin radioimmunoassay: identification of immunoreactive gamma-lipotropin in human plasma and tissue. J Clin Endocrinol Metab. 1981 Jul;53(1):1–9. doi: 10.1210/jcem-53-1-1. [DOI] [PubMed] [Google Scholar]
- Yalow R. S., Berson S. A. Size heterogeneity of immunoreactive human ACTH in plasma and in extracts of pituitary glands and ACTH-producing thymoma. Biochem Biophys Res Commun. 1971 Jul 16;44(2):439–445. doi: 10.1016/0006-291x(71)90620-6. [DOI] [PubMed] [Google Scholar]