Abstract
An important function of the seed coat is to deliver nutrients to the embryo. To relate this function to anatomical characteristics, the developing seed coat of pea (Pisum sativum L.) was examined by light‐ and cryo‐scanning electron microscopy (cryo‐SEM) from the late pre‐storage phase until the end of seed filling. During this time the apparently undifferentiated seed coat tissues evolve into the epidermal macrosclereids, the hypodermal hourglass cells, chlorenchyma, ground parenchyma and branched parenchyma. Using the fluorescent symplast tracer 8‐hydroxypyrene‐1,3,6‐trisulfonic acid, it could be demonstrated that solutes imported by the phloem move into the chlorenchyma and ground parenchyma, but not into the branched parenchyma. From a comparison with literature data of common bean (Phaseolus vulgaris L.) and broad bean (Vicia faba L.), it is concluded that in the three species different parenchyma layers, but not the branched parenchyma, may be involved in the post‐phloem symplasmic transport of nutrients in the seed coat. In pea, the branched parenchyma dies during the storage phase, and its cell wall remnants then form the boundary layer between the living seed coat parenchyma cells and the cotyledons. Using cryo‐SEM, clear images were obtained of this boundary layer which showed that many intracellular spaces in the seed coat parenchyma are filled with an aqueous solution. This is suggested to facilitate the diffusion of nutrients from the site of unloading towards the cotyledons.
Key words: Pisumsativum L., pea, legume, seed coat, anatomy, development, transport, phloem unloading, cryo‐SEM, pyranine, HPTS, apoplast
INTRODUCTION
The seed coat of legumes consists mainly of parenchyma cells with an outer layer of sclerenchyma and a vascular system embedded in it. The vasculature of the seed coat in members of the Fabaceae is variable and is probably of taxonomic significance (Corner, 1951). For example, soybean (Glycine max) and common bean (Phaseolus vulgaris), which belong to the tribe Phaseoleae, have seed coats with an extensive vascular network, whereas those of pea (Pisum sativum) and broad bean (Vicia faba) of the tribe Vicieae contain only a single chalazal vein with two lateral branches (Hardham, 1976; Thorne, 1981; Offler and Patrick, 1984, 1993; Offler et al., 1989; Patrick et al., 1995). Seed coats have two major functions: the protection of the embryo in the ripe seed and the supply of nutrients during seed development (Boesewinkel and Bouman, 1995). These nutrients are almost exclusively imported through the phloem and include the main organic nutrients, sucrose and amino acids, as well as potassium and micronutrients (Patrick, 1997; Patrick and Offler, 2001). Sucrose leaves the seed coat unaltered, but during the pre‐storage phase it is hydrolysed by extracellular invertases, at least in Vicia (Weber et al., 1997). Amino acids imported via the phloem undergo elaborate metabolic conversions in the seed coat before being released into the apoplast (Lanfermeijer et al., 1992).
Structural aspects of the nutrition of developing seeds have been studied extensively in grain legumes. Studies of early developmental stages in P. sativum (Marinos, 1970; Hardham, 1976) and V. faba (Johansson and Walles, 1994) have focused on transfer cell‐like structures, particularly those in the embryo sac and adjacent integumentary (seed coat) cells in the vicinity of the embryo. During the storage phase (seed filling), phloem‐imported nutrients are released into the apoplast and are then taken up by the cotyledons, which are the main storage organs in the seeds of grain legumes (Patrick, 1997; Patrick and Offler, 2001). It was originally thought that the sieve elements in the vascular bundles of the seed coat deliver their contents directly into the apoplast (Thorne, 1981; Wolswinkel and Ammerlaan, 1983; P. Wolswinkel, pers. comm.). However, quantitative studies of the release of sucrose, amino acids and of 14C‐labelled photosynthates, the assessment of plasmodesmatal connections between sieve elements and the various types of parenchyma cells, and the use of a fluorescent symplastic tracer have provided ample evidence that the phloem‐imported nutrients move first into seed coat parenchyma cells before being released into the apoplast (Patrick and Offler, 1995).
The aim of the present work was to relate anatomical features of the developing pea seed coat as revealed by light microscopy and cryo‐scanning electron microscopy (cryo‐SEM) to its function as a passageway for nutrients from mother plant to embryo. Anatomical changes are reported in the pea seed coat between 10 and 30 d post anthesis (dpa); to the best of our knowledge these have not been documented comprehensively in the literature. The post‐phloem symplastic route of nutrients was also investigated. To this end 8‐hydroxypyrene‐1,3,6‐trisulfonic acid (HPTS), also known as pyranine, was used as an alternative to the commonly used carboxyfluoresce (CF). HPTS has been shown to be phloem‐mobile, and because of the low membrane permeability its symplasmic transport may be less influenced by vacuolar sequestration than that of CF (Wright and Oparka, 1996).
MATERIALS AND METHODS
Plants
Pea plants (Pisum sativum L. ‘Marzia’) were grown in soil in a growth chamber with a daily regime of 16 h light at 18 °C and 8 h dark at 15 °C. Developing seeds at 10 dpa were taken from pods tagged at the time of flowering. Seed developmental stages corresponding with 15, 20 and 30 dpa were deduced from the relative water content of the cotyledons (Lanfermeijer et al., 1989).
Light microscopy
Pieces of pea seed coat tissue (a few mm3) were fixed for 5 d in a fixative containing 0·2 % glutaraldehyde, 3 % paraformaldehyde, 2 mm CaCl2, 10 mm sucrose and 25 mm Pipes adjusted to pH 7·5. The tissue was then rinsed three times for 10 min with 25 mm Pipes, pH 7·5, and dehydrated through an increasing ethanol series (30, 50, 70, 80, 96 and 100 % twice) at 15 min intervals. Ethanol was replaced with L.R. White acrylic polymer in ethanol [London Resin, JEOL (Europe), Schiphol‐Rijk, The Netherlands] in a graded series from 20 to 100 %. Tissue was embedded in plastic capsules and polymerized under ultraviolet light for 24–48 h. The entire procedure was performed at 4 °C.
Slices 1 µm thick were cut on a glass knife using a Reichert OMU3 ultramicrotome (Leica, Bensheim, Germany), and subsequently placed on slides coated with 1 % gelatine, dried and stretched at 80 °C, and stained with 0·5 % toluidine blue in water for 5–10 s. Micrographs were taken on an Olympus BX50 WI microscope equipped with a digital camera (Sony DKC5000; Olympus Nederland, Rotterdam, The Netherlands).
Cryo‐planing and cryo‐SEM
Cryo‐SEM images were obtained from fresh seeds fixed to a holder with tissue freezing medium (TBS; Electron Microscopy Sciences, Washington DC, USA) and frozen in liquid propane at –185 °C. Using a cryo‐ultra microtome (Reichert UltraCut E/FC4D; Leica, Germany) the seeds (–90 °C) were pre‐cut with a glass knife (–100 °C) to create a flat surface. The ultimate planing was done with an 8‐mm diamond knife (Histo no‐through; Drukker International, The Netherlands) at –90 °C by automatically grazing sections of 100–10 nm (Nijsse and Van Aelst, 1999). If no cryo‐fractioning was carried out, the tissue was frozen in liquid nitrogen and broken inside the cryo‐preparation chamber (T1500 HF; Oxford Instruments, Witney, England) attached to the microscope. The specimens were partly freeze‐dried for 2 min at –90 °C and 10–7 mbar. After sputtercoating with 10 nm platinum, samples were analysed in a field emission cryo‐scanning electron microscope (JEOL 6300F; Tokyo, Japan) at a temperature of –190 °C. Images were recorded digitally, and photo processing was done with Adobe PhotoShop 5.5.
Revealing the post‐phloem transport pathway using the fluorescent dye HPTS
Fully‐grown pea plants (8 weeks after germination) were cut one internode below the pod and placed in a vial containing a solution of 1 mg ml–1 8‐acetoxypyrene‐1,3,6‐trisulfonic acid, trisodium salt (Ac‐HPTS; Molecular Probes Europe, Leiden, The Netherlands), 400 mm sorbitol, 5 mm Mes/Tris, pH 5·5. The shoots were left at 100 % relative air humidity for 24–48 h. Hand‐cut slices (150–250 µm thick) of the seed coat were placed in 50 % (v/v) glycerol in water on a glass slide and viewed using an epifluorescence microscope (Olympus BX50‐WI equipped with a U‐URA reflected light illuminator and U‐MWB filter block). Photographs were taken on a Sony DKC5000 digital camera and processed using Adobe PhotoShop 5.5.
RESULTS AND DISCUSSION
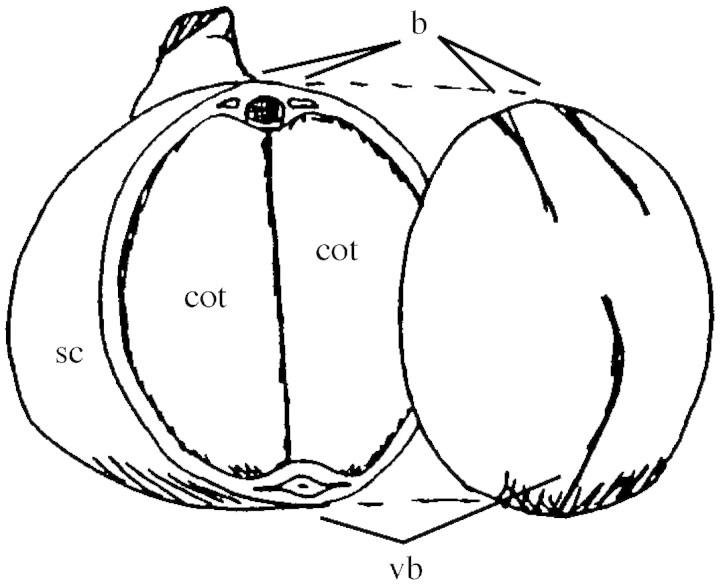
During seed development the main function of the seed coat is the release of nutrients for the embryo. These nutrients enter the seed through a single vascular bundle in the funiculus that extends into the seed coat as the chalazal vein and its two lateral branches (Fig. 1; Hardham, 1976). The chalazal vein consists of a central xylem strand surrounded by phloem elements (amphicribral bundle), and encircles about three‐quarters of the seed. The two lateral branches are much shorter, contain only phloem tissue, and run more or less parallel to the radicle of the embryo.
Fig. 1. Cut‐away view of a mature pea seed, showing the seed coat (sc) and the cotyledons (cot) of the embryo inside. Note the chalazal vascular bundle (vb) that encircles about three‐quarters of the seed and the two short side branches that contain phloem only (b).
Structure of the seed coat during development
At four different developmental stages between 10 and 30 dpa, bright‐field micrographs were made of cross‐sections through the seed coat in the chalazal vein region (Fig. 2). Cryo‐SEM micrographs gave additional information about structural aspects (Figs 3–5). At 10 dpa, the youngest stage shown, the embryo is still very small and is surrounded by liquid endosperm. Between 10 and 15 dpa cell expansion causes an increase in the size of the seed coat (Fig. 2). At 15 dpa the liquid endosperm has largely disappeared and the expanding cotyledons start to press against the seed coat and flatten its inner cell layers.
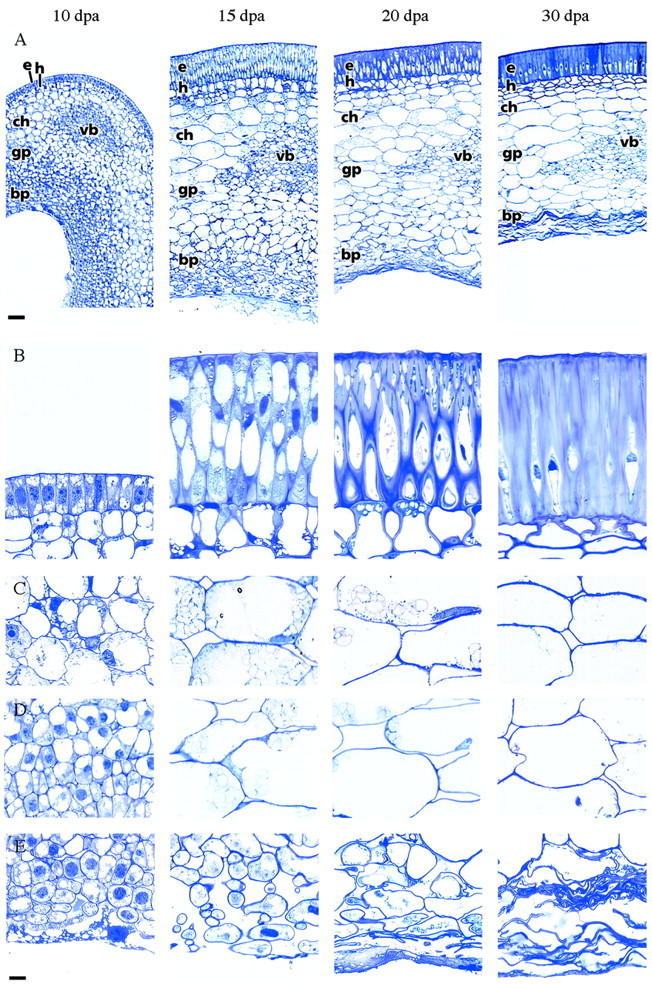
Fig. 2. Cross‐sections through the seed coat in the chalazal vein region at four different developmental stages between 10 and 30 dpa. A, Overview. B, Epidermis and hypodermis. Between 10–15 dpa the epidermis cells elongate and the hypodermis cells become hourglass shaped. Substantial cell wall formation takes place in both cell layers from approx. 20 dpa onwards. C, Chlorenchyma. Starch disappears after 20 dpa. D, Ground parenchyma. E, Branched parenchyma. Before this cell layer is compressed by the expanding cotyledons (from 20 dpa onwards), it is characterized by small, irregular cells with extensive intercellular spaces. e, Epidermis; h, hypodermis; ch, chlorenchyma; gp, ground parenchyma; bp, branched parenchyma; vb, amphicribral vascular bundle. Bars represent 100 µm (A), or 10 µm (B–E).
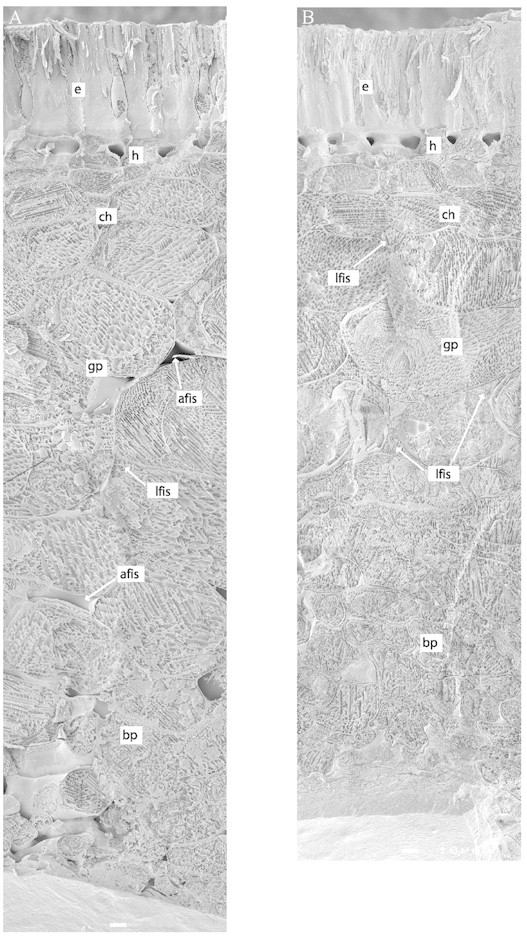
Fig. 3. Cryo‐SEM micrographs of seed coats at approx. 12 (A) and 20 dpa (B). The rapid freezing of the seed coat fixes water where it actually is in vivo, enabling a distinction to be made between water‐filled and air‐filled intercellular spaces. Note that the inner seed coat parenchyma cells get squeezed during development resulting in a mat of cell wall material. e, Epidermis; h, hypodermis; ch, chlorenchyma; gp, ground parenchyma; bp, branched parenchyma; lfis, liquid‐filled intercellular space; afis, air‐filled intercellular space. Bars represent 10 µm.
The outermost cell layer is the epidermis with a cuticle that covers the entire seed coat (Fig. 3). In young seed coats (10 dpa) the protodermal cells have a dense cytoplasm and several small vacuoles (Fig. 2B). Between 10 and 15 dpa protodermal cells elongate to about four times their original length and differentiate into the palisade of epidermal macrosclereids, which are orientated perpendicular to the seed surface (Figs 2 and 3).
The hypodermis is located just below the epidermis (Figs 2–4). In young stages (until 10 dpa) the cells in this cell layer are very similar to adjacent parenchyma cells (Fig. 2B), but they later differentiate into what are known as osteosclereids or ‘hourglass’ cells. When these cells obtain their final shape (approx. 20 dpa) they start to increase their cell wall dramatically. Osteosclereids are typical of the leguminous seed coat (Corner, 1951), but no function has been described for these cells until now.
The rest of the seed coat consists of parenchyma cells (Figs 2 and 3). Three sub‐layers can be discerned: chlorenchyma; ground parenchyma; and branched parenchyma. The parenchyma is characterized by intercellular spaces, which are particularly large in the branched parenchyma. Cryo‐SEM images revealed that the intercellular spaces are either filled with liquid or air (Figs 3–5).
The chlorenchyma consists of large cells (up to 100 µm) that contain chloroplasts (Figs 2 and 6). The photosynthetic activity of the chloroplasts in developing seeds is probably quite limited (Pate, 1984), and hypoxic conditions inside developing seeds indicate that less photosynthesis occurs than respiration (Rolletschek et al., 2002). The main function of the chloroplasts in the seed coat seems to be the transient accumulation of starch (Rochat and Boutin, 1992).
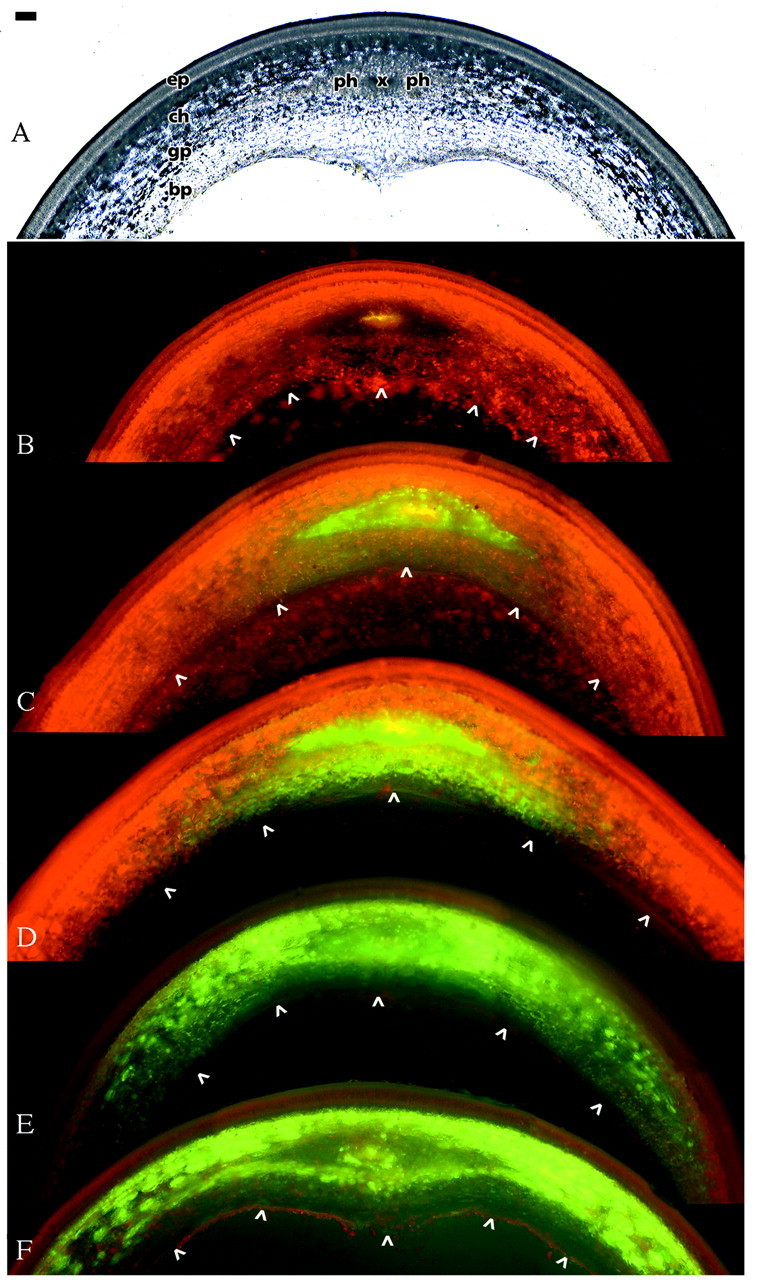
Fig. 6. Symplasmic transport of HPTS through the pea seed coat, approx. 18 dpa. A, Bright‐field micrograph of a section of fresh tissue in the chalazal vein region. B, Control treatment in which no Ac‐HPTS was applied. Note the yellow autofluorescense of the xylem and the red autofluorescence of chlorophyll, which is most intense in the chlorenchyma. C, Three hours after supply of Ac‐HPTS to the stem, HPTS was detected in the scattered sieve elements of the amphicribral vascular bundle. After 6 h (D) the dye had been transported inwardly, and after 16 h (E) laterally through chlorenchyma and ground parenchyma. F, Even 40 h after the start of the experiment, no HPTS was observed in the branched parenchyma or epidermis. The intensity of chlorophyll fluorescence in E and F is reduced because of shorter exposure times. The inner boundary of the seed coat is marked with arrow heads. Bar represents 100 µm. ep, Epidermis; ch, chlorenchyma; gp, ground parenchyma; bp branched parenchyma; x, xylem; ph, phloem.
The second parenchyma layer looks very much like the chlorenchyma, but its cells contain fewer chloroplasts (Figs 2 and 6). Since this is the most abundant cell type, it is referred to as ground parenchyma. In contrast to what has been reported for both Vicia (Offler et al., 1989) and Pisum (Tegeder et al., 1999), we could not find any cell wall ingrowths in the innermost cells of the ground parenchyma (Fig. 4).
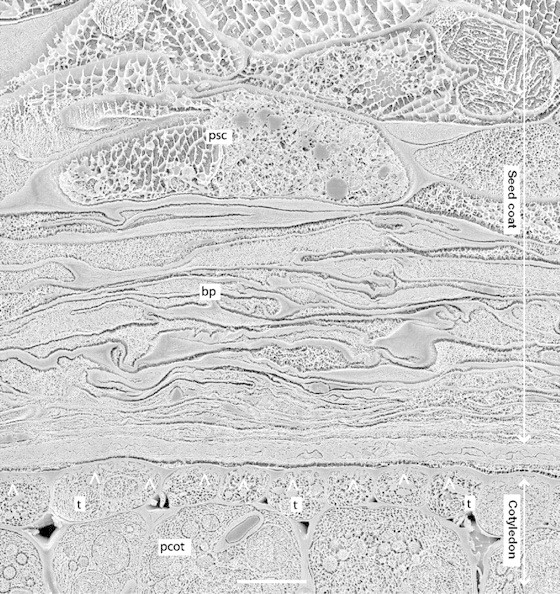
Fig. 4. Cryo‐SEM micrograph of the seed coat–cotyledon boundary, approx. 20 dpa, showing seed coat parenchyma (p), flattened branched parenchyma cells (bp), and transfer cells (t) of the cotyledonary epidermis. The layer between seed coat and cotyledon probably includes the wall of the embryo sac (Marinos, 1970). Note that cell wall invaginations are present in the epidermal cells of the cotyledon (arrow heads) but not in seed coat parenchyma cells. Bar represents 10 µm.
The ground parenchyma changes gradually into the innermost layer, the branched parenchyma, which consists of small (approx. 50 µm), irregularly shaped cells with extensive intercellular spaces. When the cotyledons of the embryo expand and approach the seed coat, the branched parenchyma and the innermost layers of the ground parenchyma deteriorate (Figs 2–4). All that remains of these cells is a compressed mat of wall material which, probably together with cell wall remnants of the embryo sac (Marinos, 1970), forms a boundary layer, about 30 µm across, between the living seed coat parenchyma and the cotyledons (Fig. 4).
The inner layer of the legume seed coat
In general, the innermost layer of the seed coat of legumes consists of irregularly shaped cells with large intracellular spaces. Such a layer is clearly present in common bean (‘branch parenchyma’, Yeung, 1983; Offler and Patrick, 1984), lima bean (‘mycoidal parenchyma’, Sterling, 1954) and soybean (‘aerenchyma’, Thorne, 1981; ‘thick‐walled parenchyma’, Miller et al., 1999). From our observations it is also unmistakably present in pea (Fig. 2A, 15 dpa, and Fig. 3). Remarkably, this layer has not been mentioned by Offler and co‐workers in their descriptions of the seed coat of Vicia (Offler et al., 1989; Offler and Patrick, 1993) and Pisum (Tegeder et al., 1999). This omission may be related to the transient character of the branched parenchyma. It could be that seed coats were examined at a stage where the branched parenchyma had already been crushed (or that the layer got lost during preparation). At any rate, the innermost layer of the pea seed coat, designated as ‘thin‐walled parenchyma transfer cells’ (Fig. 1A in Tegeder et al., 1999), could have been described equally well as branched parenchyma. In Vicia, however, a cell configuration matching that of branched parenchyma has never been observed (C. E. Offler, pers. comm.).
Symplasmic post‐phloem transport pathway
Symplasmic transport of HPTS through the pea seed coat was followed for 40 h following application of its acetate to the cut stem of the plant (Fig. 6). Ac‐HPTS was properly introduced into the phloem, and within 3 h HPTS was detected in the sieve elements of the phloem of the chalazal vein (Fig. 6C). Subsequently, the dye was first transported inwardly to the ground parenchyma near the vascular bundle (Fig. 6D). After 16 h the tracer was also detected in the chlorenchyma outward of the vascular bundle, and had moved laterally through the chlorenchyma and ground parenchyma (Fig. 6E). Even 40 h after application the fluorescent tracer was restricted to these layers and did not show up in the epidermis/hypodermis or in the branched parenchyma (Fig. 6F).
Site of unloading
The present results demonstrate the symplasmic transport of HPTS from the sieve elements of the chalazal vein into the chlorenchyma/ground parenchyma layer. This is in contrast to results obtained with seed coats of Phaseolus vulgaris and Vicia faba (Patrick et al., 1995). In Phaseolus the fluorescent tracer moved from the sieve elements of the vascular web into the ground parenchyma, but not into the chlorenchyma and branched parenchyma, whereas in Vicia the tracer was transported preferentially into the chlorenchyma and the innermost layer of the thin‐walled parenchyma. Because Patrick et al. (1995) probably used Vicia seed coats at a stage where the branched parenchyma had already collapsed we do not know whether this tissue layer takes part in the post‐phloem, symplasmic transport of nutrients. This seems improbable, however, given the close taxonomic relation between Pisum sativum and Vicia faba, and the highly similar anatomy of their seed coats.
Fluorescent tracer experiments have therefore indicated that the branched parenchyma in Phaseolus and Pisum (and probably Vicia as well) is not involved in seed coat unloading, and Weber et al. (1997) have suggested a quite different function for this parenchyma layer. During the pre‐storage phase in Vicia seeds, the extracellular invertase VfCWINV1 was found to be specifically expressed in the innermost layer of the seed coat. Adopting the terminology of Offler et al. (1989), this layer was described as ‘thin‐walled parenchyma’, but as this layer was reported to collapse at the beginning of the storage phase it seems to be homologous to the branched parenchyma of Pisum. It has been proposed that the developmentally regulated degradation of this layer initiates the storage phase through a switch from high to low ratios of hexoses to sucrose in the developing seed (Weber et al., 1997).
It is conceivable that the unloading is restricted to specialized ‘efflux cells’ (cf. Fig. 2 in Patrick and Offler, 2001). During the storage phase the innermost cell layers of the Vicia seed coat have prolific wall ingrowths, particularly on the cell walls facing the embryo. These transfer cells have been considered to be the major sites of seed coat unloading (Patrick et al., 1995). Very small cell wall ingrowths (approx. 0·5 µm) have also been observed in the innermost part of the ground parenchyma of seed coats of the pea cultivar ‘Greenfeast’ (Tegeder et al., 1999), but not in seed coats of the cultivar ‘Marzia’ used in the present work (Fig. 4). Transfer cells are also lacking in the seed coat parenchyma of Phaseolus (Offler and Patrick, 1984). It seems, therefore, that transfer cells are not required for seed coat unloading.
Final proof for the existence of specific ‘efflux cells’ must await the identification and localization of transporter(s) responsible for seed coat unloading. Unfortunately, the identification of transporters by which organic solutes are released from plant cells has remained refractory. Two opposing views have been expressed. For Vicia and Phaseolus it has been hypothesized that sucrose efflux from seed coat parenchyma cells comes about by the activity of an H+/sucrose antiporter (Walker et al., 1995; Wang et al. 1995). By contrast, experiments with Pisum have led to the proposition of the ‘supply follows demand’ model (Van Dongen et al., 2001), in which the efflux of solutes, including sucrose, glucose and amino acids, from seed coat parenchyma cells is effected by poorly selective pores in the plasma membrane (De Jong et al., 1996, 1997).
Although activities of H+‐symporters for sucrose and amino acids could not be detected in uptake experiments with isolated seed coat halves (De Jong et al., 1996, 1997), the presence of such transporters in the pea seed coat has been demonstrated unequivocally by other means. Not only has the presence of transcripts of PsSUT1 and PsAAP1 been demonstrated (genes that code for an H+/sucrose‐ and an H+/amino acid symporter, respectively; Tegeder et al., 1999, 2000), but proton symport of sucrose and amino acids has also been shown to take place in plasma membrane vesicles (De Jong and Borstlap, 2000). It is unlikely, however, that these transporters are involved in seed coat unloading because of the high solute concentration in the seed coat apoplast (see below).
Seed coat apoplast
If unloading occurs in the ground parenchyma, then assimilates need to be further transported towards the cotyledons by diffusion through the apoplast. Generally, the apoplast can be divided into three domains: cell wall; gas‐filled intercellular spaces; and liquid‐filled intercellular spaces (Canny, 1995). In roots it has been demonstrated that the presence of liquid‐filled intercellular spaces increases diffusion of solutes through the apoplast by as much as 100 times (Van der Weele et al., 1996). Similarly, it may be expected that the presence of the liquid‐filled intercellular spaces in the pea seed coat will greatly enhance the diffusion of solutes from the ground parenchyma to the cotyledons (Figs 3–5). The boundary layer between seed coat and cotyledons of compressed cell walls probably has a high porosity and may not be a real barrier for the diffusion of solutes towards the cotyledons.
Cryo‐SEM images of seed coat tissues showed black, crystalline ice with a mesh of white lines running through it. These white lines are solutes, which have been sequestered during the freezing process (Canny and Huang, 1993). The density of the mesh will be related to the original solute concentration in the compartment. Since the pattern of white lines in the liquid‐filled intercellular spaces is much the same as that in the protoplasts of adjacent cells it may be concluded that the solute concentrations in these compartments are also similar (Fig. 5). This is in line with the well‐documented phenomenon of high solute concentrations in the apoplasm of developing seeds (Patrick and Offler, 2001); in pea, this has been determined to be 400 mosm (Lanfermeijer et al., 1990).
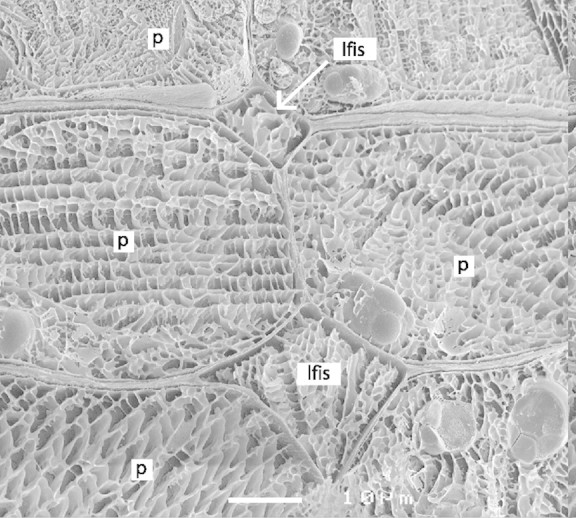
Fig. 5. Cryo‐SEM micrograph showing seed coat parenchyma cells (p) with liquid‐filled intercellular spaces (lfis). The similar mesh of the white matrix in the ice indicates a comparable solute concentration in the apoplast and in the cytosol. Bar represents 10 µm.
CONCLUSIONS
From the results of the present study and those obtained by Patrick et al. (1995), we conclude that in Phaseolus vulgaris, Vicia faba and Pisum sativum the site of unloading is in different layers of the seed coat parenchyma. The branched parenchyma is probably not involved in seed coat unloading in any of these legume species but, being the site of an extracellular invertase, this transient layer may play a crucial role in the initiation of the storage phase (Weber et al., 1997). The liquid‐filled intercellular spaces in the seed coat may be instrumental in allowing the diffusion of released nutrients at adequate rates.
ACKNOWLEDGEMENTS
We would like to thank John Patrick and Tina Offler for their critical comments and helpful discussions.
Supplementary Material
Received: 11 November 2002; Returned for revision: 16 December 2002; Accepted: 29 January 2003 Published electronically: 12 March 2003
References
- BoesewinkelFD, Bouman F.1995. The seed: structure and function. In: Kigel J, Galili G, eds. Seed development and germination. New York: Marcel Dekker, 1–24. [Google Scholar]
- CannyMJ.1995. Apoplastic water and solute movement – New rules for an old space. Annual Review of Plant Physiology and Plant Molecular Biology 46: 215–236. [Google Scholar]
- CannyMJ, Huang CX.1993. What is in the intercellular spaces of roots? Evidence from the cryo‐analytical‐scanning electron microscope. Physiologia Plantarum 87: 561–568. [Google Scholar]
- CornerEJH.1951. The leguminous seed. Phytomorphology 1: 117–150. [Google Scholar]
- De JongA, Borstlap AC.2000. A plasma membrane‐enriched fraction isolated from the coats of developing pea seeds contains H+‐symporters for amino acids and sucrose. Journal of Experimental Botany 51: 1671–1677. [DOI] [PubMed] [Google Scholar]
- De JongA, Koerselman‐Kooij JW, Schuurmans JAMJ, Borstlap AC.1996. Characterization of the uptake of sucrose and glucose by isolated seed coat halves of developing pea seeds. Evidence that a sugar facilitator with diffusional kinetics is involved in seed coat unloading. Planta 199: 486–492. [Google Scholar]
- De JongA, Koerselman‐Kooij JW, Schuurmans JAMJ, Borstlap AC.1997. The mechanism of amino acid efflux from seed coats of developing pea seeds as revealed by uptake experiments. Plant Physiology 114: 731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HardhamAR.1976. Structural aspects of the pathways of nutrient flow to the developing embryo and cotyledons of Pisum sativum L. Australian Journal of Botany 24: 711–721. [Google Scholar]
- JohanssonM, Walles B.1994. Functional anatomy of the ovule in broad bean (Vicia faba L.): Ultrastructural seed development and nutrient pathways. Annals of Botany 74: 233–244. [Google Scholar]
- LanfermeijerFC, Koerselman‐Kooij JW, Borstlap AC.1990. Effects of medium osmolarity on the release of amino acids from isolated cotyledons of developing pea seeds. Evidence for vacuolar amino‐acid release at increased turgor. Planta 181: 568–575. [DOI] [PubMed] [Google Scholar]
- LanfermeijerFC, Van Oene MA, Borstlap AC.1992. Compartmental analysis of amino‐acid release from attached and detached pea seed coats. Planta 187: 75–82. [DOI] [PubMed] [Google Scholar]
- LanfermeijerFC, Koerselman‐Kooij JW, Kollöffel C, Borstlap AC.1989. Release of amino acids from cotyledons of developing seeds of pea (Pisum sativum L.). Journal of Plant Physiology 134: 592–597. [Google Scholar]
- MarinosNG.1970. Embryogenesis of the pea (Pisum sativum). I. The cytological environment of the developing embryo. Protoplasma 70: 261–279. [Google Scholar]
- MillerSS, Bowman LAA, Gijzen M, Miki BLA.1999. Early development of the seed coat of soybean (Glycine max). Annals of Botany 84: 297–304. [Google Scholar]
- NijsseJ, van Aelst AC.1999. Cryo‐planing for cryo‐scanning electron microscopy. Scanning 21: 372–378. [DOI] [PubMed] [Google Scholar]
- OfflerCE, Patrick JW.1984. Cellular structures, plasma membrane surface areas and plasmodesmatal frequencies of seed coats of Phaseolus vulgaris L. in relation to photosynthate transfer. Australian Journal of Plant Physiology 11: 79–99. [Google Scholar]
- OfflerCE, Patrick JW.1993. Pathway of photosynthate transfer in the developing seed of Vicia faba L.: a structural assessment of the role of transfer cells in unloading from the seed coat. Journal of Experimental Botany 44: 711–724. [Google Scholar]
- OfflerCE, Nerlich SM, Patrick JW.1989. Pathway of photosynthate transfer in the developing seed of Vicia faba L. Transfer in relation to seed anatomy. Journal of Experimental Botany 40: 769–780. [Google Scholar]
- PateJS.1984. The carbon and nitrogen nutrition of fruit and seed – Case studies of selected grain legumes. In: Murray DR, ed. Seed physiology. Sydney: Academic Press. [Google Scholar]
- PatrickJW.1997. Phloem unloading: sieve element unloading and post‐sieve element transport. Annual Review of Plant Physiology and Plant Molecular Biology 48: 191–222. [DOI] [PubMed] [Google Scholar]
- PatrickJW, Offler CE.1995. Post‐sieve element transport of sucrose in developing seeds. Australian Journal of Plant Physiology 22: 681–702. [Google Scholar]
- PatrickJW, Offler CE.2001. Compartmentation of transport and transfer events in developing seeds. Journal of Experimental Botany 52: 551–564. [PubMed] [Google Scholar]
- PatrickJW, Offler CE, Wang XD.1995. Cellular pathway of photosynthate transport in coats of developing seed of Vicia faba L. and Phaseolus vulgaris L. I. Extent of transport through the coat symplast. Journal of Experimental Botany 46: 35–47. [Google Scholar]
- RochatC, Boutin JP.1992. Temporary storage compounds and sucrose‐starch metabolism in seed coats during pea seed development (Pisum sativum). Physiologia Plantarum 85: 567–572. [Google Scholar]
- RolletschekH, Borisjuk L, Koschorreck M, Wobus U, Weber H.2002. Legume embryos develop in a hypoxic environment. Journal of Experimental Botany 53: 1–9. [DOI] [PubMed] [Google Scholar]
- SterlingC.1954. Development of the seed coat of lima bean (Phaseolus lunatus L.). Bulletin of the Torrey Botanical Club 81: 271–287. [Google Scholar]
- TegederM, Offler CE, Frommer WB, Patrick JW.2000. Amino acid transporters are localized to transfer cells of developing pea seeds. Plant Physiology 122: 319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TegederM, Wang XD, Frommer WB, Offler CE, Patrick JW.1999. Sucrose transport into developing seeds of Pisum sativum L. The Plant Journal 18: 151–161. [DOI] [PubMed] [Google Scholar]
- ThorneJH.1981. Morphology and ultrastructure of maternal seed tissues of soybean in relation to the import of photosynthate. Plant Physiology 67: 1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der WeeleCM, Canny MJ, McCully ME.1996. Water in aerenchyma spaces in roots. A fast diffusion path for solutes. Plant and Soil 184: 131–141. [Google Scholar]
- Van DongenJT, Laan RGW, Wouterlood M, Borstlap AC.2001. Electrodiffusional uptake of organic cations by pea seed coats. Further evidence for poorly selective pores in the plasma membrane of seed coat parenchyma cells. Plant Physiology 126: 1688–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WalkerNA, Patrick JW, Zhang WH, Fieuw S.1995. Efflux of photosynthate and acid from developing seed coats of Phaseolus vulgaris L.: a chemiosmotic analysis of pump‐driven efflux. Journal of Experimental Botany 46: 539–549. [Google Scholar]
- WangXD, Harrington GN, Patrick JW, Fieuw S.1995. Cellular pathway of photosynthate transport in coats of developing seed of Vicia faba L. and Phaseolus vulgaris L. II. Principal cellular site(s) of efflux. Journal of Experimental Botany 46: 49–63. [Google Scholar]
- WeberH, Borisjuk L, Wobus U.1997. Sugar import and metabolism during seed development. Trends in Plant Science 2: 169–174. [Google Scholar]
- WolswinkelP, Ammerlaan A.1983. Phloem unloading in developing seeds of Vicia faba L. Planta 158: 205–215. [DOI] [PubMed] [Google Scholar]
- WrightKM, Oparka KJ.1996. The fluorescent probe HPTS as a phloem‐mobile, symplastic tracer: an evaluation using confocal laser scanning microscopy. Journal of Experimental Botany 47: 439–445. [Google Scholar]
- YeungEC.1983. Developmental changes in the branched parenchyma cells of bean‐seed coat. Protoplasma 118: 225–229. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.