Abstract
Pseudovivipary is an asexual reproductive strategy exhibited by some arctic/alpine grasses in which leafy plantlets are produced in place of seeds, with genetic conservation an advantage for stress tolerators in these nutrient‐poor habitats. Photosynthetic metabolism and the development of this reproductive system were investigated under varying nutrient availability and predicted future CO2 partial pressure (pCO2). Poa alpina var. vivipara L., grown at present ambient pCO2 or ambient plus 340 µmol mol–1 CO2 (elevated pCO2), was supplied with either 0·05 mol m–3 phosphorus and 0·2 mol m–3 nitrogen, or 0·2 mol m–3 phosphorus and 1·0 mol m–3 nitrogen. Gas exchange measurements and determination of total non‐structural carbohydrate (TNC), nitrogen and phosphorus contents revealed that parent plant leaf blade tissues experienced acclimatory loss of photosynthetic capacity after long‐term growth at elevated pCO2 (particularly so when nutrient availability was low); there were associated reductions in photosynthetic nitrogen and phosphorus use efficiencies (PNUE and PPUE). In addition, decreased PNUE and PPUE were exhibited by plantlets grown at elevated pCO2 with low nutrient availability. Decreased reproductive dry matter in this treatment also resulted from a lack of reproductive initiation in daughter tillers, and altered phenology. Pseudoviviparous P. alpina is likely to be at a disadvantage in both vegetative and reproductive phases at predicted future elevated atmospheric CO2 concentrations, particularly where nutrients are scarce and when in competition with species experiencing less acclimatory loss of photosynthetic capacity.
Key words: Alpine meadow grass, elevated pCO2, nutrient availability, photosynthetic acclimation, photosynthetic nutrient use efficiency, Poa alpina var. vivipara, pseudovivipary, prolification
INTRODUCTION
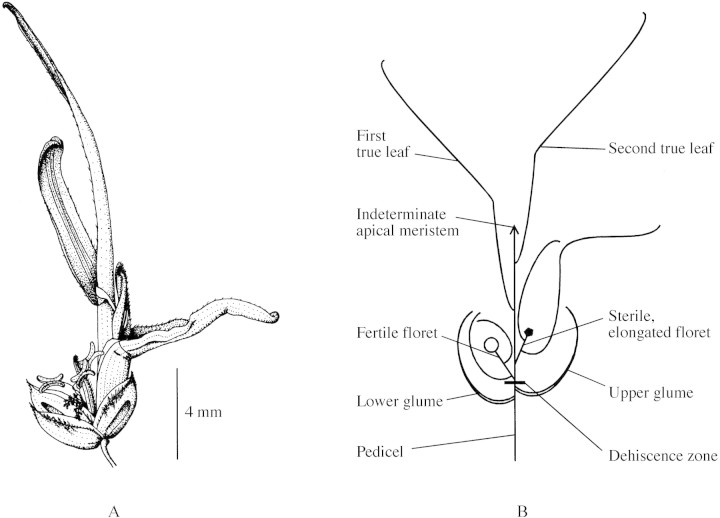
Habitually pseudoviviparous grasses reproduce asexually via the production of leafy spikelets. The upper portion of these spikelets, having reverted from sexual development back to vegetative development along the axis (a process termed prolification; Bell, 1991), may be considered as a shoot system morphologically homologous with tillers. Characteristically, spikelets retain the dehiscence zone and often some mature floral organs typical of sexual spikelets (Fig. 1; cf. ephemeral prolification—the inconsistent teratological development of leafy structures in response to abnormal temperature or disease; for a review, see Pierce, 1998). ‘Plantlets’ of habitually pseudoviviparous grasses are photosynthetically active (Lee and Harmer, 1980; Pierce et al., 2000a) and, after dehiscing from the parent plant and being dispersed (primarily by the wind), may root and establish more rapidly in a short growing season than seeds from seminiferous varieties (Harmer and Lee, 1978). Pseudovivipary is an important reproductive strategy for grasses in nutrient‐poor arctic/alpine environments, in which genetic conservation may confer selective advantage; it has been recorded in 41 species spanning 13 genera of Poaceae (Pierce, 1998). These grasses are often locally abundant, may dominate nitrophilous communities in the Arctic (Summerhayes and Elton, 1928) and, aside from ecological importance, are also major fodder species in alpine regions, e.g. Poa alpina var. vivipara L. (viviparous alpine meadow grass) (Hegi, 1930; Steiner et al., 1997).
Fig. 1. Illustration (A) and diagrammatic representation (B) of a prolificated spikelet (‘plantlet’), the propagule of the pseudoviviparous grass Poa alpina var. vivipara. Illustration by Simon Pierce.
In C3 plants such as P. alpina, vegetative growth is limited not only by the availability of inorganic nutrients, but also by atmospheric carbon dioxide partial pressure (pCO2), which is predicted to double over the next century due to the burning of hydrocarbon fuels (Watson et al., 1990). However, for many plants, including P. alpina (Baxter et al., 1994a), an initially higher rate of growth in the seedling stage is not sustained at elevated pCO2 (relative to plants grown at present day pCO2), with photosynthetic capacity and growth rates declining as photosynthetic metabolism becomes acclimated to the greater substrate availability.
During acclimation, extra photoassimilate, produced as a result of the initially higher photosynthetic rate, may cause a direct repression of transcription of genes controlling photosynthetic metabolism in flowering plants (van Oosten et al., 1994, 1997; van Oosten and Besford, 1996). However, this does not appear to contribute to the initial acclimation of C3 grasses, with source tissues exporting sufficient carbohydrates such that feedback inhibition is prevented. For example, Harmens et al. (2000) have reported that acclimation is more dependent on leaf nitrogen content in Dactylis glomerata L. Indeed, regeneration of ribulose‐1,5‐bisphosphate (Ru1,5bisP) is a fundamental limitation to photosynthesis that is dependent on nitrogen and/or Pi availability (Farquhar et al., 1980; Sharkey, 1985). Constrained by nutrient availability, longer‐term exposure to elevated pCO2 promotes a decline in photosynthetic rates to a point where the regeneration of Ru1,5bisP is once again in equilibrium with its rate of use (Stitt, 1991). If excessive amounts of substrate cannot be processed rapidly enough, then Pi will also be locked up in the form of phosphorylated intermediate compounds, further limiting photophosphorylation (Stitt, 1991). A reduction in growth and limitation of sink size curbs sink strength, resulting in decreased flux between source and sink tissues, potentially inhibiting photosynthesis as a secondary effect. Thus, feedback inhibition of photosynthetic capacity in elevated pCO2 is potentially greatest in nutrient‐limiting conditions (Arp, 1991), imposing greater constraints on growth than low nutrient availability alone. This has been reported for both vegetative D. glomerata (Harmens et al., 2000) and P. alpina (Baxter et al., 1997).
In the case of seminiferous plants, overall sink strength may increase with the production of fruit and seeds (Arp, 1991). However, the pseudoviviparous reproductive system of P. alpina is a source of carbohydrate (Pierce et al., 2000a) and, therefore, unlikely to relieve feedback inhibition. As plantlets represent vegetative shoots with a photosynthetic efficiency equal to that of parent plant leaf blade tissues (Pierce et al., 2000a), it is possible that propagules of P. alpina exhibit similar physiological responses to elevated pCO2 and low nutrient availability to those of tillers.
Other factors may also affect the reproductive potential of these clonal plants. Increased tillering of P. alpina and other graminoids in elevated pCO2 (Tissue and Oechel, 1987; Baxter et al., 1994a) may be especially pronounced under conditions of greater nutrient availability (Baxter et al., 1997). Elevated pCO2 also affects the initiation, rate of development, number and longevity of flowers of many species (Garbutt and Bazzaz, 1984), responses that are potentially mediated by nutrient availability (Lang, 1965).
Reproduction via a pseudoviviparous system, being derived from a seminiferous system, must be initiated by environmental cues (i.e. chilling and shorter day lengths for these arctic/alpine species), with reproduction occurring late in the growth season (Pierce, 1998). Thus, elevated pCO2 and nutrient availability have the potential to limit pseudoviviparous reproduction in short arctic/alpine growing seasons. This study, which aims to investigate the effect of future substrate availability on pseudoviviparous reproduction, follows the synflorescence concept of reproductive architecture in grasses [i.e. that reproductive architecture is composed of repeated co‐florescences (≈spikelets) borne in groups termed ‘paracladia’, the inflorescence or ‘main florescence’ being the distal‐most spikelet; sensu Vegetti and Anton, 1996; Vegetti and Weberling, 1996; for diagrammatic explanation, see Pierce et al., 2000b]. The nomenclature of grasses follows Hubbard (1992).
MATERIALS AND METHODS
The plant material used was the same biotype of Poa alpina var. vivipara investigated by Pierce et al. (2000a, b), originating from the Hohe Mut ridge, Oetztal, Austria (45°50′12”N, 11°2′50”E) at an altitude of 2641 m a.s.l. Plantlets were initially raised in trays of sand using one‐fifth strength Long Ashton nutrient solution (LA; Hewitt, 1966), until they possessed eight or nine fully expanded leaves, and were then transferred to the Institute of Terrestrial Ecology Solardome facility (Solardome Industries Ltd, Southampton, UK) at Abergwygregyn, Gwynedd, Wales, UK. This facility has been described by Stirling et al. (1997), and the environmental control and monitoring systems by Rafarel et al. (1995). Treatments consisted of ambient pCO2 (tracking local ambient of approx. 350 µmol mol–1 CO2) and elevated pCO2 (ambient pCO2 with an additional 340 µmol mol–1 CO2 at any point in time). Plants were cultivated in sand culture in 1‐l square black plastic pots and were supplied with either one‐fifth strength LA (modified with NaH2PO4·2H2O to provide 0·05 mol m–3 P and NH4NO3 to provide 0·2 mol m–3 N) or full‐strength LA (0·2 mol m–3 P and 1·0 mol m–3 N) twice weekly, added to pots until saturated. Plants were watered twice daily with tap water at 0600 and 1800 h using an automated sprinkler system.
Culms were tagged with the date of paracladial exsertion (i.e. the date at which the main florescence became visible), and the length of the culm was measured every second day. Harvests of plant material were conducted every 10 d following the cessation of culm elongation growth. At all harvests six plantlets were removed from the distal half of the main axis paracladial zone, and six were removed from the proximal half (paracladial zones were apportioned into halves by spikelet number after Pierce et al., 2000a). The remaining plant was divided into component organs, and these were further divided into green and non‐green portions and dried to constant weight.
Ethanol‐soluble and water‐soluble carbohydrate contents of plant tissues were determined using the phenol‐sulfuric acid method of Dubois et al. (1956). Tissue starch was determined using an enzymatic starch assay as described by Farrar (1993). Dry plant material (0·1 g sample) was digested using the Kjeldahl method (Allen, 1974; also detailed in Hind, 1993), and analysed for ammonium nitrogen and phosphate using a segmented flow autoanalyser (SANPLUS; Skalar Analytical, Breda, The Netherlands). Photosynthetic nitrogen and phosphorus use efficiencies (PNUE and PPUE, respectively) of both parent plant leaf blade and paracladial zone tissues were calculated following the method of Baxter et al. (1994b):
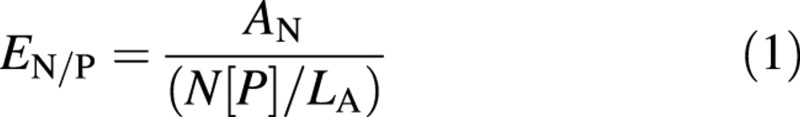
where EN/P is photosynthetic nitrogen/phosphorus use efficiencies [in g (mean structural dry weight) g–1 (tissue nitrogen or phosphorus) d–1], A is net assimilation rate (g m–2 d–1), LA is specific leaf area (m2 g–1), and N or P is the leaf nitrogen or phosphorus content [g N(P) g–1 structural d. wt]. Net assimilation rate was calculated thus:
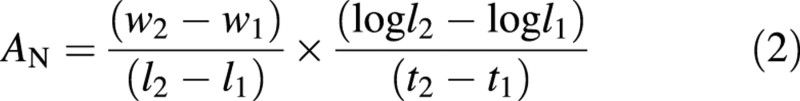
where w1 and w2 are dry weights (g), and l1 and l2 are leaf blade or plantlet area (m2) at times t1 and t2 (d), respectively.
Infrared gas analysis (IRGA; Ciras 1, PP systems, Hitchin, UK) was used to quantify photosynthetic gas exchange of the youngest fully expanded leaf of the main axis 19 d prior to paracladial exsertion. Measurements were taken in a saturating photosynthetic photon flux density (PPFD) of 1300 µmol m–2 s–1 and at leaf temperature of 20 °C. In each nutrient treatment the photosynthetic capacity of plants grown at ambient and elevated pCO2 was compared at the same calculated intracellular pCO2 to produce an index of acclimation, after Stirling et al. (1997):

where IA is the acclimation index, A′e is net photosynthetic rate per unit leaf area obtained from plants grown in 690 µmol mol–1 CO2 (elevated) and measured at the same CO2 concentration, Aa is the net photosynthetic rate obtained from plants grown and measured at 350 µmol mol–1 (ambient), and Ae is the value obtained from plants grown at ambient pCO2 but measured at elevated pCO2. An acclimation index equal to 1 indicates that no photosynthetic acclimation has occurred; IA <1 indicates a loss of photosynthetic capacity, and IA >1 indicates increased photosynthetic capacity.
RESULTS
Ambient temperature was tracked by all solardomes; mean midday temperature was 16 ± 0·1 °C between May and August (26·5 and 5·2 °C maximum and minimum, respectively), and mean midday PPFD inside the solardomes was 304 ± 13·1 µmol m–2 s–1.
Acclimatory response of photosynthesis in parent plants
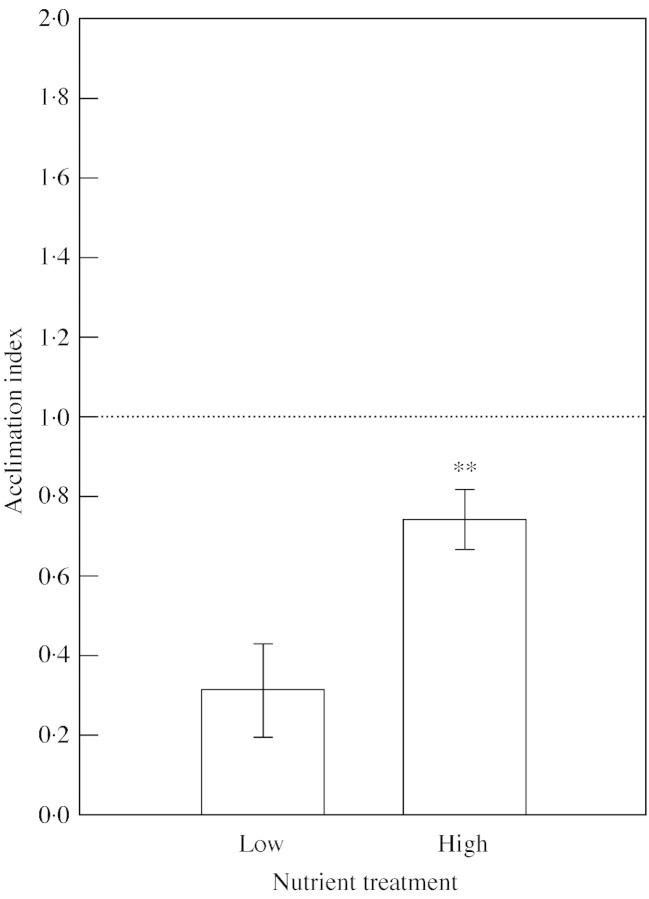
Elevated pCO2 resulted in acclimatory loss of net photosynthetic rate of fully expanded leaves of the parent plant in both nutrient treatments. However, at low nutrient availability, plants had a significantly lower photosynthetic acclimation index (i.e. had a greater acclimatory loss of photosynthetic capacity) compared with that of plants grown at ‘high’ nutrient status (0·3 ± 0·12 cf. 0·7 ± 0·08, P < 0·01; Fig. 2).
Fig. 2. Photosynthetic acclimation to elevated pCO2 of P. alpina var. vivipara plants grown at two nutrient regimes: one‐fifth strength (low) or full‐strength (high) Long Ashton nutrient solution. Data are means of six replicates ± s.e. ** Significant difference between nutrient treatment means at the P < 0·01 level determined by Student’s t‐test. An acclimation index < 1 indicates a loss of photosynthetic capacity.
Parent plant growth and dry matter accumulation
Low nutrient availability alone resulted in lower whole plant dry weights at maturity (24–27 g at high nutrient availability compared with 7–11 g at low nutrient availability; P < 0·05; data not shown), although CO2 treatments did not affect whole plant dry weight in this study.
Leaf blade metabolite concentrations in parent plants
Plants grown at ambient pCO2 with lower nutrient availability showed an increase in total non‐structural carbohydrate (TNC) content over time in parent plant leaf blades, a response not exhibited in other treatments, between which there were no differences (P < 0·05; Table ). Only the ambient pCO2 and low nutrient treatment resulted in decreases in tissue nitrogen concentration over time, and decreased tissue phosphorus concentrations were evident at elevated pCO2 (P < 0·001; Table ).
Table 1.
Total non‐structural carbohydrate (TNC) content and total nitrogen (N) and phosphorus (P) concentration of innovation zone leaf blade material at either 2 or 20 d after the cessation of culm elongation growth in Poa alpina
| Treatment | |||||
| Low nutrient | High nutrient | ||||
| Time (d) | Ambient CO2 | Elevated CO2 | Ambient CO2 | Elevated CO2 | |
| TNC (mg glucose equivalent g–1 total d. wt) | 2 | 53·0 ± 3·2a | 64·0 ± 9·1ab | 56·0 ± 6·7ab | 65.0 ± 5·4ab |
| 20 | 71·0 ± 15·6b | 64·0 ± 7·4ab | 62·0 ± 9·4ab | 59.0 ± 9·3ab | |
| N (mg g–1 total d. wt) | 2 | 24·3 ± 4·31c | 18·2 ± 0·85ab | 14·6 ± 2·86abc | 12·4 ± 2·11a |
| 20 | 16·0 ± 1·38ab | 20·8 ± 3·37bc | 20·8 ± 1·16bc | 18·2 ± 1·91abc | |
| P (mg g–1 total d. wt) | 2 | 10·4 ± 0·42c | 6·9 ± 1·23ab | 8·5 ± 1·64bc | 4·3 ± 0·62a |
| 20 | 9·8 ± 0·50c | 6·3 ± 0·19ab | 9·6 ± 0·25c | 6·3 ± 0·33ab | |
Plants were grown in either one‐fifth strength (low nutrient) or full‐strength (high nutrient) Long Ashton nutrient solution, and either 350 µmol mol–1 (ambient) or 690 µmol mol–1 (elevated) CO2. Data are means of four replicates ± s.e. Different superscripts indicate significant differences at P < 0·05 between all means for TNC data, or between treatment means and over time within each nutrient for N and P data, as determined by Tukey’s multiple comparison procedure (ANOVA) (sensu Zar, 1999).
Photosynthetic nitrogen and phosphorus use efficiencies of parent plant leaves
Nutrient and CO2 treatments had interactive effects on both photosynthetic nitrogen and phosphorus use efficiencies, with elevated pCO2 reducing PNUE in low nutrient conditions (0·1 ± 0·08 g g–1 d–1), PPUE remaining low in low nutrient conditions (0·3 ± 0·19 g g–1 d–1), and PNUE and PPUE increasing in high nutrient conditions (4·1 ± 0·87 and 11·9 ± 2·59 g g–1 d–1, respectively; P < 0·001; Table ).
Table 2.
Photosynthetic nitrogen and phosphorus use efficiencies (PNUE, PPUE; g structural d. wt g–1 N(P) d–1) of parent plant leaf blade material over an 18‐d period following cessation of culm elongation growth in Poa alpina
| Treatment | ||||
| Low nutrient | High nutrient | |||
| Nutrient | Ambient CO2 | Elevated CO2 | Ambient CO2 | Elevated CO2 |
| PNUE | 0·9 ± 0·32b | 0·1 ± 0·08a | 0·9 ± 0·51ab | 4·1 ± 0·87c |
| PPUE | 1·9 ± 0·64a | 0·3 ± 0·19a | 1·7 ± 0·92a | 11·9 ± 2·59b |
Plants were grown in either one‐fifth strength (low nutrient) or full‐strength (high nutrient) Long Ashton nutrient solution, and either 350 µmol mol–1 (ambient) or 690 µmol mol–1 (elevated) CO2. Data are means of four replicates ± s.e. Different superscripts indicate significant differences between treatment means for each nutrient use efficiency (PNUE or PPUE) at P < 0·05 determined by Tukey’s multiple comparison procedure (ANOVA) (sensu Zar, 1999).
The flowering response
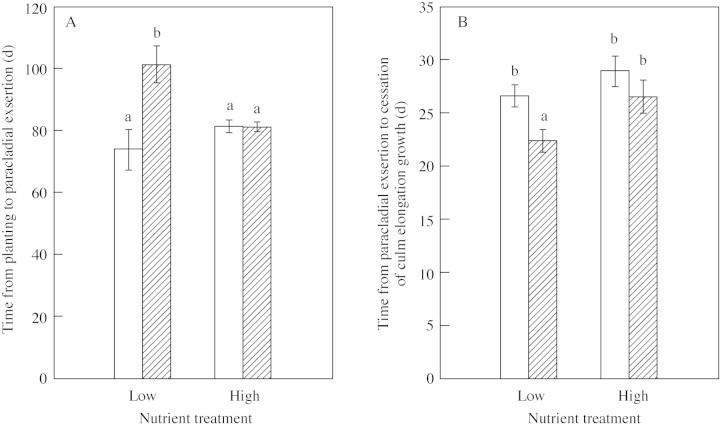
In low nutrient treatments, 27·3 and 28·6 % of plants flowered in ambient and elevated pCO2, respectively, whereas in high nutrient treatments these figures were 62·5 and 70·2 %, respectively. In plants grown in elevated pCO2 and low nutrient conditions, the time prior to initiation of reproductive growth was increased (Fig. 3A) and the period of culm elongation growth was reduced by approx. 5 d (Fig. 3B), whereas the other treatments did not affect developmental timing. Additional nutrients led independently to an increase in the number of daughter tillers in flower (Fig. 4A and B). Elevated pCO2 alone increased the number of flowering tillers in the high nutrient treatment, but interacted with low nutrient status to reduce the number of tillers in flower. This interaction was reinforced over time within all treatments except elevated pCO2 and low nutrient supply, resulting in the continued production of synflorescences (Fig. 4A and B). Total reproductive dry weight per plant was approx. 350 % higher in the high nutrient treatment compared with the low nutrient treatment at 20 d (Fig. 4C and D). There was no effect of, or interaction with, elevated pCO2, other than an apparent increase in variability in total reproductive dry weight.
Fig. 3. Developmental timing of the culm of Poa alpina: time from planting to paracladial exsertion (A) and time from paracladial exsertion to cessation of culm elongation growth (B). Plants were grown at either 350 µmol mol–1 (ambient; open bars) or 690 µmol mol–1 (elevated; hatched bars) atmospheric CO2, and two nutrient regimes: one‐fifth strength (low) or full‐strength (high) Long Ashton nutrient solution. Data are means of twelve replicates ± s.e. Different letters indicate significant differences at the P < 0·05 level determined by Tukey’s multiple comparison procedure (ANOVA) (Zar, 1999).
Fig. 4. A and B, Number of daughter tillers in flower at the time of cessation of culm elongation growth in Poa alpina. C and D, Total reproductive dry weight following the cessation of culm elongation growth. Plants were grown at either 350 µmol mol–1 (ambient; closed symbols) or 690 µmol mol–1 (elevated; open symbols) atmospheric CO2, and two nutrient regimes: one‐fifth strength (low nutrient) or full‐strength (high nutrient) Long Ashton nutrient solution. Data are means of four replicates ± s.e.
The main axis paracladial zone
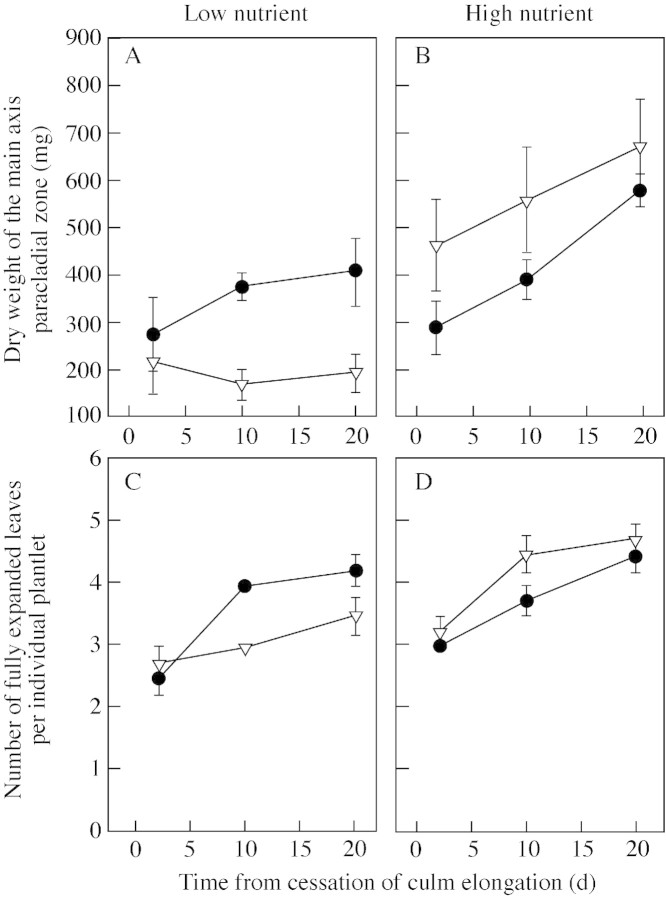
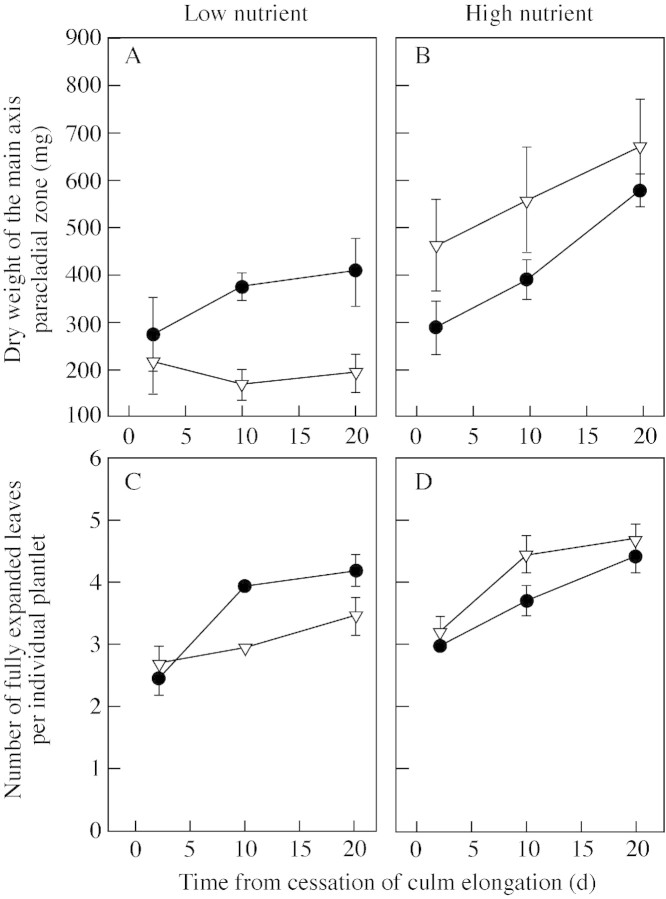
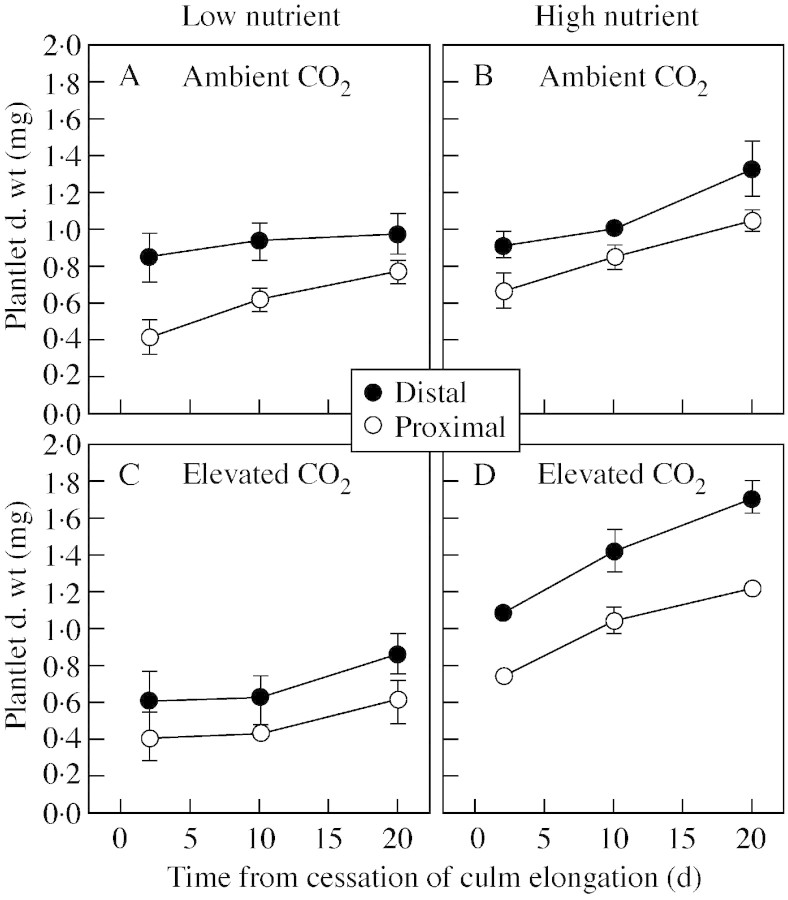
The total number of spikelets of the main synflorescence was not affected by nutrient or CO2 treatments (Table ). Total dry weight of the paracladial zone was significantly increased in high nutrient conditions compared with low nutrient conditions, with further increases over time (e.g. a difference of 211 mg in plants grown at ambient pCO2 compared with a difference of 505 mg in plants grown at elevated pCO2 at 20 d; Fig. 5A and B). Elevated pCO2 and low nutrient availability interacted to reduce significantly the dry weight of the main axis paracladial zone (P < 0·001; Table ). The number of fully expanded leaves on plantlets increased over time (Fig. 5C and D). The dry weight of distal plantlets was significantly greater than that of proximal plantlets in all treatments (P < 0·001; Fig. 6; Table A). Distal plantlets had a greater leaf area ratio (LAR) than proximal plantlets (data not shown).
Table 3.
Mean number of spikelets in the main axis paracladial zone of Poa alpina
| CO2 concentration (µmol mol–1) | ||
| Nutrient treatment | 350 | 690 |
| Low | 58·8 ± 6·44 | 60·7 ± 6·08 |
| High | 64·5 ± 3·05 | 72·0 ± 3·35 |
Plants were grown in either one‐fifth strength (low) or full‐strength (high) Long Ashton nutrient solution, and either 350 µmol mol–1 (ambient) or 690 µmol mol–1 (elevated) CO2. Data are means of 12 replicates ± s.e. Data were square‐root transformed prior to statistical analysis. No statistical differences were detected at P < 0·05 between treatments using analysis of variance (sensu Zar, 1999).
Fig. 5. A and B, Dry weight of the main axis paracladial zone after the cessation of elongation growth of the culm of Poa alpina. C and D, Number of fully expanded leaves of individual plantlets after the cessation of elongation growth of the culm (no distinction was made between distal and proximal plantlets). Plants were grown at either 350 µmol mol–1 (ambient; closed symbols) or 690 µmol mol–1 (elevated; open symbols) atmospheric CO2, and two nutrient regimes: one‐fifth strength (low nutrient) or full‐strength (high nutrient) Long Ashton nutrient solution. Data are means of four replicates ± s.e.
Table 6.
Summary of statistical significance of a balanced three‐way ANOVA of data presented in Figs 4 and 5
| Source | No. of daughter tillers in flower (Fig. 4) | Total reproductive dry weight (Fig. 4) | Dry weight of main axis paracladial zone (Fig. 5) | No. of leaves per plantlet (Fig. 5) | Senescence in paracladial zone |
| CO2 | ** | n.s. | n.s. | n.s. | n.s. |
| Nutrient | *** | *** | *** | *** | * |
| Time | *** | *** | * | *** | *** |
| CO2 × Nutrient | *** | n.s. | *** | *** | n.s. |
| CO2 × Time | n.s. | n.s. | n.s. | n.s. | n.s. |
| Nutrient × Time | n.s. | *** | n.s. | n.s. | n.s. |
| CO2 × Nutrient × Time | * | n.s. | n.s. | n.s. | n.s. |
Proportion data were arcsine‐transformed and numerical count data were square‐root transformed prior to analysis. *** P < 0·001; ** P < 0·01; * P < 0·05; n.s., not significant (P > 0·05).
Fig. 6. Dry weight of plantlets from distal and proximal halves of the paracladial zone following the cessation of culm elongation growth in Poa alpina. Plants were grown at either 350 µmol mol–1 (ambient) or 690 µmol mol–1 (elevated) atmospheric CO2, and two nutrient regimes: one‐fifth strength (low nutrient) or full‐strength (high nutrient) Long Ashton nutrient solution. Data are means of four replicates ± s.e.
Table 7.
Summary of statistical significance of a balanced four‐way ANOVA of data presented in Fig. 6.
| Source | Dry weight of distal and proximal plantlets (Fig. 6) |
| CO2 | n.s. |
| Nutrient | *** |
| Time | *** |
| Position | *** |
| CO2 × Nutrient | *** |
| CO2 × Time | n.s. |
| CO2 × Position | n.s. |
| Nutrient × Time | * |
| Nutrient × Position | n.s. |
| Nutrient × Time × Position | n.s. |
| CO2 × Nutrient × Time | n.s. |
| CO2 × Nutrient × Position | n.s. |
| CO2 × Time × Position | n.s. |
| CO2 × Nutrient × Position × Time | n.s. |
‘Position’ relates to the location of plantlets in either the distal or proximal half of the paracladial zone. Proportion data were arcsine‐transformed and numerical count data were square‐root transformed prior to analysis. *** P < 0·001; ** P < 0·01; * P < 0·05; n.s., not significant (P > 0·05).
Plantlets possessed visibly senescent leaves from the time that culm elongation growth ceased, with the proportion of senescent material in the paracladial zone increasing over time (P < 0·001; data not shown) until approx. 40 % of material in the paracladial zone was senescent at 20 d. High nutrient availability resulted in a greater proportion of senescent material (P < 0·05), with no effect of, or interaction with, the elevated pCO2 treatment (Table ).
Leaf blade metabolite concentrations in plantlets
Total non‐structural carbohydrate content of plantlets increased significantly over time in distal plantlets. Although TNC of distal plantlets did not differ among treatments, TNC of proximal plantlets was depressed at low nutrient availability and elevated pCO2 20 d after the cessation of culm elongation (P < 0·01; Table ). Phosphorus concentration of plantlet leaf tissue was significantly reduced in the elevated pCO2 treatment, compared with ambient, at 2 d following paracladial exsertion at both low and high nutrient supply (P < 0·05; Table ). Nutrient and CO2 treatments showed an interactive effect on both photosynthetic nitrogen and phosphorus use efficiencies, with elevated pCO2 decreasing PNUE and PPUE in the low nutrient treatment (0·1 ± 0·04 and 0·3 ± 0·07 g g–1 d–1, respectively), and increasing PPUE in the high nutrient treatment (2·9 ± 0·44 g g–1 d–1; P < 0·001; Table ).
Table 4.
Total non‐structural carbohydrate content (mg glucose equivalent g–1 total d. wt) of plantlets from either distal or proximal positions in the paracladial zone at two time points from cessation of culm elongation in Poa alpina
| Treatment | |||||
| Low nutrient | High nutrient | ||||
| Plantlet position | Time (d) | Ambient CO2 | Elevated CO2 | Ambient CO2 | Elevated CO2 |
| Distal | 2 | 69 ± 15·1a | 50 ± 14·9a | 78 ± 3·9a | 90 ± 26·9a |
| 20 | 171 ± 17·5b | 155 ± 19·9b | 170 ± 32·5b | 183 ± 16·3b | |
| Proximal | 2 | 163 ± 36·9ab | 117 ± 34·7a | 201 ± 28·3b | 100 ± 22·4a |
| 20 | 245 ± 12·9c | 141 ± 33·5a | 220 ± 19·6bc | 201 ± 2·9bc | |
Plants were grown in either one‐fifth strength (low nutrient) or full‐strength (high nutrient) Long Ashton nutrient solution, and either 350 µmol mol–1 (ambient) or 690 µmol mol–1 (elevated) CO2. Data are means of four replicates ± s.e. Different superscripts indicate significant differences between all means within each plantlet position (distal or proximal) at P < 0·05 determined by Tukey’s multiple comparison procedure (ANOVA) (sensu Zar, 1999).
Table 5.
Total nitrogen (N) and phosphorus (P) concentrations of the paracladial zone of Poa alpina at two time points from paracladial exsertion, and photosynthetic nitrogen and phosphorus use efficiencies (PNUE, PPUE) of the paracladial zone integrated over an 18‐d period
| Treatment | |||||
| Low nutrient | High nutrient | ||||
| Nutrient | Time (d) | Ambient CO2 | Elevated CO2 | Ambient CO2 | Elevated CO2 |
| N (mg g–1 total d. wt) | 2 | 16·1 ± 2·31ab | 14·4 ± 1·04ab | 11·4 ± 1·53a | 16·4 ± 0·75ab |
| 20 | 11·9 ± 1·81a | 15·7 ± 1·49ab | 19·9 ± 2·27bc | 12·8 ± 2·79a | |
| P (mg g–1 total d. wt) | 2 | 5·4 ± 0·50c | 3·9 ± 0·21b | 5·1 ± 0·66c | 2·7 ± 0·01a |
| 20 | 4·4 ± 0·08bc | 3·5 ± 0·16ab | 3·3 ± 0·54a | 2·8 ± 0·13a | |
| PNUE (g structural d. wt g–1 N d–1) | 0·6 ± 0·26b | 0·1 ± 0·04a | 0·4 ± 0·14ab | 0·5 ± 0·11ab | |
| PPUE (g structural d. wt g–1 P d–1) | 1·7 ± 0·57bc | 0·3 ± 0·07a | 1·0 ± 0·63ab | 2·9 ± 0·44c | |
Plants were grown in either one‐fifth strength (low nutrient) or full‐strength (high nutrient) Long Ashton nutrient solution, and either 350 µmol mol–1 (ambient) or 690 µmol mol–1 (elevated) CO2. Data are means of four replicates ± s.e. Different superscripts indicate significant differences between all means within each nutrient for N and P contents, or between treatment means for each nutrient use efficiency, PNUE or PPUE, at P < 0·05 determined by Tukey’s multiple comparison procedure (ANOVA) (sensu Zar, 1999).
DISCUSSION
Parent plant tissues
Parent plant leaf blade material of P. alpina var. vivipara exhibited acclimatory loss of photosynthetic capacity after long‐term growth at elevated pCO2, an effect that was particularly pronounced at low nutrient availability. Harmens et al. (2000) reported a positive correlation between N content and acclimation for Dactylis glomerata. However, in the present study N, P and TNC contents of parent plant leaf blade material exhibited no difference among treatments that could account for acclimation directly, and they were not present in toxic concentrations (see Marschner, 1995). Lower photosynthetic nitrogen use efficiency indicates that N was utilized less efficiently in the production of dry matter at elevated pCO2 when fewer nutrients were available. Also, photosynthetic phosphorus use efficiency remained low in this treatment, whereas both PNUE and PPUE were increased substantially by elevated pCO2 when plants were grown at higher nutrient availability (as also observed by Baxter et al., 1994b).
In addition, developmental differences were observed among treatments in the present study, indicating that CO2 concentration and nutrient availability determine the developmental age of organs, and of the main shoot system via monocarpic senescence. Indeed, elevated pCO2 may increase the developmental age of an organ by increasing the rate of cell division (e.g. Dactylis glomerata, Kinsman et al., 1997). Changes in developmental age and senescence are associated with changes in protein turnover and the size of precursor pools (Peoples and Dalling, 1988), often resulting in a lack of correlation between leaf N and Rubisco concentrations (e.g. Oryza sativa L. ‘Sasanishiki’, Makino et al., 1984). This suggests that the initial developmental response of P. alpina to elevated pCO2 may determine the subsequent deployment of resources (expressed as PNUE and PPUE), thus governing photosynthetic capacity.
Reproductive growth
Reduced reproductive dry weight observed in the low nutrient and elevated CO2 treatment resulted from both a lack of flowering of daughter tillers and the subsequent suppressed growth response of the paracladial zone(s) produced. Daughter tillers may not have reached a threshold dry mass (developmental age) above which the apical meristem could be induced into reproductive development (Wareing and Phillips, 1990), which may also explain the general lack of flowering in low nutrient treatments. Spikelet number did not differ between treatments and thus differences in dry matter accumulation within the paracladial zone reflected the performance of plantlets. Although photosynthetic acclimation in plantlet tissues was not determined directly (as suitable cuvettes for gas exchange were not available), PNUE and PPUE of plantlet tissues showed a similar response to pCO2 and nutrient availability to that of parent leaf blades, i.e. both were suppressed in low nutrient conditions with elevated pCO2, and PPUE increased in elevated pCO2 with high nutrient availability. This was reflected in the lower dry matter accumulation in the low nutrient/elevated pCO2 treatment relative to the other treatments imposed, providing further evidence (in addition to photosynthetic efficiency; Pierce et al., 2000a) that plantlets have identical photosynthetic metabolism to parent plant shoot systems.
However, the growth response of plantlets was not entirely due to the direct physiological effects of resource availability. Elevated pCO2 and low nutrient availability altered the development of the culm, with earlier senescence being promoted, and thus the physiological connection to plantlets existed for a shorter period. Earlier senescence of the culm was also associated with the earlier senescence of plantlet material. The higher, and increasing, carbohydrate contents in the paracladial zone (compared with those of the parent leaf blades) indicate that carbohydrate export was inhibited by culm senescence, potentially leading to feedback inhibition of photosynthetic metabolism in plantlet tissues. Transport in the culm xylem is likely to have occurred at this point (Pierce et al., 2000a), providing a route for the delivery of water and nutrients to photosynthetic tissues. The rapid senescence of plantlet material also indicates that plantlet health was dependent on the timing of culm senescence, an indirect effect of pCO2 and nutrient availability. Additional developmental changes included a delay in floral induction at elevated pCO2 with greater nutrient limitation. Such changes have been shown to be species‐ and biotype‐specific, with nutrient‐sufficient conditions usually resulting in either no change or earlier floral initiation (Garbutt and Bazzaz, 1984).
Plantlets produced towards the tip of the paracladial zone were consistently larger than proximal plantlets, irrespective of nutrient or CO2 availability. Such inherent paracladial heterogeneity in other grass species results from a hierarchy of phytohormonal dominance (e.g. rice, Patel and Mohapatra, 1992). The larger plantlets from distal paracladia have greater relative growth rates and are ten times more likely to establish than plantlets from proximal paracladia (Pierce et al., 2000a).
The response of an individual plant to resource availability determines its competitive abilities and, ultimately, its survival and reproductive capacity. Clearly, reproductive potential of P. alpina var. vivipara is suppressed in conditions of elevated pCO2 and low nutrient availability, as is the vegetative phase (see also Baxter et al., 1997). Therefore, where nutrients are limiting, pseudoviviparous P. alpina will be at a disadvantage compared with species that show less acclimatory loss or no change in photosynthetic capacity at predicted future pCO2.
In conclusion, leafy plantlets of pseudoviviparous P. alpina have the same photosynthetic response to resource availability as parent plant leaf blades, with both showing decreased photosynthetic nutrient use efficiencies in elevated pCO2 when nutrients are scarce, and both are equally likely to experience acclimatory loss of photosynthetic capacity. In these conditions developmental changes such as a delay in paracladial exsertion, a general lack of paracladial exsertion, and exacerbated senescence in the culm and plantlets also decrease reproductive potential. Pseudovi viparous P. alpina growing in nutrient‐poor habitats will be at both a vegetative and reproductive disadvantage compared with species that experience less loss of photosynthetic capacity during acclimation to future atmospheric CO2 concentrations.
ACKNOWLEDGEMENTS
We thank Professor Trevor Ashenden and co‐workers for assistance at the Solardome facility, CEH, Bangor. S.P. was supported by a University of Durham PhD Studentship awarded to R.B., which was held in collaboration with the Institute of Terrestrial Ecology, Bangor Research Unit, UK.
Supplementary Material
Received: 2 October 2002; Returned for revision: 3 December 2002 ; Accepted: 21 January 2003 Published electronically: 6 March 2003
References
- AllenSE.1974. Chemical analysis of ecological materials. Oxford: Blackwell. [Google Scholar]
- ArpWJ.1991. Effects of source‐sink relations on photosynthetic acclimation to elevated CO2 Plant Cell and Environment 14: 869–875. [Google Scholar]
- BaxterR, Ashenden TW, Farrar JF.1997. Effect of elevated CO2 and nutrient status on growth, dry matter partitioning and nutrient content of Poa alpina var. vivipara L. Journal of Experimental Botany 48: 1477–1486. [Google Scholar]
- BaxterR, Ashenden TW, Sparks TH, Farrar JF.1994a Effects of elevated carbon dioxide on three montane grass species. I. Growth and dry matter partitioning. Journal of Experimental Botany 45: 305–315. [Google Scholar]
- BaxterR, Gantley M, Ashenden TW, Farrar JF.1994b Effects of elevated carbon dioxide on three grass species from montane pasture. II. Nutrient uptake, allocation and efficiency of use. Journal of Experimental Botany 45: 1267–1278. [Google Scholar]
- BellAD.1991. Plant form: an illustrated guide to flowering plant morphology. Oxford: Oxford University Press. [Google Scholar]
- DuboisM, Gilles KA, Hamilton JK, Rebus PA, Smith F.1956. Colorimetric method for the determination of sugars and related substances. Analytical Chemistry 28: 330–356. [Google Scholar]
- FarquharGD, Caemmerer von S, Berry JA.1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149: 78–90. [DOI] [PubMed] [Google Scholar]
- FarrarJF.1993. Carbon partitioning. In: Hall DO, Scurlock JMO, Bolhàr‐Nordenkampf, Leegood RC, Long SP, eds. Photosynthesis and production in a changing environment: a field and laboratory manual London: Chapman and Hall, 232–246. [Google Scholar]
- GarbuttK, Bazzaz FA.1984. The effects of elevated CO2 on plants. III. Flower, fruit and seed production and abortion. New Phytologist 98: 433–446. [Google Scholar]
- HarmensH, Stirling CM, Marshall C, Farrar JF.2000. Does down‐regulation of photosynthetic capacity by elevated CO2 depend on N supply in Dactylis glomerata? Physiologia Plantarum 108: 43–50. [Google Scholar]
- HarmerR, Lee JA.1978. The germination and viability of Festuca vivipara (L.) Sm. plantlets. New Phytologist 81: 745–751. [Google Scholar]
- HegiG.1930. Alpine flowers. The most common alpine plants of Switzerland, Austria and Bavaria. London: Blackie and Son. [Google Scholar]
- HewittEJ.1966. Sand and water culture: methods used in the study of plant nutrition. 2nd edn. London and Reading: Commonwealth Agricultural Bureau, The Eastern Press. [Google Scholar]
- HindG.1993. Thylakoid components and processes. In: Hall DO, Scurlock JMO, Bolhàr‐Nordenkampf HR, Leegood RC, Long SP, eds. Photosynthesis and production in a changing environment: a field and laboratory manual London: Chapman and Hall, 283–297. [Google Scholar]
- HubbardCE.1992. Grasses: a guide to their structure, identification, uses and distribution in the British Isles. 3rdedn. London: Penguin Books. [Google Scholar]
- KinsmanEA, Lewis C, Davies MS, Young JE, Francis D, Vilhar B, Ougham HJ.1997. Elevated CO2 stimulates cells to divide in grass meristems: a differential effect in two natural populations of Dactylis glomerata Plant Cell and Environment 20: 1309–1316. [Google Scholar]
- LangA.1965. Physiology of flowering initiation. Encyclopedia of Plant Physiology 15: 1380. [Google Scholar]
- LeeJA, Harmer R.1980. Vivipary, a reproductive strategy in response to environmental stress? Oikos 35: 254–265. [Google Scholar]
- MakinoA, Mae T, Ohira K.1984. Relation between ribulose‐1,5‐bisphosphate carboxylase in rice leaves from emergence through senescence.Plant and Cell Physiology 25: 429–437. [Google Scholar]
- MarschnerH.1995. Mineral nutrition of higher plants. London: Academic Press. [Google Scholar]
- PatelR, Mohapatra PK.1992. Regulation of spikelet development in rice by hormones. Journal of Experimental Botany 43: 257–262. [Google Scholar]
- PeoplesMB, Dalling MJ.1988. The interplay between proteolysis and amino acid metabolism during senescence and nitrogen reallocation. In: Noodén LD, Leopold AC, eds. Senescence and aging in plants San Diego: Academic Press, 182–212. [Google Scholar]
- PierceS.1998. Resource allocation in the pseudoviviparous alpine meadow grass (Poa alpina L.). PhD Thesis, University of Durham, UK. [Google Scholar]
- PierceS, Stirling CM, Baxter R.2000a Architectural and physiological heterogeneity within the synflorescence of pseudoviviparous grass (Poa alpina var. vivipara L.). Journal of Experimental Botany 51: 1705–1712. [DOI] [PubMed] [Google Scholar]
- PierceS, Stirling CM, Baxter R.2000b The influence of secondary senescence processes within the culm of a pseudoviviparous grass (Poa alpina var. vivpara L.) on the supply of water to propagules. Journal of Experimental Botany 51: 1067–1075. [DOI] [PubMed] [Google Scholar]
- RafarelCR, Ashenden TW, Roberts TM.1995. An improved solardome system for exposing plants to elevated CO2 and temperature. New Phytologist 131: 481–490. [DOI] [PubMed] [Google Scholar]
- SharkeyTD.1985. Photosynthesis in intact leaves of C3 plants: physics, physiology and rate limitations. Botanical Review 51: 53–105. [Google Scholar]
- SteinerAM, Heidenreich SC, Schwarz P.1997. Verification of varieties of alpine meadow grass (Poa alpina L.) – floret morphology, chromosome number and single seed storage protein electrophoresis. Plant Varieties and Seeds 10: 129–134. [Google Scholar]
- StirlingCM, Davey PA, Williams TG, Long SP.1997. Acclimation of photosynthesis to elevated CO2 and temperature in five British native species of contrasting functional type. Global Change Biology 3: 237–246. [Google Scholar]
- StittM.1991. Rising CO2 levels and their potential significance for carbon flow in photosynthetic cells. Plant Cell and Environment 14: 741–762. [Google Scholar]
- SummerhayesVS, Elton CS.1928. Further contributions to the ecology of Spitsbergen. Journal of Ecology 16: 193. [Google Scholar]
- TissueDT, Oechel WC.1987. Response of Eriophorum vaginatum to elevated CO2 and temperature in the Alaskan tussock tundra. Ecology 68: 401–410. [Google Scholar]
- van OostenJJ, Besford RT.1996. Acclimation of photosynthesis to elevated CO2 through feedback regulation of gene expression: Climate of opinion. Photosynthesis Research 48: 353–365. [DOI] [PubMed] [Google Scholar]
- van OostenJJ, Wilkins D, Besford RT.1994. Regulation of the expression of photosynthetic nuclear genes by CO2 is mimicked by regulation by carbohydrates – a mechanism for the acclimation of photosynthesis to high CO2 Plant Cell and Environment 17: 913–923. [Google Scholar]
- van OostenJJ, Gerbaud A, Huijser C, Dijkwel PP, Chua NH, Smeekens SCM.1997. An Arabidopsis mutant showing reduced feedback inhibition of photosynthesis. Plant Journal 12: 1011–1020. [DOI] [PubMed] [Google Scholar]
- VegettiA, Anton AM.1996. The synflorescence concept in Poaceae. Flora 191: 231–234. [Google Scholar]
- VegettiA,Weberling F.1996. The structure of the paracladial zone in Poaceae. Taxon 45: 453–460. [Google Scholar]
- WareingPF, Phillips IDJ.1990. Growth and differentiation in plants. 3rd edn. Oxford: Pergamon Press. [Google Scholar]
- WatsonRT, Rohde H, Oeschger H, Siegenthaler U.1990. Greenhouse gases and aerosols. In: Houghton JT, Jenkins GJ, Ephraums JJ, eds. Climate change: the IPCC scientific assessment. Geneva: IPCC. [Google Scholar]
- ZarJH.1999. Biostatistical analysis. 4th edn. New Jersey: Prentice Hall. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.