Abstract
The morphology, ultrastructure, density and distribution of trichomes on leaves of Betula pendula, B. pubescens ssp. pubescens, B. pubescens ssp. czerepanovii and B. nana were examined by means of light, scanning and transmission electron microscopy. The composition of flavonoids in ethanolic leaf surface extracts was analysed by high pressure liquid chromatography. All taxa examined contained both glandular and non‐glandular trichomes (short and/or long hairs) but differed from each other in trichome ultrastructure, density and location on the leaf. Leaves of B. pubescens were more hairy than those of B. pendula, but the latter species had a higher density of glandular trichomes. Of the two subspecies of B. pubescens, leaves of ssp. pubescens had more short hairs on the leaf surface and four times the density of glandular trichomes of leaves of ssp. czerepanovii, whereas, in the latter subspecies, short hairs occurred largely on leaf veins, as in B. nana. The glandular trichomes were peltate glands, consisting of medullar and cortical cells, which differed structurally. Cortical cells possessed numerous small, poorly developed plastids and small vacuoles, whereas medullar cells had several large plastids with well‐developed thylakoid systems and fewer vacuoles. In B. pubescens subspecies, vacuoles of the glandular cells contained osmiophilic deposits, which were probably phenolic, whereas in B. pendula, vacuoles of glandular trichomes were characterized by the presence of numerous myelin‐like membranes. The composition of epicuticular flavonoids also differed among species. The two subspecies of B. pubescens and B. nana shared the same 12 compounds, but five of these occurred only in trace amounts in B. nana. Leaf surface extracts of B. pendula contained just six flavonoids, three of which occurred only in this species. In summary, the structure, density and distribution of leaf trichomes and the composition of epicuticular flavonoids represent good taxonomic markers for Finnish birch species.
Key words: Betula pendula, silver birch, Betula pubescens ssp. pubescens, white birch, Betula pubescens ssp. czerepanovii, mountain birch, Betula nana, dwarf birch, non‐glandular trichomes, glandular trichomes, ultrastructure, flavonoids
INTRODUCTION
The surfaces of leaves and of other plant organs are commonly covered by various non‐glandular and glandular trichomes. Scientific interest in plant trichomes is based on their functional and taxonomic importance and on the economic usefulness of some trichome‐generated products. When non‐glandular trichomes form a dense indumentum, they may serve as a mechanical barrier against various external factors, such as herbivores and pathogens, UV‐B radiation, extreme temperatures and excessive water loss (Werker, 2000). Glandular trichomes, which secrete lipophilic substances (terpenes, lipids, waxes and flavonoid aglycones), may provide chemical or physicochemical protection against various types of herbivore and pathogen by entrapping, deterring or poisoning them (Wagner, 1991). The shape, size, structure and location of trichomes, and the composition of exudates produced by them, vary greatly among species and are used in plant taxonomy to distinguish between closely related species or hybrids (Spring, 2000).
The birches (Betula L.) are common trees and shrubs of the boreal and north temperate zones of the Northern hemisphere. The taxonomy of the European members of the genus has long been in dispute because of their high morphological variability and frequent hybridization (Atkinson, 1992). Difficulties in distinguishing between closely related birch species have prompted numerous biometric and chemotaxonomic studies. The former have been based mainly on morphological characteristics of birch leaves and fruits (reviewed in Atkinson, 1992), and the latter on the composition of phenolics (particularly flavonoids) and terpenoids in birch leaves, buds, bark and stems (reviewed in Keinänen et al., 1999). The leaves and young shoots of some birch species (e.g. B. pubescens) are covered by hairs, whereas other species (e.g. B. pendula and B. papyrifera) are characterized by the presence of peltate glands on leaves and on the bark of young twigs (Lapinjoki et al., 1991). Detailed studies have been conducted on birch stem gland structure (Lapinjoki et al., 1991; Raatikainen et al., 1992), exudate composition and functional importance (Reichardt, 1981; Reichardt et al., 1984; Rousi et al., 1991; Tahvanainen et al., 1991). The resins produced by these glands have been shown to consist of a mixture of phenolics and steroidal triterpenoids (Reichardt et al., 1984; Tahvanainen et al., 1991); they act as important deterrents against mammalian herbivores, especially hares (Reichardt et al., 1984; Rousi et al., 1991). In contrast, despite the potential ecological and taxonomic importance of birch leaf trichomes, no detailed studies or among‐taxa comparisons of their morphology and ultrastructure or the composition of their exudate have been carried out to date.
The aim of the present study was to investigate whether the structure, density and distribution of leaf trichomes and the composition of epicuticular flavonoids could be used to distinguish Finnish birch taxa (Betula pendula, B. pubescens ssp. pubescens, B. pubescens ssp. czerepanovii and B. nana). The phytochemical work focused on flavonoids because they are one of the most common groups of compounds found on the leaf surface of various plants (Wollenweber et al., 1991, 1998; Stevens et al., 1995; Grayer et al., 1996; Juma et al., 2001) and because of the ecological importance of these compounds as UV radiation filters (Markham and Mabry, 1975), antioxidants (Husain et al., 1987), and feeding attractants or repellents for herbivores (Harborne, 1991). In addition, the presence of flavonoid aglycones on birch leaf surfaces has been reported earlier by Keinänen and Julkunen‐Tiitto (1998).
MATERIALS AND METHODS
Study species
Three species of birch are generally acknowledged to be native in Finland (Kallio et al., 1983): silver birch (B. pendula Roth), dwarf birch (B. nana L.) and the B. pubescens Ehrh. complex with two subspecies, white birch (B. pubescens ssp. pubescens Ehrh.) and mountain birch {B. pubescens ssp. czerepanovii (Orlova) Hämet‐Ahti [syn. B. pubescens ssp. tortuosa (Ledeb) Nyman]}. The latter subspecies is believed to be the result of introgressive hybridization between B. pubescens and B. nana (Kallio et al., 1983). Both silver birch and B. pubescens belong to subsection Albae of the genus Betula; however, silver birch is a diploid, with 28 chromosomes, whereas B. pubescens is a tetraploid, with 56 chromosomes. Silver birch and white birch are early‐succession tree species common in southern and central Finland, whereas mountain birch is a climax species at the tree line in northern Fennoscandia. Dwarf birch belongs to the Nanae subsection of genus Betula and is a diploid (2n = 28); in the south it is a shrub restricted to moist sites (e.g. bogs), but in the northern part of Fennoscandia, and especially above the tree line, it is much more common.
Experimental trees
The trees of B. pendula used in this study represent five different clones (Table ), micropropagated in 1991 from mother trees growing in southern Finland and transplanted in 1994 to the field in the Botanical Garden of the University of Turku (60°26′N, 22°10′E, (Poteri et al., 2001). At the time of this study trees were 10 years old. Trees of B. pubescens ssp. pubescens originated from seeds of mother trees from two sites in southern Finland (Table ); the trees of B. pubescens ssp. czerepanovii were from six different provenances in Norway and Finnish Lapland (Table ; Sulkinoja and Valanne, 1987). Germination and seedling growth took place in a glasshouse belonging to the Department of Biology, University of Turku, from where seedlings were transplanted into the field in the Botanical Garden of the University of Turku. At the time of this study trees were 20 and 21 years old (mountain birch and white birch, respectively). The plants of B. nana were growing wild in a bog near the Kevo Subarctic Research Station, northern Finland (Table ).
Table 1.
Origin of birch trees used for analysis of leaf trichome structure and composition of epicuticular flavonoids
| Provenance or clone | Location of mother trees | Latitude N | Longitude E |
| B. pendula | |||
| Clone 26 (V5818) | Loppi | 60°45′ | 25°02′ |
| Clone 70 (V50079) | Punkaharju | 61°48′ | 29°17′ |
| Clone 84 (K1898) | Pielavesi | 63°18′ | 26°47′ |
| Clone 86 (K1932) | Pielavesi | 63°18′ | 26°47′ |
| Clone 124 (E9661) | Kihniö | 62°08′ | 23°18′ |
| B. pubescens ssp. pubescens | |||
| Pu | Punkaharju, Saukkonen tree no E2365 (South Finland) | 61°47′ | 29°19′ |
| Kö | Kökar, Karlby, Harparnäs (South Finland) | 59°55′ | 20°55′ |
| B. pubescens ssp. czerepanovii | |||
| 39 | Varanger, Gandvik (North Norway) | 70°01′ | 29°10′ |
| 26 | Utsjoki, Petsikko (North Lapland) | 69°34′ | 27°14′ |
| 44 | Sodankylä, Vuotso (South Lapland) | 68°03′ | 27°05′ |
| 43 | Kolari, Ylläs (South Lapland) | 67°35′ | 24°13′ |
| 42 | Kolari, Teuravuoma (South Lapland) | 67°17′ | 23°48′ |
| 29 | Rovaniemi, Arctic Circle (South Lapland) | 66°33′ | 25°50′ |
| B. nana | Utsjoki, Kevo | 69°45′ | 27°01′ |
Microscopy
One leaf was collected from one short shoot of three trees of each clone or provenance in early May 2001. Very young birch leaves (approx. 1 cm in length), which were very sticky, indicating high activity of glandular trichomes, were used. Leaves of B. nana were collected in late July and were studied by light microscopy only. For light and transmission electron microscopy (TEM), fresh leaf pieces (2 mm2) were cut with a razor blade from the basal area of each leaf under a drop of 2·5 % glutaraldehyde in 0·1 m phosphate buffer. Leaf pieces were postfixed in 1 % osmium tetroxide solution, dehydrated in an ascending ethanol series, and embedded in LX 112 Epon. Sections for light microscopy were stained with toluidine blue and photographed using a Zeiss (Jena, Germany) light microscope. Sections for TEM were stained with uranyl acetate and lead citrate. Three cells from the cortical and medullar parts of one trichome per leaf were randomly selected for analysis by TEM (JEM 100 SX) operating at 80 kV at ×1500–10 000 magnification and TEMSCAN (JEM 1200 EX) at ×30 000 magnification. The presence/absence of vacuoles, plastids, myelin‐like membranes, periplasmic space, lipid droplets and osmiophilic material was recorded. The sizes of vacuoles and plastids were classified as small, medium (for vacuoles only) and large. For scanning electron microscopy (SEM), fresh leaf pieces (10 × 10 mm2) were immersed in a fixation solution of 2·5 % glutaraldehyde in 0·1 m phosphate buffer for 24 h. Samples were washed for 15–30 min with the buffer and dehydrated using the following ethanol concentrations: 25 and 50 % for 5 min, 70, 80, 95 and twice 99 % for 10 min. Samples were then critical‐point dried using CO2 (Polaron, Watford, UK), mounted on aluminium stubs, sputtered with gold–palladium under vacuum (SEM Coating Unit E 5100; Polaron) and examined using SEM (JEOL JSM – 5200) at ×75, ×150, and ×200 magnification.
Trichome number
Six young leaves (1 cm in length) from each of the five trees from each clone or provenance were sampled in early May 2001 to determine the density of glandular trichomes. Leaves were placed on microscope slides in a drop of glue, pressed against the glass, and carefully removed after 1 min. This method resulted in transparent replicas of the adaxial (upper) and abaxial (lower) leaf surface. Densities of glandular trichomes were then counted from these replicas under a light microscope from three frames (0·65 × 0·65 mm2) per leaf using systematic uniform random sampling (Kubinova, 1994). Results are expressed as means ± s.d. of counts per mm2. The exact density of non‐glandular trichomes was not calculated because of their uneven distribution within a leaf and the high variation within and between leaves, trees and provenances. Instead, the abundance of leaf hairs on leaf surfaces or veins was scored for each sample under a light microscope. Leaf hairs were classified as absent, sparse, fairly abundant and very abundant. The above scoring was conducted only for the upper leaf surface because leaf hairs were very sparse on lower side of the leaf (except on veins).
Extraction, analysis and identification of leaf surface flavonoids
Ten leaves from five trees per clone or provenance were collected in May 2001 (at the same time as leaves were sampled for microscopy). Ten leaves from each of the 30 trees of B. nana were collected on 13 Jun. 2001. After sampling, all leaves were frozen and stored at – 80 °C. Five leaves from each tree were used for chemical analyses, while the remaining five were dried at 70 °C for 48 h, and dry weight was recorded. Leaf surface flavonoids were extracted by immersing each leaf in 10 ml 95 % ethanol for 20 s. Ethanolic extracts were filtered through 0·45‐µm PTFE filters and analysed using HPLC‐DAD at 200, 280, 315 and 349 nm. The HPLC system (Merck‐Hitachi, Tokyo, Japan) consisted of a pump L‐7100, a diode array detector L‐7455, a programmable autosampler L‐7200 and an interface D‐7000. The column was Superspher 100 RP‐18 (75 × 4 mm i.d., 4 µm; Merck, Darmstadt, Germany) and the eluents CH3CN (A) and 0·05 m H3PO4 (B). The elution profile was as follows: 0–3 min, 2 % A (isocratic); 3–22 min, 2–20 % A in B (linear gradient); 22–30 min, 20–30 % A in B (linear gradient); 30–35 min, 30–45 % A in B (linear gradient); 35–37 min, 45–70 % A in B (linear gradient); 37–45 min, 70 % A (isocratic) at a flow rate 1 ml min–1.
Mass spectra of surface flavonoids of each species were acquired using HPLC–ESI–MS in the negative ion mode using a Perkin‐Elmer Sciex API 365 triple quadrupole mass spectrometer (Sciex, Toronto, Canada). Chromatographic and ESI–MS conditions were the same as those described by Salminen et al. (1999). Compounds were identified on the basis of their UV and mass spectra, and retention times when co‐eluted with authentic reference compounds. Mass spectral identification was based on the m/z value of the deprotonated molecule [M–H]—, and on the fragment ion corresponding to [M–H–CH3]—• when the compound contained a methoxyl group (Justesen, 2001).
Individual flavanones were quantified at 280 nm as naringenins, flavones at 349 nm as apigenins, luteolins or acacetins, and flavonols at 349 nm as kaempferols or quercetins, using the corresponding aglycones as external standards.
Statistical analyses
Statistical analyses were carried out using SPSS 10.0 for Windows. The chi‐square test followed by the Mann–Whitney U test was used to compare ultrastructural parameters among birch taxa. For analysis of the density of glandular trichomes, the general linear model (GLM) repeated‐measures procedure was used, with leaf side (upper or lower) as a within‐subject factor and taxa as a between‐subject factor. Following established practice in the ecological literature, the term ‘concentration’ is used to refer to the mass of a particular compound per unit of leaf mass (mg g–1 d. wt; Koricheva, 1999), although the chemically correct term would be ‘content’. Among‐species differences in total concentration of surface flavonoids were examined by univariate analysis of variance. Tukey’s multiple range test was used for comparisons among means.
RESULTS
Trichome types, number and distribution on a leaf
All birch taxa studied possessed both glandular and non‐glandular trichomes on leaves. Non‐glandular trichomes were either short or long hairs. Long hairs could exceed 1 mm in length (Fig. 1A and B), whereas the length of short hairs was 50–100 µm for all taxa (Fig. 1C and D) except for B. nana where they were half this length. The abundance of leaf hairs and their distribution within a leaf differed among the birch taxa studied (Table ). Short hairs were more abundant on the upper leaf surface of the two B. pubescens subspecies (Fig. 1C) than on B. pendula, and were absent from the leaf surface of B. nana (Table ). Instead, B. nana leaves, as well as those of B. pubescens ssp. czerepanovii, possessed numerous short hairs on veins (Fig. 1D), whereas only a few short hairs occurred on veins of B. pubescens ssp. pubescens leaves (Table ). Veins of B. pendula had no short hairs but did possess some long hairs (Fig. 1A), as did the leaf veins of the two B. pubescens subspecies (Fig. 1B). In addition, both subspecies of B. pubescens had some long hairs on their leaf surfaces (Fig. 1C), unlike other birch taxa studied (Table ).
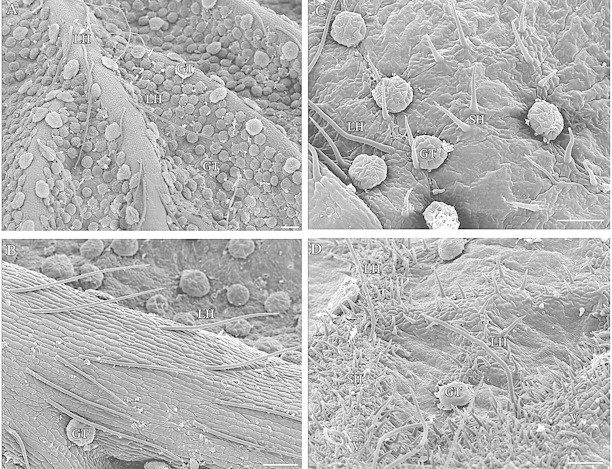
Fig. 1. SEM micrographs of non‐glandular (hairs) and glandular leaf trichomes of Betula species. A, Lower surface of B. pendula leaf. Long hairs are situated on veins and glandular trichomes occur both on the leaf surface and veins. B, Long hairs on vein of lower surface of B. pubescens ssp. pubescens leaf. C, Short hairs and glandular trichomes on upper surface of B. pubescens ssp. pubescens leaf. D, Glandular trichomes, long hairs and numerous short hairs on veins of B. pubescens ssp. czerepanovii leaf. LH, Long hair; SH, short hair; GT, glandular trichome; V, vein. Bar = 100 µm.
Table 2.
The abundance and location of different trichome types on leaves of the birch taxa studied
| Species | ||||
| B. pubescens ssp. pubescens | B. pubescens ssp. czerepanovii | B. pendula | B. nana | |
| Short non‐glandular trichomes | ||||
| Upper surface | + + + | + + | + | – |
| Veins | + | + + + | – | + + + |
| Long non‐glandular trichomes | ||||
| Upper surface | + | + | – | – |
| Veins | + + | + | + | – |
| Location of glandular trichomes | Above the epidermis | Sunken | ||
| Mean density of glandular trichomes (mm–2) ± s.d. | ||||
| Upper surface | 19 ± 12·1 | 5 ± 0·8 | 48 ± 16·2 | n.c. |
| Lower surface | 55 ± 26·2 | 14 ± 5·2 | 66 ± 13·8 | n.c. |
–, Absent; +, sparse; + +, fairly abundant; + + +, very abundant; n.c., not counted.
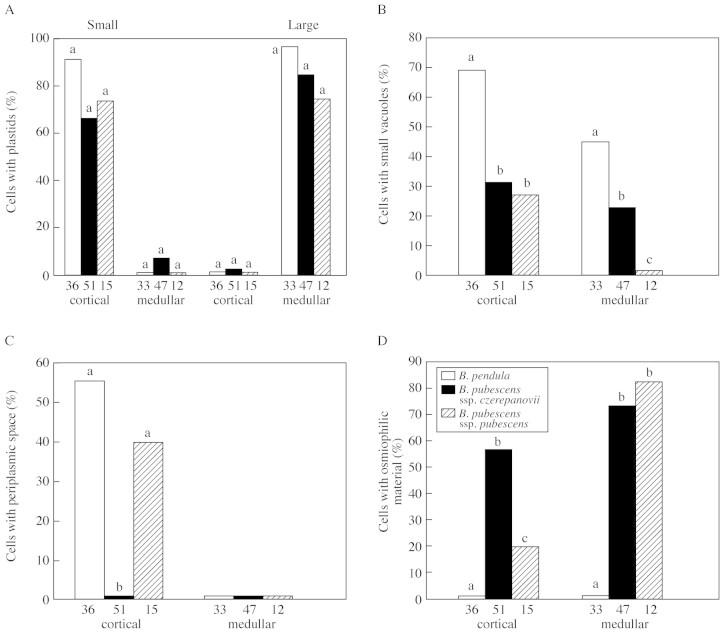
Glandular trichomes occurred on the leaf surface and on veins on both sides of the leaf (Fig. 1). The glandular trichomes of B. nana were sunk in the epidermis (Fig. 2A), whereas the trichomes of other birch species were raised above it (Fig. 2B). The density of glandular trichomes was higher on the lower than on the upper side of the leaf, and decreased in the order: B. pendula > B. pubescens ssp. pubescens > B. pubescens ssp. czerepanovii (P < 0·0001; Table ).
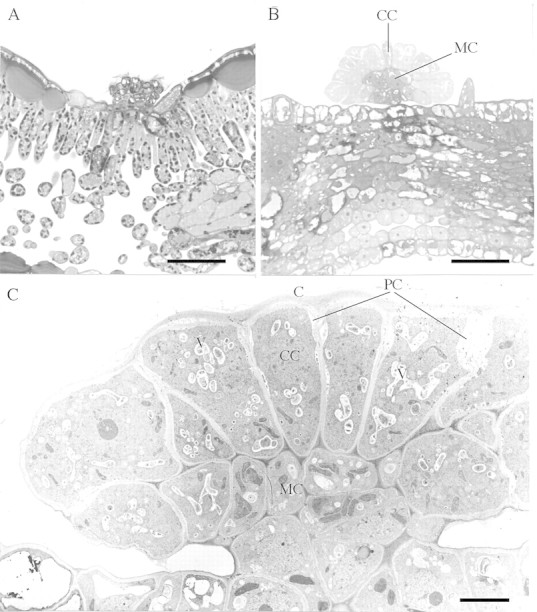
Fig. 2. Light micrographs of an aged glandular trichome of B. nana sunk in the epidermis of the upper surface of the leaf (A) and of a glandular trichome on a young leaf of B. pubescens ssp. czerepanovii (B). The cytoplasm of medullar cells (MC) stains deeply with toluidine blue, while the cytoplasm of cortical cells (CC) stains faintly. Glutaraldehyde and osmium tetroxide fixation. Bar = 50 µm. C, TEM micrograph of a glandular trichome on a young leaf of B. pendula. The trichome is tightly covered by a cuticle (C) and consists of cortical cells, which are more vacuolated (V) and have periplasmic spaces (PS), and medullar cells, which have few vacuoles and no periplasmic spaces. Bar = 5 µm.
Ultrastructure of glandular trichomes
The glandular trichomes of birch leaves belong to the peltate type and consisted of cortical and medullar cells, tightly covered by cuticle, which had no ruptures in young trichomes (Fig. 2C). The cytoplasm of medullar cells, fixed in osmium tetroxide, stained more deeply with toluidine blue than that of cortical cells (Fig. 2B). Microscopic examination revealed structural differences between cortical and medullar cells of birch glandular trichomes (Table ). The cortical cells in all taxa possessed small plastids (Fig. 3A) with homogeneous stroma, without developed internal membrane systems (Fig. 4A), whereas the medullar cells had large plastids (Fig. 3A) with well‐developed thylakoid systems (Fig. 5A and D). The cortical cells were more vacuolated and had periplasmic space, whereas medullar cells had few vacuoles and no periplasmic space (Figs 2C, 3B and C). The glandular trichomes of B. pendula had significantly more cells with small vacuoles than those in either subspecies of B. pubescens (Fig. 3B), and periplasmic space was not present in the cortical cells of B. pubescens ssp. czerepanovii (Fig. 3C).
Table 3.
Location of different cell structures in glandular trichomes of the birch taxa studied
| Species* | |||
| Structure | B. pubescens ssp. pubescens | B. pubescens ssp. czerepanovii | B. pendula |
| Nuclei with nucleoli | c, m | c, m | c, m |
| Rough endoplasmic reticulum | c, m | c, m | c, m |
| Lipid droplets | c, m | c, m | c, m |
| Small plastids | c | c | c |
| Large plastids | m | m | m |
| Small vacuoles | c | c, m | c, m |
| Periplasmic space | c | – | c |
| Myelin‐like membranes | – | – | c, m |
| Osmiophilic material | c, m | c, m | – |
c, Cortical cells; m, medullar cells; –, absent.
*B. nana was not analysed by TEM.
Fig. 3. Percentage of cortical and medullar cells of leaf glandular trichomes of Betula species with plastids (A), small vacuoles (B), periplasmic spaces (C) and osmiophilic material (D). Numbers below bars indicate the number of cells examined. Bars with different letters indicate significant differences among species (P < 0·05, Mann–Whitney U test).
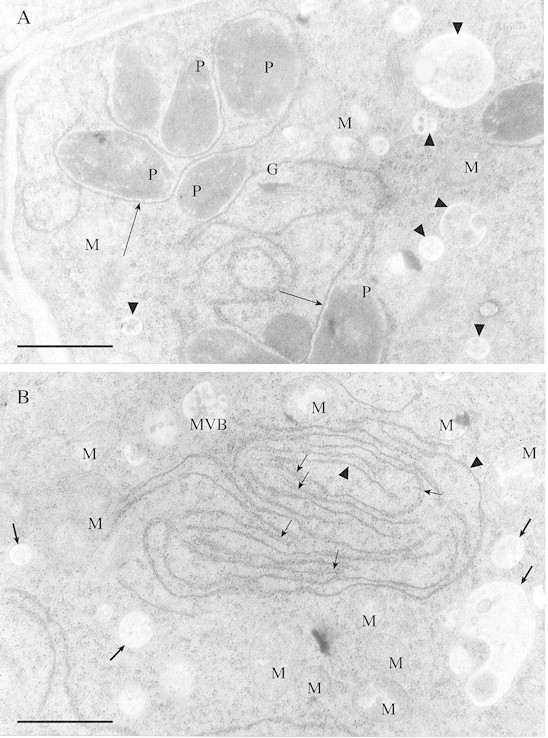
Fig. 4. TEM micrographs of cortical cells of glandular trichome of B. pubescens ssp. czerepanovii. A, Plastids (P) with homogeneous stroma, without a developed internal membrane system, are common in the cytoplasm of cortical cells. Note association of plastids with endoplasmic reticulum (arrows), Golgi apparatus (G) and mitochondria (M). Numerous vesicles (arrow heads) are present near endoplasmic reticulum and Golgi apparatus. Bar = 1 µm. B, Rough endoplasmic reticulum with double cisternae (small arrows) and a large area of cisternae fusion (arrow heads). Numerous vesicles (large arrows), a multivesicular body (which represents a vacuole containing vesicles set in a lucent matrix; MVB) and mitochondria (M) are present near rough endoplasmic reticulum. Bar = 1 µm.
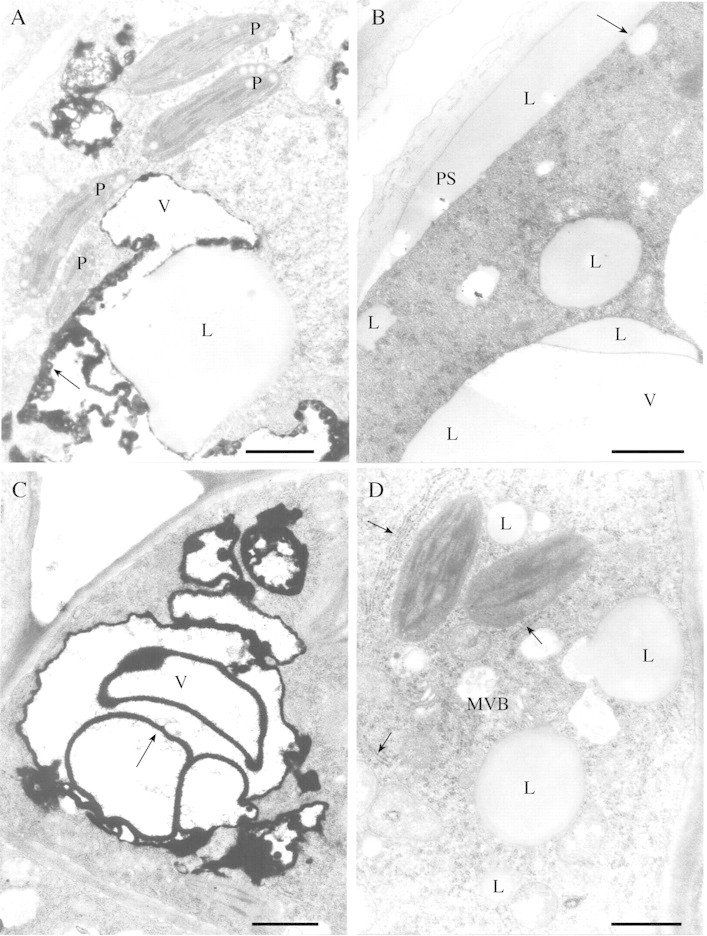
Fig. 5. TEM micrographs of leaf glandular trichomes of Betula species. Medullar cell (A) and cortical cell (B) of B. pubescens ssp. pubescens. A, Plastids (P) with well‐developed thylakoids, osmiophilic material (arrow) inside vacuoles on the border with the cytoplasm, and large lipid droplet (L). B, Lipid droplets occur in vacuoles (V) and cytoplasm. Note lipid droplet (arrow) fused with periplasmic space (PS), which contains lipid‐like material (L). C and D, Medullar cells of B. pubescens ssp. czerepanovii. C, Osmiophilic material (arrow) forms whorls inside vacuole (V). D, Plastids with well‐developed thylakoids, lipid droplets of varying size and multivesicular bodies (MVB) are present in the cytoplasm near rough endoplasmic reticulum (arrows). Bar = 1 µm.
Rough endoplasmic reticulum (RER) was present in both cortical (Fig. 4B) and medullar (Fig. 5D) cells of birch glandular trichomes. There were areas of RER with double cisternae and also large areas where cisternae had fused (Fig. 4B). Close association of RER with plastids, the Golgi apparatus and mitochondria was found (Fig. 4A). Vesicles containing substances and multivesicular bodies, which appeared as vacuoles containing vesicles set in lucent matrix, were found near the RER (Figs 4A, B and 5D). Nuclei with nucleoli occurred in both cortical and medullar cells of birch trichomes (Fig. 6).
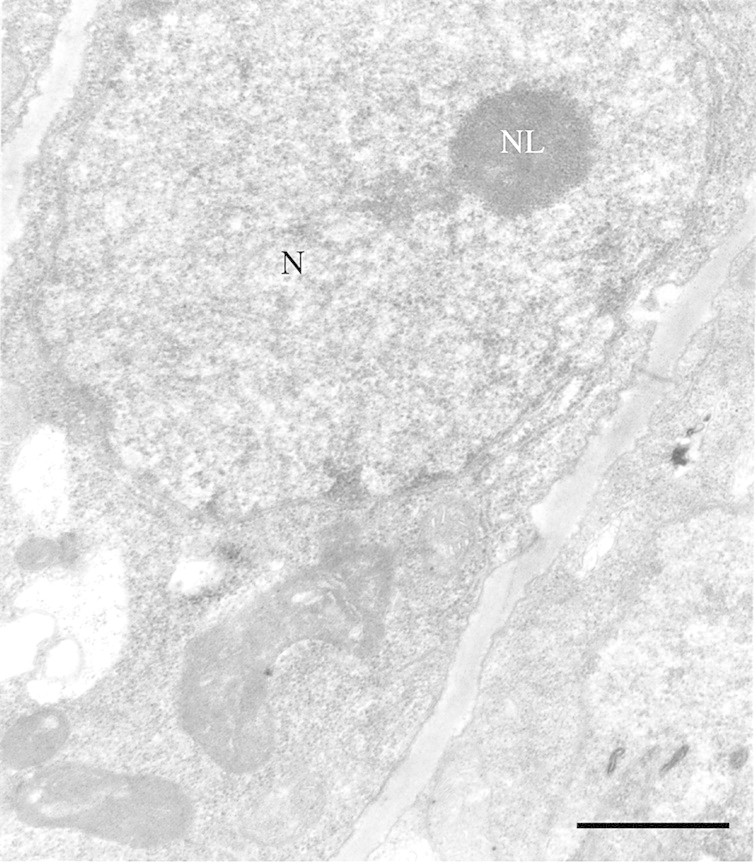
Fig. 6. TEM micrograph of nucleus (N) with nucleolus (NL) in leaf glandular trichomes of B. pubescens ssp. czerepanovii. Bar = 1 µm.
Osmiophilic material was localized in vacuoles [either on the boundary with the cytoplasm (Fig. 5A) or inside vacuoles forming whorls (Fig. 5C)], but it was absent from periplasmic and subcuticular spaces. Medullar cells of B. pubescens ssp. pubescens and B. pubescens ssp. czerepanovii possessed more osmiophilic material than did the cortical cells (P = 0·001 and 0·067, respectively; Fig. 3D), but no osmiophilic material was found in trichomes of B. pendula. Lipid droplets of varying size occurred in vacuoles (Fig. 5B) and in cytoplasm near plastids and RER (Fig. 5D) in 10–20 % of glandular trichome cells in all taxa, and there were no differences in their occurrence between cortical and medullar cells (P > 0·414). In some cases, lipid droplets were fused with periplasmic space, which commonly contained lipid‐like material (Fig. 5B). Myelin‐like membranes were numerous in small vesicles, vacuoles (Fig. 7A) and periplasmic space (Fig. 7A and B) in cortical cells of B. pendula trichomes, but were less abundant in medullar cells of this species and were completely absent from glandular trichomes of B. pubescens subspecies. Electron‐dense droplets of varying size were present in vacuoles near myelin‐like membranes (Fig. 7A).
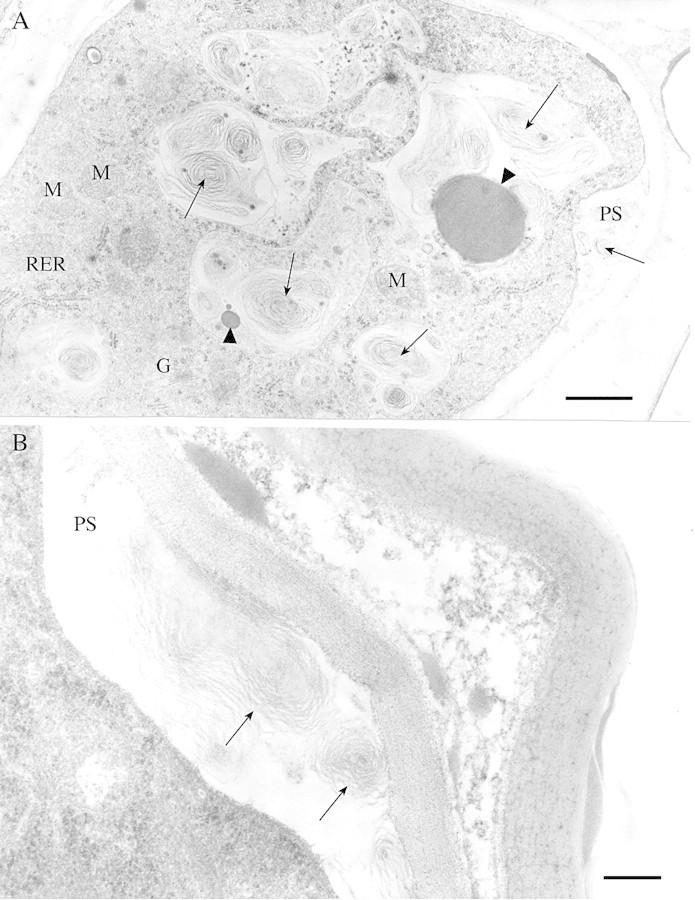
Fig. 7. Cortical cells of glandular trichomes of B. pendula. A, Numerous myelin‐like membranes (arrows) are present in vesicles, vacuoles and periplasmic space (PS). Electron‐dense droplets (arrow heads) of varying size are present in vacuoles near myelin‐like membranes. Mitochondria (M), rough endoplasmic reticulum (RER) and Golgi apparatus (G) occur in the cytoplasm. Bar = 1 µm. B, Myelin‐like membranes (arrows) occur in the periplasmic space. Bar = 200 nm.
Identification of leaf surface flavonoids
The leaf surface compounds detected in this study (Table ) were first classified on the basis of their UV spectra as flavanones (1, 7 and 13), flavonols (3, 4, 10 and 14) and flavones (2, 5, 6, 8, 9, 11, 12 and 15), although some of the compounds identified as flavones could have been flavonol 3‐methyl ethers, since these tend to have flavone‐like UV spectra. Negative ion HPLC–ESI–MS analysis revealed the molecular masses of compounds 1–13 on the basis of the most prominent m/z value corresponding to the de‐protonated molecule [M–H]—. This was confirmed for all but two compounds by the presence of [2M–H]— in addition to [M–H]—. Methoxylated flavonoids have been found to produce radical anions [M–H–CH3]—• by fragmentation of the aglycones in ESI–MS (Justesen, 2001). Accordingly, m/z values corresponding to the loss of a methyl group from the de‐protonated molecules ([M–H–CH3]—•) of 4, 7, 8, 10, 11 and 12 were observed. Therefore, these flavonoids were shown to carry at least one methyl group attached to some of their hydroxyl functions, thus forming a methoxyl group.
Table 4.
Chromatographic, UV and mass spectral properties of flavonoids and their identification
| Compound | HPLC Rt** (min) | UV spectral maxima (nm) | [M‐H]— | [2M‐H]— | [M‐H‐CH3]—• | Identification |
| 1 | 32·2 | 288 | 271 | 543 | – | Naringenin*,† |
| 2 | 33·4 | 265, 335 | 269 | 539 | – | Apigenin*,‡ |
| 3 | 33·8 | 264, 367 | 285 | 571 | – | Kaempferol* |
| 4 | 34·1 | 255, 367 | 315 | 631 | 300 | Flavonol methyl ether |
| 5 | 36·4 | 248, 266, 341 | 313 | 627 | – | Luteolin derivative‡ |
| 6 | 36·7 | 251, 271, 341 | 329 | – | – | Pentahydroxyflavone dimethyl ether |
| 7 | 36·9 | 288 | 285 | 571 | 270 | Flavanone methyl ether |
| 8 | 37·4 | 266, 329 | 283 | 567 | 268 | Acacetin*,†,‡ |
| 9 | 37·6 | 273, 331 | 313 | – | – | Tetrahydroxyflavone dimethyl ether |
| 10 | 37·7 | 264, 363 | 299 | 599 | 284 | Flavonol methyl ether |
| 11 | 37·8 | 266, 346 | 313 | 627 | 298 | Flavonol dimethyl ether |
| 12 | 38·0 | 268, 336 | 343 | 687 | 328 | Pentahydroxyflavone trimethylether |
| 13 | 38·8 | 288 | 381 | 763 | – | Flavanone† |
| 14 | 39·4 | 266, 363 | – | – | – | Kaempferol derivative† |
| 15 | 39·5 | 267, 328 | – | – | – | Apigenin derivative† |
UV spectra were recorded by HPLC‐DAD using acetonitrile and 0·05 m H3PO4 as eluents.
* Co‐chromatography with authentic standard.
** Rt, retention time.
† Identified previously from B. pubescens leaves by Keinänen and Julkunen‐Tiitto (1998).
‡ Identified previously from B. pendula leaves by Keinänen and Julkunen‐Tiitto (1998).
On the basis of the above information, and co‐chromatography with authentic standards, flavonoids 1, 2, 3 and 8 were identified as naringenin, apigenin, kaempferol and acacetin, respectively. Compounds 4, 7 and 10 were tentatively identified as flavonol methyl ether, flavanone methyl ether and flavonol methyl ether, respectively. Their UV and mass spectra would fit those of quercetin methyl ether, naringenin methyl ether and kaempferol methyl ether, but without 1H‐NMR analysis or co‐chromatography with authentic standards these structures could not be confirmed. Flavonoids at retention times similar to those of 5 and 6 have previously been classified as luteolin derivatives of B. pendula leaves (Keinänen and Julkunen‐Tiitto, 1998). Indeed, their UV spectra, with two maxima in the region 251–271 nm, were characteristic of luteolin. However, as luteolin is a tetrahydroxyflavone, the m/z value of compound 6 implied that it was a dimethyl ether of a pentahydroxyflavone derivative. Flavonoid 9 has been identified by Keinänen and Julkunen‐Tiitto (1998) as an apigenin (trihydroxyflavone) derivative. However, with a molecular mass of 314, compound 9 clearly cannot be a trihydroxyflavone, but rather must be a tetrahydroxyflavone dimethyl ether. On the basis of the molecular mass of flavonoid 11, it also has two hydroxy‐ and two methoxy‐groups. The UV spectrum (266, 346 nm) suggests that it is a 3‐methyl ether of a kaempferol derivative (and thus a flavonol dimethyl ether). Compound 12 was identified as a pentahydroxyflavone trimethyl ether. Keinänen and Julkunen‐Tiitto (1998) have identified flavonoids 13–15 as flavanone, apigenin derivative and kaempferol derivative, respectively. The present data support these findings, except that the elution order of apigenin and kaempferol derivatives was reversed. Therefore, compounds 14 and 15 were tentatively identified as a kaempferol derivative and an apigenin derivative, respectively.
Among‐species differences in the composition of leaf surface flavonoids
There were clear differences in the composition of leaf surface flavonoids in the four birch species (Table ). However, the differences between the two subspecies of B. pubescens were only quantitative: B. pubescens ssp. pubescens had a significantly higher total concentration of surface flavonoids (Table ; P = 0·0001) than B. pubescens ssp. czerepanovii. The leaf surface of B. nana contained the same 12 flavonoids as that of B. pubescens subspecies, but five of these compounds (1, 4, 7, 13 and 15) were present only in trace amounts in B. nana. As a result, the total concentration of surface flavonoids was lower in B. nana than in the two B. pubescens subspecies (Table ). Only six flavonoid compounds were found on the leaf surface of B. pendula, and three of these (5, 6 and 9) occurred only in this species. The total concentration of surface flavonoids in B. pendula was lower than that in other birch taxa examined (Table ).
Table 5.
Percentage composition and total concentration (mean ± s.d.) of epicuticular flavonoid aglycones in leaves of the birch taxa studied
| Species | |||||
| No. | Compound | B. pubescens ssp. pubescens | B. pubescens ssp. czerepanovii | B. nana | B. pendula |
| 1 | Naringenin | 1·7 | 1·3 | Trace | n.d. |
| 2 | Apigenin | 2·4 | 2·2 | 2·9 | 6·7 |
| 3 | Kaempferol | 5·6 | 5·4 | 12·6 | n.d. |
| 4 | Flavonol methyl ether | 4·6 | 5·8 | Trace | n.d. |
| 5 | Luteolin derivative | n.d. | n.d. | n.d. | 2·0 |
| 6 | Pentahydroxyflavone dimethyl ether | n.d. | n.d. | n.d. | 2·3 |
| 7 | Flavanone methyl ether | 28·6 | 15·5 | Trace | n.d. |
| 8 | Acacetin | 7·7 | 13·6 | 9·4 | 6·7 |
| 9 | Tetrahydroxyflavone dimethyl ether | n.d. | n.d. | n.d. | 67·9 |
| 10 | Flavonol methyl ether | 21·0 | 24·6 | 34·4 | n.d. |
| 11 | Flavonol dimethyl ether | 7·7 | 12·2 | 15·5 | n.d. |
| 12 | Pentahydroxyflavone trimethyl ether | 3·8 | 5·2 | 15·0 | 14·4 |
| 13 | Flavanone | 7·8 | 4·2 | Trace | n.d. |
| 14 | Kaempferol derivative | 5·1 | 6·4 | 10·1 | n.d. |
| 15 | Apigenin derivative | 4·1 | 3·7 | Trace | n.d. |
| Total concentration (mg g–1 d. wt) | 71 ± 21·7 | 22 ± 10·6 | 16 ± 4·0 | 8 ± 2·8 | |
Trace, trace amounts; n.d., not detected.
DISCUSSION
This paper represents the first detailed comparative analysis of leaf trichome morphology and ultrastructure in Finnish birch species. All taxa examined contained both glandular and non‐glandular trichomes, but the structure, density and distribution of trichomes on leaves differed among taxa. Taxa with more hairy leaves possessed fewer glandular trichomes, and vice versa. Similarly, within a single species, the upper leaf surface, which had a lower density of glandular trichomes, usually had more leaf hairs than the lower surface. The structure, density and distribution of trichomes on leaves of different birch taxa appeared to reflect the degree of their taxonomic relatedness. For instance, B. pubescens ssp. czerepanovii is believed to be the result of introgressive hybridization between B. pubescens and B. nana (Kallio et al., 1983), and its leaves displayed characteristics of both parent species having a high density of short hairs on the leaf surface, as well as long hairs, as in B. pubescens ssp. pubescens, and having numerous short hairs on veins as in B. nana.
In general, the structure of birch leaf glandular trichomes resembled that of resin glands on stems of B. pendula (Raatikainen et al., 1992): both represent peltate glands consisting of medullar and cortical cells. In all the taxa studied, cells of young birch glandular trichomes were characterized by many traits indicative of high metabolic activity, e.g. the presence of nucleoli in nuclei, the occurrence of RER, lipid droplets, numerous plastids and small vacuoles. Similar structures have been observed in glandular trichome cells of other plant species. For instance, RER has commonly been found in glandular trichomes producing terpenoids and is believed to play a main role in the biosynthesis, accumulation and secretion of terpenes (Gunning and Steer, 1975; Skubatz et al., 1995). Lipid droplets and multivesicular bodies, similar to those in birch leaf trichomes, have also been found in trichomes producing terpenes (Ascensão and Pais, 1998).
Structurally, the small, poorly developed plastids found in cortical cells of birch trichomes resembled leucoplasts in oil idioblasts of a Curcuma longa shoot (Cheniclet and Carde, 1985). Analytical and structural studies, which were performed on 45 species of higher plants, showed a very close correlation between the presence of leucoplasts in secretory cells and the amount of monoterpenes in the volatile extract (Cheniclet and Carde, 1985). Moreover, key enzymes of monoterpene biosynthesis have been found in plastids, particularly in leucoplasts (Gleizes et al., 1983; Soler et al., 1992). In the present work, cortical cells were found to possess numerous small vacuoles and periplasmic spaces filled by secreted material (Table ); periplasmic spaces have, similarly, been reported in birch resin glands (Raatikainen et al., 1992) and in leaf glandular trichomes of other species (Ascensão and Pais, 1998; Fahn, 2000). In contrast to cortical cells, medullar cells contained few vacuoles, large amounts of osmiophilic material located in vacuoles (in B. pubescens subspecies), large plastids with well‐developed thylakoids, and had no periplasmic space (Table ). Moreover, the medullar cells were more osmiophilic than cortical ones, indicating phenol‐containing areas in cytoplasm (Nielson and Griffith, 1978). The presence of phenolics in medullar cells of birch glandular trichomes has been demonstrated by fluorescence microscopy (Pääkkönen et al., 1998). These ultrastructural differences between the cortical and medullar cells may suggest functional distinction within the trichome.
The main difference among the birch species studied in terms of glandular trichome ultrastructure was that the glandular trichomes of B. pendula contained numerous myelin‐like osmiophilic membranes in small vesicles, vacuoles and in the periplasmic space, whereas those of B. pubescens subspecies possessed large amounts of osmiophilic material in vacuoles (Table ). In mammals, myelin‐like membranes, which contain mainly phospholipids, have been studied in alveolar secretory cells of the lung (Ghadially, 1988). In plants, similar structures have been found in the early secretion stage of cortical cells in the stem resin glands of B. pendula (Raatikainen et al., 1992), and it has been suggested that they represent a stage at which papyriferic acid and related triterpenes or their precursors are integrated into cytoplasmic membranes (Raatikainen et al., 1992).
The osmiophilic material, which was common in vacuoles of glandular cells of B. pubescens, is likely to represent phenolics, containing o‐dihydroxy groups (Nielson and Griffith, 1978). Osmiophilic deposits have been found exclusively in vacuoles, but not in periplasmic or subcuticular spaces, indicating that the material is not secreted with exudates, but is stored inside glandular trichome cells. In contrast to stem resin glands of the same species, leaf glandular trichomes of B. pendula lacked osmiophilic material in vacuoles (Raatikainen et al., 1992). Such an ultrastructural difference between leaf and stem glands in silver birch may indicate a different chemical composition of exudates produced by the two gland types.
Chemical analyses of birch leaf surface extracts revealed a number of flavonoid aglycones, some of which have previously been detected from the leaf surfaces of B. pendula and B. pubescens (Keinänen and Julkunen‐Tiitto, 1998) and from the bud exudates of B. nigra (Wollenweber et al., 1991). The present study demonstrates that B. pendula differs considerably from the other species in its composition of epicuticular flavonoid aglycones, whereas B. nana and the two subspecies of B. pubescens share the same compounds. The localization of flavonoid aglycones in leaf glandular trichomes has been demonstrated in several plant species (Wollenweber et al., 1992; Voirin et al., 1993; Bosabalidis et al., 1998). Flavonoid aglycones are lipophilic compounds and commonly occur along with the resinous and sticky material in which they are dissolved (Wollenweber, 1995). Therefore, they may be dissolved in lipid droplets or phospholipids of myelin‐like membranes. Moreover, myelin‐like membranes evolve from endoplasmic reticulum (Ghadially, 1988), with which are associated the enzyme complexes of phenylpropanoid and flavonoid synthesis (Hrazdina and Wagner, 1985). The presence of lipid droplets fused with periplasmic space and the occurrence of numerous vesicles and periplasmic space filled with myelin‐like membranes suggest the transport of exudates by vesicles, which is a common means of secretion of lipophilic compounds (Fahn, 2000).
In conclusion, the structure, density and distribution of trichomes on leaves and the profiles of epicuticular flavonoids differ markedly among Finnish birch species, especially between B. pubescens and B. pendula. It can be difficult to distinguish the latter species on the basis of other morphological characteristics (Atkinson, 1992); thus, ultrastructural studies of trichomes and the chemical composition of the birch leaf surface may provide a useful taxonomic tool.
ACKNOWLEDGEMENTS
We thank Marjo Helander, Lauri Kapari, Kari Saikkonen and Matti Sulkinoja for permission to use their experimental trees at the Botanical Garden of the University of Turku and at Kevo. Ritva Niemi and the staff at the Laboratory of Electron Microscopy provided advice and technical assistance with TEM and SEM, and Juha Järvenpää and Tuuli Luomahaara assisted with the chemical analyses. We also thank Lauri Pelliniemi, Erkki Haukioja and Renee Grayer for helpful comments on the manuscript, and Ellen Valle for revising the language. The study was financed by the Academy of Finland.
Supplementary Material
Received: 4 December 2002; Returned for revision: 13 January 2002; Accepted: 24 January 2003 Published electronically: 12 March 2003
References
- AscensãoL, Pais MS.1998. The leaf capitate trichomes of Leonotis leonurus: histochemistry, ultrastructure and secretion. Annals of Botany 81: 263–271. [Google Scholar]
- AtkinsonMD.1992. Betula pendula Roth (B. verrucosa Ehrh.) and B. pubescens Ehrh. Journal of Ecology 80: 837–870. [Google Scholar]
- BosabalidisA, Gabrieli C, Niopas I.1998. Flavone aglycones in glandular hairs of Origanum × intercedens Phytochemistry 49: 1549–1553. [DOI] [PubMed] [Google Scholar]
- ChenicletC, Carde JP.1985. Presence of leucoplasts in secretory cells and of monoterpenes in the essential oil: a correlated study. Israel Journal of Botany 34: 219–238. [Google Scholar]
- FahnA.2000. Structure and function of secretory cells. Advances in Botanical Research 31: 37–75. [Google Scholar]
- GhadiallyFN.1988. Ultrastructural pathology of the cell and matrix. 3rd edn. London: Butterworths. [Google Scholar]
- GleizesM, Pauly G, Carde JP, Marpeau A, Bernard‐Dagan C.1983. Monoterpene hydrocarbon biosynthesis by isolated leucoplasts of Citrofortunella mitis Planta 159: 373–381. [DOI] [PubMed] [Google Scholar]
- GrayerRJ, Bryan SE, Veitch NC, Goldstone FJ, Paton A, Wollenweber E.1996. External flavones in sweet basil, Ocimum basilicum, and related taxa. Phytochemistry 43: 1041–1047. [DOI] [PubMed] [Google Scholar]
- GunningBES, Steer MW.1975. Ultrastructure and the biology of plant cells. London: Edward Arnold. [Google Scholar]
- HarborneJB.1991. Flavonoids pigments. In: Rosenthal GA, Berenbaum MR, eds. Herbivores. Their interactions with secondary plant metabolites San Diego: Academic Press, 389–429. [Google Scholar]
- HrazdinaG, Wagner GJ.1985. Metabolic pathway as enzyme complex: evidence for synthesis of phenylpropanoids and flavonoids on membrane associated enzyme complexes. Archives of Biochemistry and Biophysics 237: 88–100. [DOI] [PubMed] [Google Scholar]
- HusainSR, Cillard J, Cillard P.1987. Hydroxyl radical scavenging activity of flavonoids. Phytochemistry 26: 2489–2492. [Google Scholar]
- JumaBF, Yenesew A, Midiwo JO, Waterman PG.2001. Flavones and phenylpropenoids in the surface exudate of Psiadia punctulata Phytochemistry 57: 571–574. [DOI] [PubMed] [Google Scholar]
- JustesenU.2001. Collision‐induced fragmentation of deprotonated methoxylated flavonoids, obtained by electrospray ionization mass spectrometry. Journal of Mass Spectrometry 36: 169–178. [DOI] [PubMed] [Google Scholar]
- KallioP, Niemi P, Sulkinoja M.1983. The Fennoscandian birch and its evolution in the marginal forest zone. In: Morisett P, Payette S, eds. Tree‐line ecology. Proceedings of the Northern Quebec Tree‐Line Conference Collection Nordicana, Laval University, Sainte Foy, Quebec, Canada, 101–111. [Google Scholar]
- KeinänenM, Julkunen‐Tiitto R.1998. High‐performance liquid chromatographic determination of flavonoids in Betula pendula and Betula pubescens leaves. Journal of Chromatography 793: 370–377. [Google Scholar]
- KeinänenM, Julkunen‐Tiitto R, Rousi M, Tahvanainen J.1999. Taxonomic implications of phenolic variation in leaves of birch (Betula L.) species. Biochemical Systematics and Ecology 27: 243–254. [Google Scholar]
- KorichevaJ.1999. Interpreting phenotypic variation in plant allelochemistry: problems with the use of concentrations. Oecologia 119: 467–473. [DOI] [PubMed] [Google Scholar]
- KubinovaL.1994. Recent stereological methods for measuring leaf anatomical characteristics: estimation of the number and size of stomata and mesophyll cells. Journal of Experimental Botany 45: 119–127. [Google Scholar]
- LapinjokiSP, Elo HA, Taipale HT.1991. Development and structure of resin glands on tissues of Betula pendula Roth. during growth. New Phytologist 117: 219–223. [Google Scholar]
- MarkhamKR, Mabry TJ.1975. Ultraviolet‐visible and proton magnetic resonance spectroscopy of flavonoids. In: Harborne JB, Marby TJ, Marby H, eds. The flavonoids London: Chapman and Hall, 45–77. [Google Scholar]
- NielsonAJ, Griffith WP.1978. Tissue fixation and staining with osmium tetroxide: the role of phenolic compounds. The Journal of Histochemistry and Cytochemistry 26: 138–140. [DOI] [PubMed] [Google Scholar]
- PääkkönenE, Günthardt‐Goerg MS, Holopainen T.1998. Responses of leaf processes in a sensitive birch (Betula pendula Roth) clone to ozone combined with drought. Annals of Botany 82: 49–59. [Google Scholar]
- PoteriM, Helander ML, Saikkonen K, Elamo P.2001. Effect of Betula pendula clone and leaf age on Melampsoridium betulinum rust infection in a field trial. Forest Pathology 31: 177–185. [Google Scholar]
- RaatikainenOJ, Taipale HT, Pelttari A, Lapinjoki SP.1992. An electron microscope study of resin production and secretion by the glands of seedlings of Betula pendula Roth. New Phytologist 122: 537–543. [DOI] [PubMed] [Google Scholar]
- ReichardtPB.1981. Papyriferic acid: a triterpenoid from Alaskan paper birch. Journal of Organic Chemistry 46: 4576–4578. [Google Scholar]
- ReichardtPB, Bryant JP, Clausen TP, Wieland GD.1984. Defence of winter‐dormant Alaska paper birch against snowshoe hares. Oecologia 65: 58–69. [DOI] [PubMed] [Google Scholar]
- RousiM, Tahvanainen J, Uotila I.1991. A mechanism of resistance to hare browsing in winter dormant European white birch (Betula pendula). The American Naturalist 137: 64–82. [Google Scholar]
- SalminenJP, Ossipov V, Loponen J, Haukioja E, Pihlaja K.1999. Characterization of hydrolysable tannins from leaves of Betula pubescens by high‐performance liquid chromatography – mass spectrometry. Journal of Chromatography 864: 283–291. [DOI] [PubMed] [Google Scholar]
- SkubatzH, Kunkell DD, Patt JM, Howald WN, Hartman TG, Meeuse BJD.1995. Pathway of terpene excretion by appendix of Sauromatum guttatum Proceedings of the National Academy of Sciences of the USA 92: 10084–10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SolerE, Feron G, Clastre M, Dargent R, Gleizer M, Ambid C.1992. Evidence for a geranyl‐diphosphate synthase located within the plastids of Vitis vinifera L. cultivated in vitro Planta 187: 171–175. [DOI] [PubMed] [Google Scholar]
- SpringO.2000. Chemotaxonomy based on metabolites from glandular trichomes. Advances in Botanical Research 31: 153–174. [Google Scholar]
- StevensJF, Hart H, Wollenweber, E.1995. The systematic and evolutionary significance of exudate flavonoids in Aeonium Phytochemistry 39: 805–813. [Google Scholar]
- SulkinojaM, Valanne T.1987. Leafing and bud size in Betula provenances of different latitudes and altitudes. Reports of the Kevo Subartic Research Station 20: 27–33. [Google Scholar]
- TahvanainenJ, Julkunen‐Tiitto R, Rousi M, Rechardt PB.1991. Chemical determinations of resistance in winter‐dormant seedlings of European white birch (Betula pendula) to browsing by the mountain hare. Chemoecology 2: 49–54. [Google Scholar]
- VoirinB, Bayet C, Colson M.1993. Demonstration that flavone aglycones accumulate in the peltate glands of Mentha × piperita leaves. Phytochemistry 34: 85–87. [Google Scholar]
- WagnerGJ.1991. Secreting glandular trichomes: more than just hairs. Plant Physiology 96: 675–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WerkerE.2000. Trichome diversity and development. Advances in Botanical Research 31: 1–35. [Google Scholar]
- WollenweberE.1995. Externally accumulated flavonoids and other lipophilic phenolics in Angiosperms. Bulletin de Liaison du Groupe Polyphenols 16: 323–324. [Google Scholar]
- WollenweberE, Mann K, Roitman JN.1991. Flavonoid aglycones from the bud exudates of three Betulaceae. Zeitschrift für Naturforschung 46: 495–497. [Google Scholar]
- WollenweberE, Stevens JF, Ivancic M.1998. Flavonoid aglycones and a thiophene derivative from Helichrysum cassianum Phytochemistry 47: 1441–1443. [Google Scholar]
- WollenweberE, Dörr M, Stelzer R, Arriaga‐Giner FJ.1992. Lipophilic phenolics from the leaves of Empetrum nigrum – chemical structures and exudate localization. Botanica Acta 105: 300–305. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.