Abstract
Architectural analysis was applied to study branch development of ‘Royal Gala’ apple trees grafted with dwarfing and non‐dwarfing rootstock/interstock combinations, which had been chosen to produce trees with a wide range of vigour. Using AMAPmod methodology, the structure of 3‐year‐old branches was described at four levels of representation: branch; annual shoot; growth unit; and node. Three types of growth units were distinguished: extension growth unit (vegetative unit with internode extension); vegetative spur with minimal internode extension; and fruiting spur or bourse. The aim of the analysis was to describe exactly how the rootstock/interstock combinations affected the structure building process. The number of extension growth units, vegetative spurs and fruiting spurs per annual shoot changed over the years, but this was not affected by rootstock/interstock combination. Compared with MM.106 rootstock, M.9 rootstock reduced the number of nodes per extension growth unit. In most cases, rootstock/interstock combination had no effect on the linear relationship between extension growth unit length and node number (R2 = 0·88). Average internode length depended on unit node number, with internodes being shorter for units with fewer nodes. Thus the difference in apple branch size induced by the rootstock/interstock combinations was mainly due to a reduction in the length and number of neoformed nodes produced on extension growth units. As percentage budbreak of axillary buds on extension growth units was not affected by rootstock/interstock combination, differences in numbers of axillary annual shoots per branch were entirely due to differences in the total numbers of nodes extended during the previous year.
Key words: Malus domestica Borkh, plant architecture, modelling, growth unit, rootstock, dwarfing, internode length, node number, neoformation
INTRODUCTION
Even though rootstocks are widely used to control apple tree size, the mechanisms involved and the specific effects on tree growth are still unclear (Atkinson and Else, 2001). The main difficulties in identifying the effects of rootstock are associated with the fact that these effects are cumulative and are superimposed over year‐to‐year trends and variations in scion development (Barritt et al., 1995; Ferree et al., 1995). Architectural analysis (Barthélémy, 1991; Godin et al., 1997), being a dynamic approach to plant development, appears well suited to investigate the rootstock effects on apple tree growth. This approach is based on the hypothesis that plant structures are ‘built’ by addition of similar constructional units, e.g. nodes, shoots and branches (White, 1979; Barlow, 1994). The primary aim of architectural analysis is to reveal the genetically determined plan of the plant construction. At any given time, plant structure is an expression of equilibrium between realization of this plan and constraints exerted by the environment. Architectural analysis can also be used to investigate the extent of architectural plasticity or, in other words, the extent to which an expression of the architecture can be altered by the external or internal environment. In this paper, architectural analysis is used to investigate the effects of rootstock on aerial architecture, which is induced via the internal environment of the apple tree.
In temperate trees, annual shoots develop from buds by emergence of successive metamers, each consisting of a node, an internode, a leaf and an axillary bud (White, 1979; Barlow, 1994). First metamers are initiated during the previous season (known as ‘preformed’) and are present in the resting bud during winter dormancy. In some species shoot growth ceases after elongation of the preformed metamers, in others it may continue as a result of initiation and elongation of new (‘neoformed’) metamers (Hallé et al., 1978; Powell, 1991). Generally the extent of neoformation decreases with plant age.
As with other species, annual shoots of apple display flushes of growth interrupted by periods of rest. The morphologically distinct growth increments corresponding to the flushes of growth are referred to as growth units (GUs) (Hallé et al., 1978; Barthélémy, 1991; deReffye et al., 1991). Each growth unit may have a ring of bud scale scars at the base or a zone of compressed internodes that morphologically marks a period of rest (Caraglio and Barthélémy, 1997).
In apple, annual shoots can be monopodial or sympodial. Monopodial shoots develop from vegetative buds containing about 12 preformed metamers (Rivals, 1965; Lauri and Terouanne, 1998). Depending on the tree age, a number of neoformed metamers may be produced. Sympodial shoots develop from mixed buds containing flower primordia in terminal positions, leaf primordia and two vegetative axillary structures (Pratt, 1988; Crabbé and Escobedo, 1991). Before winter the axillary structures comprise two or three primordia; at the time of budbreak in the following spring these axillary structures have five or six leaf primordia. Following budbreak the floral bud develops into a floral spur (bourse) with flowers in terminal positions, and each axillary structure can produce one or two flushes of growth (forming a ‘bourse shoot’) (Abbott, 1960; Pratt, 1988; Lauri and Térouanne, 1995). In the early stages of development, the vegetative structure of young apple trees is primarily built from monopodial shoots, as most buds are vegetative. As the apple tree matures, the proportion of floral buds increases, resulting in a higher proportion of sympodial shoots.
Costes et al. (2001) applied architectural analysis to examine annual shoot development and branching patterns on the main axis of two apple cultivars grafted on two different rootstocks. This study highlighted a decrease in the successive growth over 6 years and a linear relationship between the number of metamers per growth unit and the number of axillary shoots that were unchanged in the different treatments. The present study applied architectural analysis to examine the structure of 3‐year‐old branches of ‘Royal Gala’ apple trees grafted onto dwarfing and non‐dwarfing rootstock/interstock combinations, with ‘interstock’ being a clonal rootstock graft inserted as a length of stem tissue between the rootstock and the scion cultivar. Interstocks are used to induce a specific plant development response (e.g. dwarfing, overcome graft incompatibility). The model system used produced trees with a wide range of vigour from a known restricted range of rootstock genetic material by means of reciprocal application of dwarfing and non‐dwarfing rootstocks used as both rootstock and interstock.
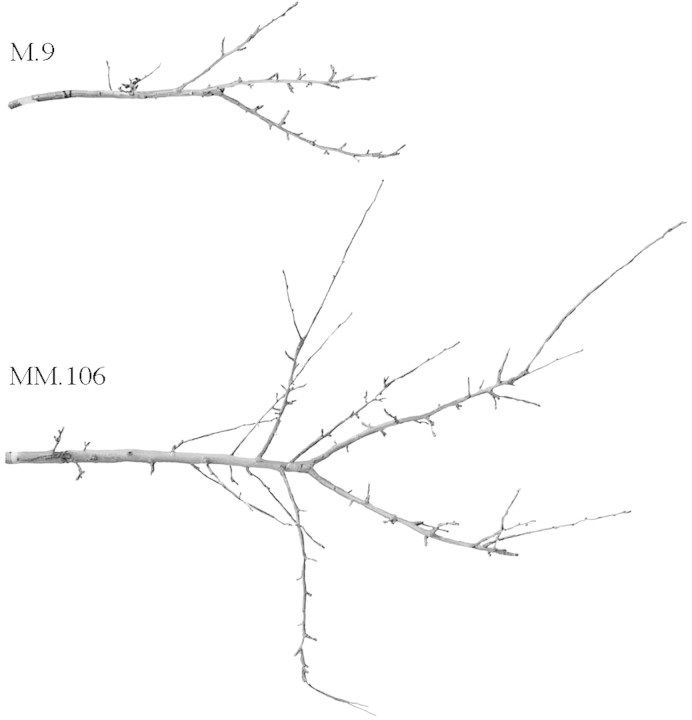
Since rootstock effects are cumulative in nature (Costes et al., 2001), the main goal was to identify the primary components and to answer the question: Where exactly was the structure building process, that leads to the dramatic differences in size and appearance of branches between dwarfed and non‐dwarfed apple trees, affected? (Fig. 1). The variables and methods of empirical modelling were chosen so as to gain an insight into quantitative aspects of the expression of dwarfing that would also form a base for future simulation modelling.
Fig. 1. Typical examples of three‐year‐old branches of ‘Royal Gala’ apple grafted on M.9 and MM.106 rootstocks.
MATERIALS AND METHODS
Plant material
In winter 1995, ‘Royal Gala’ scions were grafted onto six rootstock/interstock combinations: MM.106; MM.106/MM.106; MM.106/M.9; M.9; M.9/M.9; and M.9/MM106, where MM.106 is a non‐dwarfing rootstock and M.9 is a dwarfing rootstock (Table ). The interstock length was standardized at 30 cm. This was grafted onto the clonal rootstock, cutting at a height of 20 cm. The rootstock was planted to a depth of 15 cm, so that the above‐ground portion of the rootstock was standardized at 5 cm with the 30 cm of interstock above that. These were carefully standardized because it is known that the length of dwarfing interstock and different methods of planting affect growth modification by rootstocks in apple. In the treatments with no interstock, the clonal rootstock cutting at a height of 50 cm was planted to a depth of 15 cm, so that the above‐ground portion of the rootstock was standardized at 35 cm.
Table 1.
Rootstock/interstock model system
| Treatment | Symbol | Rootstock | Interstock | No. of graft unions |
| 1 | ▪ | MM.106 | – | 1 |
| 2 | • | MM.106 | MM.106 | 2 |
| 3 | ▴ | MM.106 | M.9 | 2 |
| 4 | □ | M.9 | – | 1 |
| 5 | ○ | M.9 | M.9 | 2 |
| 6 | ▵ | M.9 | MM.106 | 2 |
Grafted trees were grown in a field nursery in a completely randomized block design for rootstock treatments until winter 1997, when they were planted into an orchard block at the HortResearch Hawkes Bay Research Centre, New Zealand (39°40′S, 176°53′E). Trees were planted in two rows at 4·5 × 2·5 m spacing, with a single wire trellis as support, in a complete randomized block design with six replicates per treatment. In the first year after grafting, young axillary shoots near the tip of the primary growth axis were removed to promote development of the central leader. Subsequently, trees received no pruning apart from the occasional removal of physically damaged shoots. No tree cropping was allowed in the first year after planting (third year from grafting), and standard commercial fruit thinning practices were applied in the fourth and fifth year. In winter 2000, when the trees had completed in their fifth season of growth after grafting, one 3‐year‐old branch, located directly on the main axis of each tree was collected for analysis.
Branch structure representation
A dynamic interpretation of the architecture of the tree can be made without long‐term observation once the correlation between growth and structure is established (Tomlinson, 1984). General botanical (Caraglio and Barthélémy, 1997; Lauri and Terouanne, 1998) rather than horticultural terminology for branch structure representation was used because it allowed the establishment of correlations between structural units comprising branches and the time‐frame of branch development.
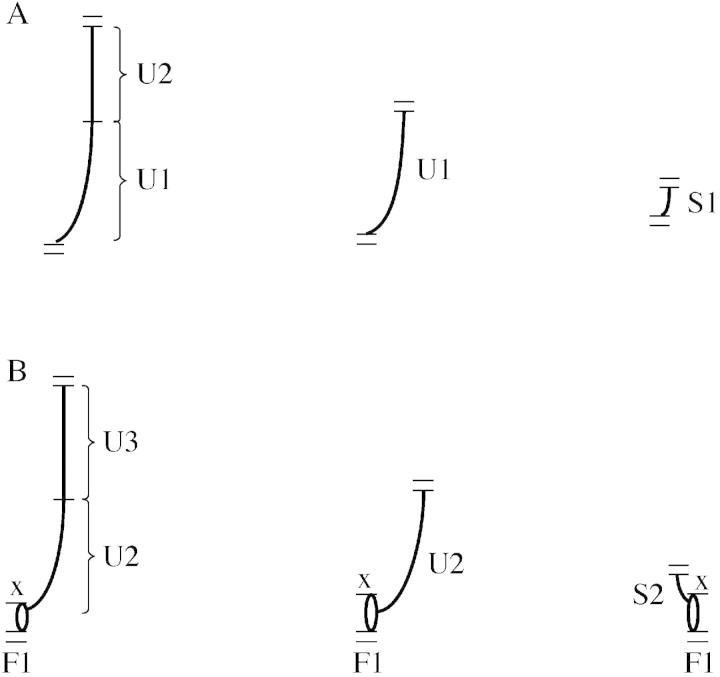
The structure of 3‐year‐old branches (B) was described in terms of annual shoots (A, denoted A1 to A3, with the index representing the year of shoot development), consisting of GUs, each representing a section developed during a single growth flush (Hallé and Martin, 1968; Barthélémy, 1991). To identify GUs, the morphological marker of a ring of bud scale scars or compressed internodes at the base was used. Three types of GUs were distinguished: a vegetative spur (S) with minimal internode extension and a total length <2·5 cm (Boyes, 1922; Barlow, 1994); a vegetative growth unit (U) with internode extension and a total length ≥2·5 cm; and a fruiting spur or bourse (F) (Costes et al., 1997). Possible compositions of monopodial and sympodial annual shoots are illustrated in Fig. 2. AMAPmod methodology (Godin et al., 1997) was used to represent the topological structure of the branches using multi‐scale tree graphs (MTGs) with four levels of organization: B, A, GU and internode (I). Apart from topological relationships within the branch each MTG contained the basal diameter of the branch, and length and number of nodes for each U.
Fig. 2. Possible compositions of (A) monopodial and (B) sympodial annual shoots. F, floral spur (bourse); S, vegetative spur; U, vegetative extension growth unit. Index indicates growth flush number. =, limit of annual growth; –, limit of growth unit (flush); X, termination of apical meristem.
Data analysis and modelling
The MTG database was explored using AMAPmod software and modelling language (Godin and Guédon, 1999), and the following variables were extracted: (1) the cross‐sectional area at the base of each branch and total number of annual shoots per branch; (2) the number of each type of growth unit per annual shoot; and (3) the number of nodes, (4) total length and (5) number of axillary shoots per extension growth unit.
ANOVA was used to test differences in branch cross‐sectional area between rootstock/interstock treatments. Treatment effects on variables 2–5 were analysed using non‐parametric Kruskal–Wallis ANOVA, since these variables were not normally distributed. In our experiments the majority of A2 and A3 were sympodial and had only one extension growth unit (U2 in Fig. 2B). In general the number of nodes per growth unit decreases with plant age, hence treatment comparisons had to be done on a per year basis. Since there were more shoots in the third year, we used (all) U2 from sympodial A3 to compare distributions of number nodes per U2 (n) between treatments. The number of ‘eligible’ U2 per treatment (sample size) varied between 20 and 50 depending on rootstock treatment vigour.
The same U2 samples were used to analyse the effects of rootstock treatments on total length (L) and average internode length (l). A linear model with treatment as a factor and n as a covariate was fitted to L. Internode length was highly variable; however, the data suggest that unit average internode length depended on its number of nodes. Sub‐populations S(n) of U2 with a given number of nodes n were considered and it was verified that internode length for each S(n) was normally distributed and the standard deviation σ was independent of n [the mean size of subpopulation S(n) was 14]. Mean internode length l̄(n) for S(n) depended on n. Total length, number of nodes and average internode length of individual growth units are by definition related by an expression l = L/n, hence the assumption that the same expression should hold for model values of these variables, namely
l̂(n) = L̂(n)/n(1)
To verify this assumption we fitted a model l̄(n) = a + kl̂(n) and tested the hypothesis {a = 0, k = 1} by fitting more parsimonious models and using the F‐statistic. Acceptance of the hypothesis {a = 0, k = 1} would mean that l̂(n), given by eqn (1), represented mean internode length l̄(n) without bias.
As one of the goals of this study was to distinguish between primary and secondary effects of rootstock/interstock combinations on branch growth, tests were carried out on the hypothesis that apparent differences in treatment medians for internode length of U2 were in fact induced by the differences in the corresponding node number distributions. Using l̂(n) and σ, a relationship between internode length distribution and the corresponding node number distribution for different treatments was established; for a detailed description of the method see Seleznyova et al. (2002).
Finally, a linear relationship was established (PROC GENMOD with Poisson distribution and identity link function; SAS, 1990) between the total number of extended metamers per branch in year 2 and the number of axillary annual shoots developed from these metamers in year 3. There was insufficient variation in the total number of extended metamers per branch in year 1 to establish a similar relationship between this variable and the number of axillary annual shoots developed in year 2.
RESULTS
Branch composition and cross‐sectional area
Rootstock/interstock combination affected the cross‐sectional area at the branch base, ranging from 6·3 cm2 for MM.106/MM.106 to 2·2 cm2 for M.9/M.9 (Table ). Branch cross‐sectional area at the end of the third growing season was better correlated (R2 = 0·88, P = 0·006) with the number of vegetative extension growth units than with the number of annual shoots (R2 = 0·80, P = 0·016) developed during this season.
Table 2.
Mean cross‐sectional area at the base of 3‐year‐old branches for different rootstock/interstock combinations
| Rootstock/interstock | MM.106/– | MM.106/MM.106 | MM.106/M.9 | M.9/MM.106 | M.9/– | M.9/M.9 |
| Area (cm2) | 5·0ab | 6·3a | 3·4bc | 3·5bc | 2·6c | 2·2c |
Superscript letters indicate significant differences at P < 0·05.
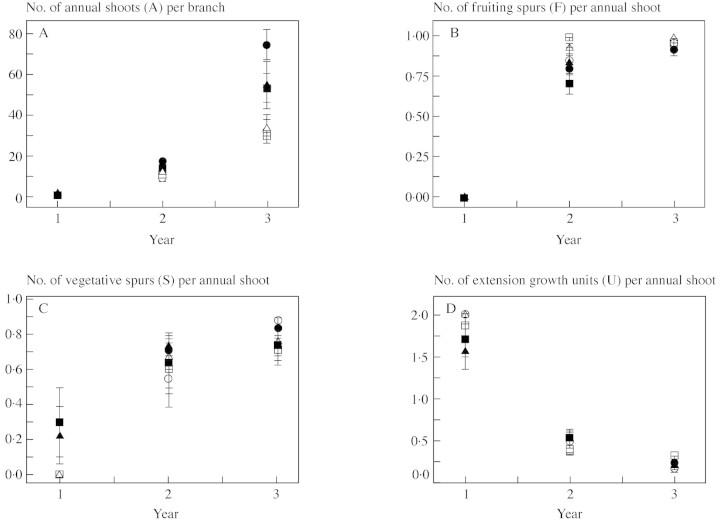
The numbers of annual shoots per branch [NA(Y)] in years 2 and 3 were affected by the rootstock/interstock combination, in the following order: MM.106/MM.106 > MM.106/M.9 > M.9/MM.106 > M.9/M.9 (Fig. 3A). Differences in medians for NA(2) and NA(3) between individual treatments were not always significant. However, the corresponding differences between extreme treatments MM.106/MM.106 and M.9/M.9 were significant (P < 0·05) for both years. Rootstock effect on NA(2) and NA(3), regardless of presence and type of interstock, was highly significant (P < 0·01 and P < 0·001, respectively).
Fig. 3. Branch and shoot characteristics. Symbols represent treatment means and vertical bars represent standard errors. The key to symbols is given in Table 1.
Annual shoot composition
Annual shoot composition, measured in numbers of fruiting spurs NF(Y), vegetative spurs NS(Y) and vegetative extension growth units NU(Y) changed markedly over the years (Fig. 3B–D). All A1 were monopodial [NF(1) = 0; Fig. 3B], and most of them had two extension growth units [non‐zero values of NS(1) for treatments with MM.106 rootstock are due to the presence of some summer flush axillary shoots, each comprising a vegetative spur]. In year 2, the proportion of sympodial (floral) shoots increased markedly, reaching an average of 0·95 by year 3 (Fig. 3B). The number of vegetative spurs per annual shoot increased over the years, while the number of vegetative extension growth units decreased (Fig. 3C and D). Shoot composition in years 2 and 3 did not differ between individual treatments, the only exception was the higher (P < 0·05) value in year 2 of NF(2) for treatment 4 than for treatment 1 (M.9 and MM.106, respectively). This indicates earlier transition to flowering for scions on M.9 than for those on MM.106 rootstock.
Composition of extension growth units
Rootstocks dramatically affected the shape of the node number distribution for extension units U2 from sympodial shoots A3 (Fig. 4A and B). Judging by the cumulative distribution function (probability of U2 having number of nodes ≤n), the presence of any interstock affected the shape of the node number distribution only when MM.106 was used as rootstock (Fig. 4C). The median value for number of nodes per U2 was lower when M.9 was used as rootstock, while the presence of any interstock affected the median only with MM.106 as rootstock (Table ). Note that because the axillary structures giving rise to extension growth of sympodial shoots contain only five or six primordia just before budbreak (Crabbé and Escobedo, 1991), it can therefore be concluded from our data that the differences in the numbers of nodes per U2 between treatments were due to the effect of rootstock/interstock combination on the extent of neoformation.
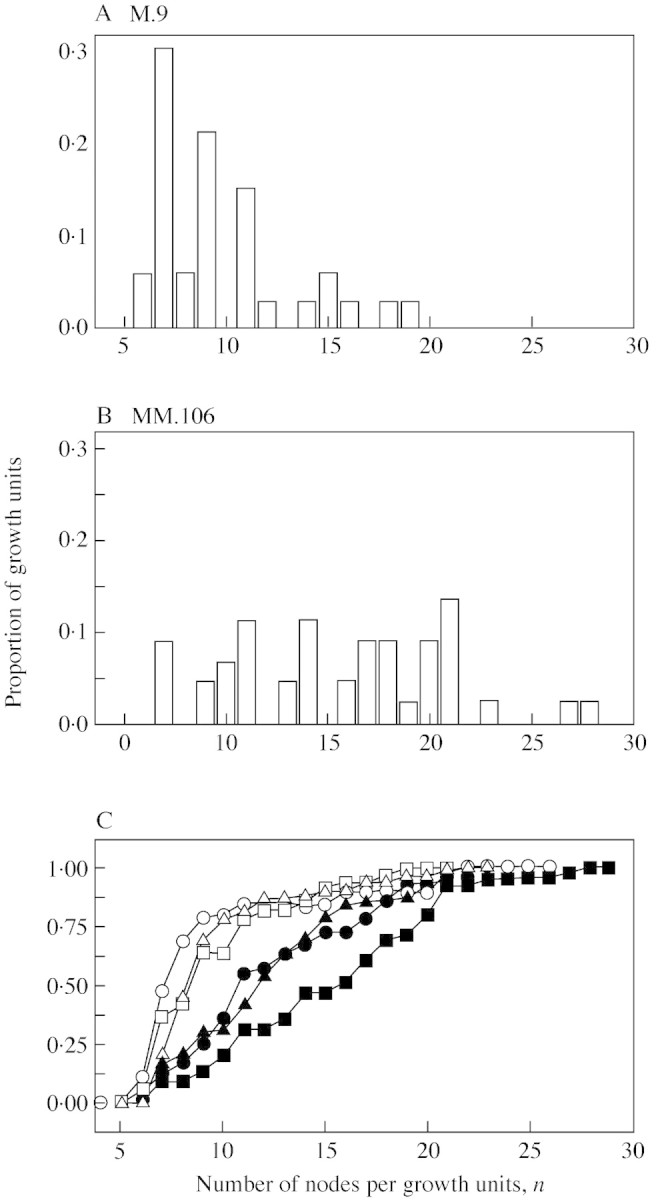
Fig. 4. Effect of treatment on node number distribution for vegetative extension units (U2, Fig. 2B) from sympodial annual shoots. Observed node number distributions from scions grown on (A) M.9 and (B) MM.106. (C) Observed cumulative distribution function (proportion of U2 having number of nodes ≤n). The key to symbols is given in Table 1.
Table 3.
Treatment mean and median values of number of nodes and internode length of vegetative growth units (U2, Fig. 2B) from sympodial shoots developed in year 3
| Rootstock/interstock | Node number | Internode length (mm) | ||
| combination | Mean | Median | Mean | Median |
| MM.106 | 15·6 | 16a | 18·7 | 18a |
| MM.106/MM.106 | 12·7 | 11b | 14·2 | 15b |
| MM.106/M.9 | 12·5 | 12b | 14·9 | 15b |
| M.9/MM.106 | 9·8 | 9c | 14·6 | 15bc |
| M.9 | 9·8 | 9c | 11·7 | 11c |
| M.9/M.9 | 9·6 | 8c | 11·1 | 9c |
Superscript letters indicate significant differences at P < 0·01, according to a Kruskal–Wallis test.
Total length and internode length of extension growth units
Growth unit length was measured in millimetres, and all coefficients in the models correspond to this choice of unit. It was not useful to compare treatment effects on the length of extension growth units due to the wide range of values found, irrespective of treatment (Fig. 5A). The linear relationship (R2 = 0·88) between the total length of U2 and the number of nodes
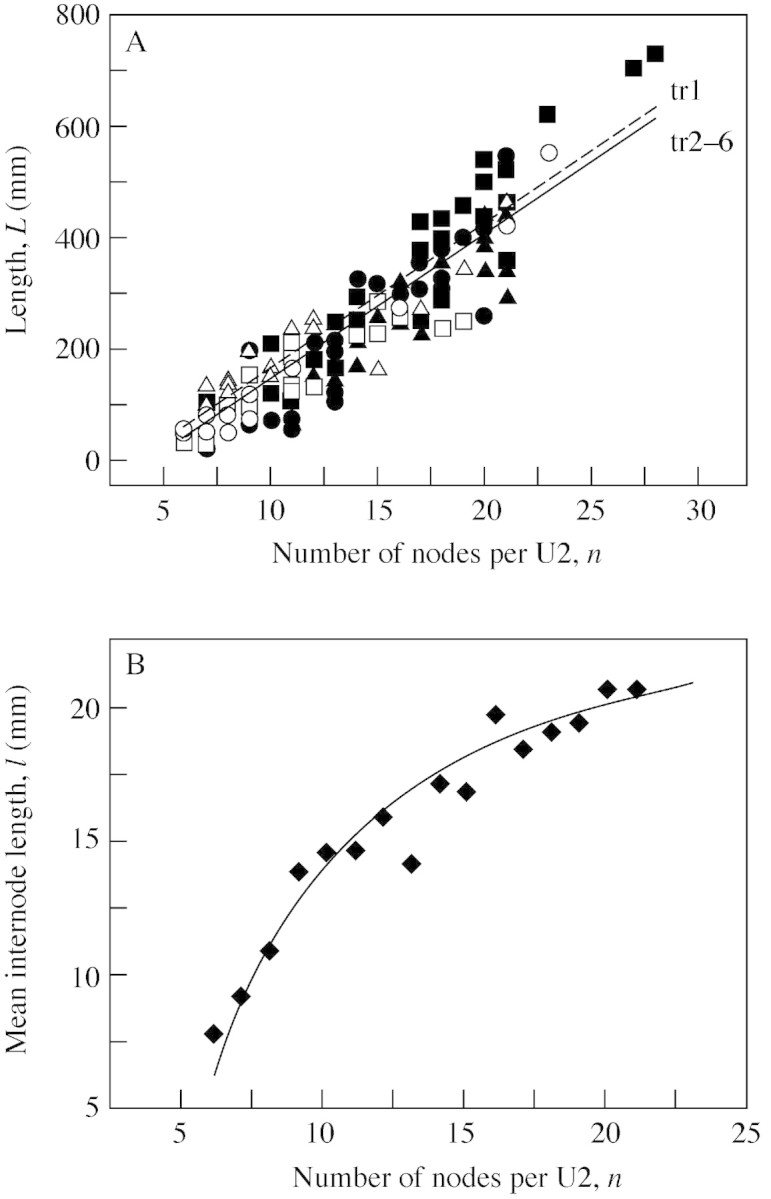
Fig. 5. Effect of treatment on total length L and internode length l of vegetative extension growth units U2 (Fig. 2B) from sympodial shoots. (A) Relationship between unit length, L and number on nodes n; symbols (key given in Table 1) represent data, and line graphs represent linear model L̂(n) fitted to the data (R2 = 0·88); treatment effect was significant (P = 0·004) for treatment 1. (B) Symbols represent l̄(n) mean internode length for sub‐populations of U2 with a given n, and line graphs represent model for average per unit internode length l̂(n).
L̂(n) = – 120 + tr + 26n n ≥ 6(2)
where tr = 22 for treatment 1 and tr = 0 for other treatments, was consistent for all treatments, except for treatment 1 (Fig. 5A). Although the treatment effect on L̂(n) was significant (P = 0·004), it was very small; hence it was neglected in the following calculations. It was confirmed that the model for internode length l̂(n) = L̂(n)/n , calculated from eqn (2) (Fig. 5B), represented l̄(n) without bias (R2 = 0·93) and that the standard deviation of l(n) was independent of n, σ = 3·7.
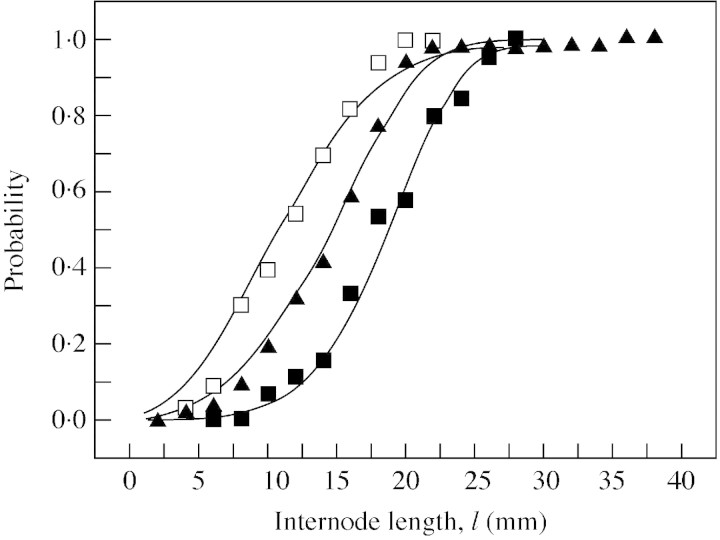
Treatment median values for internode length mainly followed a similar pattern to median values for node number (Table ). Model cumulative distribution functions for internode length, calculated from corresponding node number distributions using l̂(n) and σ, showed good agreement with their measured counterparts (Fig. 6). Note that when sub‐populations of shoots with a given number of nodes were compared, no effect of rootstock/interstock combination on internode length was found. Differences in (total) treatment medians for internode length of U2 were therefore related to differences in the corresponding node number distributions.
Fig. 6. Cumulative distribution function for internode length l. Symbols (key given in Table 1) represent observed probabilities of internode length being ≤l and line graphs represent models obtained from the corresponding node number distributions (Fig. 4A and B) and relationship l̂(n) (for clarity only three treatments are represented).
Proportion of budbreak for axillary buds
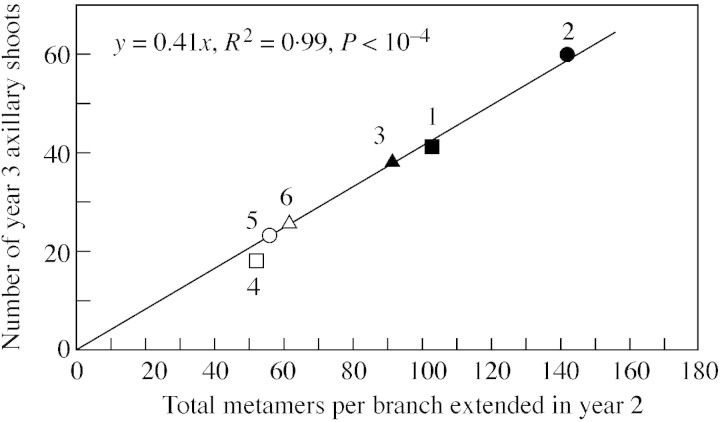
Across all treatments, the number of extended internodes (metamers) per branch in year 2 was linearly related (R2 = 0·99, P < 0·001) to the number of axillary shoots that developed from these metamers in year 3 (Fig. 7). Hence rootstock/interstock treatments did not affect the proportion of budbreak (i.e. the slope of the linear relationship between these variables, 0·41). The range of data was not sufficient to obtain a similar relationship between the number of metamers extended in year 1 and the number of axillary shoots developed in year 2 (the numbers of extended metamers per branch produced in year 1 were similar for all treatments). The proportion of axillary budbreak in year 2 (0·34) was not significantly different from that in year 3 (P = 0·1).
Fig. 7. Axillary bud outgrowth from extended metamers. Number of axillary shoots from U as a function of number of metamers extended during the previous year (on per branch basis). Symbols represent measured values; the treatment number is given next to the corresponding symbol. The line represents linear model fitted to the data. The slope of the line 0·41 gives the proportion of axillary bud break in spring of year 3.
DISCUSSION
The subject of this paper is an old horticultural problem, namely the effect of rootstock on fruit tree size. However, the approach to the problem is relatively new as it used the concepts of plant architecture and the recently developed AMAPmod methodology for mathematical representation and analysis of plant structures. The advantage of this methodology is that it enables interpretation of plant development based on analysis of its structure. Because general botanical rather than horticultural terminology was used, this approach is not restricted to apple trees and it can be adapted to study environmental effects on plants in general.
Development of ‘Royal Gala’ branches was followed, using a model system comprising dwarfing and non‐dwarfing rootstock/interstock combinations. The focus of this study was to describe exactly how the rootstock/interstock combinations affected the structure building process of the branches. For each annual growth cycle, percentage of budbreak, proportion of floral annual shoots, and numbers of vegetative extension growth units and vegetative spurs per annual shoot were considered. Because extension growth units play an important role in the building of the spatial structure of the tree, average internode length and number of nodes per extension growth unit were also considered.
In this study, percentage of budbreak was not affected by rootstock/interstock combination. Hirst and Ferree (1995) reported similar results with ‘Delicious’ apple cultivar grown on a range of rootstocks, showing that the number of growing points (both on a per parent shoot basis and a per metre basis) was not affected by rootstocks. In their study of the branching patterns on the main axis of ‘Rome Beauty’ and ‘Starkrimson’ apple, grafted onto two different rootstocks, Costes et al. (2001) found that the total number of laterals per annual shoot was not affected by the rootstock. However, comparison of these results should be taken with caution because the measures of branching used in these studies (percentage of budbreak, number of laterals per annual shoot and number of laterals per unit length) are not equivalent. Indeed, the number of laterals (growing points) per shoot is a product of percentage of budbreak and the number of axillary buds (equal to the number of nodes), and the latter was found to be considerably affected by rootstock in the present study. The number of growing points per unit length equals percentage of budbreak divided by internode length. Hence treatments producing shoots with shorter internodes will have a higher growing point density even when the percentage budbreak is not affected.
All branches in our experiment developed from vegetative shoots; the majority of shoots in the second year were floral, with a significantly higher proportion of flowering for the treatment with M.9 rootstock. Costes et al. (2001) found that flowering was not affected by the rootstock, but the difference between these results could be due to use of different scions and to the location of flowers. Costes et al. (2001) considered terminal flowering along the main axes, while the present data are related to flowering of axillary buds forming higher order axes.
The balance between vegetative spurs and extension growth units changed over the years but was not affected by rootstock/interstock treatment. The number of nodes produced by the extension growth units was significantly less for treatments with M.9 rootstock. Note that, for this comparison, vegetative extension growth units from sympodial annual shoots (only about 5 % of annual shoots were monopodial in year 3) were used. According to Crabbé and Escobedo (1991), these units originate from vegetative axillary structures within floral buds and have only five or six leaf primordia at the time of budbreak of the parent buds. Comparison of these values with node number distributions obtained in this study suggests that neoformation (initiation of new metamers) in treatments with M.9 rootstock stopped very soon after budbreak (of the parent buds), while it continued for much longer in treatments with MM.106 rootstock. This is consistent with a previous study on ‘Starkrimson’ and ‘Rome Beauty’, which demonstrated a reduction in neoformation when grafted on M9 (Costes et al., 2001). In future, it is important to determine exactly when this rootstock effect occurs to identify the actual mechanism(s) involved in the dwarfing response. In earlier works, the capacity for shoot neoformation has been interpreted as a plastic trait that enables plants to respond to current growing conditions, including position in the crown, site, water availability and pruning treatments (Remphrey and Powell, 1984; Remphrey and Steeves, 1984; Davidson and Remphrey, 1994). The present findings support this interpretation, adding another aspect to the understanding of architectural plasticity.
Final shoot length is determined by the number of nodes and length of internodes. Either, or both, components may contribute to expression of dwarfing. According to Brown et al. (1994), the most prominent morphological feature associated with dwarfed mutants is a reduction in internode length. In contrast, the dwarfing apple rootstock (M.9) affected the number of nodes per shoot but not internode length. Poll (1973) pointed out that, in apple, internode length varies considerably, even within the same cultivar, depending on shoot length, and suggested that shoot length be taken into account when comparing internode length between cultivars. Poll argued that a comparison of the mean internode of different cultivars, which disregards the correlations between shoot length and internode length, may give misleading results in plant breeding research and other studies. This conclusion is in agreement with the findings of this study. Indeed, the mean internode length for populations of shoots with a given number of nodes was the same for all treatments. On the other hand, the treatment mean values of internode length were much lower for treatments with M.9 rootstock, with differences being related to differences in node number distribution.
Previous studies on the effects of dwarfing rootstocks on apple tree growth have usually recorded whole tree parameters, such as tree height, trunk cross‐sectional area and annual total shoot growth, often to help quantify the effects of rootstocks on tree photosynthesis and cropping efficiency (e.g. Tustin et al., 2001). In the present studies, cross‐sectional area measured at the base of 3‐year‐old branches best correlated with the number of extension growth units developed in the third year. This reflects the cumulative effect of rootstock over 3 years and agrees with previous work by Costes et al. (2001).
Finally, the difference in overall branch size between treatments was due mostly to the reduced number of neoformed nodes, and hence the shorter internodes, on vegetative extension growth units for treatments with M.9 rootstock. Reduction in the number of nodes resulted in reduction in the number of axillary annual shoots in the following year. The nature of cumulative effects over time means that even a small shift in node number can have a dramatic effect on branch development. This is in agreement with previous conclusions that the relative proportions of preformation and neoformation can significantly affect the overall architecture of the plant (Remphrey and Steeves, 1984; Davidson and Remphrey, 1994).
ACKNOWLEDGEMENTS
We thank research and field technical staff at HortResearch Hawkes Bay for the tree management and assistance with data collection. This research was funded by the New Zealand Foundation for Research Science and Technology Contract No. CO6X0005.
Supplementary Material
Received: 2 October 2002; Returned for revision: 5 December 2002; Accepted: 3 February 2003 Published electronically: 28 March 2003
References
- AbbottDL.1960. The bourse shoot as a factor in the growth of apple fruits. Annals of Applied Biology 48: 434–438. [Google Scholar]
- AtkinsonC, Else M.2001. Understanding how rootstocks dwarf fruit trees. The Compact Fruit Tree 34: 46–49. [Google Scholar]
- BarlowPW 1994. From cell to system: repetitive units of growth in the development of roots and shoots. In: Iqbal M, ed. Growth patterns in vascular plants Portland, USA: Dioscorides Press, 19–58. [Google Scholar]
- BarrittBH, Konishi BS, Dilley MA.1995. Performance of three apple cultivars with 23 dwarfing rootstocks during 8 seasons in Washington. Fruit Varieties Journal 49: 158–170. [Google Scholar]
- BarthélémyD.1991. Levels of organization and repetition phenomena in seed plants. Acta Biotheoretica 39: 309–323. [Google Scholar]
- BoyesD1922. Notes on the characters of apple tree shoots. The Journal of Pomology and Horticultural Science 3: 36–46. [Google Scholar]
- BrownCL, Sommer HE, Wetzstein H 1994. Morphological and histological differences in the development of dwarf mutants of sexual and somatic origin in diverse woody taxa. Trees 9: 61–66. [Google Scholar]
- CaraglioY, Barthélémy D.1997. Revue critique des termes relatifs à la croissance et à la ramification des tiges des végétaux vasculaires. In: Bouchon J, de Reffye P, Barthélémy D, eds. Modélisation et simulation de l’architerture des végétaux Science Update. Paris: INRA Editions, 11–87. [Google Scholar]
- CostesE, Godin C, Guédon Y.1997. A methodology for the exploration of fruit tree structures. Acta Horticulturae 451: 709–715. [Google Scholar]
- CostesE, Salles JC, Garcia G.2001. Growth and branching pattern along the main axis of two apple cultivars grafted on two different rootstocks. Acta Horticulturae 557: 131–138. [Google Scholar]
- CrabbéJ, Escobedo Alvarez J.1991. Activités méristématiques et cadre temporal assurant la transformation florale des bourgeons chez le Pommier (Malus domestica Borkh. cv. Golden Delicious). In: Edelin C, ed. L’arbre: biologie et développement. Montpellier: Naturalia Monspeliensa, n° hors serié, 369–379. [Google Scholar]
- DavidsonCG, Remphrey WR.1994. Shoot neoformation in clones of Fraxinus pennsylvanica in relation to genotype, site and pruning treatments. Trees 8: 205–212. [Google Scholar]
- de ReffyeP, Elguero E, Costes E 1991. Growth units construction in trees: a stochastic approach. Acta Biotheoretica 39: 325–342. [Google Scholar]
- FerreeDC, Hirst PM, Schmid JC, Dotson PE.1995. Performance of three apple cultivars with 22 dwarfing rootstocks during 8 seasons in Ohio. Fruit Varieties Journal 49: 171–178. [Google Scholar]
- HalléF, Martin R.1968. Etude de la croissance rythmique chez Hevea brasiliensis Müll.‐Arg. (Euphorbiacées‐Crotonoïdées). Adansonia 8: 475–503. [Google Scholar]
- HalléF, Oldeman RAA, Tomlinson PB.1978. Tropical trees and forests: an architectural analysis. Berlin: Springer‐Verlag. [Google Scholar]
- HirstPM, Ferree DC.1995. Rootstock effects on shoot morphology and spur quality of ‘Delicious’ apple and relationships with precocity and productivity. Journal of the American Society for Horticultural Science 120: 622–634. [Google Scholar]
- GodinC, Guédon Y.1999. AMAPmod introduction and reference manual Version 1.2. CIRAD: Marie‐Hélène Lafond. [Google Scholar]
- GodinC, Guédon Y, Costes E, Caraglio Y.1997. Measuring and analysing plants with AMAPmod software. In: Michalewicz MT, ed. Plants to ecosystems. advances in computational life sciences Australia: CSIRO Publishing, 53–84. [Google Scholar]
- LauriPE, Térouanne E.1995. Analyse de la croissance primaire de rameaux de pommier (Malus × domestica Borkh.) au cours d’une saison de végétation. Canadian Journal of Botany 73: 1471–1489. [Google Scholar]
- LauriPE, Térouanne E.1998. The influence of shoot growth on the pattern of axillary development on the long shoots of young apple trees (Malus domestica Borkh.). International Journal of Plant Science 159: 283–296. [Google Scholar]
- PollL.1973. The relationship between internode length and shoot length in apple. Horticultural Research 13: 83–88. [Google Scholar]
- PowellGR.1991. Preformed and neoformed extension of shoots and sylleptic branching in relation to shoot length in Tsuga canadensis Trees 5: 107–116. [Google Scholar]
- PrattC.1988. Apple flower and fruit: morphology and anatomy. Horticultural Reviews 10: 273–308. [Google Scholar]
- RemphreyWR, Powell GR.1984. Crown architecture of Larix laricina saplings: shoot preformation and neoformation and their relationship to shoot vigour. Canadian Journal of Botany 62: 2181–2192. [Google Scholar]
- RemphreyWR, Steeves TA.1984. Shoot ontogeny in Arctostaphylos uva‐ursi (bearberry): the annual cycle of apical activity. Canadian Journal of Botany 62: 1925–1932. [Google Scholar]
- RivalsP.1965. Essai sur la croissance des arbres et sur leurs systèmes de floraison. Journal d’Agriculture Tropicale et de Botanique Appliquée 12: 655–686. [Google Scholar]
- SAS Institute Inc.2000. SAS OnlineDoc, Version 8, (http://v8doc. sas.com/sashtml/), Copyright (c) 2000, SAS Institute Inc., Cary, NC, USA. [Google Scholar]
- SeleznyovaAN. Thorp TG, Barnett AM, Costes E.2002. Quantitative analysis of shoot development and branching patterns in Actinidia Annals of Botany 89: 471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TomlinsonPB 1984. Tree architecture. American Scientist 71: 141–149. [PubMed] [Google Scholar]
- TustinDS, Cashmore WM, Bensley RB.2001. Pomological and physiological characteristics of Slender Pyramid central leader apple (Malus domestica) planting systems grown on intermediate vigour, semi‐dwarfing, and dwarfing rootstocks. New Zealand Journal of Crop and Horticultural Science 29: 195–208. [Google Scholar]
- WhiteJ.1979. The plant as a metapopulation. Annual Review of Ecological Systems 10: 109–145. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.