Abstract
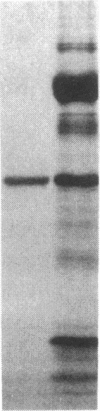
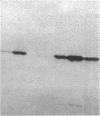
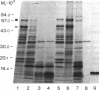
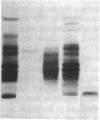
A 33-kD glycoprotein, known as the "prostate-specific antigen," was purified to homogeneity from human seminal plasma. The prostatic protein was identified as a serine protease, and its NH2-terminal sequence strongly suggests that it belongs to the family of glandular kallikreins. The structural protein of human seminal coagulum, the predominant protein in seminal vesicle secretion, was rapidly cleaved by the prostatic enzyme, which suggests that this seminal vesicle protein may serve as the physiological substrate for the protease. The prostatic enzyme hydrolyzed arginine- and lysine-containing substrates with a distinct preference for the former. All synthetic substrates tested were poor substrates for the enzyme. Synthetic Factor XIa substrate (pyro-glutamyl-prolyl-arginine-p-nitroanilide), and the synthetic kallikrein substrate (H-D-prolyl-phenylalanyl-arginine-p-nitroanilide) were hydrolyzed with maximum specific activities at 23 degrees C of 79 and 34 nmol/min per mg and Km values of 1.0 and 0.45 mM, respectively. Synthetic substrates for plasmin, chymotrypsin, and elastase were either not hydrolyzed by the enzyme at all, or only hydrolyzed very slowly.
Full text
PDF
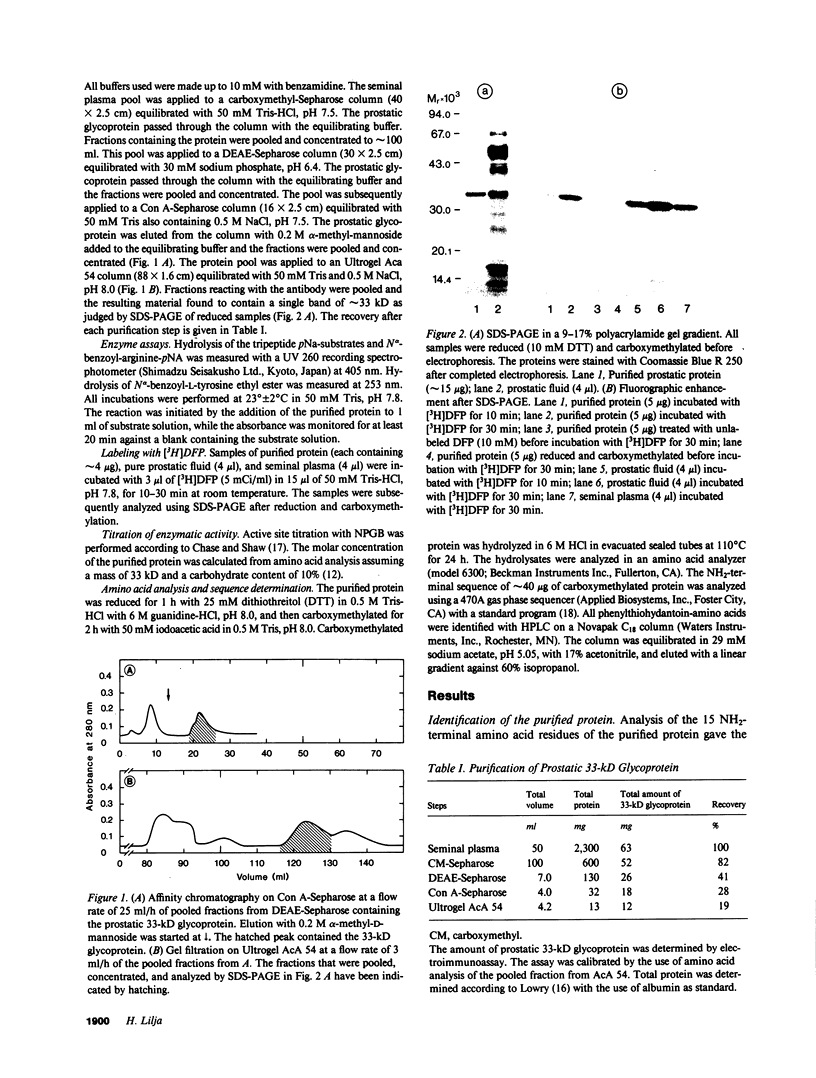
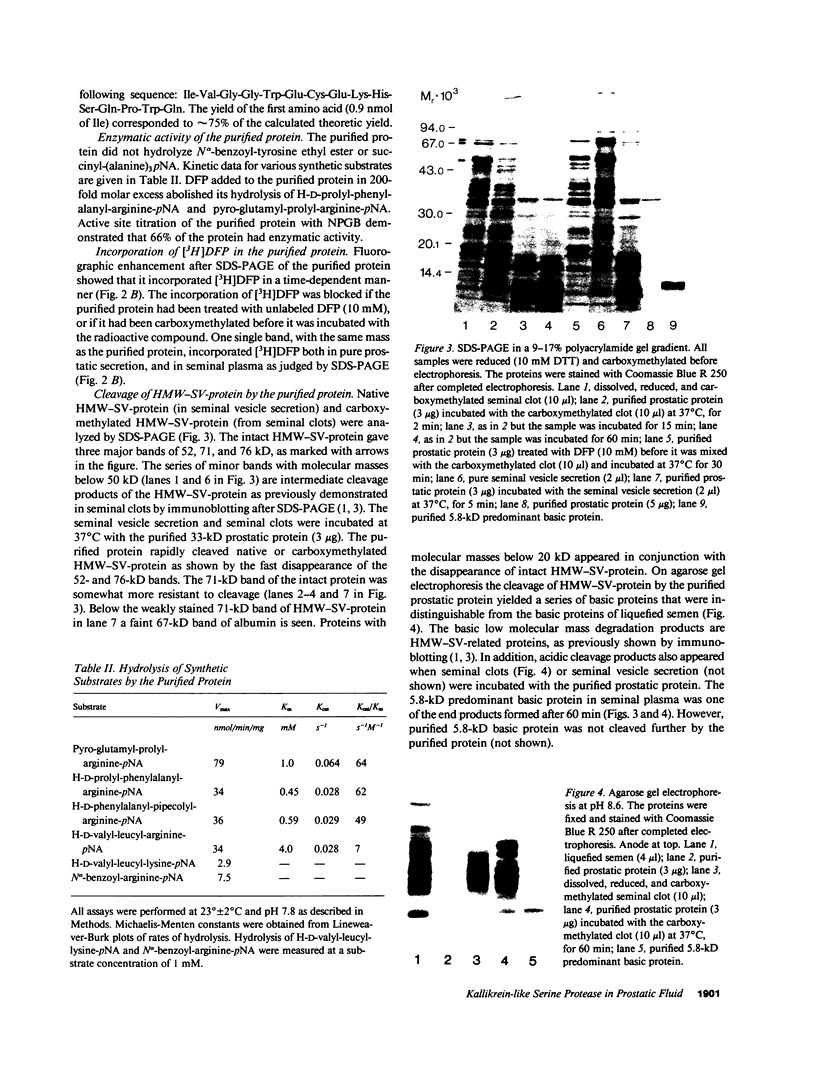


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ban Y., Wang M. C., Watt K. W., Loor R., Chu T. M. The proteolytic activity of human prostate-specific antigen. Biochem Biophys Res Commun. 1984 Sep 17;123(2):482–488. doi: 10.1016/0006-291x(84)90256-0. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaistitvanich N., Boonsaeng V. Molecular structure of human seminal coagulum: the role of disulfide bonds. Andrologia. 1983 Sep-Oct;15(5):446–451. doi: 10.1111/j.1439-0272.1983.tb00167.x. [DOI] [PubMed] [Google Scholar]
- Chapdelaine P., Dubé J. Y., Frenette G., Tremblay R. R. Identification of arginine esterase as the major androgen-dependent protein secreted by dog prostate and preliminary molecular characterization in seminal plasma. J Androl. 1984 May-Jun;5(3):206–210. doi: 10.1002/j.1939-4640.1984.tb02395.x. [DOI] [PubMed] [Google Scholar]
- Chase T., Jr, Shaw E. p-Nitrophenyl-p'-guanidinobenzoate HCl: a new active site titrant for trypsin. Biochem Biophys Res Commun. 1967 Nov 30;29(4):508–514. doi: 10.1016/0006-291x(67)90513-x. [DOI] [PubMed] [Google Scholar]
- Han Y. N., Kato H., Iwanaga S., Suzuki T. Primary structure of bovine plasma high-molecular-weight kininogen. The amino acid sequence of a glycopeptide portion (fragment 1) following the C-terminus ot the bradykinin moiety. J Biochem. 1976 Jun;79(6):1201–1222. doi: 10.1093/oxfordjournals.jbchem.a131175. [DOI] [PubMed] [Google Scholar]
- Han Y. N., Komiya M., Iwanaga S., Suzuki T. Studies on the primary structure of bovine high-molecular-weight kininogen. Amino acid sequence of a fragment ("histidine-rich peptide") released by plasma kallikrein. J Biochem. 1975 Jan 1;77(1?):55–68. [PubMed] [Google Scholar]
- Hermodson M. A., Ericsson L. H., Neurath H., Walsh K. A. Determination of the amino acid sequence of porcine trypsin by sequenator aalysis. Biochemistry. 1973 Aug 14;12(17):3146–3153. doi: 10.1021/bi00741a002. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Isaacs W. B., Coffey D. S. The predominant protein of canine seminal plasma is an enzyme. J Biol Chem. 1984 Sep 25;259(18):11520–11526. [PubMed] [Google Scholar]
- Jeppsson J. O., Laurell C. B., Franzén B. Agarose gel electrophoresis. Clin Chem. 1979 Apr;25(4):629–638. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazure C., Leduc R., Seidah N. G., Thibault G., Genest J., Chrétien M. Amino acid sequence of rat submaxillary tonin reveals similarities to serine proteases. Nature. 1984 Feb 9;307(5951):555–558. doi: 10.1038/307555a0. [DOI] [PubMed] [Google Scholar]
- Lilja H., Jeppsson J. O. Amino acid sequence of the predominant basic protein in human seminal plasma. FEBS Lett. 1985 Mar 11;182(1):181–184. doi: 10.1016/0014-5793(85)81179-0. [DOI] [PubMed] [Google Scholar]
- Lilja H., Laurell C. B., Jeppsson J. O. Characterization of the predominant basic protein in human seminal plasma, one cleavage product of the major seminal vesicle protein. Scand J Clin Lab Invest. 1984 Sep;44(5):439–446. doi: 10.3109/00365518409083835. [DOI] [PubMed] [Google Scholar]
- Lilja H., Laurell C. B. Liquefaction of coagulated human semen. Scand J Clin Lab Invest. 1984 Sep;44(5):447–452. doi: 10.3109/00365518409083836. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Ramasharma K., Sairam M. R., Chrétien M. Partial amino acid sequence of a human seminal plasma peptide with inhibin-like activity. FEBS Lett. 1984 Feb 13;167(1):98–102. doi: 10.1016/0014-5793(84)80840-6. [DOI] [PubMed] [Google Scholar]
- Tschesche H., Mair G., Godec G. The primary structure of porcine glandular kallikreins. Adv Exp Med Biol. 1979;120A:245–260. doi: 10.1007/978-1-4757-0926-1_25. [DOI] [PubMed] [Google Scholar]