Abstract
• Background and aims Control of diseases in the key tropical staple, cassava, is dependent on resistant genotypes, but the innate mechanisms are unknown. The aim was to study phenylpropanoids and associated enzymes as possible defence components.
• Methods Phenylalanine ammonia‐lyase (PAL), phenylpropanoids and peroxidases (POD) were investigated in elicited cassava suspension cells and leaves. Yeast elicitor was the most effective of several microbial and endogenous elicitors. Fungitoxicity was determined against the cassava pathogens Fusarium solani, F. oxysporum and the saprotroph Trichoderma harzianum.
• Key results A single and rapid (≥2–3 min) oxidative burst, measured as hydrogen peroxide, occurred in elicited cells. PAL activity was induced maximally at 15 h and was preceded by PAL mRNA accumulation, which peaked at 9 h. Symplasmic POD activity increased four‐fold in cells, 48 h post‐elicitation. POD isoforms (2–7 isoforms, pI 3·1–8·8) were detected in elicited and unelicited cells, extracellular medium and leaves but two extracellular isoforms were enhanced post‐elicitation. Also expression of a cassava peroxidase gene MecPOD1 increased in elicited cells. Only anionic forms oxidized scopoletin, with highest activity by isoform pI 3·6, present in all samples. Unidentified phenolics and possibly scopolin increased post‐elicitation, but there was no enhancement of scopoletin, rutin or kaempferol‐3‐O‐rutinoside concentration. Fungal germ tube elongation was inhibited more than germination by esculetin, ferulic acid, quercetin and scopoletin. T. harzianum was generally more sensitive than the pathogens and was inhibited by ≥50 µg mL–1 of ferulic acid and quercetin and ≥10 µg mL–1 of scopoletin.
• Conclusions Phenolic levels in cells were not enhanced and were, theoretically, too low to be inhibitory. However, in combination and when oxidized they may contribute to defence, because oxidation of esculetin and scopoletin by peroxidase and of esculetin by tyrosinase enhanced their fungitoxicity up to 20‐fold.
Key words: Cassava, Manihot esculenta, phenylalanine ammonia‐lyase, peroxidase, oxidative burst, phenylpropanoids, kaempherol‐3‐rutinoside, rutin, scopoletin, scopolin, yeast elicitor, plant defence
INTRODUCTION
Cassava (Manihot esculenta: Euphorbiaceae) is extensively cultivated in many tropical and sub‐tropical countries and in some is the principal source of daily carbohydrates (Cock, 1985). Constraints on this staple crop include 25 different pathogens comprising a wide range of bacteria, viruses and fungi (Lozano, 1986). Usually, the only practicable and sustainable means of disease control is by deployment of resistant or tolerant genotypes. However, in contrast to many well‐studied temperate crop or model species, the mechanisms of resistance in this plant to any pathogen have not been characterized. The interaction between elicitors of defence responses and plant cells has been much used as a tool to explore the potential resistance reactions to pathogens, but has not been exploited in cassava.
An almost ubiquitous feature of plant responses to incompatible pathogens or to elicitors is the activation of phenylpropanoid metabolism in which phenylalanine ammonia‐lyase (PAL; EC 4·3.1·5) catalyses the first committed step of the core pathway of general phenylpropanoid metabolism. Branch pathways lead to the synthesis of compounds that have diverse functions in plants, notably in defence, such as cell wall strengthening and repair (e.g. lignin and suberin), antimicrobial activity (e.g. furanocoumarin, pterocarpan and isoflavonoid phytoalexins), and as signalling compounds such as salicylic acid (Hammerschmidt, 1999). The resulting phenolics are often converted into more reactive species by phenol oxidases and peroxidases (Mayer and Harel, 1979; Heath, 1980).
PAL genes can be regulated developmentally, induced by wounding, by low temperatures, by other stress conditions and by pathogen attack (Collinge and Slusarenko, 1987; Wu and Lin, 2002). PAL induction has been linked to defence responses that involve phenylpropanoids in numerous diseases. Typically, accumulation of PAL activity and mRNA is more rapid, higher and longer lasting in incompatible plant–pathogen interactions (Cui et al., 1996). Inhibitors of PAL decrease resistance and associated changes in phenolic synthesis and incorporation into host cell walls, as in Mla1 resistance of barley to powdery mildew and Eucalyptus calophylla to Phytophthora cinnamomi (Cahill and McComb, 1992; Zeyen et al., 1995).
Marked increases in PAL synthesis and corresponding mRNA occur in response to microbial or endogenous elicitors in many plant–pathogen systems (Sharan et al., 1998). In particular, an elicitor derived from yeast induces PAL and the consequent accumulation of phytoalexins and other secondary metabolites in numerous plant species, including alfalfa, tobacco, Lupinus albus, apple, Solanum khasianum and soybean (Fahrendorf et al., 1995; Mühlenbeck et al., 1996; Wojtaszek et al., 1997; Baier et al., 1999; Borejsza‐Wysocki et al., 1999). This readily obtained, effective elicitor was deployed in this study.
Plant peroxidases (EC 1·11·17) are ubiquitous, heme‐containing glycoproteins that catalyze oxidation of diverse organic and inorganic substances at the expense of hydrogen peroxide (H2O2). Higher plants have a number of peroxidase (POD) isoenzymes that are usually classified as anionic, neutral and cationic, based on their isoelectric points (Barz et al., 1990). Typically there are 10–20 isoforms, some arising from divergent genes, which can differ by more than 50 % in peptide sequence, but some originate from post‐translational modification of the same gene product (Zapata et al., 1995; Lagrimini et al., 1997). Peroxidase gene families are well described from many species, such as rice and parsley (Kawalleck et al., 1995; Chittoor et al., 1997). The increase of peroxidases after infection or elicitation often correlates with the appearance of new isoforms (Ludwig‐Müller et al., 1994; Adam et al., 1995; Chittoor et al., 1997). Anionic and neutral peroxidases are mainly cell wall‐bound, and cationic forms are confined to the central vacuole (Perrey et al., 1989; Kawalleck et al., 1995), so the former are those mainly linked with defence because of their location. The roles in defence include oxidation of hydroxy‐cinnamyl alcohols into free radical intermediates, phenol oxidation, cross‐linking of polysaccharides and of extensin monomers, lignification and suberization (Chittoor et al., 1997). Some forms can also generate active oxygen species as part of the oxidative burst during incompatible interactions (Adam et al., 1995; Hiraga et al., 2001). Increase in peroxidase activity during incompatible plant–pathogen/elicitor interactions is well documented and some peroxidases have been spatially and temporally associated with inhibition of pathogen growth (Adam et al., 1995; Kawalleck et al., 1995; Milosevic and Slusarenko, 1996; Chittoor et al., 1997). Therefore, determination of peroxidase levels, isoforms and locations in cassava suspension cells and leaves challenged with yeast elicitor was undertaken to investigate their role as modifiers of the potential antimicrobial activity of cassava phenylpropanoids.
MATERIALS AND METHODS
Plant cell cultures and leaves
Stem stakes of cassava (Manihot esculenta Crantz) cultivar ‘MCol 22’ were provided by the Centro Internacional de Agricultura Tropical (CIAT) in Cali, Colombia, and were propagated in a glasshouse. The basal medium of Murashige and Skoog (MS) was used for the induction and maintenance of plantlets, callus and cell suspension cultures of cassava. Cell suspension cultures were grown in (MS) medium, supplemented with the growth regulator 2,4‐D (1 mg L–1), kinetin (1 mg L–1), plus 2 % glucose (Stamp and Henshaw, 1982). The cultures were incubated at 120 r.p.m. at 25 °C in darkness. Growth was determined by measurement of packed cell volume (pellet volume after centrifugation at 2500 g in 50 mL graduated conical centrifuge tubes).
Preparation of elicitors
Yeast cell wall glucan elicitor was prepared from baker’s yeast, autoclaved in citrate buffer, then the supernatant was precipitated with 50 % (v/v) (precipitate discarded), then 95 % (v/v) ethanol, and freeze‐dried (Schumacher et al., 1987). Cell wall glucan preparations from Colletotrichum lindemuthianum obtained by the method of Dixon and Lamb (1979) were kindly provided by R. Dixon (Samuel Roberts Noble Foundation, OK, USA) and G. P. Bolwell (Royal Holloway College, University of London, England). The carbohydrate content of glucan elicitor preparations was determined by the phenol–sulphuric acid method and concentrations are expressed as glucose equivalents (Dubois et al., 1956). Oligogalacturonic acid elicitor was prepared by the method of Nothnagel et al. (1983) and generously donated by Dr F. Cervone (Dipartimento di Biologia Vegetale, Università di Roma ‘La Sapienza’, Italy) and Dr J. Dow (Purdue University, USA). Glutathione (GSH) was from Sigma (Poole, UK).
Treatment of cassava material with elicitors
Cassava suspension cells were treated with elicitors (water controls) after 5 d of subculture. After elicitation cells were harvested at time intervals, washed twice with 100 mL water on a porous‐glass funnel with filter paper (Whatman No.1) then frozen in liquid nitrogen and stored at –80 °C. For some experiments, extracellular fluids were also analysed. Elicited cells to be analysed for production of peroxide were treated differently (see below). Leaf discs (1 cm diameter) were immersed in elicitor solution and incubated at 25 °C in the dark. Discs were collected at time intervals, frozen in liquid nitrogen and stored at –80 °C.
Enzyme extraction and PAL activity assay from cassava suspension cells
Crude enzyme extracts were prepared according to Hahlbrock and Ragg (1975), by homogenising 2 g of frozen cassava cells with 4 mL of 0·1 m sodium borate buffer pH 8·8 with an Ultra‐turrax (IKA) homogenizer for 30 min at 0 °C; The slurry was centrifuged at 4 °C for 15 min at 20 000 g and the supernatant passed through pre‐packed PD 10 columns (Sephadex G25 M). The reaction mixture consisted of 200 µL of the extract made up to 0·5 mL with 0·1 m sodium borate buffer (pH 8·8) and then added to an equal volume of the same buffer containing 0·02 m of l‐phenylalanine or d‐phenylalanine. The d‐isomer is not degraded during the reaction and serves as a blank. The extinction coefficient of cinnamic acid is 16596 L mol–1 cm–1. Incubation was at 30 °C, and A290 nm was measured after 0, 15, 30 and 60 min. One unit (U) of enzyme activity was defined as the amount of enzyme forming 1 pmol of trans‐cinnamic acid from l‐phenylalanine per min per mg of protein. The experiment was carried out in triplicate in three independent sets of experiments.
Enzyme extraction from cassava suspension cells and POD activity assay
Suspension cells (0·5 g) were ground for 15 min in a pestle and mortar on ice with 5 mL of 50 mM phosphate buffer, pH 6·0, 0·15 % polyvinylpyrrolidone (PVP, insoluble), 1 mm EDTA, 1 mm dithiothreitol (DTT), and 0·5 mm α‐toluenesulfonyl fluoride 99 %. Debris was removed by centrifugation (4 °C, 45 min, 15 000 g). A standard curve was made with horseradish peroxidase (HRP) (Sigma, Type II). Peroxidase activity was assayed in the supernatant by the method of Van Gestelen et al. (1997). The 3·0 mL reaction volume contained 50 mm phosphate buffer (pH 6·0), 100 mm 3,5‐dichloro‐2‐hydroxybenzenesulfonic acid, 10 mm 4‐aminoantipyrine, and 150 µL of enzyme extract (approx. 0·05 mg mL–1 protein). After incubation at 25 °C for 5 min, the reaction was started by addition of 30 µl of 100 mm H202, and the reaction followed at A510 nm. Three replicates were performed from three independent experiments.
Electrofocusing methods for POD isoforms
Symplasmic peroxidases.
Extracts obtained as above from 3 g of cells were passed through a PD 10 column (Sephadex G25 M) then concentrated for about 2 h with polyethylene glycol 35 000 (PEG) in dialysis tubing to minimum volume and then dissolved in 100 µL of 50 mM phosphate buffer pH 6·0 at 4 °C.
Apoplastic peroxidases.
After elicitation, cells were left for 15 min to sediment. 15 mL of extracellular fluids were collected into Falcon tubes by filtration through three layers of miracloth membrane, and then dialysed (Medicell International Size 2, internal diameter 18/32, molecular cut‐off 12–14 kDa) for 2 d against excess 10 mm phosphate buffer, pH 6·0. The samples were concentrated for 6 h in dialysis tubing surrounded by PEG to minimum volume, then dissolved in 100 µL of phosphate buffer at 4 °C.
Electrofocusing conditions for peroxidase isoforms.
Peroxidase isoforms were separated over pH 3·5–9·5 in Ampholine PAG polyacrylamide gel by the method of Manchenko (1994). After running, the pI markers (pI 3–10) were cut from the gel and stained with Coomassie blue. The remainder of the gel was used for detection of POD isoforms by direct staining in 100 mL of 50 mm phosphate buffer (pH 6·0), with 100 mm 3,5‐dichloro‐2‐hydroxybenzensulfonic acid, 10 mm 4‐aminoantipyrine and 100 mm H2O2 until red‐brown bands appeared. Peroxidase isoforms with activity toward scopoletin were obtained by immersing gels in 0·1 mm scopoletin dissolved in 0·1 m phosphate buffer, pH 6·0 (Gutiérrez et al., 1995).
Protein determination
Protein was determined by the Bradford method (Stoscheck, 1990) with bovine serum albumin (BSA) as a standard.
Oxidative burst: H2O2 estimation
The method of Glazener et al. (1991) was used to estimate H202‐scavenging by luminol (N‐[4‐aminobutyl]‐N‐ethylisoluminol)‐dependent chemiluminescence in cassava suspension cells challenged with elicitor(s). Suspension cells were collected by centrifugation (200 g, 5 min) and washed with 150 mL of assay medium containing 0·5 mm of CaCl2, 0·5 mm K2SO4, 175 mm mannitol and 0·5 mm MES (2‐[N‐morpholino] ethanesulfonic acid) buffer pH 5·8–6·0. Cell aggregates were removed by filtration through a 5 µm pore plastic mesh then cells were suspended at 0·05 g mL–1 of assay medium and preincubated for 2–3 h at 180 r.p.m. at 27 °C before use. Luminol‐dependent chemiluminescence was measured with a luminometer (LUCY 1; Labtech) equipped with automatic sample dispensers. After addition of elicitors, 40 µL of suspension cells were dispensed into black microtitre plates containing100 µL of 50 mm pH 9·0 CHES buffer (2‐[N‐cyclohexylamino] ethanesulfonic acid) and 60 µL of peroxidase‐luminol mixture was dispensed into each well to give final concentrations of 0·03 and 0·07 mg mL–1, respectively. Total counts were measured over 30 s and converted to H202 equivalents.
Northern hybridization for PAL and POD transcripts
PAL (GenBank accession number AY036011) and POD (AY033386) cDNA probes were isolated from a post‐harvest physiological deterioration library constructed in λgt10 from roots of cassava cultivar ‘Mnga 1’ (Beeching et al., 1997). PAL transcripts were identified by northern hybridization. The probe consisted of a 1·4 kb coding region of the PAL cDNA, excised by EcoRI, purified by agarose gel electrophoresis and labelled with α32P using an oligo‐labelling kit (Pharmacia Biotech). The probe MecPOD1 consisted of a 600 bp coding region of the POD cDNA and was previously isolated in this laboratory. MecPOD1 shows highest similarity (up to 65 %) with adzuki bean (Vincula angularis) cationic peroxidase. Total RNA was extracted from cassava suspension cells, using the SV total RNA isolation system kit from Promega. Northern hybridization protocol was according to Sambrook et al. (1989). All lanes were loaded with 10 µg of total RNA. The amount of RNA was determined spectrophotometrically based on the assumption that 1·0 A260 = 40 µg mL–1. All samples were adjusted to 1 µg µL–1 RNA concentration. Equal loading of RNA was monitored. RNA markers were obtained from New England Biolabs and were stained with methylene blue according to Herrin and Schmidt (1988).
HPLC analysis of phenolics
Cassava cell suspensions were comminuted with a mortar and pestle in the presence of liquid nitrogen. 1 g of cells was suspended in 5 mL of ethanol 95 % (v/v), homogenized for 3 min in an Ultra‐turrax (IKA), then incubated at 48 °C for 1 h. For cell suspension supernatants, 15 mL of medium was completely dried for 2 d in a speed‐vacuum concentrator, and stored at –20 °C. 1 cm diameter discs from cassava leaves were ground with liquid nitrogen, then extracted with ethanol (1:10 w/v). Resulting suspensions were homogenized for 3 min then incubated at 48 °C for 1 h. Samples were centrifuged (5 min, 3000 g) then filtered (Whatman No.1), the supernatant recovered and evaporated to dryness at 40 °C. Samples, from cell suspensions, supernatants or leaf discs, were then prepared for HPLC analysis as detailed by Buschmann et al. (2000). Chlorophyll was removed from 200 µL samples in methanol by addition of 200 µL of water at 0 °C; chlorophyll precipitated after about 4 h, when samples were centrifuged (2 min, 4 °C, 10 000 g) then filtered (HPLC technology, PTFE 0·22 µm, 1·3 mm), transferred into brown glass sample vials and stored at –20 °C. Phenolics were identified by their retention times, by co‐chromatography with reference compounds and by UV spectroscopy, as detailed by Buschmann et al. (2000). Quantification was based on reference compounds as external standards; standards were obtained from Sigma, except kaempferol 3‐O‐rutinoside (Roth Ltd, Germany).
Bioassays for antifungal activity
Fungal cultures.
Fusarium solani and F. oxysporum from cassava were supplied by CIAT and cultured for 3 d at 25 °C on potato dextrose agar plates (PDA). Trichoderma harzianum was cultured for 4 d on malt agar plates. Conidial suspensions were made by flooding plates with sterile 1/6 strength Czapek Dox liquid medium pH 6 and adjusting to approx. 2·0 × 105 spores mL–1.
Spore germination.
Fungitoxicity of phenolic compounds was tested against spore germination on glass slides. Compounds were dissolved in absolute ethanol to give 10, 50, 100, 200, 500 and 1000 µg mL–1. 50 µL of each solution was added to wells in Teflon™ microscope slides (BDH), evaporated, then 25 µL of spore suspensions were added. Conditions and assessment of germination and germ tube length were as described by Williams et al. (2002). In addition, crude extracts were tested by applying to thin layer chromatography (TLC) silica plates and determining toxicity to spores and to mycelial growth by spraying plates with fungal spores in nutrient solution; following incubation inhibitory zones were visualized as areas where mycelium failed to colonize the silica gel (Williams et al., 2002). Phenolic solutions up to 1 mg mL–1 were also added to sterile 13 mm antibiotic assay discs (Whatman, Maidstone, UK) and applied to newly established fungal cultures on agar plates; zones of inhibition would indicate toxicity to mycelial growth.
Fungitoxicity of oxidized phenolics.
A preliminary survey was made of potential enzymic oxidation of major phenolics from cassava. Phenolics (250 µL of 100 µg mL–1) in 60 mm phosphate buffer pH 6·0 were added to microtitre plates with hydrogen peroxide (at 0·1–1 % to establish optimum concentration) and 20 µL horseradish peroxidase (Sigma, Type II) or tyrosinase (Sigma), which was added without peroxide. Controls comprised combinations of all reagents except the phenolics. Plates were assessed visually and at A420 nm to determine enzyme‐mediated oxidation (evident as colour change).
Spores of F. solani, F. oxysporum and T. harzianum (100 µL of 2·0 × 105 spores mL–1) were added to 50 µL mL–1 phenolics (those shown previously to undergo oxidation) with 1:10 (v/v) peroxidase (plus H2O2 at 1, 0·05 or 0·02 % final concentrations) or tyrosinase, and after 30 min, centrifuged for 15 min at 2000 g. Spores were resuspended in 900 µL sterile solution of 15 % Czapek Dox, pH 6, and germination in 25 µL samples was determined on Teflon™ microscope slides as described above.
RESULTS
Evaluation of elicitor activity based on induction of the oxidative burst
A rapid, transient production of reactive oxygen species, is one of the earliest detectable responses to stress in plant cells (Bolwell, 1995), and is an indicator of the activity of potential elicitors. Several microbial and endogenous elicitors were tested for ability to induce production of extracellular H2O2. All elicitors were effective and caused a rapid and transient single burst starting after only 2–3 min with maximum production of 8 to >10 µm H2O2 after 5–35 min (Fig 1). Yeast cell wall glucan elicitor gave the fastest and highest response. It also induced other defence‐related genes (Cooper et al., 2001) and was used in subsequent experiments.
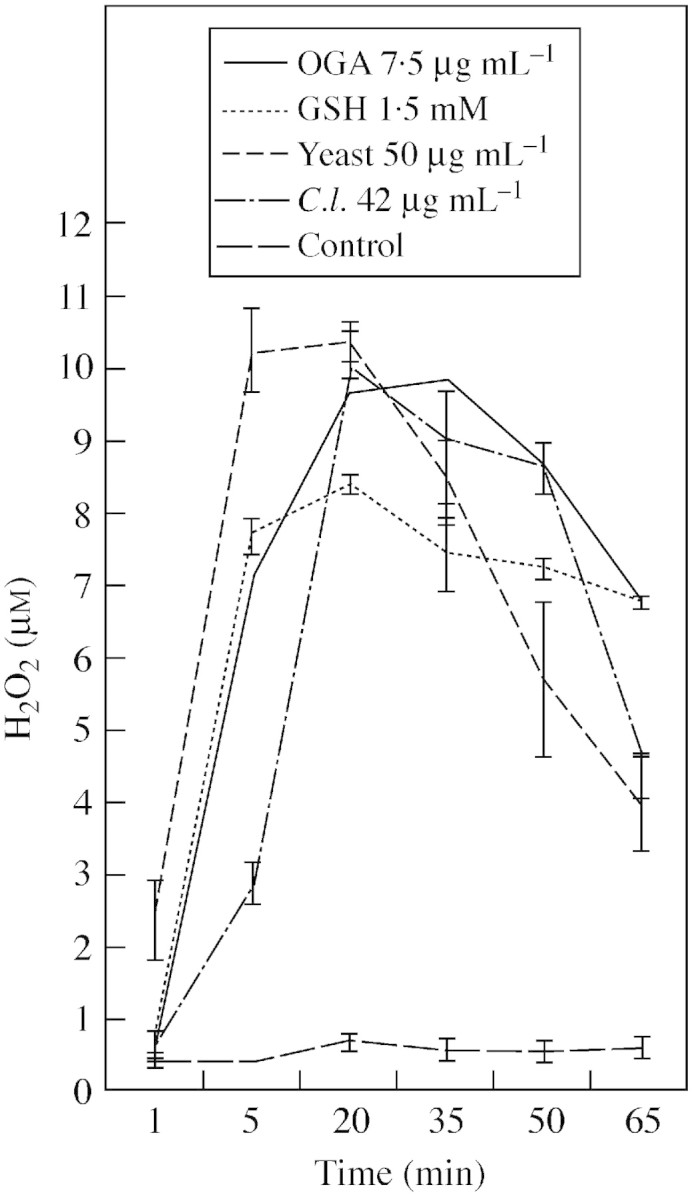
Fig. 1. Oxidative burst from cassava suspension cells challenged with microbial and endogenous elicitors. Extracellular hydrogen peroxide was measured by luminometry. OGA (oligogalacturonic acid 7·5 µg mL–1), GSH (glutathione 1·5 mm), C.l. (Colletotrichum lindemuthianum elicitor 42 µg mL–1), yeast (yeast elicitor 50 µg mL–1). Amounts of fungal elicitors are glucose equivalents. Error bars represent s.e. of three independent replicates.
Elicitation of phenylalanine ammonia lyase activity and transcripts
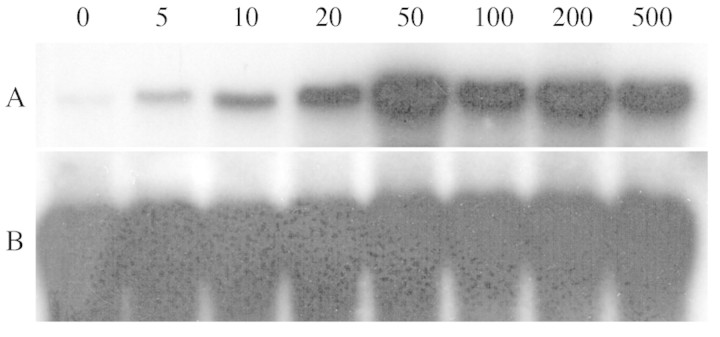
A range of concentrations of yeast elicitor was used to elicit PAL mRNA accumulation in cell suspension cultures (Fig. 2). All concentrations were effective but 50 µg mL–1 elicitor proved optimal and was used in subsequent experiments.
Fig. 2. Dose‐dependent induction of PAL mRNA by yeast elicitor in cassava suspension cells. Northern blot of cassava cell suspension RNA purified 3 h after elicitation with 5–500 µg mL–1 glucose equivalents of glucan cell wall from yeast, and probed with the cassava PAL1 cDNA clone. (A) RNA from elicited cells probed with PAL cDNA; (B) the same RNA probed with 18S rDNA gene probe to check equal loading. RNA size‐markers on the original gel indicated a size of approx. 2·5 kb for the PAL mRNA.
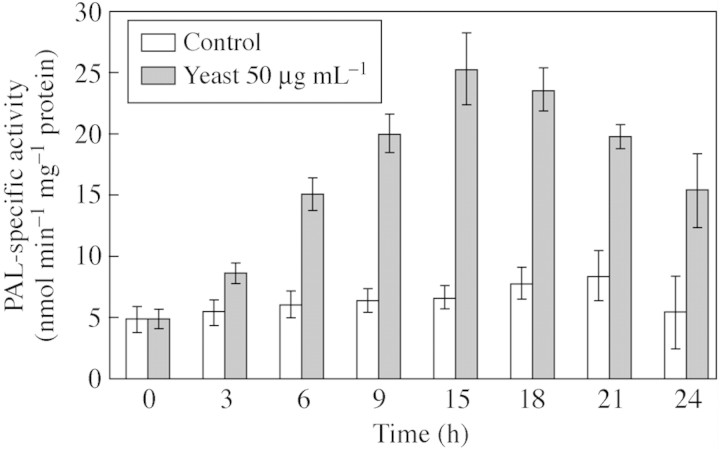
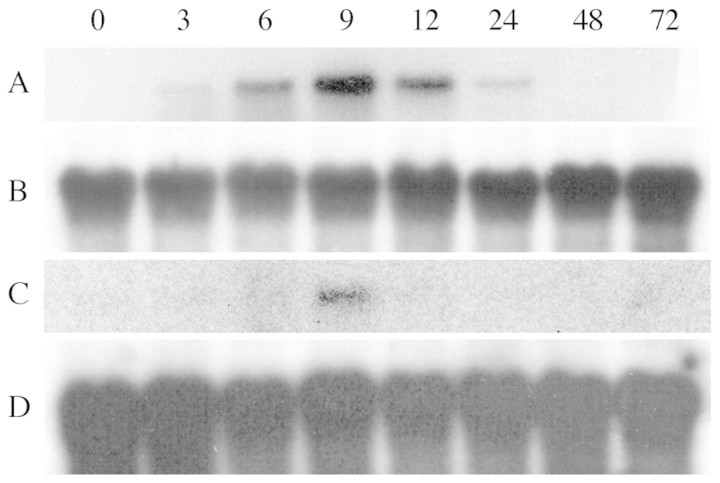
There was a progressive increase of PAL activity in cells after yeast elicitor treatment, with up to a four‐fold increase relative to controls (Fig. 3). The maximum peak occurred at 15 h after elicitation. Northern blotting revealed PAL mRNA accumulated within 3 h and peaked at 9 h (Fig. 4). The overall profile over time mirrors that of changes in PAL activity, but the mRNA peak preceded that of enzyme activity by approx. 6 h, suggesting that the increases in PAL activity were due to expression of the corresponding PAL gene(s). No PAL expression was detected in elicited cassava leaf discs.
Fig. 3. Temporal induction of PAL in cassava suspension cells treated with yeast elicitor. Cassava cell suspensions (5 d growth) were treated with 50 µg mL–1 of glucan cell wall from yeast (solid bars) or control cells (open bars). Results are the means ± s.e. of three replicates and are representative of three experiments.
Fig. 4. Accumulation of PAL mRNA in elicited cassava cells. Northern blot of cassava cell suspension RNA purified over a time course after elicitation and probed with the cassava PAL1 cDNA clone. (A) RNA from cells elicited with 50 µg mL–1 of glucan cell wall from yeast, probed with PAL1 cDNA; (C) RNA from control cells probed with PAL1 cDNA. The same RNA probed with a 18S rDNA gene probe to serve as a loading control in: (B) elicited cells; and (D) in control cells.
Identification and quantification of phenolic compounds in cassava cells and leaves
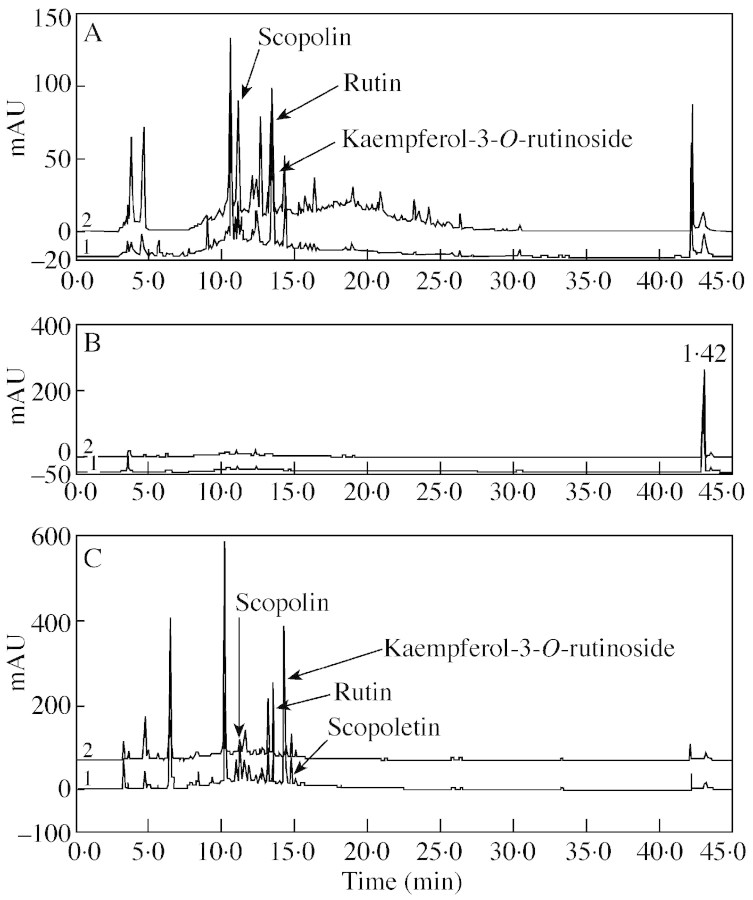
The induction of PAL in elicited cells suggested that phenolics might be produced during elicitation. This was investigated by HPLC of extracts from cassava cells, the medium in which they had been grown, and from leaves. Three phenolics, scopolin, rutin and kaempferol‐3‐O‐rutinoside, were identified in the suspension cells. Scopolin was the only compound that sometimes increased on elicitation (Fig. 5A). In contrast, the medium in which the cells had been grown did not contain detectable phenolics (Fig. 5B). In cassava leaves there was a change in abundance of some compounds between elicited and control samples, but no change in the number of detectable compounds (Fig. 5C). Scopoletin in addition to scopolin, rutin and kaempferol‐3‐O‐rutinoside were readily identifiable. However, a compound eluting at 6·5 min, which decreased on elicitation, and one eluting at 10·5 min, which increased, were not characterized.
Fig. 5. HPLC detection of phenolics in elicited cassava cells and tissues. In all graphs the upper line corresponds to elicited cassava cells or leaves and the lower line to control cassava cells or leaves. (A) Elicited and sterile distilled water (SDW)‐treated control cassava cells at 48 h; (B) elicited and SDW‐treated medium at 48 h; and (C) elicited and SDW‐treated cassava leaves at 48 h. Measurements at 280 nm.
Quantification of the four identified phenolics was made by HPLC with reference to pure samples according to Buschmann et al. (2000). Amounts were substantially greater in leaves than in cells and there was no consistent increase following elicitation. The maximum concentrations (nmol g–1 f. wt) found in elicited cells and leaves (bracketed figures) are: scopoletin, 0·07 (4·2); scopolin, 0·71 (6·0); rutin, 1·29 (9·6); and kaempferol‐3‐O‐rutinoside 1·0 (20·5).
Antimicrobial activity of four major cassava phenolic compounds
Crude extracts from elicited and unelicited cassava cells showed fungitoxicity to spore germination by the slide bioassay and on the TLC plate bioassay with extracts equivalent to >50 mg f. wt mL–1 (data not shown). Therefore, the fungitoxicity of the phenolic compounds identified in the cassava leaves and cells (scopoletin and rutin) and phenolics previously identified in cassava roots undergoing post‐harvest deterioration (esculin, quercetin, ferulic acid; Buschmann et al., 2000) was tested. Preliminary experiments ruled out the toxicity of rutin and kaempferol‐3‐O‐rutinoside.
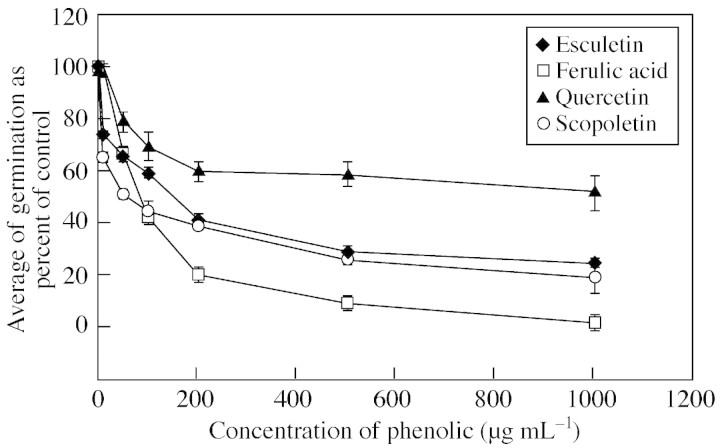
Figure 6 shows a typical dose–response curve for germ tube growth of F. oxysporum. Germ tube growth was reduced to 50 % of the control value by 100–200 µg mL–1 esculetin (0·6–1·2 µM), 50–100 µg mL–1 ferulic acid (0·25–0·5 µm), >1000 µg mL–1 quercetin (3·3 µm) and 50–100 µg mL–1 scopoletin (0·26–0·52 µm), but germination inhibition required >1 mg mL–1 of all phenolics (data not shown). Germ tube growth was generally more sensitive than germination and T. harzianum was usually more susceptible to phenolics than were the two pathogenic species. Trichoderma harzianum germ tube growth was inhibited by ≥50 µg ml–1 ferulic acid and quercetin and ≥10 µg ml–1 of scopoletin. None of the four phenolic compounds tested inhibited mycelial growth of the three fungi (data not shown).
Fig. 6. Dose–response curve for germ tube growth of F. oxysporum treated with the four phenolic compounds.
Effect of oxidation on toxicity of phenolics
The major phenolics identified and tested above proved not to be highly antimicrobial. However, during infection and damage phenols and oxidases, such as peroxidase and tyrosinase, become decompartmentalized and could oxidize phenolic compounds to form antimicrobial quinones. In this study, peroxidase increased following elicitation (see below). Scopoletin and esculetin were readily oxidized by peroxidase and esculin by tyrosinase in vitro (data not shown), as evident from the formation of coloured products. Therefore fungal spores were subjected to enzymatically oxidized esculetin and scopoletin (50 µg mL–1).
With T. harzianum, inhibition was evident with scopoletin‐ and esculetin‐oxidized derivatives; germination and germ tube growth were unaffected in all controls, including those containing hydrogen peroxide. In contrast to the native pure esculetin, which had no effect on germ tube growth at >1 mg mL–1 and required >100 µg mL–1 to effect 50 % inhibition of germination (Fig 6), following oxidation by peroxidase germination and germ tube growth were completely inhibited at 50 µg mL–1; whereas, the product(s) resulting from tyrosinase activity reduced germination and germ tube growth to 28 and 19 %, respectively. The quinone deriving from 50 µg mL–1 scopoletin was more toxic to germination (reduced to 26 %) than the native compound, which required >200 µg mL–1 to produce half the effect (Fig 6). Similar trends occurred with the cassava pathogens but the degree of inhibition was less, with the oxidized forms again much more effective than the parent molecule, but typically resulting in 30–60 % inhibition of germination and germ tube growth (data not shown).
Elicitation of peroxidase activity and transcripts
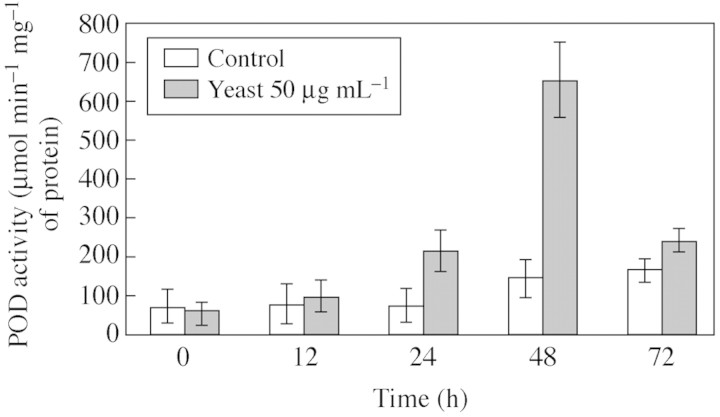
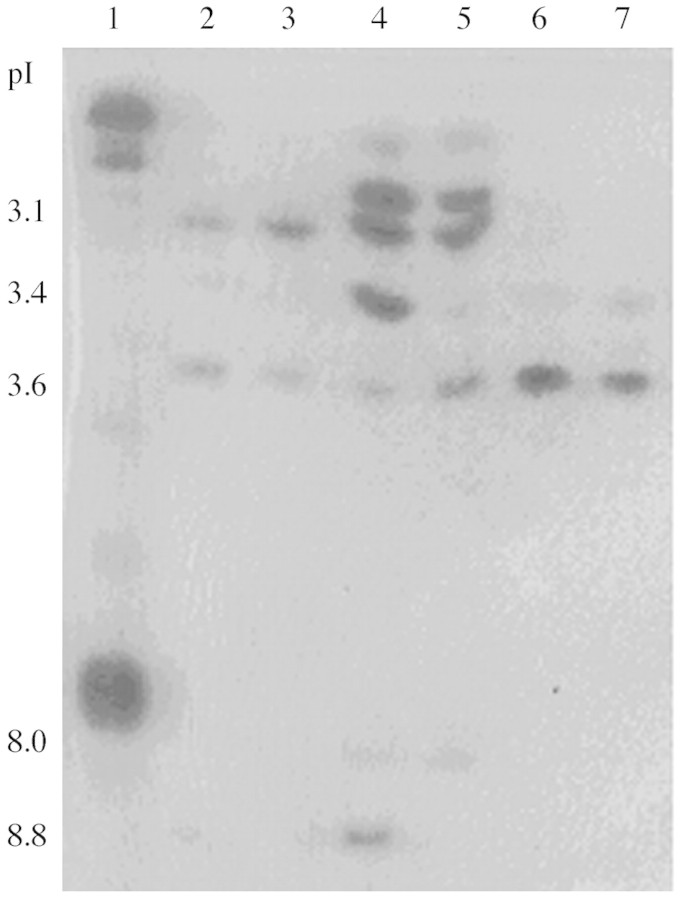
Yeast elicitor, triggered up to a four‐fold increase of peroxidase activity in cells, which peaked at 48 h (Fig. 7). In contrast, peroxidase activity in the extracellular medium increased two‐fold 12 h after elication and then decreased two‐fold by 24 h (data not shown). Different peroxidase isoforms were revealed from these two locations and in leaves (Fig. 8). Suspension cells contained three or four POD isoforms, pIs 3·1, 3·4, 3·5 and 8·8; those of pI 3·1 and 3·5 predominated. However, there was no discernible difference in either isoform number or their intensity between control and elicited cells. At least seven isoforms of pI (2·9, 3·0, 3·1, 3·4, 3·6, 8·8 and 8·8) were detected in the extracellular medium. Two isoforms (pI 8·8, but especially isoform pI 3·4) were enhanced in the elicited medium compared with the control. Two of the previously detected isoforms pIs, 3·4 and 3·6, were present in leaves and no difference was evident between the elicited and control leaves. Isoform pI 3·6 was present in all the samples.
Fig. 7. Peroxidase activity in elicited and control cassava cells. Results are the means ± s.e of three replicates and representative of three experiments.
Fig. 8. Separation by isoelectric focussing of peroxidase isoforms of cassava cells, extracellular medium and leaves at 48 h after elicitation. Approximately 5 µg of cassava protein in 20 µL of extraction buffer was loaded into each lane. pI, isoelectric point. Lane 1, horseradish peroxidase II (positive control); Lane 2, cassava elicited cells; Lane 3, cassava control cells; Lane 4, extracellular medium from elicited cells; Lane 5, extracellular control medium; Lane 6, cassava elicited leaves; and Lane 7, cassava control leaves (SDW‐treated).
Peroxidase isoforms from cassava suspension cells and leaves were tested for their capacity to oxidize scopoletin to a potentially more toxic form, as described earlier with a commercial peroxidase, by immersing isoelectric focusing gels with resolved POD isoforms in hydrogen peroxide and scopoletin (Fig. 9). All isoforms of horseradish peroxidase were able to oxidize scopoletin to a blue product (which later turned yellow), but only anionic peroxidases from cassava oxidized scopoletin. In particular, isoform pI 3·6, which was present in all samples including leaves, was highly effective in scopoletin oxidation.
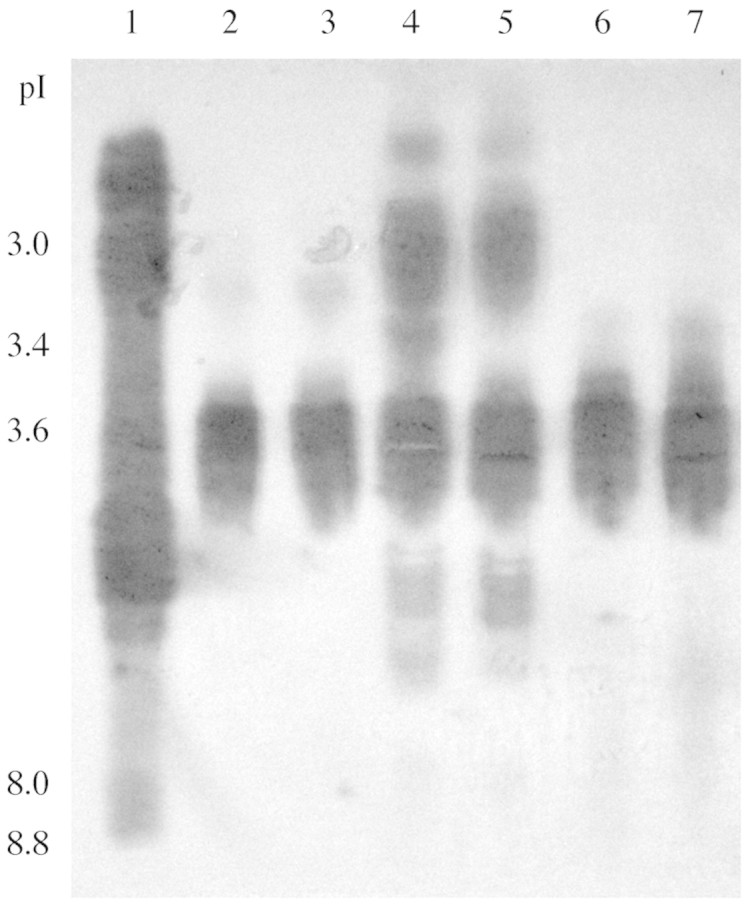
Fig. 9. Identification of cassava peroxidase isoforms with activity toward scopoletin. IEF gels were prepared as described for Fig. 8, then immersed in scopoletin as described in Materials and Methods. Coloured areas indicated formation of oxidized product corresponding to peroxidase isoforms.
Peroxidase changes during the time course of elicitation may derive from activation or from de novo synthesis of one of several isoforms. Therefore, it was of interest to investigate whether a representative corresponding gene (or genes) was activated. Total RNA was probed in northern blots with a cassava cDNA probe MecPOD1. Northern blots obtained with cassava cells after yeast elicitor challenge showed that expression of MecPOD1 started after 6 h and was maximal and similar after 12 and 24 h (Fig 10). No expression was observed in control cells, which shows that the non‐induced activity is due to expression of peroxidase genes other than those coded by MecPOD1. Similarly, MecPOD1 started to be induced 3 h after elicitation in leaves with maximal expression after 24 and 48 h.
Fig. 10. Expression of the MecPOD1 gene in cassava suspension cells and leaves after elicitation. Northern blots of cassava RNA purified after elicitation and probed with cassava MecPOD1. 10 µg of total RNA was loaded per lane. (A) POD in elicited cells; (B) POD in control cells; (C) POD in elicited leaves; (D) POD in control leaves; and (E) 18S rDNA in elicited leaves.
Discussion
A range of microbial and endogenous elicitors were tested for their ability to induce an oxidative burst in cassava suspension cells. All were effective but yeast elicitor always produced the most rapid and highest generation of H2O2, and of several other defence‐related genes (data not shown). Therefore, yeast elicitor was chosen, rather than better‐characterized but harder to obtain elicitors, to investigate phenylpropanoids and two associated enzymes.
Generation of H2O2 was rapid and in the form of a single burst, as was detected in soybean suspension cells treated with oligalacturonide elicitor (Chandra and Low, 1995). A second phase occurs following interactions with incompatible pathogens (Baker and Orlandi, 1995; Legendre et al., 1993) and this was displayed by cassava cells when exposed to incompatible Erwinia amylovora (Cooper et al., 2001). The peroxide produced may perform many defence‐related functions (Baker and Orlandi, 1995), but in this context may contribute, as co‐substrate, to the oxidation of phenolic compounds by peroxidase.
Maximal PAL activity in elicited cells occurred after 9 h. Similar kinetics have been found in cells and tissues of many resistant plant–pathogen interactions ranging from potato leaves and Phytophthora infestans to Arabidopsis suspension cells and elicitors (Fritzmeier et al., 1987; Davis and Ausubel, 1989). Other members of gene families linked with phenylpropanoid biosynthesis may be activated later with different kinetics, including cinnamic acid 4‐hydroxylase (C4H), chalcone isomerase (CHI), isoflavone reductase (IFR) and caffeic acid 3‐O‐methyltransferase (COMT) (Ni et al., 1996).
No PAL expression was detected in elicited cassava leaves. Nevertheless, PAL activity and mRNA is induced in cassava leaves following infiltration with the incompatible plant pathogenic bacterium Pseudomonas syringae p.v. phaseolicola (R. Day and R. M. Cooper, unpubl. data), and Pereira et al. (1999) also obtained PAL activity and transcription of the PAL gene MEPAL in cassava leaves inoculated with incompatible Xanthomonas cassavae. It is feasible that there was insufficient exposure of leaf cells following immersion of leaf discs in elicitor solution.
Visible browning of elicited cassava cells may be indicative of enhanced phenylpropanoid metabolism but is more probably a consequence of phenolic oxidation. Browning of elicited suspension cells has often been noted (e.g. Fritzmeier et al., 1987) but has sometimes been associated with cell death or decreased growth rate (Davis and Ausubel, 1989). However, elicitation did not decrease growth of challenged cassava suspension cells. Increased phenylpropanoid metabolism following infection or elicitor challenge has been found in many plants (Corchete et al., 1993; Butland et al., 1998). For example, alfalfa suspension cells challenged with yeast elicitor produced the isoflavonoid phytoalexin medicarpin (Dixon et al., 1995). In other plants yeast elicitor has induced isoflavonoids and alkaloids, some of which were antimicrobial (Schumacher et al., 1987; Borejsza‐Wysocki et al., 1999).
Phenolic compounds identified in cassava cells and leaves included the coumarin scopolin and its aglycone scopoletin, and the flavonoids kaempferol‐3‐O‐rutinoside and rutin. Whilst some unidentified phenolics increased in elicited cells, none of these characterized phenolics, other than possibly scopolin, gave a clear increase after elicitation. This general pattern concurs with data of Davis and Ausubel (1989) and others (Jones, 1984) that PAL synthesis and enhanced phenolic accumulation are not invariably correlated. Phenolics were not detected by HPLC in the culture medium.
Some of these compounds and other phenolics have been reported in cassava to increase during wounding (scopoletin, scopolin, catechin, esculetin), post‐harvest physiological deterioration (ferulic acid, esculetin, esculin, quercetin) or with defence responses to mealybug infestation (kaempferol‐3‐O‐rutinoside, rutin) (Tanaka et al., 1983; Wheatley and Schwabe, 1985; Calatayud et al., 1994; Prawat et al., 1995; Beeching et al., 1999; Buschmann et al., 2000). Scopoletin accumulates in many plants after infection and has been implicated as a phytoalexin in another member of the Euphorbiaceae, Hevea brasiliensis infected with Microcyclus ulei (García et al., 1995) and with Corynespora cassiicola (Breton et al., 1997). Confusingly, scopoletin has also been described as a phytoanticipin, in that it may be a defence compound mobilized from preformed precursors (Gutiérrez‐Mellado et al., 1996).
The toxicities of four available major phenolics from cassava were tested. The greater sensitivity of T. harzianum to the four phenolics than either F. oxysporum or F. solani may reflect that T. harzianum is a saprotroph, while the others are cassava‐adapted pathogens. Tolerance to, or degradation of, antimicrobials from a host plant is often a key component of pathogenicity (Mansfield, 2000). Detoxification of scopoletin to scopolin, or by degradation, has been reported for several pathogens (García et al., 1995).
Other studies have shown the fungitoxic effect of scopoletin (Ahl Goy et al., 1993; García et al., 1995; Breton et al., 1997; Valle et al., 1997). However, the efficacy of any antimicrobial agent depends upon it being at an appropriate concentration and at the right time and place in vivo (Williams et al., 2002). Typically, compounds with a key role in resistance are active at ≤10–4 m (Mansfield, 2000), but many preformed phenolics do not possess this level of activity (Heath, 1980). Nevertheless, increase in scopoletin up to 35 nmol g–1 f. wt and scopolin to 4 nmol g–1 f. wt in leaves of the hybrid Nicotiana glutinosa × Nicotiana debneyi has been associated with resistance against Cercospora nicotianae and Phytophthora parasitica var. nicotianae (Goy et al., 1993). Levels of the four identified phenolics in cassava cells were much lower than these reports; amounts were 0·07–1·29 nmol g–1 f. wt in elicited cells and 4·2–20·5 nmol g–1 f. wt in elicited leaves. These levels also contrast with the higher scopoletin levels of 34–124 nmol g–1 f. wt in deteriorating cassava roots (Buschmann et al., 2000). In addition, H. brasiliensis leaves accumulated up to 2 mm of scopoletin and this concentration was strongly inhibitory to germ tube elongation and conidial germination of leaf pathogens, including Colletotrichum gloeosporioides and C. cassiicola (García et al., 1995). Fliniaux et al. (1997) found that cell suspensions of different plant species vary greatly (0·001–5 mg g–1) in the levels of scopolin and scopoletin they contain. Scopoletin has been reported to be inhibitory at approx. 2 mm to various fungi (Breton et al., 1997; Valle et al., 1997).
Critical information on fungitoxicity of the other phenolic compounds was not available; the few studies conducted with, for example, kaempferol, quercetin and rutin, used unstated levels or high and fixed concentrations, which does not allow for calculation of minimum inhibitory concentrations (e.g. El‐Gammal and Mansour, 1986). However, as a generalization, phenolics in their unoxidized state are not highly antimicrobial (Heath, 1980). Therefore we investigated peroxidase, a defence‐related enzyme with the capability of producing the more reactive, oxidized quinone forms.
Following elicitation, POD gene expression is often later than that of PAL, and for longer. Here, peak activity at 48 h followed maximum expression of a cassava peroxidase gene MecPOD1 at 12–24 h in elicited cassava cells and leaves. In elicited Arabidopsis cells, POD mRNA was maximal at 24–48 h (Davis and Ausubel, 1989). Nevertheless, MecPOD1 expression was first detectable much earlier after 3 h in leaves and 6 h in suspension cells. In elicitor‐treated cassava suspension cells, symplasmic POD activity increased to a maximum at 48 h after elicitation.
Of the seven POD isoforms detected from cassava cells, anionic forms predominated and were mainly extracellular. Only two appeared to be up‐regulated after elicitation, an isoform of pI 3·4 and, unusually, a cationic form of pI 8·8. POD genes from a family can be differentially regulated in response to various cues and in different tissues, as in rice and in bean (Adam et al., 1995; Chittoor et al., 1997). It is significant, perhaps, that only anionic isoforms in cassava oxidized one of the major cassava phenolics, scopoletin, and the most effective isoform pI 3·6 was present in suspension cells, leaf cells and extracellularly. Also, in tobacco suspension cells a particular isoperoxidase, A3, was associated with the oxidation of scopoletin (Reigh et al., 1975). However, the predicted amino acid sequence of the partial POD cDNA clone, MecPOD, used here in the northern analyses, has a theoretical pI and high homology with a cationic peroxidase. Therefore, it does not correspond to the anionic peroxidases with activity towards scopoletin. While POD isoforms were predominantly extracellular, as was the co‐substrate H2O2, it is paradoxical that potential phenolic substrates for these enzymes were found mostly within the cells.
Furthermore, in cassava roots the increased peroxidase activity during PPD has been associated with scopoletin oxidation in planta (Wheatley and Schwabe, 1985). In these roots we found that mRNA for extensin (a cDNA clone MeHRGP1) showed a similar pattern of expression to that of MecPOD1 12–24 h after wounding (Han et al., 2001). The cross‐linking of extensin monomers by peroxidase may be yet another facet of the multi‐component defences of cassava to pathogenic and biotic stress.
ACKNOWLEDGEMENTS
R.G.V. would like to thank the University of Bath, Department of Biology and Biochemistry, COLCIENCIAS (Colombian Science Council) and CIF (Centro Internacional de Física, Bogotá‐Colombia), and R.D. acknowledges the ODA (now DFID) and the EC for support. This publication is in part an output from a research project (R8156 Crop Post‐Harvest Programme) funded by the United Kingdom Department for International Development (DFID) for the benefit of developing countries. The views expressed are not necessarily those of DFID. We would like to thank Dr Kim Reilly for the cassava POD probe and Dr Han Yuanhuai for the PAL probe.
Supplementary Material
Received: 12 September 2003; Returned for revision: 5 November 2003; Accepted: 13 February 2003. Published electronically: 14 May 2004
References
- AdamAL, Bestwick CS, Barna B, Mansfield JW.1995. Enzymes regulating the accumulation of active oxygen species during the hypersensitive reaction of bean to Pseudomonas syringae pv. phaseolicola Planta 197: 240–249. [Google Scholar]
- Ahl GoyPA, Signer H, Reist R, Aichholz R, Blum W, Schmidt E, Kessmann H.1993. Accumulation of scopoletin is associated with the high disease resistance of the hybrid Nicotiana glutinosa × Nicotiana debneyi Planta 191: 200–206. [Google Scholar]
- BaierR, Schiene K, Kohring B, Flaschel E, Niehaus K.1999. Alfalfa and tobacco cells react differently to chitin oligosaccharides and Sinorhizobium meliloti nodulation factors. Planta 210: 157–164. [DOI] [PubMed] [Google Scholar]
- BakerJC, Orlandi EW.1995. Active oxygen in plant pathogenesis. Annual Review of Phytopathology 33: 299–321. [DOI] [PubMed] [Google Scholar]
- BarzW, Beimen A, Dräger B, Jaques U, Otto C, Süper E, Upmeier B.1990. Turnover and storage of secondary products in cell cultures. In: Charlwood, BV, Rhodes MJC, eds. Secondary products from plant tissue culture Proceedings of the Phytochemical Society of Europe. Dordrecht: Kluwer, 79–101. [Google Scholar]
- BeechingJR, Buschmann H, Gómez‐Vásquez R, Han Y, Iglesias C, Li H, Reilly K, Rodriguez MX.1999. An abiotic stress response in cassava: post‐harvest physiological deterioration. In: Smallwood MF, Calvert CM, Bowles DJ, eds. Plant responses to environmental stress Oxford: BIOS, 205–210. [Google Scholar]
- BeechingJR, Han Y, Cooper RM.1997. Physiological deterioration in cassava: towards a molecular understanding. African Journal of Root and Tuber Crops 2: 99–105. [Google Scholar]
- BolwellGP.1995. The origin of the oxidative burst in plants. Free Radical Research 23: 517–532. [DOI] [PubMed] [Google Scholar]
- Borejsza‐WysockiW, Lester C, Attygalle AB, Hrazdina G.1999. Elicited cell suspension cultures of apple (Malus × domestica) cv. liberty produce biphenyl phytoalexins. Phytochemistry 50: 231–235. [Google Scholar]
- BretonF, Sanier C, D’auzac J.1997. Scopoletin production and degradation in relation to resistance of Hevea brasiliensis to Corynespora cassiicola. Journal of Plant Physiology 151: 595–602. [Google Scholar]
- BuschmannH, Rodriguez MX, Tohme J, Beeching JR.2000. Accumulation of hydroxycoumarins during post‐harvest deterioration of tuberous roots of cassava (Manihot esculenta Crantz). Annals of Botany 86: 1153–1160. [Google Scholar]
- ButlandSL, Chow ML, Ellis BE.1998. A diverse family of phenylalanine ammonia‐lyase genes expressed in pine trees and cell cultures. Plant Molecular Biology 37: 15–24. [DOI] [PubMed] [Google Scholar]
- CahillDM, McComb JA.1992. A comparison of changes in phenylalanine ammonia lyase activity, lignin and phenolic synthesis in the roots of Eucalyptus calopyhlla (field resistant) and E marginata (susceptible) when infected with Phytophthora cinnamomi. Physiological and Molecular Plant Pathology 40: 315–332. [Google Scholar]
- CalatayudPA, Tertuliano M, Leru B.1994. Seasonal‐changes in secondary compounds in the phloem sap of cassava in relation to plant genotype and infestation by Phenacoccus manihoti (homoptera, Pseudococcidae). Bulletin of Entomological Research 84: 453–459. [Google Scholar]
- ChandraS, Low PS.1995. Role of phosphorylation in elicitation of the oxidative burst in cultured soybean cells. Proceedings of the National Academy of Sciences, USA 92: 4120–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ChittoorJM, Leach JE, White FF.1997. Differential induction of a peroxidase gene family during infection of rice by Xanthomonas oryzae pv. oryzae Molecular Plant–Microbe Interactions 10: 861–871. [DOI] [PubMed] [Google Scholar]
- CockJH.1985.Cassava: new potential for a neglected crop. Boulder: Westfield Press, 191 pp. [Google Scholar]
- CollingeDB, Slusarenko AJ.1987. Plant gene expression in response to pathogens. Plant Molecular Biology 9: 389–410. [DOI] [PubMed] [Google Scholar]
- CooperRM, Kemp B, Day R, Gomez‐Vasquez R, Beeching JR.2001. Pathogenicity and resistance in Xanthomonas blight of cassava. In: DeBoer SH, ed. Plant pathogenic bacteria. Dortrecht: Kluwer, 319–323. [Google Scholar]
- CorcheteMP, Diez JJ, Valle T.1993. Phenylalanine ammonia‐lyase activity in suspension cultures of Ulmus pumila and U campestris treated with spores of Ceratocystis ulmi Plant Cell Reports 13: 111–114. [DOI] [PubMed] [Google Scholar]
- CuiY, Magill J, Frederiksen R, Magill C.1996. Chalcone synthase and phenylalanine ammonia lyase mRNA following exposure of sorghum seedlings to three fungal pathogens. Physiological and Molecular Plant Pathology 49: 187–199. [Google Scholar]
- DavisKD, Ausubel FM.1989. Characterization of elicitor‐induced defences in suspension cultured cells of Arabidopsis Molecular Plant–Microbe Interactions 2: 363–368. [Google Scholar]
- DixonRA, Harrison MJ, Paiva NL.1995. The isoflavonoid phytoalexin pathway: from enzymes to genes to transcription factors. Physiologia Plantarum 93: 385–392. [Google Scholar]
- DixonRA, Lamb CJ.1979. Stimulation of de novo synthesis of L‐phenylalanine ammonia‐lyase in relation to phytoalexin accumulation in Colletotrichum lindemuthianum elicitor‐treated cell suspension cultures of French bean (Phaseolus vulgaris). Biochemica et Biophysica Acta 586: 453–463. [DOI] [PubMed] [Google Scholar]
- DuboisM, Gilles KA, Hamilton JK, Rebers PA, Smith F.1956. Colorimetric method for determination of sugars and related substances. Analytical Chemistry 28: 350–356. [Google Scholar]
- El‐GammalAA, Mansour RMA.1986. Antimicrobial activities of some flavonoid compounds. Zentralbiology Mikrobiology 141: 561–565. [DOI] [PubMed] [Google Scholar]
- FahrendorfT, Ni WT, Shorrosh BS, Dixon RA.1995. Stress responses in alfalfa (Medicago sativa L.). Transcriptional activation of oxidative pentose‐phosphate pathway genes at the onset of the isoflavonoid phytoalexin response. Plant Molecular Biology 28: 885–900. [DOI] [PubMed] [Google Scholar]
- FritzmeierK‐H, Cretin C, Kombrink E, Rohwer F, Taylor J, Schel D, Hahlbrook K.1987. Transient induction of phenylanine ammonia lyase and 4‐coumarate:CoA ligase mRNAs in potato leaves infected with virulent or avirulent races of Phytophthora infestans Plant Physiology 85: 34–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FliniauxMA,GilletManceau F, Marty D, Macek T, Monti JP, Jacquin Dubreuil A.1997. Evaluation of the relation between the endogenous scopoletin and scopolin level of some solanaceous and papaver cell suspensions and their ability to bioconvert scopoletin to scopolin. Plant Science 123: 205–210. [Google Scholar]
- GarcíaD, Sanier C, Macheix, J, D’Auzac J.1995. Accumulation of scopoletin in Hevea brasiliensis infected by Microcyclus ulei (P. Henn.) V. ARX and evaluation of its fungitoxicity for three leaf pathogens of rubber tree. Physiological and Molecular Plant Pathology 47: 213–223. [Google Scholar]
- GlazenerJA, Orlandi EW, Harmon GL, Baker J.1991. An improved method for monitoring active oxygen in bacteria‐treated suspension cells suing luminol‐dependent chemiluminescence. Physiological and Molecular Plant Pathology 39: 123–133. [Google Scholar]
- GoyPA, Signer H, Reist R, Aichholz R, Blum W, Schmidt E, Kessmann H.1993. Accumulation of scopoletin is associated with the high disease resistance of the hybrid Nicotiana glutinosa × Nicotiana debneyi Planta 191: 200–206. [Google Scholar]
- GutiérrezMC, Parry A, Tena M, Jorrin J, Edwards R.1995. Abiotic elicitation of coumarin phytoalexins in sunflower. Phytochemistry 38: 1185–1191. [Google Scholar]
- Gutiérrez‐MelladoM, Edwards R, Tena M, Cabello F, Serghini K, Jorrín J.1996. The production of coumarin phytoalexins in different plant organs of sunflower (Helianthus annuus L.). Journal of Plant Physiology 149: 261–266. [Google Scholar]
- HahlbrockK, Ragg H.1975. Light‐induced changes of enzyme activities in parsley cell suspension cultures. Archives of Biochemistry and Biophysics 166: 41–46. [DOI] [PubMed] [Google Scholar]
- HammerschmidtR.1999. Phytoalexins: what have we learned after 60 years? Annual Review of Phytopathology 37: 285–306. [DOI] [PubMed] [Google Scholar]
- HanYH, Gomez‐Vasquez R, Reilly K, Li HY, Tohme J, Cooper RM, Beeching JR.2001. Hydroxyproline‐rich glycoproteins expressed during stress responses in cassava. Euphytica 120: 59–70. [Google Scholar]
- HeathMC.1980. Reaction of nonsuscepts to fungal pathogens. Annual Review of Phytopathology 18: 211–236. [Google Scholar]
- HerrinDL, Schmidt GW.1988. Rapid, reversible staining of Northern blots prior to hybridization. BioTechniques 6: 196–198. [PubMed] [Google Scholar]
- HiragaS, Sasaki K, Ito H, Ohashi Y, Matsui H.2001. A large family of class III plant peroxidases. Plant and Cell Physiology 42: 462–468. [DOI] [PubMed] [Google Scholar]
- JonesDH.1984. Phenylalanine ammonia‐lyase: regulation of its induction, and its role in plant development. Phytochemistry 23: 1349–1359. [Google Scholar]
- KawalleckP, Scmelzer E, Hahlbrock K, Somssich EI.1995. Two pathogen‐responsive genes in parsley encode a tyrosine‐rich hydroxyproline‐rich protein (HRGP) and an anionic peroxidase. Molecular General Genetics 247: 444–452. [DOI] [PubMed] [Google Scholar]
- KombrinkE, Hahlbrock K.1986. Responses of cultured parsley cells to elicitors from phytopathogenic fungi. Plant Physiology 81: 216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LagriminiLM, Gingas V, Finger F, Rothstein S, Liu T‐T.1997. Characterisation of antisense transformed plants deficient in the tobacco anionic peroxidase. Plant Physiology 114: 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LegendreL, Rueter S, Heinstein PF, Low P.1993. Characterization of the oligogalacturonide‐induced oxidative burst in cultured soybean (Glycine max) cells. Plant Physiology 102: 233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LozanoJC.1986. Cassava bacterial blight‐a manageable disease. Plant Disease 12: 1089–1093. [Google Scholar]
- Ludwig‐MullerJ, Thermann P, Hilgenberg W.1994. Peroxidase and chitinase isoenzyme activities during root infection of Chinese cabbage with Plasmodiophora brassicae Physiologia Plantarum 90: 661–670. [Google Scholar]
- MansfieldJW.2000. Antimicrobial compounds and resistance. The role of phytoalexins and phytoanticipins. In: Fraser RSS, van Loon LC, eds. Mechanisms of resistance to plant diseases. Dortrecht: Kluwer, 325–370. [Google Scholar]
- ManchenkoGP.1994.Handbook of detection of enzymes on electrophoretic gels. London: CRC Press. [Google Scholar]
- MayerAM, Harel E.1979. Polyphenol oxidases in plants. Phytochemistry 18: 193–215. [DOI] [PubMed] [Google Scholar]
- MilosevicN, Slusarenko AJ.1996. Active oxygen metabolism and lignification in the hypersensitive response in bean. Physiological and Molecular Plant Pathology 49: 143–158. [Google Scholar]
- MühlenbeckU, Kortenbusch A, Barz, W.1996. Formation of hydroxycinnamoylamides and α‐hydroxyacetovanillone in cell cultures of Solanum khasianum Phytochemistry 42: 1573–1579. [Google Scholar]
- NiW, Fahrendorf T, Ballance GM, Lamb CJ, Dixon RA.1996. Stress responses in alfalfa (Medicago sativa L.). XX. Transcriptional activation of phenylpropanoid pathway genes in elicitor‐induced cell suspension cultures. Plant Molecular Biology 30: 427–438. [DOI] [PubMed] [Google Scholar]
- NothnagelEA, McNeil M, Albersheim P, Dell A.1983. Host–pathogen interactions. XXII. A galacturonic acid oligosaccharide from plant cell walls elicits phytoalexins. Plant Physiology 71: 916–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PereiraLF, Goodwin PH, Erickson L.1999. The role of a phenylalanine ammonia lyase gene during cassava bacterial blight and cassava bacterial necrosis. Journal of Plant Research 112: 51–60. [Google Scholar]
- PerreyR, Hauser MT, Wink M.1989. Cellular and subcellular localization of peroxidase isoenzymes in plants and cell suspension cultures from Lupinus polyphyllus Zeitschrift für Naturforschung 44c: 931–936. [Google Scholar]
- PrawatH,Mahidol C, Ruchirawat S, Prawat U, Tuntiwachwuttikul P, Tooptakong Uet al.1995. Cyanogenic and non‐cyanogenic glycosides from Manihot esculenta Phytochemistry 40: 1167–1173. [DOI] [PubMed] [Google Scholar]
- ReighDL, Wender SH, Smith EC.1975. Scopoletin: kinetic nature of isoperoxidase A3 catalysed oxidation and its possible relation to the physiological action of this naturally occurring growth factor. Physiologia Plantarum 34: 44–46. [Google Scholar]
- SambrookJ, Fritsch EF, Maniatis T.1989.Molecular cloning: a laboratory manual. Cold Spring Harbor: Cold Spring Harbor Press. [Google Scholar]
- SchumacherHM, Gundlach H, Fiedlar F, Genk MH.1987. Elicitation of benzophenanthridine alkaloid synthesis in Escholtzia cell cultures. Plant Cell Reports 6: 410–413. [DOI] [PubMed] [Google Scholar]
- SharanM, Taguchi G, Gonda K, Jouke T, Shimosaka M, Hayashida N, Okazaki M.1998. Effects of methyl jasmonate and elicitor on the activation of phenylalanine ammonia‐lyase and the accumulation of scopoletin and scopolin in tobacco cell cultures. Plant Science 132: 13–19. [Google Scholar]
- StampJA, Henshaw GG.1982. Somatic embryogenesis in cassava. Zeitschrift Fur Pflanzenphysiologie 105: 183–187. [Google Scholar]
- StoscheckCM.1990. Increased uniformity in the response of the Coomassie blue G protein assay to different proteins. Analytical Biochemistry 184: 111–116. [DOI] [PubMed] [Google Scholar]
- TanakaY, Data ES, Hirose S, Taniguchi T, Uritani I.1983. Biochemical changes in secondary metabolites in wounded and deteriorated cassava roots. Agricultural Biological Chemistry 47: 693–700. [Google Scholar]
- ValleT, López LJ, Hernández JM, Corchete P.1997. Antifungal activity of scopoletin and its differential accumulation in Ulmus pumila and Ulmus campestris cell suspension cultures infected with Ophiostoma ulmi spores. Plant Science 125: 97–101. [Google Scholar]
- Van GestelenP, Asard H, Caubergs RJ.1997. Solubilization and separation of a plant plasma membrane NADPH‐O2–‐synthase from other NAD(P)H‐oxidoreductases. Plant Physiology 115: 543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WheatleyCC, Schwabe WW.1985. Scopoletin involvement in post‐harvest physiological deterioration of cassava root (Manihot esculenta Crantz). Journal of Experimental Botany 36: 783–791. [Google Scholar]
- WilliamsJ, Hall SA, Hawkesford MJ, Beale MH, Cooper RM.2002. Elemental sulfur and thiol accumulation in tomato and defence against a fungal pathogen. Plant Physiology 128: 150–159. [PMC free article] [PubMed] [Google Scholar]
- WojtaszekP, Stobiecki M, Bolwell GP.1997. Changes in the composition of exocellular proteins of suspension‐cultured Lupinus albus cells in response to fungal elicitors or CuCl2 Journal of Experimental Botany 48: 2015–2021. [Google Scholar]
- WuJY, Lin LD.2002. Ultrasound‐induced stress responses of Panax ginseng cells: Enzymatic browning and phenolics production. Biotechnology Progress 18: 862–866. [DOI] [PubMed] [Google Scholar]
- ZapataJM, Calderon AA, Barcelo AR.1995. Peroxidase isoenzyme patterns in cell‐cultures derived from cotyledon, stem, leaf and fruit from grapevine (Vitis vinifera cv. monastrell). Annals of Botany 75: 443–448. [Google Scholar]
- ZeyenRJ, Bushnell WR, Carver TLW, Robbins MP, Clark TA, Boyles DA, Vance CP.1995. Inhibiting phenylalanine ammonia lyase and cinnamyl alcohol dehydrogenase suppresses Mla1 (HR) but not mlo5 (non‐HR) barley powdery mildew resistance. Physiological and Molecular Plant Pathology 47: 119–140. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.