Abstract
• Background and Aims This study on reproductive biology examines the stigmatic morphology of 12 Brazilian Malpighiaceae species with regard to their pollination and breeding system.
• Methods The species were studied in natural populations of a semi‐deciduous forest fragment. Style tips were processed for observation by SEM and pollen‐tube growth was analyzed under fluorescence microscopy. The breeding system was investigated by isolating flowers within waterproof bags. Floral visitors were recorded through notes and photographs.
• Key Results Flowers are yellow, pink or white, protogynous, herkogamous and sometimes lack oil glands. While Banisteriopsis pubipetala has functional female flowers (with indehiscent anthers), 11 species present hermaphrodite flowers. Stigmas of these species may be terminal, with a slightly concave surface, or internal, consisting of a circular cavity with a large orifice, and are covered with a thin, impermeable cuticle that prevents pollen from adhering, hydrating, or germinating. Malpighiaceae have a special type of ‘wet’ stigma, where a secretion accumulates under the cuticle and is released by mechanical means—mainly rupture by pollinators. Even though six species show a certain degree of self‐compatibility, four of them present a form of late‐acting self‐incompatibility, and the individual of B. pubipetala is agamospermous. Species of Centris, Epicharis and Monoeca bees pollinate these flowers, mainly collecting oil. Some Epicharis and Monoeca species collected pollen by vibration. Paratetrapedia and Tetrapedia bees are pollen and oil thieves.
• Conclusions The Malpiguiaceae species studied are pollinator‐dependent, as spontaneous self‐pollination is limited by herkogamy, protogyny and the stigmatic cuticle. Both the oil‐ and pollen‐collecting behaviours of the pollinators favour the rupture of the stigmatic cuticle and the deposition of pollen on or inside the stigmas. As fruit‐set rates in natural conditions are low, population fragmentation may have limited the sexual reproduction of these species.
Key words: Malpighiaceae, Banisteriopsis spp., Dicella bracteosa, Heteropterys intermedia, Mascagnia spp., Stigmaphyllon lalandianum, Tetrapterys spp., pollination, reproduction, stigma morphology, oil‐collecting bees, Monoeca
INTRODUCTION
Malpighiaceae are predominantly tropical plants, and approx. 85 % of the currently recognized species occur in the New World (Anderson, 1979; 1990). Flowers of neotropical Malpighiaceae are relatively similar in terms of general morphology, especially as regards attraction, orientation and reward of pollinators. However, their androecia and gynoecia present considerable diversity (Anderson, 1979; 1990).
Malpighiaceae are pollinated by female Apidae bees of the tribes Centridini and Tapinotaspidini (Monoeca spp.), which gather the oil produced by the calyx glands, called elaiophores (Buchmann, 1987; Frankie et al., 1989; Sazima and Sazima, 1989; Vogel, 1990; Pedro, 1994). Since it is used as larval provision and to waterproof the earthen brood‐cell walls, this oil is very important (Neff and Simpson, 1981; Anderson, 1979; 1990; Buchmann, 1987; Sazima and Sazima, 1989; Vogel, 1988; 1990; Vinson et al., 1997).
The pollination of a neotropical Malpighiaceae species was first described by Hauman‐Merck (1913), who considered these calyx glands as nectaries. Later, Vogel (1974) proved that the reward produced by these glands was oil and established a new pollination system, the ‘oil‐flower syndrome’. This stimulated studies of the floral morphology and pollination systems of the neotropical Malpighiaceae from several ecosystems (Gottsberger, 1986; Rêgo and Albuquerque, 1989; Sazima and Sazima, 1989; Simpson, 1989; Teixeira and Machado, 2000).
Although a few genera or species of this family have had their breeding system studied (Bawa, 1974; Ruiz and Arroyo, 1978; Steiner, 1985; Barros, 1992; Teixeira and Machado, 2000), further details regarding their stigmatic morphology and its function are so far unknown. Since the morphological and physiological stigmatic features affect the reproductive biology and breeding systems (Heslop‐Harrison, 2000), this information is quite important.
The purpose of the present contribution was therefore twofold: (1) to describe the reproductive biology and stigmatic morphology of four species of Banisteriopsis, three of Mascagnia, two of Tetrapterys, and one each of Dicella, Heteropterys and Stigmaphyllon; and (2) to discuss the stigma morphology of these species in the light of their pollination and breeding systems.
MATERIALS AND METHODS
Study area and plant species
Field observations were made in 1994 and from 1996 to 1998 in the Santa Genebra Reserve, a 250‐ha semi‐deciduous forest, close to an urban area in Campinas (22°49′S, 47°7′W; altitude 670 m), São Paulo, Brazil. This forest fragment has been affected by anthropogenic disturbances (e.g. cultivation, human dwellings) since the 1950s. Included within its boundaries are areas of secondary vegetation and swamp forest. Additional information on vegetation type and climate can be found in Morellato and Leitão‐Filho (1995; 1996) and Figueiredo and Sazima (2000).
The Malpighiaceae species studied are lianas commonly found at the forest edge. Their inflorescences are racemose (11 species) or cymose (Stigmaphyllon lalandianum), and aggregated in dichasial units. Species names and herbaria numbers are listed in the Appendix. Vouchers of these species are deposited at the herbaria of the Universidade Estadual de Campinas (UEC) and Universidade Federal de Mato Grosso do Sul (CGMS/UFMS), in Campo Grande.
General flower features and stigma morphology
Flowers and stigmas were examined in situ and in the laboratory. Plant material was fixed in either 70 % alcohol or FAA. Style tips were dehydrated through an ethanol series, dried by the critical‐point method, mounted on stubs, and coated with gold–palladium (Dulberger et al., 1994). Details of the stigmatic morphology were studied by optical, stereo and/or scanning electron microscopy.
Floral biology observations were made to verify the events of anthesis, as stigma receptivity, anther dehiscence and oil production. Stigma receptivity was checked with a magnifying glass. Pollen viability was estimated by cytoplasmic staining, using the aceto‐carmine technique (Radford et al., 1974).
Breeding system
The study of the breeding system of 11 species (all but Heteropterys intermedia) was performed by isolating flowers in waterproof bags to verify the occurrence of fertilization in spontaneous and hand self‐pollination, and/or cross‐pollination. Self‐pollinations were made with the help of a brush. Cross‐pollinated flowers were not emasculated, and pollinations were performed by rubbing anthers onto stigmas. Pollinations were made in the morning, and to verify that the stigmatic cuticle was ruptured, stigmas were examined with a magnifying glass. Flowers visited by pollinators were tagged and monitored for open pollination (control). Fruit set was recorded after full development. The number of plants per species used in each treatment is summarized in Table 1.
Table 1.
Pollen viability and results of fruit set (%) in 11 species of neotropical Malpighiaceae from a semi‐deciduous forest in south‐eastern Brazil, after emasculation, and self‐, cross‐ and open pollinations
| Pollen | Compatibility | Self‐pollination | Cross‐ | Open pollination | ||||
| Species | n | viability (%) | system | Emasculation | spontaneous | hand | pollination | (control) |
| Banisteriopsis adenopoda | 6 | 89 | SC | 0 (0/63) | 2·5 (4/159) | 24·5 (42/171) | 16·7 (25/150) | 8·9 (20/225) |
| B. lutea | 3 | 57 | SC | 0 (0/87) | 5·5 (2/36) | 1·8 (1/54) | ||
| B. muricata | 3 | 84 | SC | 0 (0/66) | 1·7 (2/117) | 38·0 (16/42) | 2·7 (3/108) | |
| B. pubipetala | 1 | 22 | AG | 42·5 (51/120) | 18·0 (26/144) | |||
| Dicella bracteosa | 12 | 85 | SI | 0 (0/42) | 0 (0/45) | 5·2 (2/38) | 0 (0/41) | |
| Mascagnia anisopetala | 10 | 87 | SC | 1·7 (2/117) | 0·7 (2/261) | 3·3 (6/183) | 4·0 (8/198) | |
| M. cordifolia | 8 | 77 | SI | 0 (0/36) | 42·8 (18/42) | 0 (0/57) | ||
| M. sepium | 6 | 73 | SI | 0 (0/36) | 0 (0/9) | 11·7 (6/51) | ||
| Stigmaphyllon lalandianum | 14 | 91 | SI | 0 (0/45) | 0 (0/117) | 7·6 (8/105) | 4·9 (8/162) | |
| Tetrapterys guilleminiana | 5 | 74 | SC | 0 (0/90) | 13·0 (9/69) | 5·2 (3/57) | 3·3 (4/120) | |
| T. phlomoides | 1 | 87 | SC | 0 (0/60) | 12·9 (17/132) | 5·9 (96/102 | ||
Figures in parentheses indicate the number of samaras matured/carpels (fruits/functional carpels for Dicella bracteosa) used in each treatment.
n = number of individuals per species; SC = self‐compatible, SI = self‐incompatible, AG = agamospermous.
Pollen‐tube growth was examined in at least five pistils of self‐pollinated and/or cross‐pollinated flowers of eight species (Banisteriopsis adenopoda, B. lutea, B. pubipetala and Heteropterys intermedia were omitted). Pistils were fixed in FAA at different intervals after pollination and analyzed under fluorescence microscopy (Martin, 1959) as for pollen‐tube growth. This also established when and where any possible incompatibility reactions may occur (cf. Seavey and Bawa, 1986).
Visitors
Floral visitors were recorded over 37 days, totalling 140 h of observations. Flowers were surveyed for visitors at different times of the day, from early morning (0700 h) to late afternoon (1700 h). The behaviour of visitors was recorded through notes and photographs (cf. Sazima and Sazima, 1989). Bee specimens were collected with nets and/or waterproof bags. They were subsequently conditioned in plastic vials, identified and deposited as vouchers at the Laboratório de Abelhas of the Universidade de São Paulo.
RESULTS AND DISCUSSION
General flower features and stigmatic morphology
Flower morphology of the studied species is very similar to that of other neotropical Malpighiaceae, and readers interested in more details on the morphology, shape and variation of flower parts should refer to Anderson (1979; 1990), Gates (1982), Anderson (1997), Vogel (1990) and Barroso et al. (1991). Eight of these species present yellow flowers (Fig. 1A, B), while one has white or pinkish flowers and the other three have pink ones (Fig. 1C). These flowers, which last 1 d (except for B. pubipetala, which last 2 d), range from small (<14 mm in diameter; e.g. H. intermedia, M. anisopetala and T. guilleminiana) to relatively large (18–20 mm in diameter; e.g. Banisteriopsis spp. and Stigmaphyllon lalandianum), and give off a sweet odour.

Fig. 1. (A–F) Flowers of Mascagnia sepium and Banisteriopsis lutea showing the different flag petals, and the presence (A) or lack (B) of elaiophores (arrow). Scale bars = 2·5 mm. (C) Elaiophores of M. cordifolia with oil accumulated under the transparent cuticle (arrow). Scale bar = 1·0 mm. Arrangements of styles/stigmas and stamens in flowers of (D) B. adenopoda, (E) Tetrapterys guilleminiana, and (F) Dicella bracteosa. Scale bars = 5·0 mm.
The oil produced by the elaiophores accumulates under a transparent cuticle (Fig. 1C), a characteristic of most neotropical Malpighiaceae (Vogel, 1974; 1990; Simpson and Neff, 1981; Subramanian et al., 1990; Cocucci et al., 1996), and is the main reward for pollinators. However, B. lutea (Fig. 1B) and some individuals of B. muricata and H. intermedia have eglandular flowers that offer only pollen as a reward (Sazima and Sazima, 1989). Eglandular flowers are found within different taxonomic levels of neotropical Malpighiaceae (Anderson, 1990) and pollen‐collecting bees are the presumed pollinators (Anderson, 1979). However, Sazima and Sazima (1989) found that the eglandular morphs of Heteropterys intermedia and Banisteriopsis muricata, and even the eglandular species Banisteriopsis lutea, were only pollinated by oil‐collecting bees, and that such morphs and species can act as deceptive blossoms within the ‘oil‐flower’ category, and turn into ‘pollen‐flowers’ for some bee species, thus improving their chances of pollination.
In the species studied, the flag petal, known to orientate and support the pollinators (Hauman‐Merck, 1913; Anderson, 1979; Gottsberger, 1986), varies in position, form and/or limb size, length and claw thickness (Fig. 1A, B). The limb can display stripes or spots of different colours (B. lutea, Fig. 1B) or hues (B. adenopoda, B. muricata, M. anisopetala; see Anderson, 1979; 1990; Lobreau‐Callen, 1989).
The arrangement of stamens and pistils in the centre of the flower (Fig. 1A, B, D–F) favours the contact of pollinators with both structures simultaneously during their visits. Anthers are versatile, extrorse and open longitudinally (Fig. 1D–F), usually 30 min to 1 h after floral anthesis, when stigmas are already receptive. Thus, the flowers are protogynous (except B. pubipetala, see below). Pollen is released near the styles, just below the stigmas (Figs 1D–F, 2A), and its viability varies between 57 and 94 % (Table 1). These species are hermaphrodite and display the ‘homomorphic approach herkogamy’ type; i.e. flower visitors contact the stigmas first and pollen is picked up subsequently, or when visitors depart (Webb and Lloyd, 1986; Endress, 1994), which favours cross‐pollination (Webb and Lloyd, 1986). Banisteriopsis pubipetala has pseudo‐hermaphrodite (functionally female) flowers since their anthers are indehiscent. Hermaphroditism is a more common condition in neotropical Malpighiaceae (Anderson, 1990), even though pseudo‐hermaphroditism has been reported in various genera and species (Anderson, 1981; Anderson, 1982; Steiner, 1985; M.R.Sigrist, pers. obs.). Although indehiscent, the anthers of B. pubipetala do bear pollen but its viability is low (22 %), a feature that may be related to agamospermy (Table 1) and was also recorded for some Peixotoa species (Anderson, 1982).
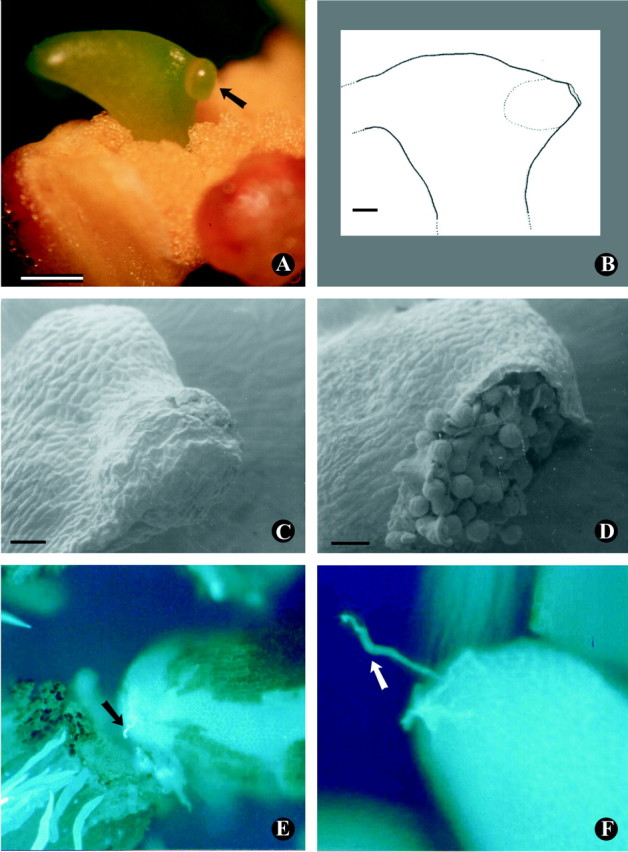
Fig. 2. (A–F) Stigmas of Malpighiaceae species. Stigmaphyllon lalandianum: (A) secretion accumulated below the transparent stigmatic cuticle (arrow), and (B) internal stigma with an almost circular cavity and a large orifice. Scale bars = 5·0 mm and 1·0 mm, respectively. Mascagnia cordifolia: (C) stigma of an unpollinated flower, note the intact cuticle and the absence of pollen on it, and (D) of a pollinated flower, note the large orifice and the numerous pollen grains. Scale bars = 50 µm. Pollen tubes penetrating into ovules of Banisteriopsis muricata flowers 72 h after (E) self‐ and (F) cross‐pollination (arrows). Magnification ×125 and ×312·5, respectively.
The gynoecium of the studied Malpighiaceae comprises three (eleven species) or two (Dicella bracteosa) superior uniovulate functional carpels, connate at base. In D. bracteosa one of the two ovules aborts so that only one ovule matures into a seed (W. R. Anderson, pers. comm.). Styles may be arranged in three different ways: (1) separated and disposed in a more or less broad triangle (nine species, Fig. 1D); (2) slightly separated but aligned (H. intermedia and Tetrapterys guilleminiana, Fig. 1E); or (3) grouped (D. bracteosa, Fig. 1F). Species of Banisteriopsis and T. phlomoides have large terminal stigmas (Fig. 1D), with a slightly concave stigmatic surface. Stigmas of D. bracteosa (Fig. 1F), H. intermedia, Mascagnia spp., S. lalandianum (Fig. 2A) and T. guilleminiana are internal, consisting of an almost circular cavity (Fig. 2B) with relatively large orifices (Fig. 2B, D). Orifices at the end of the stylar canal have also been reported in many other plant species (Heslop‐Harrison and Shivanna, 1977; Dulberger et al., 1994). According to Anderson (1979) and Barroso et al. (1991), the stigmas of neotropical Malpighiaceae vary from minute to fairly large, and from terminal to internal; when they are internal, the styles may bear apical‐dorsal extensions or appendages like those of S. lalandianum (Fig. 2A).
In species with internal stigmas, they are orientated towards the flower centre (Fig. 1E); D. bracteosa was the only one to present stigmas orientated towards the sepals near the flag petal (Fig. 1F). Such stigma orientations favour deposition of a large pollen load inside the stigmatic cavity (Fig. 2D). Stigmas are covered with a thin, continuous membrane (stigmatic cuticle), which is impermeable (M. C. B. Pinheiro, pers. comm.) and protects its secretion (Fig. 2A). Pre‐anthesis buds present small amounts of stigmatic secretion and their cuticle is whitish. However, in buds immediately before anthesis stigmas are receptive, swollen, with abundant secretion, and the cuticle is transparent (Fig. 2A). At that point in time, the stigmatic cuticle may function as an adaptation against excessive water loss, and temporarily protects the fluid layer below (Heslop‐Harrison, 2000).
The stigmatic cuticle needs to be ruptured for pollen to adhere and hydrate, which is an essential prelude to germination (Heslop‐Harrison and Heslop‐Harrison, 1985). In Malpighiaceae the stigmatic cuticle is easily broken but its rupture needs mechanical factors, usually floral visitors, and mainly pollinators (Fig. 2D). The stigmas of flowers isolated in waterproof bags displayed intact cuticles and no pollen (Fig. 2C). Malpighiaceae species have a special type of ‘wet’ stigma (cf. Heslop‐Harrison and Shivanna, 1977; Heslop‐Harrison, 2000), a feature already reported for many Leguminosae (Heslop‐Harrison and Heslop‐Harrison, 1983; Lord and Heslop‐Harrison, 1984).
Breeding system
The individual of Banisteriopsis pubipetala showed high fruit set in both emasculated and control flowers (Table 1), which is characteristic of agamospermous species. Marked differences in fruit set between emasculated and control flowers of this individual may be related to less intense predation of the emasculated flowers, which remained bagged for about 1 week after its manipulation, whereas control flowers were not bagged. This species is widely distributed over South America with male‐sterile and male‐fertile populations scattered throughout its range (W. R. Anderson, pers. comm.), and in this case agamospermy cannot be extended for the whole species. Agamospermy is uncommon in Malpighiaceae, and has been reported in only a few species, for example Hiptage madablota (Grant, 1981) and five species of Peixotoa (Anderson, 1982).
Six species showed a certain degree of self‐compatibility: Banisteriopsis adenopoda, B. lutea, B. muricata, Mascagnia anisopetala, Tetrapterys guilleminiana and T. phlomoides, which had low fruit set after self‐pollination (Table 1), suggesting an unimportant degree of self‐compatibility. However, populations of B. muricata in Costa Rica (Bawa, 1974) and Venezuela (Ruiz and Arroyo, 1978) showed high levels of fruit set after manual self‐pollination. Self‐compatibility seems to be common in neotropical Malpighiaceae, and has already been reported for some species of Byrsonima, Banisteriopsis, Galphimia, Heteropterys, Malpighia and Peixotoa (Bawa, 1974; Barros, 1992; A. A. A. Barbosa and H. B. N. Borges, pers. comm.), and certainly in cleistogamic species of Aspicarpa, Camarea, Gaudichaudia and Janusia (Anderson, 1980). Spontaneous self‐pollination was not frequent and occurred in B. adenopoda, M. anisopetala and T. guilleminiana (Table 1). In most of the studied Malpighiaceae, however, spontaneous self‐pollination is limited by herkogamy, protogyny and the stigmatic cuticle (the latter being a mechanical system that prevents selfing), and may well be a significant determinant in the breeding behaviour of potentially self‐fertile species (Heslop‐Harrison and Heslop‐Harrison, 1983).
Since they produced no fruits after self‐pollination, Dicella bracteosa, M. cordifolia, M. sepium and S. lalandianum were considered as self‐incompatible (Table 1). These species present some kind of late‐acting self‐incompatibility (Seavey and Bawa, 1986), since self‐tubes were observed penetrating into the ovules in a similar way to cross‐tubes (Fig. 2E, F). This type of self‐tube behaviour has been reported in self‐incompatible species of other angiosperm families (Gibbs et al., 1999; Gribel et al., 1999). In neotropical Malpighiaceae, self‐incompatibility has been reported for Byrsonima coccolobifolia (Barros, 1992), B. sericea (Teixeira and Machado, 2000), Camarea affinis, Heteropterys sp. (A. A. A. Barbosa, pers. comm.) and Spachea membranacea (Steiner, 1985), but the category of incompatibility has not been determined yet (cf. Seavey and Bawa, 1986).
Our results show that the majority of the species are pollinator‐dependent. However, most species have low fruit set even in open (control) and/or cross‐pollinations (Table 1), self‐compatible species included. These results diverge from those of Bawa (1974), Ruiz and Arroyo (1978) and Barros (1992), where control and/or cross‐pollination fruit‐set rates were higher. The low reproductive success in the studied species suggests that inbreeding depression may be occurring in these populations (see Bawa et al., 1985; Futuyma, 1993). This suggestion is supported by the fact that the study area is a relatively small forest fragment, isolated a long time ago (Morellato and Leitão‐Filho, 1996).
Pollination and visitors
Flowers of the studied species were visited by female bees of the family Apidae (Table 2). Species of Centris, Epicharis (Centridini) and Monoeca (Tapinotaspidini) pollinated the flowers, collecting mainly oil (98 % of visits) (Fig. 3A). During the visits these bees behaved as described by Vogel (1974), and as further confirmed by other studies (Rêgo and Albuquerque, 1989; Sazima and Sazima, 1989; Gaglianone, 2000). The oil‐collecting behaviour favours the rupture of the stigmatic cuticle and the exposure of the stigmatic surface or cavity, promoting the deposition of a large load of pollen on or inside the stigmas (Fig. 2D).
Table 2.
Visiting bee species and the rewards collected from the flowers of neotropical Malpighiaceae from a semi‐deciduous forest in south‐eastern Brazil
| Bee species | Rewards | Plant species |
| Centris (Xanthemisia) bicolor Lepeletier | O | Blu, Dbr, Man, Sla, Tph |
| Centris (Melanocentris) collaris Lepeletier | O | Bad, Bmu, Dbr, Man, Sla, Tgu, Tph |
| Centris (Melanocentris) discolor Smith | O | Bad, Dbr, Man |
| Centris (Melanocentris) mocsaryi Friese | O | Bad, Bmu, Dbr, Man, Mco, Sla, Tgu |
| Centris (Centris) aenea Lepeletier | O | Dbr |
| Centris (Paremisia) fuscata Lepeletier | O | Bad, Tgu |
| Centris (Heterocentris) labrosa Friese | O | Dbr |
| Centris (Centris) nitens Lepeletier | O | Dbr, Mco |
| Centris (Hemisiella) tarsata Smith | O | Blu, Dbr, Mse |
| Centris (Hemisiella)trigonoides Lepeletier | O | Dbr, Sla |
| Epicharis (Epicharis) affinis Friese | O/P | Bad, Bmu, Dbr, Hin, Man, Sla, Tgu, Tph |
| Epicharis (Triepicharis) analis Lepeletier | O | Bmu, Dbr |
| Epicharis (Epicharis) flava Friese | O/P | all, except Mco |
| Epicharis (Triepicharis) schrottkyi Friese | O/P | Bad, Bmu, Dbr, Mco, Sla |
| Epicharis (Epicharitides) obscura Friese | O | Bad, Bmu, Sla |
| Monoeca sp.1 | O/P | Bad, Bmu, Dbr |
| Monoeca sp.2 | O | Bad, Man, Sla |
| Monoeca sp.3 | O | Hin |
| Paratetrapedia (P.) velutina Friese | O/P | Sla, Tgu |
| Paratetrapedia (Lophopedia) sp.1 | O/P | Bad, Dbr, Sla |
| Paratetrapedia (L.) sp.2 | O/P | Bad, Bmu, Bpu, Dbr, Hin, Mco, Sla |
| Paratetrapedia (L.) sp.3 | O/P | Bad |
| Tetrapedia (T.) diversipes Klug | O/P | Bad, Hin, Mco, Sla, Tgu |
O, oil; P, pollen; Bad, B. adenopoda; Blu, B. lutea; Bmu, B. muricata; Bpu, B. pubipetala; Dbr, D. bracteosa; Hin, H. intermedia; Man, M. anisopetala; Mco, M. cordifolia; Mse, M. sepium; Sla, S. lalandianum; Tgu, T. guilleminiana; Tph, T. phlomoides.

Fig. 3.Epicharis schrottkyi pollinator of Stigmaphyllon lalandianum and of the eglandular morph of Banisteriopsis muricata while collecting (A) oil and (B) pollen by vibration. Scale bars = 5·0 mm.
According to Vogel (1990), Centridini bees are the main pollinators of the New World Malpighiaceae, plants that evolved under the selective impact of these oil‐collecting bees. Vogel (1990) claimed that the role of the Monoeca species in Malpighiaceae pollination was doubtful. In the present study, however, Monoeca bees were pollinators of six species (Table 1); moreover Monoeca sp.3 was the most frequent pollinator of H. intermedia. Monoeca species adopt an appropriate intrafloral posture to pollinate Malpighiaceae, probably because they have tarsal combs located on the ‘inner’ side of their basitarsi, similarly to Centridini bees (see Neff and Simpson, 1981). Based only on putative oil‐collecting organs of female bees, Neff and Simpson (1981) suggested that the Monoeca‐Centridini lineage might represent an early split of generalized oil‐collecting bees that specialized on taxa with epithelial elaiophores such as those found in the modern Malpighiaceae.
Four species of bees, Epicharis affinis (on B. muricata and S. lalandianum), E. flava (on S. lalandianum), E. schrottkyi (on B. adenopoda, B. muricata and S. lalandianum, Fig. 3B) and Monoeca sp.1 (on B. adenopoda), collected pollen by vibration (see Sazima and Sazima, 1989). When collecting pollen, the bees adopted an intrafloral posture similar to that of oil‐collecting, but for their fully extended hindlegs (Fig. 3B; see Sazima and Sazima, 1989, for behavioural details). Pollen‐collecting by vibration generally occurs in flowers with poricidal anthers, although it has been recorded for many non‐poricidal species of diverse angiosperm families (Vogel, 1978; Buchmann, 1985; Oliveira and Sazima, 1990). Buzz‐pollination by Epicharis and Centris species has been reported for various Malpighiaceae species (Sazima and Sazima, 1989; Barros, 1992; Pedro, 1994; Gaglianone, 2000); however, this behaviour is reported here for the first time for the Monoeca species.
Paratetrapedia (Tapinotaspidini) and Tetrapedia (Tetrapedinini) bees collected oil and sometimes pollen. To collect oil, after landing, they crawled on the underside of the flower, where they scraped the oil glands. For pollen‐collecting (18 % of visits), they landed on anthers and stigmas and grasped the filament or style with their mandibles. While they were collecting pollen they could rupture the stigmatic cuticle and deposit small loads of pollen. However, these bees are basically pollen and oil thieves. Vogel (1990) mentioned that Paratetrapedia and Tetrapedia species were found on Malpighiaceae as illegitimate flower visitors, but suggested that they might be legitimate pollinators of small‐flowered genera.
ACKNOWLEDGEMENTS
We thank W. R. Anderson and an anonymous referee for valuable suggestions in the manuscript, H. Dolder for help in scanning electron microscopy, F. L. Modesto for help with the illustrations, I. de F. Bressan for technical assistance, M. C. H. Mamede and S. R. de M. Pedro for taxonomic identification, Núcleo de Apoio à Pesquisa Eletrônica Aplicada à Pesquisa Agropecuária (NAP‐MEPA, Esalq‐USP) for the use of the Zeiss DSM 940A SEM and Fundação José Pedro de Oliveira for providing permission to research in the reserve. Financial support was provided by CNPq. This contribution is part of the first author’s PhD thesis.
APPENDIX.
The following species were recorded and included in this study
| Species | Herbaria numbers |
| Banisteriopsis adenopoda (A. Juss.) B. Gates | UEC 066311, 090721, 090722; CGMS 05986, 05994, 05995 |
| B. lutea (Griseb.) B. Gates | UEC 090557, 090558, CGMS 05988 |
| B. muricata (Cav.) Cuatrec. | UEC 066310, 090560; CGMS 05987 |
| B. pubipetala (A. Juss.) Cuatrec. | UEC 090568 090570, 090708, 090709, 090710, 090726; CGMS 0985 |
| Dicella bracteosa (A. Juss.) Griseb. | UEC 066305, 090719; CGMS 05984, 05993 |
| Heteropterys intermedia Griseb. | UEC 066302, 090555, 090566; CGMS 05983, 05992 |
| Mascagnia anisopetala (A. Juss.) Griseb. | UEC 066309, 090723, 090725; CGMS 05982, 05991 |
| M. cordifolia (A. Juss.) Griseb. | UEC 066308, 090714, 090715; CGMS 05980 |
| M. sepium (A. Juss.) Griseb. | UEC 090711, 090712, 090713; CGMS 05978 |
| Stigmaphyllon lalandianum A. Juss. | UEC 090717, 090718; CGMS 05977, 05989, 05990 |
| Tetrapterys guilleminiana A. Juss. | UEC 066304, 090499, 090720; CGMS 05796 |
| T. phlomoides (Spreng.) Nied. | UEC 066306, 090559; CGMS 05979 |
Received: 8 August 2003; Returned for revision: 19 September 2003; Accepted: 18 February 2004. Published electronically: 11 June 2004
References
- AndersonC.1982. A monograph of the genus Peixotoa (Malpighiaceae). Contributions from the University of Michigan Herbarium 15: 1–92. [Google Scholar]
- AndersonC.1997. Monograph of Stigmaphyllon (Malpighiaceae). Systematic Botany 51: 1–313. [Google Scholar]
- AndersonWR.1979. Floral conservatism in neotropical Malpighiaceae. Biotropica 11: 219–223. [Google Scholar]
- AndersonWR.1980. Cryptic self‐fertilization in Malpighiaceae. Science 207: 892–893. [DOI] [PubMed] [Google Scholar]
- AndersonWR.1981. Malpighiaceae: the botany of Guayana Highland. Part XI. Memoirs of the New York Botanical Garden 32: 21–305. [Google Scholar]
- AndersonWR.1990. The origin of the Malpighiaceae – the evidence from morphology. Memoirs of the New York Botanical Garden 64: 219–224. [Google Scholar]
- BarrosMAG.1992. Fenologia da floração, estratégias reprodutivas e polinização de espécies simpátricas do gênero Byrsonima Rich (Malpighiaceae). Revista Brasileira de Biologia 52: 343–353. [Google Scholar]
- BarrosoGM, Peixoto AL, Ichaso CLF, Costa CG, Guimarães, EF, Lima, HC.1991.Sistemática de angiospermas do Brasil. Vol. 2. Viçosa: UFV Imprensa Universitária. [Google Scholar]
- BawaKS.1974. Breeding systems of tree species of a lowland tropical community. Evolution 28: 85–92. [DOI] [PubMed] [Google Scholar]
- BawaKS, Perry DR, Beach JH.1985. Reproductive biology of tropical lowland rain forest trees. I. Sexual systems and incompatibility mechanisms. American Journal of Botany 72: 331–345. [Google Scholar]
- BuchmannSL.1985. Bees use vibration to aid pollen collecting from non‐poricidal flowers. Journal of the Kansas Entomological Society 58: 517–525. [Google Scholar]
- BuchmannSL.1987. The ecology of oil flowers and their bees. Annual Review of Ecology and Systematics 18: 343–369. [Google Scholar]
- CocucciAA, Holgado AM, Anton AM.1996. Estudio morfológico y anatómico de los eleóforos pedicelados de Dinemandra ericoides, malpigiácea endémica del desierto de Atacama, Chile. Darwiniana 34: 183–192. [Google Scholar]
- DulbergerR, Smith MB, Bawa, KS.1994. The stigmatic orifice in Cassia, Senna, and Chamaecrista (Caesalpiniaceae): morphological variation, function during pollination, and possible adaptative significance. American Journal of Botany 81: 1390–1396. [Google Scholar]
- EndressPK.1994.Diversity and evolutionary biology of flowers. Cambridge: Cambridge University Press. [Google Scholar]
- FigueiredoRA, Sazima M.2000. Pollination biology of Piperaceae species in southeastern Brazil. Annals of Botany 85: 455–460. [Google Scholar]
- FrankieGW, Vinson SB, Williams H.1989. Ecological and evolutionary sorting of 12 sympatric species of Centris bees in Costa Rican dry forest. In: Bock JH, Linhart YB, eds. The evolutionary ecology of plants Boulder: Westview Press, 535–549. [Google Scholar]
- FutuymaDJ.1993.Biologia evolutiva, 2nd edn. Ribeirão Preto: Sociedade Brasileira de Genética/CNPq. [Google Scholar]
- GaglianoneMC.2000. Interações de Epicharis (Apidae, Centridini) e flores de Malpighiaceae em µm ecossistema de cerrado. Anais do Encontro sobre Abelhas 4: 246–252. [Google Scholar]
- GatesB.1982.Banisteriopsis, Diplopterys (Malpighiaceae). Flora Neotropica Monograph 30: 1–237. [Google Scholar]
- GibbsPE, Oliveira PE, Banchi MB.1999. Postzygotic control of selfing in Hymenaea stigonocarpa (Leguminosae – Caesalpinioideae), a bat‐pollinated tree of the Brazilian cerrado. International Journal of Plant Sciences 160: 72–78. [Google Scholar]
- GottsbergerG.1986. Some pollination strategies in neotropical savannas and forests. Plant Systematics and Evolution 152: 29–45. [Google Scholar]
- GrantV.1981.Plant speciation. New York: Columbia University Press. [Google Scholar]
- GribelR, Gibbs PE, Queiroz AL.1999. Flowering phenology and pollination biology of Ceiba pentandra (Bombacaeae) in Central Amazonia. Journal of Tropical Ecology 15: 247–263. [Google Scholar]
- Hauman‐MerckL.1913. Observations sur la pollination d‘une Malpighiacée du genre Stigmaphyllon Recherche de l’Institut Botanique de l’Université de Bruxelles 9: 21–27. [Google Scholar]
- Heslop‐HarrisonJ, Heslop‐Harrison Y.1983. Pollen‐stigma interaction in the Leguminosae: the organization of the stigma in Trifolium pratense L. Annals of Botany 51: 571–583. [Google Scholar]
- Heslop‐HarrisonJ, Heslop‐Harrison Y.1985. Surfaces and secretions in the pollen‐stigma interaction: a brief review. Journal of Cell Science Supplement 2: 287–300. [DOI] [PubMed] [Google Scholar]
- Heslop‐HarrisonY.2000. Control gates and micro‐ecology: the pollen‐stigma interaction in perspective. Annals of Botany Supplement (A) 85: 5–13. [Google Scholar]
- Heslop‐HarrisonY, Shivanna KR.1977. The receptive surface of the angiosperm stigma. Annals of Botany 41: 1233–1258. [Google Scholar]
- Lobreau‐CallenD.1989. Les Malpighiaceae et leurs pollinisateurs. Coadaptation ou coévolution? Bulletin du Museum National d’Histoire Naturelle 11: 79–94. [Google Scholar]
- LordEM, Heslop‐Harrison Y.1984. Pollen‐stigma interaction in the Leguminosae: stigma organization and the breeding system in Vicia faba L. Annals of Botany 54: 827–836. [Google Scholar]
- MartinFW.1959. Staining and observing pollen tubes in the style by means of fluorescence. Stain Technology 34: 125–128. [DOI] [PubMed] [Google Scholar]
- MorellatoLPC, Leitão Filho HF.1995.Ecologia e preservação de uma floresta tropical urbana: Reserva de Santa Genebra. Campinas: Editora da Unicamp. [Google Scholar]
- MorellatoLPC, Leitão Filho HF.1996. Reproductive phenology of climbers in a southeastern Brazilian forest. Biotropica 28: 180–191. [Google Scholar]
- NeffJL, Simpson BB.1981. Oil‐collecting structures in the Anthophoridae (Hymenoptera): morphology, function, and use in systematics. Journal of the Kansas Entomological Society 54: 95–123. [Google Scholar]
- OliveiraPEAM, Sazima M.1990. Pollination biology of two species of Kielmeyera (Guttiferae) from Brazilian vegetation. Plant Systematics and Evolution 172: 35–49. [Google Scholar]
- PedroSRM.1994. Interações entre abelhas e flores em uma área de cerrado no NE do estado de São Paulo: abelhas coletoras de óleo (Hymenoptera: Apoidea: Apidae). Anais do Encontro sobre Abelhas 1: 243–255. [Google Scholar]
- RadfordAE, Dickison WC, Massey JR, Bell CR.1974.Vascular plant systematics. New York: Harper & Row Publishers. [Google Scholar]
- RêgoMAG, Albuquerque PMC.1989. Comportamento das abelhas visitantes de murici, Byrsonima crassifolia (L.) Kunth, Malpighiaceae. Boletim Museu Paraense Emilio Goeldi Nova Serie Zoologica 5: 179–193. [Google Scholar]
- RuizTZ, Arroyo MTK.1978. Plant reproductive ecology of a secondary deciduous tropical forest in Venezuela. Biotropica 10: 221–230. [Google Scholar]
- SazimaM, Sazima I.1989. Oil‐gathering bees visit flowers of eglandular morphs of the oil‐producing Malpighiaceae. Botanica Acta 102: 106–111. [Google Scholar]
- SeaveySR, Bawa KS.1986. Late‐acting self‐incompatibility in angiosperms. Botanical Review 52: 195–219. [Google Scholar]
- SimpsonBB.1989. Pollination biology and taxonomy of Dinamandra and Dinemagonum (Malpighiaceae). Systematic Botany 14: 408–426. [Google Scholar]
- SimpsonBB, Neff JL.1981. Floral rewards: alternatives to pollen and nectar. Annals of the Missouri Botanical Garden 68: 301–322. [Google Scholar]
- SteinerKE.1985. Functional dioicism in the Malpighiaceae: Spachea membranacea Cuatr. American Journal of Botany 72: 1537–1543. [Google Scholar]
- SubramanianRB, Arumugasamy K, Inamdar JA.1990. Studies in the secretory gland of Hiptage sericea (Malpighiaceae). Nordic Journal of Botany 10: 57–62. [Google Scholar]
- TeixeiraLAG, Machado IC.2000. Sistema de polinização e reprodução de Byrsonima sericea DC (Malpighiaceae). Acta Botanica Brasilica 14: 347–357. [Google Scholar]
- VinsonSB, Williams HJ, Frankie GW, Shrum G.1997. Floral lipid chemistry of Byrsonima crassifolia (Malpighiaceae) and a use of floral lipids by Centris bees (Hymenoptera: Apidae). Biotropica 29: 76–83. [Google Scholar]
- VogelS.1974. Ölblumen und Ölsammelnde Bienen. Tropische und Subtropische Pflanzenwelt 7: 285–547. [Google Scholar]
- VogelS.1978. Evolutionary shifts from reward to deception in pollen flowers. Linnean Society Symposium Series 6: 89–96. [Google Scholar]
- VogelS.1988. Die Ölblumen‐Symbiosen – Parallelismus und andere Aspekte ihrer Entwicklung in Raum und Zeit. Zeitschrift fuer Zoologische Systematik und Evolutionsforschung 26: 341–362. [Google Scholar]
- VogelS.1990. History of the Malpighiaceae in the light of pollination ecology. Memoirs of the New York Botanical Garden 55: 130–142. [Google Scholar]
- WebbCJ, Lloyd DG.1986. The avoidance of interference between the presentation of pollen and stigmas in angiosperms. II. Herkogamy. New Zealand Journal of Botany 24: 163–178. [Google Scholar]


